Key Clinical Message
A T217M heterozygous mutation in the SLC22A12 gene caused renal hypouricemia; this patient with IgA nephropathy had no findings other than IgA nephropathy on renal biopsy. Hypouricemia was susceptible to oxidative stress, but IgA nephropathy in the patient with hypouricemia could be treated with steroid pulse therapy without adverse events.
Keywords: IgA nephropathy, renal hypouricemia, steroid pulse therapy, urate transporter
1. INTRODUCTION
Renal hypouricemia (RHUC) is a rare inherited disorder characterized by impaired urate reabsorption in the proximal tubule resulting in low urate levels. RHUC is caused by genetic abnormalities in URAT1 and GLUT9, the transporters responsible for uric acid reabsorption in the proximal tubules. 1 Uric acid kidney stone or acute kidney injury may develop, especially after exercise. 2 We have recently encountered a patient with RHUC caused by a heterozygous mutation in T217M in the gene SLC22A12 encoding URAT1.
IgA nephropathy is the most common form of glomerulonephritis worldwide and is prevalent in Japan and other Asia‐Pacific countries. 3 The pathology is characterized by granular deposition of mainly IgA and complement C3 in the glomerular mesangium. To date, there have been few reports of hypouricemia associated with IgA nephropathy. Steroid treatment causing increased oxidative stress was considered to have the potential to increase the incidence of adverse events in hypouricemic patients with reduced tolerance to oxidative stress. We present here a rare case of RHUC incidentally affected by IgA nephropathy, to show characteristic clinical course and histological findings.
2. CASE HISTORY/EXAMINATION
A 48‐year‐old man presented for evaluation of hypertension. Examination revealed his systolic blood pressure 160 mm Hg or above, and he was already prescribed antihypertensive medication. At the same time, he was found to have proteinuria of more than 2.0 g/gCr. He was started additionally on taking losartan for elevated blood pressure and persistent proteinuria. As his blood pressure decreased, his urinary protein slightly improved, however, his proteinuria remained positive, hence, he was referred to a nephrologist at another hospital. Since the urinalysis showed protein 2+ (1.05 g/gCr) and urinary occult blood 2+, he was referred to our university hospital for renal biopsy to determine an accurate diagnosis. Furthermore, laboratory tests showed uric acid 1.0 mg/dL and fractional excretion of uric acid 48.7% (standard value 5.5%–11.1%), indicating hypouricemia and increased uric acid excretion in urine. According to the medical interview, he has been disliking physical activities and avoiding strenuous exercises since childhood. He had no episodes of acute renal failure. On kidney US stones were found in the bladder. Therefore, he was suspected of having RHUC.
3. METHODS (DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT)
In addition to renal biopsy, he was scheduled for genetic testing to identify an inherited disorder associated with RHUC. In a clinical setting, he was diagnosed with RHUC on admission because of persistent hypouricemia and a markedly elevated uric acid excretion rate (Table 1). Genetic testing revealed T217M heterozygous and R172L heterozygous mutations. Since the R172L heterozygous mutation has never been reported as a pathogenic mutation, T217M heterozygous mutation in URAT1 could be the cause of RHUC. In the meantime, his renal biopsy showed IgA nephropathy with an Oxford classification of M0S1E0T0 (Figure 1A–F). Given these results, he was diagnosed with RHUC associated with IgA nephropathy. His renal biopsy revealed active glomerular changes, including crescent formations. Because of persistent urinary protein, tonsillectomy and steroid pulse therapy were scheduled. He underwent tonsillectomy 4 months after the renal biopsy. He was then started steroid pulse therapy. Methylprednisolone 500 mg was intravenously administered for 3 days, followed by oral administration of 30 mg of glucocorticoid for 4 days, and the total three courses were repeated. In this patient, steroid pulse therapy might cause fatigue and worsen renal function due to its strong adverse reactions. Therefore, we evaluated changes in Chalder's fatigue scale score, changes in serum creatinine concentration and changes in uric acid excretion rate between before and after intravenous methylprednisolone administration. The results demonstrated that Chalder's fatigue scale scores did not change between before and after steroid pulse therapy. In addition, serum creatinine level did not worsen by steroid pulse therapy. Only uric acid excretion rate showed an increasing tendency after steroid pulse therapy (Table 2).
