Abstract
The carotenoid lutein is thought to play a role in the human eye and to protect against age-related macular degeneration. Lutein transport in the human intestine has not been characterized. We examined lutein transport processes using Caco-2 TC-7 monolayers as a model for human intestinal epithelium. Purified lutein was mixed with phospholipids, lysophospholipids, cholesterol, mono-olein, oleic acid and taurocholate to obtain lutein-rich mixed micelles that mimicked those found under physiological conditions. The micelles were added to the apical side of Caco-2 TC-7 cell monolayers for 30 min or 3 h at 37 °C. Absorbed lutein, i.e. the sum of lutein recovered in the scraped cells and in the basolateral chamber, was quantified by HPLC. Transport rate was measured (i) as a function of time (from 15 to 60 min), (ii) as a function of micellar lutein concentration (from 1.5 to 15 μM), (iii) at 4 °C, (iv) in the basolateral to apical direction, (v) after trypsin pretreatment, (vi) in the presence of β-carotene and/or lycopene, (vii) in the presence of increasing concentrations of antibody against SR-BI (scavenger receptor class B type 1) and (viii) in the presence of increasing concentrations of a chemical inhibitor of the selective transfer of lipids mediated by SR-BI, i.e. BLT1 (blocks lipid transport 1). The rate of transport of lutein as a function of time and as a function of concentration was saturable. It was significantly lower at 4 °C than at 37 °C (approx. 50%), in the basal to apical direction than in the opposite direction (approx. 85%), and after trypsin pretreatment (up to 45%). Co-incubation with β-carotene, but not lycopene, decreased the lutein absorption rate (approx. 20%) significantly. Anti-SR-BI antibody and BLT1 significantly impaired the absorption rate (approx. 30% and 57% respectively). Overall, these results indicate that lutein absorption is, at least partly, protein-mediated and that some lutein is taken up through SR-BI.
Keywords: Caco-2 TC-7 cells, carotenoid, cluster determinant 36 (CD36), lutein, scavenger receptor class B type I (SR-BI), xanthophyll
Abbreviations: BLT1, blocks lipid transport 1; CD36, cluster determinant 36; DMEM, Dulbecco's modified Eagle's medium; FBS, foetal bovine serum; SR-BI, scavenger receptor class B type I
INTRODUCTION
Lutein is a food microconstituent of the carotenoid family, more specifically the xanthophyll family, a subclass of carotenoids possessing oxygenated groups. In the human diet, it is found mainly in dark-green leafy vegetables and egg yolks [1,2]. It is not at present considered as a nutrient [3], because sufficient evidence is not available to show that it sustains or enhances physiological functions and/or prevents diseases. Nevertheless, interest in lutein is growing because it selectively accumulates in the retina [4,5], where it may protect photoreceptors against light-initiated oxidative damage [6–8], and since it has been associated with a low incidence of age-related macular degeneration [9,10]. Furthermore, a recent study has shown that the visual function of patients with atrophic age-related macular degeneration is improved with lutein supplementation [11].
Scarce data are available on lutein digestion in humans. Although a recent study focused on the fate of lutein in the human gastrointestinal tract [12], the characteristics of lutein transport across human enterocytes have not been investigated. Early results on β-carotene [13] suggested that carotenoids are absorbed by a passive process. However, several observations suggest that this may not be the case. First, there is a very marked inter-individual variability in the absorption of carotenoids [14] that cannot easily be explained by a passive diffusion process. Secondly, recent studies suggested that SR-BI (scavenger receptor class B type I) and the CD36 (cluster determinant 36) may be involved in the uptake of lipids, with little structural discrimination of the lipid molecules [15,16], suggesting that they may mediate an uptake of lipophilic microconstituents, such as carotenoids or tocopherols. Thirdly, a scavenger receptor with significant sequence identity with the mammalian class B scavenger receptors SR-BI and CD36, which mediates the cellular uptake of carotenoids, has recently been described in Drosophila [17].
The objective of the present study was to characterize lutein transport by human enterocytes and to assess the role of SR-BI in lutein uptake.
MATERIALS AND METHODS
Chemicals
All-trans-lutein (96% pure) and echinenone (98% pure), used as internal standard for HPLC lutein analysis, were gifts from F. Hoffmann–La Roche (Basel, Switzerland). 2-Oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (phosphatidylcholine), 1-palmitoyl-sn-glycero-3-phosphocholine (lysophosphatidylcholine), mono-olein, free cholesterol, oleic acid, sodium taurocholate, pyrogallol, mouse monoclonal anti-(human CD36) IgM and anti-(mouse IgM) and anti-(mouse IgG) conjugated to alkaline phosphatase were purchased from Sigma–Aldrich (St Quentin-Fallavier, France). Mouse monoclonal IgG raised against the external domain (amino acids 104–294) of human SR-BI, also known as CLA-1, was purchased from BD Transduction Laboratories (Lexington, KY, U.S.A.). Mouse polyclonal IgG raised against the C-terminal domain (amino acids 495–509) of human SR-BI was a gift from N. Domingo (UMR 476 INSERM/1260 INRA, Marseille, France). BLT1 (blocks lipid transport 1), a chemical inhibitor of lipid transport mediated by SR-BI, was purchased from Chembridge (San Diego, CA, U.S.A.). Sulpho-NHS-LC biotin [where sulpho-NHS-LC stands for sulphosuccinimidyl-6-(biotinamido)hexanoate] was purchased from Uptima-Interchim (Montluçon, France). N-Acetyl-β-D-glucopyranoside (manufactured by Calbiochem) and streptavidin–agarose solution (manufactured by Oncogene Research Products) were obtained from VWR International SAS (Strasbourg, France). DMEM (Dulbecco's modified Eagle's medium) containing 4.5 g/l glucose and trypsin/EDTA (500 and 200 mg/l respectively) were purchased from BioWhittaker (Fontenay-sous-Bois, France), FBS (foetal bovine serum) was from Biomedia (Issy-les-Moulineaux, France), and non-essential amino acids, penicillin/streptomycin, PBS and PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBSCM) were from Gibco BRL (Cergy-Pontoise, France). Protease inhibitor cocktail was a gift from F. Tosini (Avantage Nutrition, Marseille, France).
