Abstract
In semen, the gel proteins SgI and SgII (semenogelins I and II) are digested by PSA (prostate-specific antigen), resulting in liquefaction and release of motile spermatozoa. Semen contains a high concentration of Zn2+, which is known to inhibit the protease activity of PSA. We characterized the binding of Zn2+ to SgI and SgII and found evidence that these proteins are involved in regulating the activity of PSA. Intact SgI and SgII and synthetic semenogelin peptides were used in the experiments. Binding of Zn2+ was studied by radioligand blotting, titration with a zinc (II) fluorophore chelator and NMR analysis. A chromogenic substrate was used to measure the enzymatic activity of PSA. SgI and SgII bound Zn2+ with a stoichiometry of at least 10 mol (mol of protein)−1 and with an average dissociation constant of approx. 5 μM per site. Moreover, Zn2+-inhibited PSA was activated by exposure to SgI or SgII. Since both proteins have high affinity for Zn2+ and are the dominating proteins in semen, they probably represent the major Zn2+ binders in semen, one function of which may be to regulate the activity of PSA. The system is self-regulating, and PSA is maintained in an active state by its substrate.
Keywords: kallikrein, prostate-specific antigen (PSA), reproduction, semenogelin, seminal plasma, zinc
Abbreviations: PSA, prostate-specific antigen; SgI, semenogelin I; PSgI, peptide derived from SgI
INTRODUCTION
SgI and SgII (semenogelins I and II) are secreted by the seminal vesicles and constitute the major proteins in human semen. After ejaculation, SgI, SgII and fibronectin aggregate to form a gelatinous mass that is liquefied within 5–20 min which releases the trapped spermatozoa. Liquefaction occurs through cleavage of the semenogelins by PSA (prostate-specific antigen) [1–4]. SgI and SgII are encoded by two different genes located 11.5 kbp apart on the long arm of chromosome 20 [5]. These proteins have 80% similarity in amino acid sequence and contain similar repeats of 60 amino acid residues. SgI (50 kDa) has six such repeats and SgII (63 kDa), which also exists in a glycosylated form, contains eight such repeats [6]. In addition to participating in gel formation, SgI and SgII can serve as substrates for transglutaminase and activators of sperm hyaluronidase. The latter function suggests that these proteins are involved in the degradation of the envelope around the ovum during sperm penetration (fertilization) [7,8]. Most of the literature on the semenogelins has discussed their role in reproduction. However, these proteins have now been found in tissues that are not related to the reproductive organs (e.g. kidney, trachea and the gastro-intestinal tract), although it is not yet clear what functions they perform in those locations [9].
In semen, the semenogelins are the main substrates for PSA, which is a 33 kDa glycoprotein secreted by the prostate. PSA is a member of the human kallikrein family and the dominating serine protease in semen. It has chymotrypsin-like specificity [2,3], and its activity is inhibited by Zn2+ [10].
Zn2+ has several biological functions that are regulatory, structural and catalytic in nature, as illustrated, respectively, by the role of this metal ion in neurotransmission [11], the many DNA-binding proteins that contain zinc fingers [12] and alcohol dehydrogenase [13]. The mean concentration (S.D.) of Zn2+ in seminal plasma is 2 (1.2) mM [14], which is approx. 100 times higher than the reference interval in blood plasma (10–18 μM) [15]. The role for the high Zn2+ level in seminal plasma has not been satisfactorily explained. Several studies have attempted to correlate Zn2+ concentration in semen with fertility and various characteristics of the spermatozoa, but the results are inconclusive [16]. The main fraction of Zn2+ in semen originates from the prostate and appears in a low-molecular-mass form in complex with citrate in the prostate secretion [17]. However, in the mixture of the secretions from the prostate and seminal vesicles, Zn2+ is found in a high- or intermediate-molecular-mass form. This difference indicates that Zn2+ is transferred from a low-molecular-mass ligand to a high-molecular-mass ligand that has greater affinity for this ion [18]. Analysis of secretion from the seminal vesicles has indicated that Zn2+ is bound by proteins with molecular masses of 14–70 kDa; during liquefaction, the fraction of Zn2+ bound to the proteins with a higher molecular mass decreases rapidly, and after 15 min, the Zn2+ is only bound to proteins of <25 kDa [19]. Interestingly, these molecular-mass characteristics of Zn2+-binding proteins match with those in the intact and PSA-digested forms of SgI and SgII, an observation that requires further investigation.
In the present study, we characterized the Zn2+-binding properties of SgI and SgII. Moreover, we propose that the semenogelins help to regulate the activity of PSA by controlling the concentration of free Zn2+.
EXPERIMENTAL
Materials
Human semen specimens were collected from healthy volunteer sperm donors (through masturbation) at the fertility laboratory (Malmö University Hospital, Malmö, Sweden). Sample collection and purification of SgI and SgII were performed essentially as previously described by Malm et al. [4], but, instead of alkylating the proteins, a reducing agent (30 mM dithiothreitol) was included in all buffers during purification. Liquefaction of the seminal plasma was achieved by keeping the samples of ejaculate at room temperature (∼22 °C) for at least 1 h, after which the spermatozoa were removed by centrifugation, and the samples were stored at −70 °C. The concentrations of the purified proteins were calculated from amino acid analysis after performing acid hydrolysis (24 h in 6 M HCl at 110 °C in vacuo) with a Beckman 6300 amino acid analyser. Cleavage of purified semenogelins by PSA was accomplished as described by Malm et al. [10].