TABLE 1.
Laboratory data on admission.
| WBC | 8220 | /μL | P | 3.4 | mg/dL | RF | <3.0 | U/mL | Glucose | − | ||||
| Hb | 16.0 | g/dL | UN | 20.5 | mg/dL | SS‐A | 0.49 (−) | U/mL | Ketones | − | ||||
| Plt | 25.9 × 104 | /μL | Cr | 0.93 | mg/dL | SM | 2.71 (−) | U/mL | Occult Blood | ± | ||||
| PT‐INR | 0.97 | eGFR | 69.6 | mL/min/1.73m2 | Anti dsDNA Ab | 0.78 (−) | U/mL | Sediment | ||||||
| APTT | 29.8 | s | UA | 1.2 | mg/dL | PR3‐ANCA | 0.84 (−) | IU/mL | Red Cells | 5–9 | /HPF | |||
| DDimer | <0.5 | μg/dL | CK | 208 | U/L | MPO‐ANCA | 0.66 (−) | IU/mL | White Cells | <1 | /HPF | |||
| TP | 7.3 | g/dL | Amy | 56 | U/L | Anti GBM Ab | <1.50 (−) | U/mL | Epithelial Casts | <1 | /HPF | |||
| Alb | 4.4 | g/dL | Fe | 93 | μg/dL | ASO | 38 | IU/mL | RBC form | Dysmorphic | ||||
| T. Bil | 0.68 | mg/dL | CRP | 0.23 | mg/dL | ACE | 12.2 | U/L | RBC casts | − | ||||
| AST | 37 | U/L | sIL‐2R | 337.6 | U/mL | Cryogobulin | (−) | Urinary Chemistry | ||||||
| ALT | 60 | U/L | HbA1c | 5.5 | % | HBs Ag | <0.005 (−) | IU/mL | U‐TP | 0.99 | g/gCr | |||
| γ‐GT | 51 | U/L | CH50 | >60 | U/mL | HBs Ab | <3.0 (−) | mIU/mL | 1232.6 | mg/day | ||||
| LD | 247 | U/L | C3 | 123.2 | mg/dL | HCV Ab | <1.0 (−) | U‐UN | 673 | mg/dL | ||||
| LDL‐C | 192 | mg/dL | C4 | 25.9 | mg/dL | Urinalysis | U‐Cr | 81.6 | mg/dL | |||||
| Na | 137 | mmol/L | IgG | 1239.5 | mg/dL | pH | 6.0 | U‐Na | 109 | mmol/L | ||||
| K | 4.0 | mmol/L | IgA | 286.1 | mg/dL | Specific gravity | 1.016 | U‐UA | 50.3 | mg/dL | ||||
| Cl | 103 | mmol/L | IgM | 47.0 | mg/dL | Screening dipstick test | NAG | 6.0 | U/L | |||||
| Ca | 9.5 | mg/dL | ANA | <40 | Fold | Protein | 2+ | β2MG | 0.204 | μg/mL | ||||
Abbreviations: ACE, angiotensin converting enzyme; Alb, albumin; ALT, alanine aminotransferase; Amy, amylase; ANA, antinuclear antibody; ANCA, anti‐neutrophil cytoplasmic antibody; anti dsDNA Ab, anti‐double strand DNA antibody; anti GBM Ab, anti‐glomerular basement membrane antibody; APTT, activated partial thromboplastin time; ASO, anti‐streptolysin O; AST, aspartate aminotransferase; C3, c3 complement; C4, c4 complement; Ca, calcium; CH50, homolytic complement activity; CK, Creatine kinase; Cl, chlorine; Cr, creatinine; CRP, C‐reactive protein; eGFR, estimated Glomerular Filtration Rate; Fe, serum iron; Hb, hemoglobin; HbA1c, hemoglobin A1c; K, potassium; LD, lactate dehydrogenase; LDL‐C, Low density lipoprotein cholesterol; Na, sodium; NAG, N‐Acetyl‐β‐D‐glucosaminidase; P, phosphorus; Plt, platelet; PT‐INR, prothrombin Time‐International Normalized Ratio; RBC, red blood cell; RF, rheumatoid factor; sIL‐2R, Soluble‐Interleukin‐2‐Receptor; SM, anti‐Sm antibody; T.Bil, total bilirubin; TP, total protein; UA, uric acid; UN, urea nitrogen; WBC, white blood cell; β2MG, β2‐microglobuli.γ‐GT, γ‐Glutamyl Transpeptidase.