Cell culture
Caco-2 clone TC-7 cells [18,19] were a gift from Dr M. Rousset (U178 INSERM, Villejuif, France). Cells, passages 18–70, were grown in 25 cm2 flasks (manufactured by TPP, Trasadingen, Switzerland, and obtained from VWR International SAS) in the presence of DMEM supplemented with 20% heat-inactivated FBS, 1% non-essential amino acid and 1% antibiotics (complete medium). Cells were incubated at 37 °C in a humidified atmosphere of air/CO2 (90:10, v/v) and the medium was changed every 48 h. Monolayers were subcultured with a 7-day passage frequency when they reached a confluence of approx. 80% by treatment with 0.25% trypsin/EDTA. For each experiment, cells were seeded at a density of 25×104 cells/well, and grown on transwells (6-well plate, 24 mm diameter, 1 μm-pore-size polycarbonate membrane; Becton Dickinson, le Pont-de-Chaix, France). Apical chamber received 1 ml of complete medium (conductivity=13.7±0.3 mS, as measured by a conductivity meter; model CDM3; Radiometer, Copenhagen, Denmark) and the basolateral chamber received 2 ml of complete medium during the first 7 days. For the last 14 days, the complete medium was still used in the basolateral chamber, whereas an FBS-free medium (conductivity=13.6±0.3 mS) was used in the apical chamber. Media were changed every day for 21 days to obtain confluent differentiated cell monolayers. Before each experiment, the integrity of the cell monolayers was checked by measuring trans-epithelial electrical resistance with a voltohmmeter equipped with a chopstick electrode (Millicell ERS; Millipore, Saint Quentin en Yvelines, France).
Preparation of lutein-rich micelles
For delivery of lutein to cells, mixed micelles were prepared as described previously [20] with some modifications to mimic the physiological conditions better. For example, the ratio of lysophosphatidylcholine/phosphatidylcholine was similar to that found in human duodenum during digestion [21]. Appropriate volumes of the following compounds were transferred to glass bottles to obtain the following final concentrations: 0.04 mM phosphatidylcholine, 0.16 mM lysophosphatidylcholine, 0.3 mM mono-olein, 0.1 mM free cholesterol, 0.5 mM oleic acid and 0.5–30 μM lutein [12]. Stock solution solvents were carefully evaporated under nitrogen. The dried residue was solubilized in DMEM containing 5 mM taurocholate and vigorously mixed by sonication at 25 W (Branson 250W sonifier; Danbury, CT, U.S.A.) for 3 min. The mixtures obtained were sterilized and filtered by passing them through a presterilized 0.22 μm filter (Millipore), and the resultant solutions were optically clear. HPLC analysis showed no significant degradation of lutein after sonication, and normal lutein absorption spectrum of micellar lutein showed that lutein was present as monomers and not as crystals in micelles. Micelle stability was checked before and after storage at −80 °C with a Malvern High Performance particle sizer (Malvern, Worcs., U.K.). This control showed no effect of deep-freezing on particle size. The concentration of lutein in the micellar solutions was measured before each experiment.
Identification of transport characteristics
At the beginning of each experiment, cell monolayers were washed twice with 0.5 ml of PBS. The apical or basolateral side of the cell monolayers received the lutein-rich micelles (1 or 2 ml respectively), whereas the other side received the normal medium. Cell monolayers were incubated at either 37 or 4 °C, depending on the experiment. The incubation time was chosen after a preliminary experiment to measure the maximal absorption rate, and to obtain sufficient amounts of absorbed lutein for accurate measurements. After the incubation period, media from each side of the membrane were harvested. Cell monolayers were washed twice with 0.5 ml of ice-cold PBS containing 10 mM taurocholate to eliminate adsorbed lutein, scraped and collected in 1 ml of PBS. All the samples were stored at −80 °C under nitrogen with 0.5% pyrogallol as a preservative before lutein extraction and HPLC analysis. Aliquots of cell samples without pyrogallol were used to estimate protein concentrations with a bicinchoninic acid kit (Pierce, Montluçon, France). To ensure that cells were not damaged, particularly when micelles were added in the basolateral medium, the integrity of the monolayers was checked by measuring trans-epithelial electrical resistance after experiments during preliminary tests.
Involvement of carrier protein(s) in lutein transport
Trypsin-sensitivity experiments were performed as described previously [22]. Apical surface of monolayers of cells was pretreated with trypsin at 0.5 mg/ml for 10 min. The cells were then rinsed three times with 0.5 ml of PBS, the trans-epithelial electrical resistance was measured to ensure the integrity of tight junctions, and the uptake experiments were performed within 5 min.
Competition studies
Lutein absorption was measured after lutein-rich mixed micelles were mixed either with carotenoid-free mixed micelles or with physiological concentrations of mixed micelles containing other carotenoids (0.2 μM β-carotene and/or 0.13 μM lycopene).