Peptides
A Milligen 9050 Plus synthesizer (Applied Biosystems, Foster City, CA, U.S.A.) and standard Fmoc (9-fluorenylmethyloxycarbonyl) chemistry were used to generate semenogelin peptides, which were subsequently purified by reversed-phase HPLC. Five peptides were derived from SgI: PSgI(28–37) (QKGKQQTESK), PSgI(99–113) (HFHRVVIHHKGGKAH), PSgI(190–199) (SHQNKGHYQN), PSgI(410–439) (LDIVIIEQEDDSDRHLAQHLNNDRNPLFT) and PSgI(421–430) (DSDRHLAQHL). Four peptides representing SgII were synthesized: PSgII(28–37) (QKDQQHTKSK), PSgII(99–113) (HFHMIVIHHKGGQAH), PSgII(530–559) (SHNIVITEHEVAQDDHLTQQYNEDRNPIST) and PSgII(541–550) (AQDDHLTQQY).
65Zn2+ blotting
Semenogelins (intact proteins and fragments) were subjected to SDS/PAGE (12% gel) using a Mini PROTEAN II system (Bio-Rad Laboratories, Hercules, CA, U.S.A.) under reducing conditions. The proteins were subsequently electroblotted on to a PVDF membrane (Millipore, Bedford, MA, U.S.A.) and visualized by staining with Coomassie Brilliant Blue R250. For the radioligand blotting, the membrane was incubated for 1 h at room temperature with 10 mM Tris/HCl (pH 7.5). Thereafter the buffer was changed to TK buffer [10 mM Tris/HCl (pH 7.5) and 100 mM KCl] containing 65Zn (0.2 μCi 65ZnCl2/ml with specific radioactivity 6.65 Ci/mmol; PerkinElmer LifeSciences, Boston, MA, U.S.A.) and the membrane was incubated for 15 min at room temperature. Next, the membrane was washed twice for 15 min in TK buffer without adding Zn2+ and then dried and scanned using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA, U.S.A.).
Chelator experiments
The fluorescence of the Zn2+-specific chelator Fluo-Zin 2 (Molecular Probes, Eugene, OR, U.S.A.) was recorded using a Fluoro-Max-3 spectrometer (Jobin Yvon, Edison, NJ, U.S.A.) and excitation and emission wavelengths of 490 and 523 nm respectively. The starting volume for the titrations was 800 μl. The storage buffer for SgI and SgII was diluted by a factor of at least 1000 when using a Centricon 30YM filtration unit (Millipore) to change the buffer to 20 mM Hepes (pH 7.0) containing 2 M urea. Thereafter the proteins were diluted to appropriate concentrations for each titration curve. The Fluo-Zin 2 chelator was dissolved according to the manufacturer's instructions to give a final concentration of 15 μM in the starting solution. A 1.6 μl sample of 500 μM zinc acetate in 20 mM Hepes (pH 7.0) was added at each step of the titration, and the fluorescence was recorded. This was repeated 15 times, resulting in a final Zn2+ concentration of approx. 15 μM, equimolar to the Zn2+ chelator. The titrations were repeated with different concentrations of SgI, SgII and peptides. The urea concentration in the cuvette was kept at 50 mM for SgI and 70 mM for SgII, and the peptides were measured in a corresponding buffer without urea. The stoichiometry was estimated by using Caligator software [20]. This software uses a Levenberg–Marquardt non-linear fitting routine to find the best-matched curve for the registered data, and it compensates for the change in volume and concentrations caused by the addition of samples.
NMR analysis
1H NMR spectra were recorded on a Varian Inova Unity Plus spectrometer, operating at 600 MHz using a 1.4 s solvent presaturation pulse. Each peptide (100 μM) was dissolved in 2 mM sodium acetate buffer in 2H2O (pH 6.5), and Zn2+ was added in steps of 50 μM, and an NMR spectrum was recorded at each titration point.
Gel filtration
All peptides were analysed by gel filtration using two serially coupled 5 ml Sephadex G25 Superfine columns (Amersham Biosciences, Uppsala, Sweden) connected to a Bio Logic HR FLPC system (Bio-Rad Laboratories). Each peptide (300 μl, concentration 200 μM) was applied to the columns that had been equilibrated with 2 mM sodium acetate (pH 6.5) and 100 mM NaCl, with or without the addition of 0.2 mM zinc acetate. The flow rate was 2 ml/min, and the absorbance was monitored at 214 nm.
PSA activation
The proteolytic activity of PSA was studied using the synthetic substrate MeO-Suc-Arg-Pro-Tyr-p-nitroanilide (S-2586; Chromogenix, Milano, Italy) at a final concentration of 1 mM in the presence of 40 μM zinc acetate and intact SgI (0.05–3.2 μM), intact SgII (0.05–3.2 μM), semenogelin peptides (12.5–200 μM) or EGTA (5-40 μM). The samples were preincubated for 2 min in Linbro/Titrek EIA microtitre plate wells, after which cleavage was initiated by adding PSA (final concentration of 0.65 μM). Formation of p-nitroaniline was recorded for 15 min at 37 °C using a microplate reader (Molecular Devices, Sunnyvale, CA, U.S.A.). The final volume was 100 μl and the cleavage buffer (pH 7.5) comprised 50 mM Tris, 150 mM NaCl and 200 mM urea.