FIGURE 1.
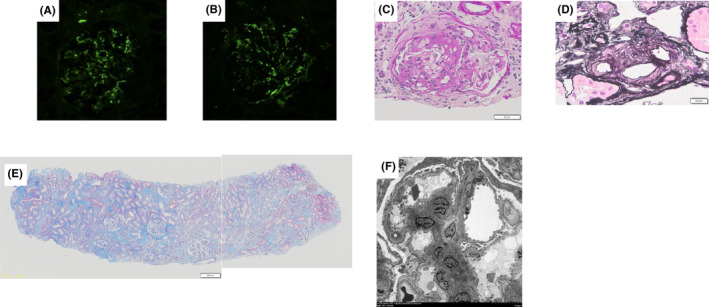
Kidney biopsy findings. (A) shows prominent mesangial IgA deposits for immunofluorescent stain of IgA. (B) shows prominent mesangial C3 deposits for immunofluorescent stain of C3. (C) shows a fibrocelluar crescent in the upper half of glomerulus for periodic acid–schiff stain. (D) shows a hyaline like changes in arterioles for periodic acid‐methenamine silver stain. (E) shows the limited fibrosis around sclerosing glomeruli in Masson Trichrome stain. (F) shows that electron dense deposits are present in the mesangial region of electron micrographs.
TABLE 2.
Changes of parameters before and after steroid pulse therapy.
| Pre‐1st pulse | Post‐1st pulse | Pre‐2nd pulse | Post‐2nd pulse | Pre‐3rd pulse | Post‐3rd pulse | |
|---|---|---|---|---|---|---|
| FEUA (%) | 55.8 | 58.6 | 48.5 | 53.4 | 50.7 | 51.1 |
| Cr (mg/dL) | 1.05 | 0.92 | 1.08 | 0.93 | 1.11 | 0.91 |
| Chalder's fatigue scale score | 17 | 17 | 15 | 14 | 14 | 15 |
Abbreviations: Cr, creatinine; EUA, fractional excretion of uric acid.
4. CONCLUSION AND RESULTS (OUTCOME AND FOLLOW‐UP)
He was discharged on the 17th day of hospitalization without any adverse events or renal dysfunction during treatment. The glucocorticoid was tapered off in the outpatient clinic. Currently, this patient is doing well with no acute renal failure due to hypouricemia, no worsening of renal function after remission, moreover, no recurrence of urinary protein.
5. DISCUSSION
Approximately 90% of uric acid filtered by the glomerulus is reabsorbed in the renal tubules. Loss of function of the transporters that reabsorb uric acid in the tubules due to genetic abnormalities results in RHUC. Two genetic abnormalities, URAT1 and GLUT9, are very well known as causes of RHUC, and the disease is classified into two forms based on these abnormalities. Its prevalence has been reported to be as low as 0.12%–0.72% 4 , 5 Complications have been reported as postexercise acute renal failure and kidney stones 6 , 7 Multiple genetic mutations of URAT1/SLC22A12 have been reported, indicating a high rate of W258X and R90H mutations among them. 8 Indeed, in another of our patient, W258X mutation was found (Table 3). In the present case, T217M mutation and R172L mutation were identified. The T217M mutation was previously reported as possible pathogenic variant but only found with a compound heterozygous mutation of W258X and T217M. 8 The R172L mutation has never been reported to be pathogenic. Therefore, in our patient, RHUC could be caused by a heterozygous mutation in T217M alone. However, when the R172L mutation is confirmed as a pathogenic mutation in the future, it remains possible that this case was a compound heterozygous mutation of T217M and R172L (Table 3). The accumulation of further cases will be required.
TABLE 3.