Involvement of scavenger receptors in lutein transport
Test for the presence of SR-BI and CD36 in Caco-2 TC-7 cells
Centrifuged Caco-2 cells scraped from different wells were homogenized in 200 μl of PBS and lysed by sonication. Protein concentrations of cell lysates were measured and 20 μg of proteins was used for Western-blot analysis. Proteins were separated by SDS/PAGE using 12% polyacrylamide gels under reducing conditions as described by Laemmli [23]. Proteins were electrophoretically transferred on to PVDF membranes as described by Burnette [24]. The blotting membrane was incubated with the monoclonal mouse anti-(human CD36) IgM or the monoclonal mouse anti-(human SR-BI) IgG raised against the external domain at 1/1000 dilution. For visualization, monoclonal anti-mouse IgM or IgG was used as secondary antibody at 1/5000 dilution.
Test for the localization of SR-BI in apical or basolateral membranes of Caco-2 TC-7 cells
Biotinylation of cell monolayers was performed as described by Cai et al. [25] with minor changes. On the day of the experiment, Caco-2 TC-7 culture dishes were placed on ice and both apical and basolateral sides were washed three times with ice-cold PBS-CM. Sulpho-NHS-LC biotin was added to either the apical or basolateral side (0.5 mg/ml in PBS-CM). Cells were incubated for 30 min at room temperature (25±3 °C) with the biotin solution. Cells were then washed once with 20 mM Tris-buffered saline (pH 8.0) to quench free biotin and twice with PBS-CM to remove any remaining biotinylation reagent. Cell monolayers were scraped in 500 μl of PBS containing protease inhibitors and lysed using 1% Triton X-100 and 1.75% N-octyl-β-D-glucopyranoside. Protein concentrations of cell lysates were measured and 20 μg of protein was then incubated with an equal volume of immobilized streptavidin for 16 h at 4 °C. The supernatant, free of biotinylated surface proteins, was analysed by dot-blotting, as the specificity of the antibody had been checked by Western-blot analysis. Aliquots of the supernatant were directly blotted on to PVDF membranes, which were incubated with the monoclonal mouse anti-(human SR-BI) IgG raised against the external domain at 1/1000 dilution. For visualization, monoclonal anti-mouse IgG was used as a secondary antibody at 1/5000 dilution.
Lutein transport inhibition by anti-(human SR-BI) antibody
Cell monolayers were incubated for 2 min with anti-(human SR-BI) antibody raised against the external domain before lutein-rich micelles were added. Different concentrations of the antibody were used, ranging from 0 to 5.5 μg/ml. Anti-(human SR-BI) antibody raised against the C-terminal domain, which is located at the internal side of the apical membrane, was used as a control at 3.75 μg/ml [26].
Lutein transport inhibition by BLT1
BLT1 cytotoxicity was controlled before performing the inhibition experiments. Cells were seeded at a density of 10000 cells/well, and grown on 96-well plate for 5 days before experiment. Then they received either DMSO (control) or BLT1 at different concentrations (up to 20 μM) for 180 min. Cytotoxicity was assessed by a CellTiter 96 Aqueous One Solution assay (Promega, Madison, WI, U.S.A.). These control experiments showed that BLT1 was not toxic up to 10 μM (results not shown). The effect of BLT1 on lutein transport was assessed as follows: cell monolayers were pretreated with either DMSO (control) or BLT1 at different concentrations ranging from 0.01 to 10 μM for 1 h. Then, cells received lutein-rich mixed micelles with BLT1 at the preincubation concentration [27].
Lutein extraction
Lutein was extracted from 500 μl aqueous samples using the following method. The carotenoid echinenone was used as internal standard and was added to the samples in 500 μl ethanol. The mixture was extracted twice with two volumes of hexane. The hexane phases obtained after centrifugation (500 g, 5 min, room temperature) were evaporated to dryness under nitrogen, and the dried extract was dissolved in 100 μl of methanol/dichloromethane (65:35, v/v). A volume of 10–60 μl was used for HPLC analysis.
Lutein analysis
We have developed a reversed-phase isocratic HPLC method for the assay of carotenoids [28]. Lutein was separated using a 250 mm×4.6 mm inner-diameter reversed-phase C18, 5 μm Zorbax column (Interchim), and a guard column. The mobile phase was 70% acetonitrile, 20% dichloromethane and 10% methanol. The flow rate was 1.5 ml/min and the column was kept at a constant temperature (30 °C). The HPLC system was composed of a Waters 2690 separation module and a Waters 2996 photodiode array detector (Waters, Saint Quentin en Yvelines, France). Carotenoids were detected at 450 nm and identified by retention time and spectral analysis (300–500 nm) compared with pure (>95%) standards of lutein and echinenone. Quantification was performed using Waters Millennium 32 software (version 3.05.01) comparing peak area with standard reference curves. All solvents used were HPLC grade from SDS (Peypin, Bouches-du-Rhône, France).
Calculations and statistical analysis
Absorbed lutein was estimated as lutein found in scraped cells plus lutein found on the opposite side of the cell monolayer (basolateral side when micellar lutein was added to the apical side, and vice versa). Lutein absorption rate was expressed as pmol of lutein absorbed·min−1·(mg of protein)−1. Results are expressed as means±S.E.M. Differences between more than two groups of unpaired data underwent the non-parametric Kruskal–Wallis test. The non-parametric Mann–Whitney U test was used as a post hoc test when the Kruskal–Wallis test showed significant differences between groups. Differences between only two groups of unpaired data were tested by the Mann–Whitney U test. Values of P<0.05 were considered significant. All statistical analyses were performed using Statview software version 5.0 (SAS Institute, Cary, NC, U.S.A.). Relationships between two variables were examined by regression analysis using KaleidaGraph version 3.6 (Synergy Software, Reading, PA, U.S.A.).