RESULTS
65Zn2+ blotting
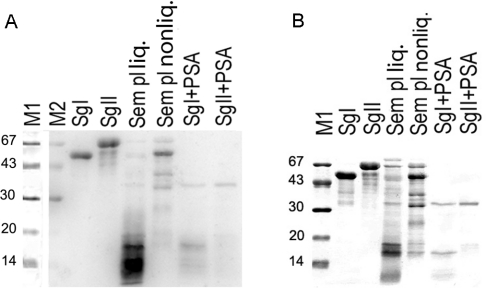
Autoradiography showed that 65Zn2+ was bound by purified intact SgI and SgII subjected to gel electrophoresis and blotted on to a PVDF membrane (Figure 1). Bands at corresponding positions were observed for non-liquefied seminal plasma. Digestion of the purified intact SgI and SgII by PSA resulted in the disappearance of the original bands and appearance of a broad faint smear at 6–20 kDa indicating that the proteolytic fragments of SgI and SgII bind Zn2+; a similar pattern was seen for the liquefied seminal plasma. The components that bound Zn2+ were found in the low-molecular-mass area, and there were no visible bands with mobility corresponding to intact SgI and SgII. The known Zn2+-binding protein PSA was clearly visible as a band at 30 kDa in the lanes representing PSA-cleaved purified SgI and SgII.
Figure 1. Purified semenogelin proteins (SgI and SgII), and seminal plasma separated by SDS/PAGE (12% gel) and (A) visualized by radio-ligand blotting (65ZnCl2) and (B) staining with Coomassie Blue.
M1, low-molecular-mass standard stained with Coomassie Blue; M2, low-molecular-mass standard as a part of the radio-ligand blot; SgI, 5 μg of purifed SgI; SgII, 5 μg of purifed SgII; Sem pl liq., 0.4 μl of liquefied seminal plasma; Sem pl nonliq., 0.04 μl of non-liquefied seminal plasma; SgI+PSA, 5 μg of SgI digested by PSA; SgII+PSA, 5 μg of SgII digested by PSA.
Chelator study of Zn2+ binding to SgI and SgII
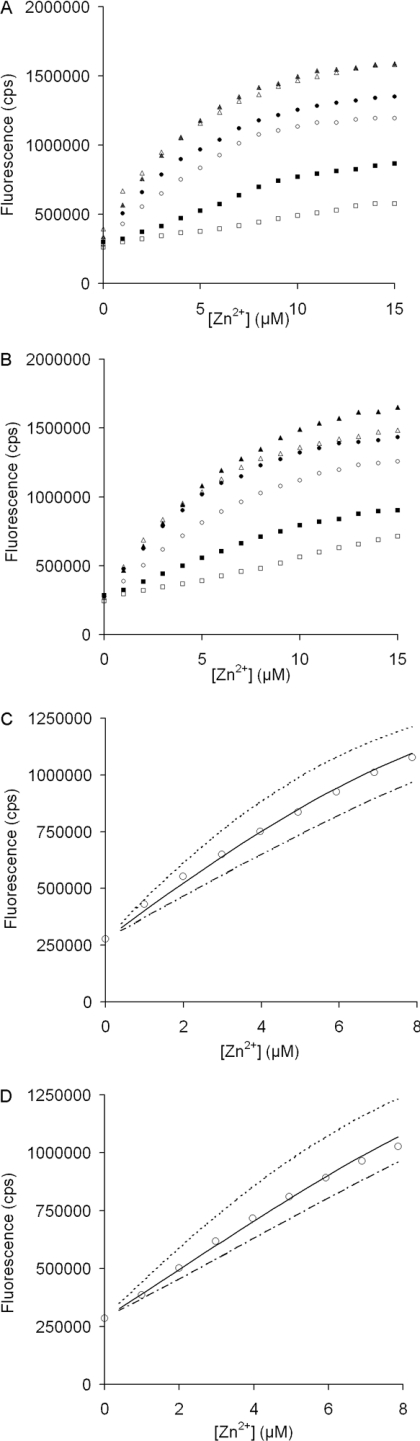
The zinc(II) fluorophore chelator FluoZin-2 was used to quantify the specific binding of Zn2+ to intact SgI and SgII. Fluorescence was recorded during titrations of FluoZin-2 with Zn2+ in the absence or presence of SgI or SgII. The results indicated that the higher the concentration of SgI or SgII in the titration, the more Zn2+ was needed to saturate FluoZin-2 and the lower the slope of fluorescence plotted versus Zn2+ concentration (Figure 2). This observation indicates that there was competition for Zn2+ between the semenogelins and the chelator, which would have occurred only if the proteins and the chelator have similar affinity for Zn2+. However, due to precipitation of the protein at high Zn2+ concentration, it was not possible to obtain complete titration curves, which are required to accurately determine the Zn2+-dissociation constant (KD) for each protein. Nonetheless, the linear appearance of the curves for SgI and SgII suggests that both proteins have a KD similar to that of the chelator for Zn2+ (i.e. ∼5 μM) [21]. The first nine to ten titration data points were analysed with the Caligator software, to estimate the stoichiometry of Zn2+ binding for SgI and SgII, assuming that the change in fluorescence between free and Zn2+-bound chelator was the same in the presence and absence of the proteins. Only a lower limit of the stoichiometry could be determined, which was similar for the two proteins and was approximated to at least 10 mol of Zn2+ (mol of protein)−1. After changing from the storage buffer, the protein–chelator solutions could have contained a maximum concentration of 1 μM EDTA or EGTA. However, Zn2+ titrations of 15 μM FluoZin-2 in the presence of 1 μM EDTA or EGTA gave similar results as provided by titrations performed with FluoZin-2 alone (results not shown). Thus Zn2+ binding to EDTA or EGTA was not detected at these levels. Binding between the chelator and the protein could alter their affinity for Zn2+. Therefore, in the presence of 1 mM EDTA, we recorded the fluorescence of 15 μM chelator and 0.30 μM SgI separately and after mixing, and the procedure was repeated for 0.28 μM SgII. The fluorescence responses were similar for the different setups, which indicates that there was no provable binding between the chelator and the intact proteins.