Clinical data and SLC22A12 mutations of the patients with Renalhypouricemia.
| No. | Gender | History | Complication | SLC22A12 Mutations (Amino Acid) | UA (mg/dL) |
|---|---|---|---|---|---|
| 1 | M | W258X | 0.7 | ||
| 2 | M | W258X | 0.6 | ||
| 3 | M | W258X | 0.7 | ||
| 4 | M | W258X | 0.8 | ||
| 5 | F | W258X | 0.5 | ||
| 6 | M | ARS, NS | IgAN | W258X | 1.0 |
| 7 | M | CRF | W258X | 1.8 | |
| 8 | F | CRF | W258X | 2.0 | |
| 9 | F | HSPN | W258X | 0.5 | |
| 10 | M | IgAN | W258X | 0.7 | |
| 11 | M | W258X | 0.5 | ||
| 12 | M | W258X/T217M | 0.9 | ||
| 13 | M | ARF | W258X/Q297X | 0.7 | |
| 14 | M | W258X/Frameshift | 0.8 | ||
| 15 | M | W258X/R90H | 0.7 | ||
| 16 | M | W258X/R90H | 0.6 | ||
| 17 | M | Proteinuria | W258X/R90H | 0.7 | |
| 18 | M | W258X/R90H | 1.1 | ||
| 19 | M | W258X/R90H | 0.4 | ||
| 20 | F | Urolithiasis | W258X/V138M | 0.6 | |
| 21 | M | Urolithiasis | W258X/V138 | 0.5 | |
| 22 | F | W258X/G164S | 0.8 | ||
| 23 | F | W258X/Frameshift | 0.6 | ||
| 24 | F | W258X/Q382L | 0.8 | ||
| 25 | F | W258X | 0.9 | ||
| 26 | F | W258X | 1.7 | ||
| 27 | M | ARF | Urolithiasis | W258X | 0.6 |
| 28 | F | W258X | 2.0 | ||
| 29 | F | W258X | 0.6 | ||
| 30 | F | M430T | 1.5 | ||
| 31 | F | Urolithiasis | 2.0 | ||
| 32 | F | 1.5 | |||
| 33 | F | W258X | 2.0 | ||
| 34 | M | IgAN, urolithiasis | T217M | 1.2 |
Abbreviations: ARF, acute renal failure; CRF, chronic renal failure; HSPN, Henoch‐Schoenlein purpura; IgAN, IgA nephropathy; NS, nephrotic syndrome; UA, uric acid.
Few cases of IgA nephropathy complicated by RHUC have been reported. 9 It is known that RHUC is complicated by acute renal failure after exercise. There have been reports of findings consistent with acute tubular necrosis, such as thickening of the basement membrane of the proximal tubules, fibrosis of the interstitium, and atrophy of the brush border, as well as evidence of uricemia in the tubular lumen. 9 , 10 On the other hand, in a case of RHUC without acute renal failure, it was reported that the microvasculature and glomeruli did not show any changes. 11 In the present case, the patient had no history of acute renal failure, and based on previous reports, we hypothesized that there would be little tissue changes in the microvasculature other than glomerular changes due to IgA nephropathy. Actually, renal biopsy demonstrated little changes by RHUC other than glomerular changes and fibrosis around sclerotic glomeruli presumably due to IgA nephropathy, such as mesangial cell proliferation and fibrous crescent. The patient had always averted a higher intensity exercise such as anaerobic exercises. Perhaps because of the fatigue caused by exercise, he had not been engaged in strenuous exercise that could have caused acute renal failure. This may reflect the tissue changes in our case. It has been known that uric acid has a role of antioxidant substance as well as oxidative stress. 12 In this point, it might be expected that there would be histological changes influenced by oxidative stress in the renal tissues. As for the microvessels, there were few abnormal lesions in the microvessels, although arteriolar hyalinosis was observed in only a small portion of the arterioles. In the present case, we couldn't find the distinctive features of arterioles due to hypouricemia in the kidney tissues. Several studies have shown a J‐shaped relationship between uric acid and a risk of cardiovascular events. The risk increased in those studies even in the group with lower uric acid levels, with a J‐shaped curve. 13 Further studies for influences of hypouricemia on intra‐renal vasculature are needed.