RESULTS
Incorporation of lutein in mixed micelles
Increasing amounts of lutein, corresponding to theoretical final concentrations of 1.5–30 μM, were added to the lipid mixture during micelle preparation, and lutein concentration recovered in the mixed micelles was measured. Incorporation of lutein increased linearly up to 5 μM. Then the efficiency of incorporation gradually fell to reach approx. 47% at 30 μM (results not shown). The maximum lutein concentration that could be incorporated in mixed micelles was approx. 14 μM.
Effect of incubation time on lutein uptake, and choice of incubation times
Lutein absorbed by the differentiated monolayers increased in a curvilinear way up to 60 min incubation (results not shown). To be close to the initial absorption rate, we should have worked at incubation time shorter than 15 min; however, no sufficient amount of lutein would have been accurately measured. Note that previous studies were performed for 16 h incubation [29]. We decided to work at 30 min incubation, because the amount of lutein recovered in the cells could be accurately measured (approx. 600 pmol of lutein absorbed/mg of protein) and because this time represents approximately the time during which the bowel content stays in contact with duodenal cells during digestion [30]. Finally, note that some experiments were performed at 180 min (approx. 1 nmol of lutein absorbed/mg of protein) to increase differences between groups.
Effect of micellar lutein concentration on lutein absorption rate
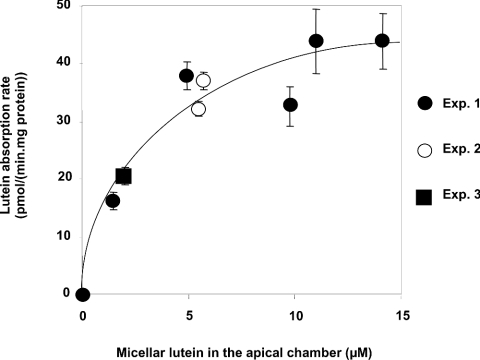
Absorption rate of lutein in cells sharply increased with increasing micellar lutein concentrations up to 2 μM and then levelled out for higher concentrations, indicating that the lutein uptake by cells was saturable (Figure 1). The best fit was an hyperbolic curve: y=ax/(x+b), R2=0.94. We calculated the apparent Vmax and K values, where Vmax represents maximal absorption rate and K the concentration of lutein in mixed micelles necessary to reach Vmax/2. We found an apparent Vmax of 51.5 pmol·min−1·(mg of protein)−1 and an apparent K of 2.78 μM.
Figure 1. Effect of micellar lutein concentration on lutein absorption by differentiated Caco-2 TC-7 cell monolayers at 37 °C.
The apical side received the FBS-free medium containing lutein-rich mixed micelles and the basolateral side received the complete medium. Incubation time was 30 min. Results are means±S.E.M. for three or four assays.
Effect of temperature
Table 1 shows that lutein absorption rate was significantly lower at 4 °C compared with that at 37 °C (38–69% decrease). It is noteworthy that, in contrast with what was observed at 37 °C, the rate of lutein uptake was not saturable at 4 °C and exhibited a linear increase with increasing micellar lutein concentration (results not shown).
Table 1. Effects of temperature and transport direction on rate of lutein transport by Caco-2 TC-7 monolayers.
Lutein uptake [pmol·min−1·(mg of protein)−1] was measured at 37 °C after apical micellar lutein delivery (control=100%). Lutein uptake at 4 °C, and when micellar lutein was added in the basolateral chamber, were compared with control under the same experimental conditions. Incubation time was 30 min. Results are means±S.E.M. for three assays. *P<0.05: value calculated for a given experimental condition was significantly different from that measured for the control.
| Uptake (% of control) | ||
|---|---|---|
| Lutein concentration (μM) | 4 °C | Basolateral to apical |
| 5.0 | 37.9±2.9* | 13.1±2.7* |
| 9.7 | 46.0±4.0* | Not detectable |
| 14.2 | 68.9±3.0* | 17.3±11.5* |
Effect of transport direction
Table 1 shows a significantly lower (approx. 80%) transport rate when micellar lutein was supplied at the basolateral side compared with the apical side.
Effect of trypsin pretreatment
Lutein absorption, from 5 μM lutein-rich micelles, was significantly decreased when the cell monolayers were pretreated with trypsin. The percentages of inhibition were 22.0±2.6% after 30 min incubation and 45.0±7.4% after 3 h incubation (results not shown).
Effect of co-incubation with β-carotene and/or lycopene
Lutein absorption was significantly decreased when lutein was co-incubated with β-carotene (approx. 20%) or with β-carotene and lycopene, but not with lycopene alone (Table 2).
Table 2. Effects of other carotenoids (β-carotene and lycopene) on lutein absorption.
The apical side received FBS-free medium containing lutein-rich micelles, lutein and lycopene-rich micelles, lutein and β-carotene-rich micelles or lutein plus β-carotene- and lycopene-rich micelles. The basolateral side received the complete medium. Incubation time was 180 min. Results are means±S.E.M. for three assays. *P<0.05 for significant difference from the control (0.90 μM lutein alone).
| Concentration | Absoprtion (% of control) |
|---|---|
| 0.90 μM lutein+0.13 μM lycopene | 89.7±7.4 |
| 0.90 μM lutein+0.20 μM β-carotene | 71.7±2.0* |
| 0.90 μM lutein+0.13 μM lycopene+0.20 μM β-carotene | 77.9±3.4* |
Involvement of the scavenger receptors SR-BI and CD36
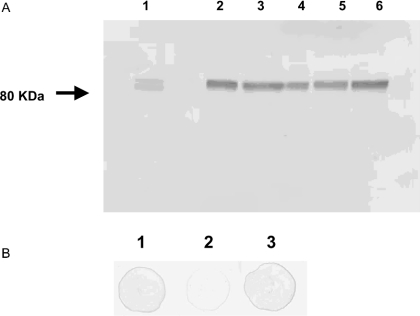
Immunoblot analysis showed that SR-BI was expressed in the Caco-2 TC-7 cells for all the passages studied (Figure 2A). Only one band was observed, demonstrating that the antibody was specific to the protein. In contrast, CD36 was not detected under the same conditions (results not shown). Figure 2(B) reveals that a large part of SR-BI was located at the apical side of the cells, as described previously in Caco-2 [25] and in intestinal cells [15,31].