Figure 2. Fluorescence of 15 μM FluoZin-2 titrated with Zn2+ in the presence of different amounts of intact semenogelins.
(A) Zn2+ titrations with the following concentrations of SgI: 0 (▲), 0.075 (△), 0.15 (●), 0.30 (○), 0.60 (■) and 1.2 (□) μM. (B) Zn2+ titrations with the following concentrations of SgII: 0 (▲), 0.074 (△), 0.14 (●), 0.28 (○), 0.56 (■) and 1.1 (□), μM. (C) Computer fitting to the first nine steps in the 0.30 μM SgI Zn2+-titration. With a KD of approx. 5 μM, the three fitting curves represent a stoichiometry of 1 (…), 10 (−) and 20 (---). (D) Computer fitting to the first nine steps in the 0.28 μM SgII Zn2+-titration. With a KD of approx. 5 μM, the three fitting lines represent a stoichiometry of 1 (…), 12 (−) and 20 (---).
Chelator study of Zn2+ binding to the peptides
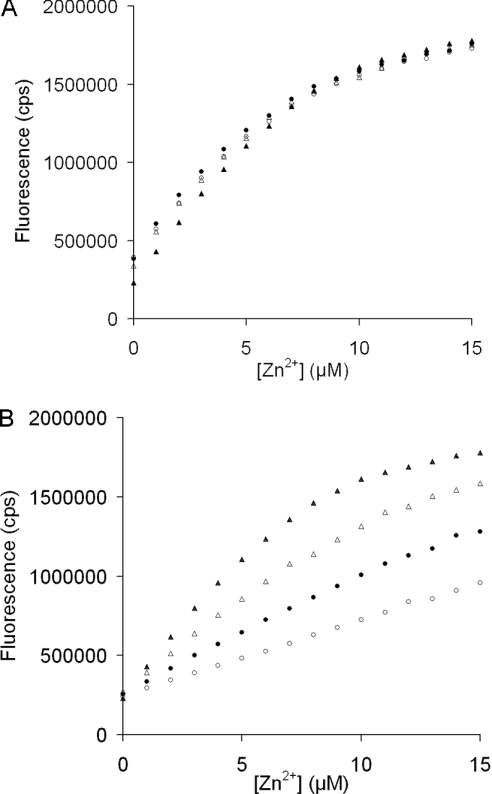
The chelator method was used to identify which of the peptides corresponding to different parts of SgI and SgII were capable of binding Zn2+. The presence of PSgI(28–37) did not change the fluorescence response during a Zn2+ titration and produced curves identical to those obtained with FluoZin-2 alone (Figure 3). This pattern was typical for the non-Zn2+-binding peptides, which should not have competed with the Zn2+ chelator. However, several peptides markedly affected the fluorescence response, as exemplified by PSgI(421–430) (Figure 3). Stoichiometry for Zn2+ binding to these peptides could not be ascertained with sufficient certainty, but the shape of the titration curves enabled a rough estimation of the KD values (Table 1).
Figure 3. Fluorescence of 15 μM FluoZin-2 titrated with Zn2+ in the presence of (A) PSgI(28–37) and (B) PSgI(421–430).
The peptides were used at concentrations of 0 (▲), 8 (△), 16 (●) and 32 (○) μM.