Tonsillectomy alone or with steroid pulse therapy is frequently performed in Japan because multiple studies have reported improved kidney survival and remission of proteinuria and hematuria. 14 , 15 Hence, one of the highly effective treatments for IgA nephropathy is tonsillectomy plus steroid pulse therapy. 16 Since the high doses of glucocorticoid are administered in a single dose in this steroid pulse therapy, various adverse reactions such as elevated blood pressure, elevated blood sugar and so on, can easily occur. We have previously reported that steroid pulse therapy for IgA nephropathy causes increased oxidative stress, which impairs vascular endothelial function. 17 Since patients with RHUC are vulnerable to strong oxidative stress, steroid pulse therapy in this patient might have the potential to cause fatigue and worsen renal function due to severe adverse effects. To confirm the effect of steroid pulse therapy on the patient, we evaluated the changes of fatigue level between before and after steroid pulse therapy using the Chalder's fatigue scale, 18 which quantifies subjective fatigue, and found that, contrary to our expectations, little changes were observed. Although there are several possible causes of post‐exercise acute renal failure, including renal ischemia due to renal vasospasm, 19 soluble UA and monosodium UA crystals elicit an immune response through activation of NLRP3, and tubular Na‐K‐ATPase is reported to cause tubular mitochondrial damage due to ATP consumption, contributing to the cause of exercise‐induced acute kidney injury. 20 In the current case, the uric acid excretion rate showed an increasing trend between before and after steroid pulse therapy. On the other hand, no obvious increase in Cr was observed. Thus, the increase in uric acid excretion due to short‐term steroid pulse therapy did not cause deterioration of renal function. These results demonstrate that steroid pulse therapy for IgA nephropathy complicated by RHUC did not demonstrate any burdensome enough to cause adverse events. The patient is currently on an outpatient steroid tapering regimen and is doing well, with no signs of renal dysfunction or recurrence of proteinuria. However, the effects of long‐term steroid use are unknown, and since this is the only case with hypouricemia who received steroid pulse therapy, further studies will be warranted to determine the impacts of steroids on IgA nephropathy patients with RHUC.
AUTHOR CONTRIBUTIONS
Yoshimasa Sakurabu: Data curation; investigation; writing – original draft. Haruhito A. Uchida: Conceptualization; data curation; project administration. Toshihisa Tahara: Data curation. Tomohiko Asakawa: Data curation. Haruka Yamasaki: Data curation. Katsuyoshi Katayama: Data curation. Shugo Okamoto: Data curation. Yasuhiro Onishi: Data curation. Natsumi Matsuoka‐Uchiyama: Data curation. Keiko Tanaka: Data curation. Hidemi Takeuchi: Data curation. Kenji Tsuji: Data curation. Ryoko Umebayashi: Data curation. Yuki Ohashi: Data curation. Kimiyoshi Ichida: Data curation. Jun Wada: Supervision.
FUNDING INFORMATION
No funds, grants, or other support was received.
CONFLICT OF INTEREST STATEMENT
None of the author and co‐authors has any conflicts of interest or any financial ties to disclose.
ETHICS STATEMENT
This case report has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Declaration of Helsinki.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Sakurabu Y, Uchida HA, Tahara T, et al. A case of renal hypouricemia due to T217M mutation in SLC22A12 incidentally associated with IgA nephropathy. Clin Case Rep. 2024;12:e9368. doi: 10.1002/ccr3.9368
DATA AVAILABILITY STATEMENT
All data generated or analyzed in this case report are included in this published article.