Figure 2. (A) Immunoblot analysis of Caco-2 TC-7 cells using monoclonal anti-(human SR-BI) antibody, and (B) expression of SR-BI protein in differentiated Caco-2 TC-7 cells grown on filters.
(A) Lane 1, HeLa lysate used as a standard for SR-BI and as a mass marker (80 kDa). Lane 2, differentiated Caco-2 TC-7 passage 18; lane 3, passage 22; lane 4, passage 54; lane 5, passage 67; lane 6, passage 70. (B) Dot-blot of remaining proteins from 20 μg of cell extract of differentiated cells after biotinylation of apical (2) or basolateral (3) membranes and elimination of biotinylated proteins with streptavidin-agarose. Control was performed without biotinylation (1).
Table 3 shows that the addition of anti-(human SR-BI) antibody raised against the external domain significantly diminished lutein apical absorption rate. The percentage of diminution increased with increasing concentrations of antibody up to 2.27 μg/ml IgG and then levelled out at higher concentrations. The best-fitting curve was obtained by a hyperbolic regression analysis: diminution of absorption rate (%)=ax/(x+b), R2=0.86 (results not shown). The maximum diminution percentage was estimated at 35% (a). The concentration of antibody at which 50% of the maximum effect was observed (b) was 1.25 μg/ml. In contrast, the addition of anti-(human SR-BI) antibody raised against the intracellular C-terminal domain at 3.75 μg/ml did not significantly diminish lutein apical absorption rate. Although the inhibition of lutein uptake by an antibody raised against the extracellular domain of SR-BI may be a non-specific phenomenon, a convincing complementary result on the involvement of SR-BI in lutein absorption is that increasing concentrations of a specific chemical inhibitor of SR-BI (BLT1) in the apical medium significantly decreased lutein absorption by 57% (Table 4).
Table 3. Effects of anti-(human SR-BI) antibody on rate of lutein absorption by differentiated Caco-2 TC-7 monolayers.
The apical sides of the cell monolayers were preincubated with the anti-(human SR-BI) antibody, raised against the external domain (AC1), at different concentrations (from 0 to 5.50 μg/ml) and then received FBS-free medium containing lutein-rich mixed micelles at 5 μM. The basolateral sides received the complete medium. Anti-SR-BI antibody raised against the C-terminal domain was used as a control (AC2). Incubation time was 180 min. Results are means±S.E.M. for three or four assays from three independent experiments. *P<0.05 for significant difference from the control (assay performed without antiboby).
| Antibody | Concentration (μg/ml) | Inhibition (%) |
|---|---|---|
| AC1 | 1.13 | 10.1±4.3 |
| 2.27 | 27.8±7.2* | |
| 3.42 | 23.3±6.7* | |
| 4.55 | 24.9±2.0* | |
| 5.50 | 32.3±3.5* | |
| AC2 | 3.42 | 6.30±0.7 |
Table 4. Effects of BLT1 on rate of lutein absorption by differentiated Caco-2 TC-7 monolayers.
The apical sides of the cell monolayers were preincubated with BLT1 (a chemical inhibitor of the transfer of lipids by SR-B1) at different concentrations and then received FBS-free medium containing lutein-rich mixed micelles at 0.90 μM plus BLT1 at the concentration indicated. The basolateral sides received the complete medium. Incubation time was 180 min. Reults are means±S.E.M. for three to six assays from two independent experiments. *P<0.05 for significant difference from the control (lutein absorption rate without BLT1). Negative inhibition indicates that these groups of cells absorbed more than the control.
| BLT1 concentration (μM) | Inhibition (%) |
|---|---|
| 0.01 | −3.4±1.6 |
| 0.1 | −12.4±4.8 |
| 1 | 51.6±3.1* |
| 10 | 57.1±6.9* |
DISCUSSION
The Caco-2 TC-7 model was selected for two main reasons. First, it is the model most frequently employed to evaluate the intestinal transport of several nutrients, including fatty acids [32], cholesterol [15,16,33] and carotenoids [29,34–36], and gives reproducible values that closely correlate with in vivo data [37]. Secondly, Caco-2 cells spontaneously differentiate into polarized absorptive cell monolayers and display morphological and biochemical characteristics similar to human enterocytes after differentiation. The clone TC-7 was chosen because it is more homogeneous than the parent Caco-2 cell line [38] and it possesses β-carotene 15,15′-dioxygenase activity [39].