Table 1. Zn2+ binding characteristics of the semenogelin peptides.
| Peptide | His* | Glu* | Asp* | Gln* | KD (μM)† | NMR spectroscopy‡ | Gel filtration§ Difference in elution volume | Change of peak shape |
|---|---|---|---|---|---|---|---|---|
| PSgI(28–37) | 0 | 1 | 0 | 3 | n.d. | − | 0.17 | − |
| PSgI(99–113) | 5 | 0 | 0 | 0 | ∼5 | + | 3.6∥ | + |
| PSgI(190–199) | 2 | 0 | 0 | 2 | >5 | + | 0.18 | − |
| PSgI(410–439) | 2 | 2 | 5 | 2 | ∼5 | + | I¶ | I¶ |
| PSgI(421–430) | 2 | 0 | 2 | 1 | ∼5 | + | 2.6 | + |
| PSgII(28–37) | 1 | 0 | 1 | 3 | n.d. | − | 0.0 | − |
| PSgII(99–113) | 5 | 0 | 0 | 1 | ∼5 | + | −0.22∥ | + |
| PSgII(530–559) | 3 | 3 | 3 | 3 | ≫5 | + | 0.10 | + |
| PSgII(541–550) | 1 | 0 | 2 | 3 | ≫5 | + | 0.18 | − |
* Results indicate number of residues in each peptide.
† Dissociation constant (KD) for Zn2+ approximated from the chelator experiments; n.d., Zn2+ binding not detected.
‡ The Zn2+ binding capacity was scored as undetectable (−) or detectable (+) based on NMR results.
§ All peptides were subjected to gel filtration with and without added Zn2+, and the elution volume (ml) for each peptide without Zn2+ was subtracted from the elution volume with Zn2+. The change in shape of the elution peak in the presence of Zn2+ is classified as undetectable (−) or detectable (+).
∥ The area under the elution peak was significantly reduced in the presence of Zn2+ when compared with the absence of Zn2+.
¶I indicates peptide insoluble under the gel-filtration conditions.
NMR analysis
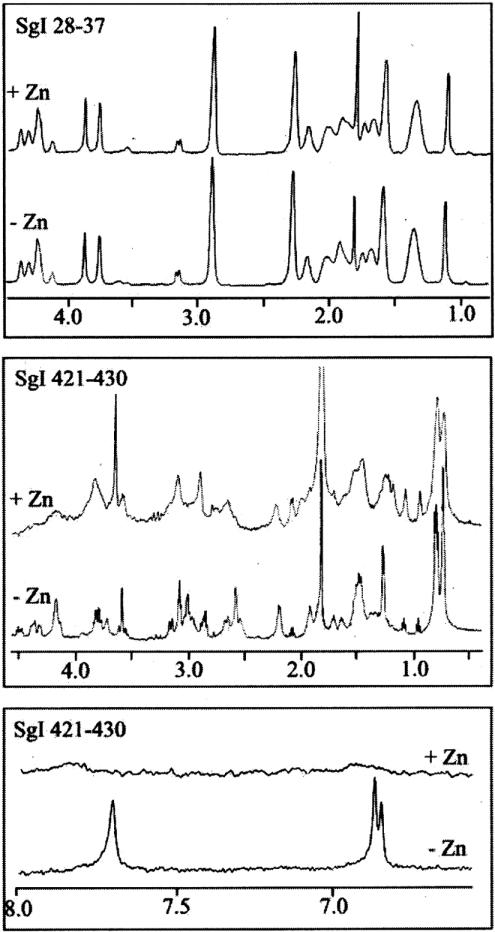
The binding of Zn2+ to semenogelin peptides was also studied by 1H NMR spectroscopy. Spectra were recorded for each peptide in the presence of 0, 50, 100 and 150 μM Zn2+. Interpretation of the spectra indicated that all the peptides except PSgI(28–37) and PSgII(28–37) were capable of binding Zn2+ (Table 1). A typical spectrum for a Zn2+-binding peptide is illustrated for PSgI(421–430) in Figure 4. It can be seen that exposure to Zn2+ resulted in a change in chemical shift, amplitude and line width of the resonance peaks in the area around histidine residue (7–8 p.p.m.). The presence of Zn2+ also caused significant changes in the spectral region corresponding to α-protons and aliphatic side chains (0–5 p.p.m.), but it did not induce any spectral changes for PSgI(28–37) (Figure 4), which was thus considered to be a non-Zn2+-binding peptide. Addition of Zn2+ led to precipitation of PSgI(410−439).
Figure 4. 1H NMR spectra of PSgI(28–37) and PSgI(421–430) in the presence and absence of Zn2+.
Gel filtration
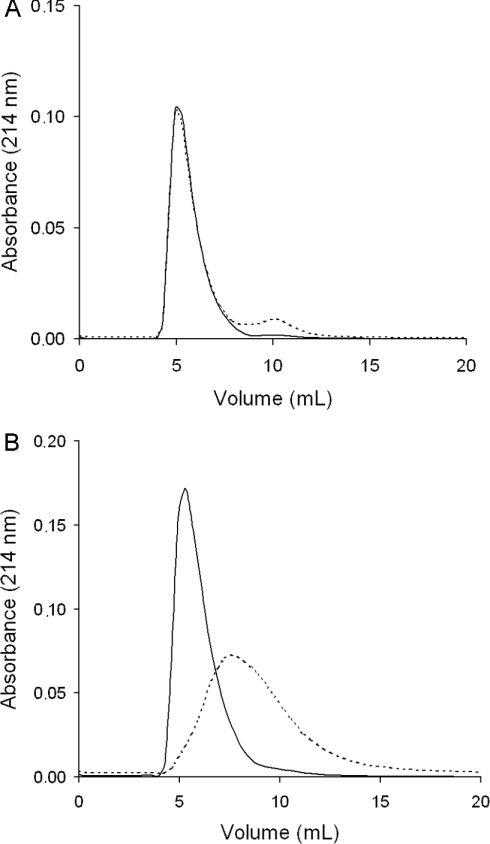
Experiments performed to elucidate the impact of Zn2+ on the elution volumes of the peptides in gel filtration revealed a typical pattern for the Zn2+-binding peptides, which is exemplified by PSgI(421–430) (Figure 5). The pattern induced by exposure to Zn2+ included an increased elution volume and a more heterogeneous peak. As expected, the chromatogram of the non-Zn2+-binding peptide PSgI(28–37) was not affected by the presence of Zn2+. The changes in elution pattern caused by Zn2+ are summarized for all peptides in Table 1. For PSgI(99–113) and PSgII-(99–113), Zn2+ significantly decreased the area under the elution peak. Accordingly, we compared absorbance spectra of those two peptides with and without added Zn2+ present, which was recorded at 200–300 nm. The spectra were not different, hence it appears that precipitation caused the loss of material in the chromatography system for PSgI(99–113) and PSgII(99–113).