REFERENCES
- 1. Perdomo‐Ramirez A, Cordoba‐Lanus E, Trujillo‐Frias CJ, et al. Pathogenic variants of SLC22A12 (URAT1) and SLC2A9 (GLUT9) in Spanish patients with renal Hypouricemia: founder effect of SLC2A9 variant c.374C>T; p.(T125M). Int J Mol Sci. 2023;24(9):8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazzierli T, Cirillo L, Palazzo V, Ravaglia F, Becherucci F. Clinical features suggesting renal hypouricemia as the cause of acute kidney injury: a case report and review of the literature. J Nephrol. 2023;36(3):651‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiryluk K, Freedberg DE, Radhakrishnan J, et al. Global incidence of IgA nephropathy by race and ethnicity: a systematic review. Kidney360. 2023;4(8):1112‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Peenen HJ. Causes of hypouricemia. Ann Intern Med. 1973;78(6):977‐978. [DOI] [PubMed] [Google Scholar]
- 5. Hisatome I, Ogino K, Kotake H, et al. Cause of persistent hypouricemia in outpatients. Nephron. 1989;51(1):13‐16. [DOI] [PubMed] [Google Scholar]
- 6. Ishikawa I, Saito Y, Shinoda A, Onouchi Z. Evidence for patchy renal vasoconstriction in man: observation by CT scan. Nephron. 1981;27(1):31‐34. [DOI] [PubMed] [Google Scholar]
- 7. Erley CM, Hirschberg RR, Hoefer W, Schaefer K. Acute renal failure due to uric acid nephropathy in a patient with renal hypouricemia. Klin Wochenschr. 1989;67(5):308‐312. [DOI] [PubMed] [Google Scholar]
- 8. Ichida K, Hosoyamada M, Hisatome I, et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan‐influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15(1):164‐173. [DOI] [PubMed] [Google Scholar]
- 9. Tofuku Y, Nakashima A, Koni I. A case of renal hypouricemia associated with IgA nephropathy—with special reference to changes in serum uric acid and uric acid clearance in a clinical course exceeding 15 years. Nihon Jinzo Gakkai Shi. 1994;36(3):277‐283. [PubMed] [Google Scholar]
- 10. Takeda Y, Fujimoto T, Uyama H, et al. Two cases of exercise‐induced acute renal failure with idiopathic renal hypouricemia. Nihon Jinzo Gakkai Shi. 2001;43(5):384‐388. [PubMed] [Google Scholar]
- 11. Ito O, Hasegawa Y, Sato K, et al. A case of exercise‐induced acute renal failure in a patient with idiopathic renal hypouricemia developed during antihypertensive therapy with losartan and trichlormethiazide. Hypertens Res. 2003;26(6):509‐513. [DOI] [PubMed] [Google Scholar]
- 12. Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta. 2008;392(1–2):1‐7. [DOI] [PubMed] [Google Scholar]
- 13. Kamei K, Konta T, Hirayama A, et al. Associations between serum uric acid levels and the incidence of nonfatal stroke: a nationwide community‐based cohort study. Clin Exp Nephrol. 2017;21(3):497‐503. [DOI] [PubMed] [Google Scholar]
- 14. Kawamura T, Yoshimura M, Miyazaki Y, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin a nephropathy. Nephrol Dial Transplant. 2014;29(8):1546‐1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawasaki Y, Takano K, Suyama K, et al. Efficacy of tonsillectomy pulse therapy versus multiple‐drug therapy for IgA nephropathy. Pediatr Nephrol. 2006;21(11):1701‐1706. [DOI] [PubMed] [Google Scholar]
- 16. Hotta O, Miyazaki M, Furuta T, et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis. 2001;38(4):736‐743. [DOI] [PubMed] [Google Scholar]
- 17. Uchida HA, Nakamura Y, Kaihara M, et al. Steroid pulse therapy impaired endothelial function while increasing plasma high molecule adiponectin concentration in patients with IgA nephropathy. Nephrol Dial Transplant. 2006;21(12):3475‐3480. [DOI] [PubMed] [Google Scholar]
- 18. Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147‐153. [DOI] [PubMed] [Google Scholar]
- 19. Ishikawa I. Acute renal failure with severe loin pain and patchy renal ischemia after anaerobic exercise in patients with or without renal hypouricemia. Nephron. 2002;91(4):559‐570. [DOI] [PubMed] [Google Scholar]
- 20. Hosoya T, Uchida S, Shibata S, Tomioka NH, Matsumoto K, Hosoyamada M. Xanthine oxidoreductase inhibitors suppress the onset of exercise‐induced AKI in high HPRT activity Urat1‐Uox double knockout mice. J Am Soc Nephrol. 2022;33(2):326‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this case report are included in this published article.