Classical experiments on absorption rate were performed [40]. The first experiment showed that the rate of lutein uptake was saturable under physiological conditions (37 °C, 30 min). It can be described by a hyperbolic equation. The estimated K value obtained was 2.78 μM, and thus saturation would be reached at approx. 5.56 μM lutein. This is noteworthy, as we recently measured lutein concentration in the human duodenal lumen at 5–7 μM after a spinach-rich meal [12]. Such saturation of the rate of lutein uptake is a first argument for a protein-mediated transport of this carotenoid. Indeed, it can be explained by a limited number of receptors/transporters capable of handling the molecule. The second experiment showed that the rate of uptake was significantly impaired at 4 °C. Although numerous biological processes can be slowed down at low temperature, and although it is possible that changes in temperature may have affected the partition coefficient of the monomer phase of lutein into the micelles thus affecting the absorption, this observation is a second argument supporting a facilitated process. The simplest explanation for this effect is that one or more receptors/transporters were, at least partly, impaired at low temperature. The fact that a fraction of lutein was still absorbed at 4 °C, plus the finding that, at this temperature, absorption rate increased linearly as a function of micellar lutein concentration, suggests that a fraction of lutein may be absorbed by passive diffusion. The third experiment dealt with the direction of transport. The finding that the absorption rate was significantly lower from the basolateral side to the apical side than in the opposite direction is a further argument in favour of a transporter preferentially distributed in the apical membrane. This hypothesis is supported by the fact that a trypsin pretreatment of the apical side of the monolayers significantly decreased lutein absorption rate. The hypothesis of a transporter is also supported by the competition between lutein and β-carotene for absorption. Although it is not sure that a significant competition could have been observed at a lower, more physiological, time of incubation, i.e. 30 min, this last result is in agreement with human studies in which β-carotene significantly affected lutein absorption [41–43]. The fact that lycopene did not significantly affect lutein absorption can be explained by the lower concentration of lycopene that could have been incorporated in micelles (0.13 μM instead of 0.2 μM for β-carotene) or by the fact that, in contrast with β-carotene and lutein, this carotenoid has no β-ionone rings. The strongest evidence suggesting that the transport of lutein is protein-mediated has come from the use of antibody raised against SR-BI and from the use of a specific chemical inhibitor of SR-BI. We have chosen BLT1 because it inhibits lipid transport by SR-BI in nanomolar concentrations [27] and it does not cross-react with other transporter proteins such as ABC proteins [44]. The finding that the flux of lutein was significantly decreased when cells were treated with the antibody or BLT1 strongly suggests that SR-BI is involved in the transport of lutein. The preferential transport from the apical side together with the preferential localization of SR-BI at the apical side adds a further argument on the involvement of this protein in lutein transport. The fact that we did not manage to inhibit lutein absorption fully suggests that one or more way of transport might be involved (passive diffusion or other transporters).
Our results agree well with recent data suggesting that β-carotene uptake by intestinal cells is a facilitated process [29], but seems at variance with previous results suggesting that carotenoids are absorbed by passive diffusion [13,34,45]. There may be several explanations for the discrepancy between our results and those of During et al. [29], and the results of Hollander and Ruble [13]. First, the data were obtained with another model (perfusion of intestinal loops in the rat). Secondly, the authors concluded that β-carotene absorption was passive because absorption rate was apparently non-saturable. However, a careful check of their data shows that the possibility that a plateau was reached from approx. 7 μM β-carotene in the distal part of the intestine cannot be ruled out. Other authors [34] concluded that carotenoid absorption is passive because a linear relationship between the lipophilicity of carotenoids and their relative uptake was observed in Caco-2 cells. Nevertheless this can be explained, as suggested by the authors themselves, by the fact that molecules that are more lipophilic will partition faster into the lipid cell membrane than less lipophilic ones [34]. It can also be explained by a positive relationship between carotenoid lipophilicity and affinity for a receptor/transporter, which is consistent with the fact that SR-BI preferentially facilitates the uptake of molecules that are more hydrophobic than free cholesterol [16], and lutein is more lipophilic than free cholesterol (octanol/water partition coefficients of 14.8 and 8.7 respectively). This critical analysis of the literature thus shows that our results do not really conflict with previous results.
The observation that the apical uptake of lutein is partly mediated by SR-BI is not surprising. SR-BI functions as a port or docking receptor and exhibits low lipid specificity. It apparently mediates transport of cholesteryl esters, triacylglycerols, phospholipids, free cholesterol [15,33], and is involved in the metabolism of α-tocopherol [46], which, like the carotenoids, is an isoprenoid. It has recently been shown in Drosophila that a scavenger receptor, which has significant sequence identity with the mammalian class B scavenger receptors SR-BI and CD36 and called ‘ninaD’, is essential in the cellular uptake of lutein [17]. It is therefore probable that this receptor is involved in the metabolism of lutein in humans.
The physiopathological consequences of the identification of a protein-mediated transport of lutein in enterocytes are not known, but three main possibilities can be suggested. First, interindividual variations in transporter(s) expression or efficiency, due to genetic polymorphisms, may explain the very marked interindividual variations in lutein bioavailability [47]. Secondly, individuals who partly or fully lack these receptors/transporters may malabsorb lutein and thus be at higher risk of age-related macular degeneration. Thirdly, because SR-BI is regulated by hormones, such as oestrogen [48], lutein absorption may be regulated by hormonal stimuli. These hypotheses need to be tested by appropriate experiments.
In summary, the results obtained show that the transport of lutein in Caco-2 TC-7 cells is, at least partly, facilitated and involves the scavenger receptor SR-BI. Further work is required to evaluate the implications of this mechanism for lutein metabolism and the claimed protective effect of lutein on age-related macular degeneration.
Acknowledgments
We thank I. Crenon, C. Kreuser and B. Kerfelec for helpful technical suggestions and comments in the preparation of the manuscript, and B. Gleize and J. Kaloustian for valuable help and technical assistance.