Figure 5. Gel filtration of the semenogelin peptides PSgI(28–37) (A) and PSgI(421–430) (B) in the presence (---) and absence (−) of Zn2+.
PSA activation
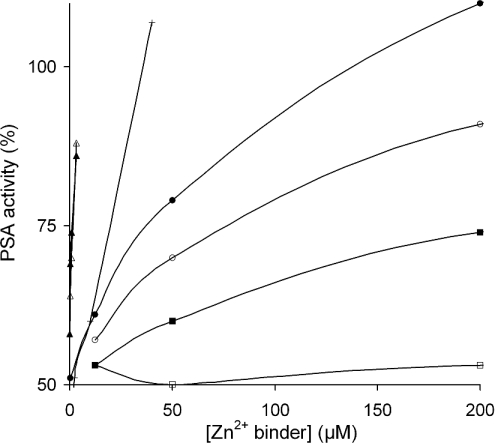
The semenogelins bind substantial amounts of Zn2+ in semen, and they are thereby involved in controlling the levels of free Zn2+. Consequently, SgI and SgII have the potential to indirectly modulate the enzymatic activity of PSA because that protease is inhibited by Zn2+. In the light of this knowledge, we studied the ability of SgI, SgII and the peptides to activate PSA in the presence of Zn2+. A chromogenic substrate was employed to measure the activity of PSA, and since SgI and SgII are also substrates for PSA, the chromogenic substrate was used at a concentration 1000 times higher than that of semenogelins. SgI and SgII were found to be similar regarding their capacity to activate PSA, which indicates that both proteins can bind Zn2+ equally well (Figure 6). The peptides differed significantly in terms of their ability to activate PSA, and as expected, the non-Zn2+-binding peptide PSgI(28–37) did not activate PSA at all. EGTA was included as a positive control.
Figure 6. Activation of Zn2+-inhibited PSA by the addition of increasing amounts of different Zn2+ binders.
The activity was studied using a synthetic chromogenic substrate for PSA. The following Zn2+ binders were used: SgI (▲), SgII (△), EGTA (+), PSgI(99–113) (●), PSgI(421–430) (○), PSgI(190–199) (■) and PSgI(28–37) (□).
DISCUSSION
Our results clearly show that SgI and SgII bind Zn2+ with high affinity at several sites, and that this ability allows the two proteins to regulate the activity of PSA. SgI, SgII and fragments thereof were identified as Zn2+ binders by three complementary methods: 65Zn2+ blotting, competition using a Zn2+-specific fluorescent chelator as a competitor for Zn2+ and NMR spectroscopy. Use of the chelator made it possible to estimate the KD and stoichiometry for the intact proteins. Both intact and PSA-cleaved purified SgI and SgII were included in the 65Zn2+ blot, and the Zn2+-binding patterns that were observed corresponded to those of non-liquefied and liquefied seminal plasma. Thus, the most abundant proteins in seminal vesicle secretion, SgI and SgII, were recognized as Zn2+ binders.
As gel-forming proteins, the semenogelins have an inherent ability to aggregate and form macrocomplexes. One of the aggregation mechanisms depends on the formation of disulphide links [22]. Therefore Zn2+ blotting was performed under reduced conditions enabling the proteins to enter the gel and produce distinct bands. In addition, the semenogelins are not stable under physiological conditions. The most effective stabilizing agent in that context is urea. Therefore we included that compound in the chelator study of the intact proteins, but kept the concentration as low as possible (50–70 mM). At an indicated level of urea, the semenogelins are known to function as substrates for both PSA and transglutaminase [7,10]. However, peptides representing parts of SgI and SgII also proved to bind Zn2+, and could be studied in a more physiological environment without urea. The observation of Zn2+ binding to reduced and SDS-denatured semenogelins by 65Zn2+ blotting as well as to semenogelins and peptide fragments in a more native state with the chelator study indicates that the tertiary structure is not of major importance for Zn2+ binding.
NMR analysis of the peptides identified the same Zn2+ binders and non-binders as in the chelator study. The NMR results also show that the presence of histidine or glutamic residue in the sequence does not guarantee Zn2+ binding, and many proteins contain histidine residues without being known as Zn2+ binders. Apparently, the sequence of a peptide has to allow folding around the Zn2+ ion to provide several ligands, or the peptide–peptide repulsion has to be small enough to ensure that Zn2+ has access to ligands on more than one peptide molecule. The design of the present study does not enable a characterization of individual Zn2+-binding sites in the semenogelins. However, it is known that Zn2+ is often co-ordinated by four ligands. Each of the amino acids histidine, glutamic acid, aspartic acid and cysteine has the potential to act as a ligand [13]. The most distinctive feature of the Zn2+-binding peptides, we studied, was the abundance of histidine (Table 1), and NMR spectra revealed pronounced changes in the region around this amino acid. Furthermore, SgI and SgII contain numerous histidine residues (∼7%) that are fairly evenly distributed along their primary structures. Thus, it seems that histidine is an important component of the Zn2+-binding sites and the abundance of histidine residues in the intact proteins agrees well with the estimated stoichiometry of at least 10 mol of Zn2+·(mol of protein)−1.
In the gel filtration experiments, Zn2+ increased the elution volumes for some of the peptides, which suggests that binding of this metal ion structurally alters the molecules so that they have a smaller Stokes' radius. This can be regarded as indirect proof of Zn2+ binding and supports the results of the chelator experiments and the NMR analysis. However, it was not possible to ascertain whether Zn2+ affects the structure of intact SgI and SgII, because the proteins precipitated extensively after the addition of increasing amounts of Zn2+. Indeed, it was the problem with precipitations and the instability of the intact semenogelins under physiological conditions that made it necessary to use the peptides in the gel filtration. Notwithstanding, addition of Zn2+ also caused three of the peptides with high affinity for Zn2+ (see Table 1) to precipitate to different extents. A plausible explanation for this observation is that Zn2+ induces the semenogelin molecules to bind to each other to form insoluble multimeric complexes. Such binding could occur if two or more proteins contribute to a common Zn2+ site, or if Zn2+ binding simply favours a structure in the proteins and/or peptides that makes them more prone to aggregate.
The results discussed so far clearly show that SgI and SgII have the capacity to bind Zn2+ in vitro. Two important questions are whether Zn2+ binding occurs with similar affinity and stoichio- metry in vivo and, if so, whether such binding has biological implications. The pH and total salt concentration under which Zn2+ binding was confirmed do not differ much from conditions in vivo. Furthermore, the peptides representing parts of the intact protein were able to bind Zn2+ under more physiological conditions, without urea. Concentrations of Zn2+, SgI and SgII are higher in seminal plasma when compared with most of the other fluids and tissues. A physiological SgI concentration of 50 mg/ml [4] corresponds to 1 mM. Thus, if we suppose that each SgI molecule contains at least ten Zn2+-binding sites, each with an average KD value of approx. 5 μM, then SgI alone would have the capacity to capture 10 mM Zn2+ (five times the actual Zn2+ concentration in seminal plasma). The average KD per Zn2+ site is approximately the same for the semenogelins and PSA [10], whereas SgI and SgII have a molar excess (by a factor of 160) of Zn2+ sites when compared with PSA. This high Zn2+-binding capacity of the semenogelins in seminal plasma, and the finding that these proteins can activate Zn2+-inhibited PSA in vitro support the hypothesis that SgI and SgII modulate PSA activity by controlling the level of free Zn2+. PSA must be able to exert its protease effects to cleave the semenogelins and thereby dissolve the coagulum formed after ejaculation to release the trapped spermatozoa so that they can reach the ovum. The crystal structure of stallion seminal plasma PSA, which shares extensive sequence similarity with human PSA, was recently reported [23]. This structure contains a Zn2+ ion that is co-ordinated by Asp-91, His-101 and His-234 near the catalytic triad residues and the entrance to the specificity pocket is blocked by the kallikrein loop [23]. It is thus possible that chelation of this inhibitory Zn2+ ion by the semenogelins triggers a conformational transition which facilitates the substrate access to the catalytic site. The fact that PSA is maintained in an active state by its substrates is an intriguing example of a self-limited system. Initially, the major Zn2+-binding proteins in semen, SgI and SgII, have the capacity to keep the level of free Zn2+ low enough to avoid inhibition of PSA. However, since SgI and SgII are continually broken down into smaller fragments, it is easy to imagine that the number of Zn2+ sites gradually decreases, which in turn increases the availability of Zn2+ for PSA and eventually more or less inhibits the protease.
In conclusion, we have found that Zn2+ is bound by SgI, SgII and fragments thereof. The intact semenogelins have the same KD (∼5 μM) and stoichiometry [>10 mol of Zn2+·(mol of protein)−1]. The abundancy of these proteins in semen and their high Zn2+-binding capacity suggest that SgI and SgII are responsible for the largest proportion of Zn2+ binding in semen. Moreover, we propose that the Zn2+-binding sites in SgI and SgII play a physiological role in regulating the enzymatic activity of PSA.