References
- 1.Chug-Ahuja J. K., Holden J. M., Forman M. R., Mangels A. R., Beecher G. R., Lanza E. The development and application of a carotenoid database for fruits, vegetables, and selected multicomponent foods. J. Am. Diet. Assoc. 1993;93:318–323. doi: 10.1016/0002-8223(93)91559-9. [DOI] [PubMed] [Google Scholar]
- 2.Sommerburg O., Keunen J. E., Bird A. C., van Kuijk F. J. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br. J. Ophthalmol. 1998;82:907–910. doi: 10.1136/bjo.82.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrich S., Lee K. W., Xu X., Wang H. J., Murphy P. A. Defining food components as new nutrients. J. Nutr. 1994;124:1789S–1792S. doi: 10.1093/jn/124.suppl_9.1789S. [DOI] [PubMed] [Google Scholar]
- 4.Chan C., Leung I., Lam K. W., Tso M. O. The occurrence of retinol and carotenoids in human subretinal fluid. Curr. Eye Res. 1998;17:890–895. doi: 10.1076/ceyr.17.9.890.5141. [DOI] [PubMed] [Google Scholar]
- 5.Bone R. A., Landrum J. T., Friedes L. M., Gomez C. M., Kilburn M. D., Menendez E., Vidal I., Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997;64:211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 6.Junghans A., Sies H., Stahl W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001;391:160–164. doi: 10.1006/abbi.2001.2411. [DOI] [PubMed] [Google Scholar]
- 7.Krinsky N. I. Possible biologic mechanisms for a protective role of xanthophylls. J. Nutr. 2002;132:540S–542S. doi: 10.1093/jn/132.3.540S. [DOI] [PubMed] [Google Scholar]
- 8.Rapp L. M., Maple S. S., Choi J. H. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest. Ophthalmol. Vis. Sci. 2000;41:1200–1209. [PubMed] [Google Scholar]
- 9.Snodderly D. M. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am. J. Clin. Nutr. 1995;62:1448S–1461S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 10.Landrum J. T., Bone R. A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 11.Richer S., Stiles W., Statkute L., Pulido J., Frankowski J., Rudy D., Pei K., Tsipursky M., Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial) Optometry. 2004;75:216–230. doi: 10.1016/s1529-1839(04)70049-4. [DOI] [PubMed] [Google Scholar]
- 12.Tyssandier V., Reboul E., Dumas J. F., Bouteloup-Demange C., Armand M., Marcand J., Sallas M., Borel P. Processing of vegetable-borne carotenoids in the human stomach and duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G913–G923. doi: 10.1152/ajpgi.00410.2002. [DOI] [PubMed] [Google Scholar]
- 13.Hollander D., Ruble P. E. Beta-carotene intestinal absorption: bile, fatty acid, pH, and flow rate effects on transport. Am. J. Physiol. 1978;235:E686–E691. doi: 10.1152/ajpendo.1978.235.6.E686. [DOI] [PubMed] [Google Scholar]
- 14.Borel P., Grolier P., Mekki N., Boirie Y., Rochette Y., Le Roy B., Alexandre-Gouabau M. C., Lairon D., Azais-Braesco V. Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J. Lipid Res. 1998;39:2250–2260. [PubMed] [Google Scholar]
- 15.Hauser H., Dyer J. H., Nandy A., Vega M. A., Werder M., Bieliauskaite E., Weber F. E., Compassi S., Gemperli A., Boffelli D., et al. Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry. 1998;37:17843–17850. doi: 10.1021/bi982404y. [DOI] [PubMed] [Google Scholar]
- 16.Werder M., Han C. H., Wehrli E., Bimmler D., Schulthess G., Hauser H. Role of scavenger receptors SR-BI and CD36 in selective sterol uptake in the small intestine. Biochemistry. 2001;40:11643–11650. doi: 10.1021/bi0109820. [DOI] [PubMed] [Google Scholar]
- 17.Kiefer C., Sumser E., Wernet M. F., Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvini S., Charbonnier M., Defoort C., Alquier C., Lairon D. Functional characterization of three clones of the human intestinal Caco-2 cell line for dietary lipid processing. Br. J. Nutr. 2002;87:211–217. doi: 10.1079/BJNBJN2001507. [DOI] [PubMed] [Google Scholar]
- 19.Chantret I., Rodolosse A., Barbat A., Dussaulx E., Brot-Laroche E., Zweibaum A., Rousset M. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J. Cell Sci. 1994;107:213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 20.Homan R., Hamelehle K. L. Phospholipase A2 relieves phosphatidylcholine inhibition of micellar cholesterol absorption and transport by human intestinal cell line Caco-2. J. Lipid Res. 1998;39:1197–1209. [PubMed] [Google Scholar]
- 21.Armand M., Borel P., Pasquier B., Dubois C., Senft M., Andre M., Peyrot J., Salducci J., Lairon D. Physicochemical characteristics of emulsions during fat digestion in human stomach and duodenum. Am. J. Physiol. 1996;271:G172–G183. doi: 10.1152/ajpgi.1996.271.1.G172. [DOI] [PubMed] [Google Scholar]
- 22.Ho S. Y., Storch J. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am. J. Physiol. Cell. Physiol. 2001;281:C1106–C1117. doi: 10.1152/ajpcell.2001.281.4.C1106. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Burnette W. N. ‘Western blotting’: electrophoretic transfer of proteins from sodium dodecyl sulfate–polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 25.Cai L., Eckhardt E. R., Shi W., Zhao Z., Nasser M., de Villiers W. J., van der Westhuyzen D. R. Scavenger receptor class B type I reduces cholesterol absorption in cultured enterocyte CaCo-2 cells. J. Lipid Res. 2004;45:253–262. doi: 10.1194/jlr.M300303-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Jourdheuil-Rahmani D., Charbonnier M., Domingo N., Luccioni F., Lafont H., Lairon D. Biliary anionic peptide fraction and apoA-I regulate intestinal cholesterol uptake. Biochem. Biophys. Res. Commun. 2002;292:390–395. doi: 10.1006/bbrc.2002.6664. [DOI] [PubMed] [Google Scholar]