References
- 1.Lilja H., Oldbring J., Rannevik G., Laurell C. B. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J. Clin. Invest. 1987;80:281–285. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lilja H., Abrahamsson P. A., Lundwall A. Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J. Biol. Chem. 1989;264:1894–1900. [PubMed] [Google Scholar]
- 3.Robert M., Gibbs B. F., Jacobson E., Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physiological substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry. 1997;36:3811–3819. doi: 10.1021/bi9626158. [DOI] [PubMed] [Google Scholar]
- 4.Malm J., Hellman J., Magnusson H., Laurell C. B., Lilja H. Isolation and characterization of the major gel proteins in human semen, semenogelin I and semenogelin II. Eur. J. Biochem. 1996;238:48–53. doi: 10.1111/j.1432-1033.1996.0048q.x. [DOI] [PubMed] [Google Scholar]
- 5.Ulvsback M., Lazure C., Lilja H., Spurr N. K., Rao V. V., Loffler C., Hansmann I., Lundwall A. Gene structure of semenogelin I and II. The predominant proteins in human semen are encoded by two homologous genes on chromosome 20. J. Biol. Chem. 1992;267:18080–18084. [PubMed] [Google Scholar]
- 6.Lilja H., Lundwall A. Molecular cloning of epididymal and seminal vesicular transcripts encoding a semenogelin-related protein. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4559–4563. doi: 10.1073/pnas.89.10.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peter A., Lilja H., Lundwall A., Malm J. Semenogelin I and semenogelin II, the major gel-forming proteins in human semen, are substrates for transglutaminase. Eur. J. Biochem. 1998;252:216–221. doi: 10.1046/j.1432-1327.1998.2520216.x. [DOI] [PubMed] [Google Scholar]
- 8.Robert M., Gagnon C. Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein. Cell. Mol. Life Sci. 1999;55:944–960. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundwall A., Bjartell A., Olsson A. Y., Malm J. Semenogelins I and II, the predominant human seminal plasma proteins, are also expressed in non-genital tissues. Mol. Hum. Reprod. 2002;8:805–810. doi: 10.1093/molehr/8.9.805. [DOI] [PubMed] [Google Scholar]
- 10.Malm J., Hellman J., Hogg P., Lilja H. Enzymatic action of prostate-specific antigen (PSA or hK3): substrate specificity and regulation by Zn(2+), a tight-binding inhibitor. Prostate. 2000;45:132–139. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Frederickson C. J., Suh S. W., Silva D., Thompson R. B. Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 12.Hanas J. S., Hazuda D. J., Bogenhagen D. F., Wu F. Y., Wu C. W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J. Biol. Chem. 1983;258:14120–14125. [PubMed] [Google Scholar]
- 13.Auld D. S. Zinc coordination sphere in biochemical zinc sites. Biometals. 2001;14:271–313. doi: 10.1023/a:1012976615056. [DOI] [PubMed] [Google Scholar]
- 14.Arver S., Sjoberg H. E. Calcium fractions in seminal plasma and functional properties of human spermatozoa. Acta Physiol. Scand. 1982;116:159–165. doi: 10.1111/j.1748-1716.1982.tb07125.x. [DOI] [PubMed] [Google Scholar]
- 15.Burtis C. A., Aschwood E. R. Philadelphia: W. B. Saunders Company; 1999. Tietz Textbook of Clinical Chemistry. [Google Scholar]
- 16.Shivaji S., Scheit K.-H., Bhargava P. M. New York: John Wiley & Sons; 1990. Proteins of Seminal Plasma and Secretions of the Male Reproductive Tract. [Google Scholar]
- 17.Arver S. Zinc and zinc ligands in human seminal plasma. III. The principal low molecular weight zinc ligand in prostatic secretion and seminal plasma. Acta Physiol. Scand. 1982;116:67–73. doi: 10.1111/j.1748-1716.1982.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 18.Arver S., Eliasson R. Zinc and zinc ligands in human seminal plasma. II. Contribution by ligands of different origin to the zinc binding properties of human seminal plasma. Acta Physiol. Scand. 1982;115:217–224. doi: 10.1111/j.1748-1716.1982.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 19.Frenette G., Tremblay R. R., Dube J. Y. Zinc binding to major human seminal coagulum proteins. Arch. Androl. 1989;23:155–163. doi: 10.3109/01485018908986838. [DOI] [PubMed] [Google Scholar]
- 20.Andre I., Linse S. Measurement of Ca2+-binding constants of proteins and presentation of the CaLigator software. Anal. Biochem. 2002;305:195–205. doi: 10.1006/abio.2002.5661. [DOI] [PubMed] [Google Scholar]
- 21.Linse S. Calcium binding to proteins studied via competition with chromophoric chelators. Methods Mol. Biol. 2002;173:15–24. doi: 10.1385/1-59259-184-1:015. [DOI] [PubMed] [Google Scholar]
- 22.Lilja H., Laurell C. B. The predominant protein in human seminal coagulate. Scand. J. Clin. Lab. Invest. 1985;45:635–641. doi: 10.3109/00365518509155271. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho A. L., Sanz L., Barettino D., Romero A., Calvete J. J., Romao M. J. Crystal structure of a prostate kallikrein isolated from stallion seminal plasma: a homologue of human PSA. J. Mol. Biol. 2002;322:325–337. doi: 10.1016/s0022-2836(02)00705-2. [DOI] [PubMed] [Google Scholar]