- 27.Nieland T. J., Penman M., Dori L., Krieger M., Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15422–15427. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyan B., Azais-Braesco V., Cardinault N., Tyssandier V., Borel P., Alexandre-Gouabau M. C., Grolier P. Simple method for clinical determination of 13 carotenoids in human plasma using an isocratic high-performance liquid chromatographic method. J. Chromatogr. B Biomed. Sci. Appl. 2001;751:297–303. doi: 10.1016/s0378-4347(00)00488-6. [DOI] [PubMed] [Google Scholar]
- 29.During A., Hussain M. M., Morel D. W., Harrison E. H. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 2002;43:1086–1095. doi: 10.1194/jlr.m200068-jlr200. [DOI] [PubMed] [Google Scholar]
- 30.Bernier J., Adrian J., Vidon N. Les Aliments dans le Tube Digestif. Paris: Doin Editeurs; 1988. La motricité de I'intestin grêle et le transit intestinal des aliments; pp. 99–119. [Google Scholar]
- 31.Lobo M. V., Huerta L., Ruiz-Velasco N., Teixeiro E., de la Cueva P., Celdran A., Martin-Hidalgo A., Vega M. A., Bragado R. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J. Histochem. Cytochem. 2001;49:1253–1260. doi: 10.1177/002215540104901007. [DOI] [PubMed] [Google Scholar]
- 32.Trotter P. J., Ho S. Y., Storch J. Fatty acid uptake by Caco-2 human intestinal cells. J. Lipid Res. 1996;37:336–346. [PubMed] [Google Scholar]
- 33.Play B., Salvini S., Haikal Z., Charbonnier M., Harbis A., Roussel M., Lairon D., Jourdheuil-Rahmani D. Glucose and galactose regulate intestinal absorption of cholesterol. Biochem. Biophys. Res. Commun. 2003;310:446–451. doi: 10.1016/j.bbrc.2003.08.150. [DOI] [PubMed] [Google Scholar]
- 34.Sugawara T., Kushiro M., Zhang H., Nara E., Ono H., Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001;131:2921–2927. doi: 10.1093/jn/131.11.2921. [DOI] [PubMed] [Google Scholar]
- 35.Garrett D. A., Failla M. L., Sarama R. J., Craft N. E. Accumulation and retention of micellar beta-carotene and lutein by Caco-2 human intestinal cells. J. Nutr. Biochem. 1999;10:573–581. doi: 10.1016/s0955-2863(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 36.Ferruzzi M. G., Failla M. L., Schwartz S. J. Assessment of degradation and intestinal cell uptake of carotenoids and chlorophyll derivatives from spinach puree using an in vitro digestion and Caco-2 human cell model. J. Agric. Food Chem. 2001;49:2082–2089. doi: 10.1021/jf000775r. [DOI] [PubMed] [Google Scholar]
- 37.Artursson P., Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991;175:880–885. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 38.Gres M. C., Julian B., Bourrie M., Meunier V., Roques C., Berger M., Boulenc X., Berger Y., Fabre G. Correlation between oral drug absorption in humans, and apparent drug permeability in TC-7 cells, a human epithelial intestinal cell line: comparison with the parental Caco-2 cell line. Pharm. Res. 1998;15:726–733. doi: 10.1023/a:1011919003030. [DOI] [PubMed] [Google Scholar]
- 39.During A., Albaugh G., Smith J. C. J. Characterization of beta-carotene 15,15′-dioxygenase activity in TC7 clone of human intestinal cell line Caco-2. Biochem. Biophys. Res. Commun. 1998;249:467–474. doi: 10.1006/bbrc.1998.9160. [DOI] [PubMed] [Google Scholar]
- 40.Artursson P., Karlsson J., Ocklind G., Schipper N. Studying transport processes in absorptive epithelia. In: Shaw A. J., editor. Epithelia Cell Culture: a Practical Approach. Oxford: IRL Press, Oxford University Press; 1995. pp. 111–133. [Google Scholar]
- 41.Kostic D., White W. S., Olson J. A. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am. J. Clin. Nutr. 1995;62:604–610. doi: 10.1093/ajcn/62.3.604. [DOI] [PubMed] [Google Scholar]
- 42.van den Berg H. Effect of lutein on beta-carotene absorption and cleavage. Int. J. Vitam. Nutr. Res. 1998;68:360–365. [PubMed] [Google Scholar]
- 43.van den Berg H., van Vliet T. Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am. J. Clin. Nutr. 1998;68:82–89. doi: 10.1093/ajcn/68.1.82. [DOI] [PubMed] [Google Scholar]
- 44.Nieland T. J., Chroni A., Fitzgerald M. L., Maliga Z., Zannis V. I., Kirchhausen T., Krieger M. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J. Lipid Res. 2004;45:1256–1265. doi: 10.1194/jlr.M300358-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Moore A. C., Gugger E. T., Erdman J. W. J. Brush border membrane vesicles from rats and gerbils can be utilized to evaluate the intestinal uptake of all-trans and 9-cis beta-carotene. J. Nutr. 1996;126:2904–2912. doi: 10.1093/jn/126.11.2904. [DOI] [PubMed] [Google Scholar]
- 46.Mardones P., Strobel P., Miranda S., Leighton F., Quinones V., Amigo L., Rozowski J., Krieger M., Rigotti A. Alpha-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J. Nutr. 2002;132:443–449. doi: 10.1093/jn/132.3.443. [DOI] [PubMed] [Google Scholar]
- 47.Borel P. Factors affecting intestinal absorption of highly lipophilic food microconstituents (fat-soluble vitamins, carotenoids and phytosterols) Clin. Chem. Lab. Med. 2003;41:979–994. doi: 10.1515/CCLM.2003.151. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava R. A. Scavenger receptor class B type I expression in murine brain and regulation by estrogen and dietary cholesterol. J. Neurol. Sci. 2003;210:11–18. doi: 10.1016/s0022-510x(03)00006-6. [DOI] [PubMed] [Google Scholar]