Abstract
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are effective for glycemic control, with many also demonstrating cardiovascular (CV) benefit, in people with type 2 diabetes (T2D). This study aimed to find a consensus on the barriers and strategies for the optimal use of GLP-1 RAs in people with T2D and high CV risk or established cardiovascular disease (CVD) in Spain.
Methods
A two-round Delphi survey (53 questions) was conducted among members of four national scientific societies in Spain, including physicians experienced in the management of people with T2D. The degree of consensus was evaluated with a 7-point Likert scale, establishing consensus when ≥ 70% of the panelists agreed (6–7) or disagreed (1–2).
Results
A total of 97 physicians participated in the first round (endocrinology: 34%, family and community medicine: 21%, internal medicine: 23%, and cardiology: 23%), and 96 in the second round. The main barriers identified were: therapeutic inertia and late use of GLP-1 RAs; lack of a comprehensive approach to CV risk; lack of knowledge on the usefulness of GLP-1 RAs in CVD prevention and treatment; and economic/administrative barriers. Strategies with a highest consensus included: the need to establish simple protocols that integrate awareness of CV risk monitoring; training professionals and patients; and the use of new technologies.
Conclusion
Physicians identified clinical, healthcare, and economic/administrative barriers that limit the use of GLP-1 RAs in people with T2D and high CV risk or established CVD in Spain, highlighting the importance of integrating these therapies according to clinical practice guidelines.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02938-2.
Keywords: Type 2 diabetes, GLP-1 receptor agonists, Cardiovascular risk, Cardiovascular prevention, Clinical guidelines
Key Summary Points
| Why carry out this study? |
| Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are effective for glycemic control, with many also demonstrating cardiovascular (CV) benefit, in people with type 2 diabetes (T2D). |
| GLP-1 RAs are underprescribed in people with T2D and high CV risk or established cardiovascular disease (CVD) in Spain. |
| This study investigated the potential barriers to and strategies for the optimal use of GLP-1 RAs in people with T2D with high CV risk or established CVD in Spain. |
| What was learned from the study? |
| The traditional stepwise approach to T2D management, the costs for patients not meeting required medical criteria to obtain funded GLP-1 RA prescriptions, the administrative procedures for obtaining prescription authorization, and not considering CV risk as a switching treatment factor, were the main barriers that limited the use of GLP-1 RAs in people with T2D and high CV risk or established CVD. |
| Potential solutions include establishing simple protocols that integrate awareness of CV risk monitoring, training professionals and patients, and the use of new technologies. |
| It is important to integrate the use of therapies with CV benefit into the treatment of patients with T2D in accordance with clinical practice guidelines. |
Introduction
In people with type 2 diabetes (T2D), cardiovascular disease (CVD) is a highly prevalent and well-established risk factor for morbidity and mortality. Approximately one-third of people with T2D develop CVD, and CVD is estimated to be the cause of death in almost 50% of people with T2D [1, 2]. In Spain, > 90% of people with T2D are at high or very high cardiovascular (CV) risk [3]. CVD in people with T2D has a significant clinical and economic impact compared with people with T2D without CVD, mainly due to hospitalizations [4]. For these reasons, primary and secondary prevention of CVD in people with T2D should be a priority when considering any T2D treatment [4].
Current clinical guidelines indicate that CV risk factors should be assessed in all people with T2D, since their control is essential for the optimal management of T2D [5–9]. In this regard, based on the results of the CV outcome trials, the latest American Diabetes Association (ADA)-European Association for the Study of Diabetes (EASD) consensus recommends for the first time the use of sodium-glucose cotransporter-2 inhibitors (SGLT-2is) or glucagon-like peptide 1 receptor agonists (GLP-1 RAs) with proven CV benefit as first-line glucose-lowering therapy in people with T2D with established CVD, indicators of high CV risk, or established kidney disease or heart failure, independently of glycated hemoglobin (HbA1c) levels or previous metformin use [9]. This treatment approach of considering cardiorenal protection even in early disease stages had already been proposed by the American College of Cardiology in 2019 and has since been included in new updates of various Spanish consensus documents [10–12].
GLP-1 RAs increase glucose-dependent insulin secretion and glucagon suppression, slow gastric emptying, and reduce appetite and body weight, with the overall effect of decreasing HbA1c levels without generally increasing hypoglycemia risk. Also, many GLP-1 RAs can reduce the occurrence of major adverse CV events, CV mortality, and the risk of all-cause mortality, [13, 14]. However, recent studies have shown that use of GLP-1 RAs is limited in most countries [15]. Some studies have highlighted the urgency of implementing guidelines to ensure that people with T2D and high CV risk benefit from these therapies [7, 16–19]. In Spain, current legislation restricts public reimbursement of GLP-1 RAs to the treatment of people with T2D and obesity [body mass index (BMI) ≥ 30 kg/m2] and only in combination with other glucose-lowering drugs [20]. Additionally, observational studies in Spain have shown that the use of GLP-1 RAs is suboptimal and mainly restricted to advanced and complex patient types: with BMI > 35 kg/m2; HbA1c levels > 8.0%; comorbidities; and often at an advanced stage of diabetes (> 9 years since diagnosis) [21–24]. A recent study demonstrated that GLP-1 RAs are scarcely used in primary care and mostly for people with T2D and obesity [25].
Prior Delphi studies in Spain have shown that no consensus was reached regarding the preferential use of GLP-1 RAs in patients with established CVD [26, 27]. However, a recent study of family physicians revealed that the presence of CVD or high CV risk was a critical factor in deciding T2D therapy [25]. The barriers that could limit the wider use of GLP-1 RAs in people with T2D and CV risk are poorly defined and could have patient, clinician, and system-level origins [16, 21–24]. However, there are still major uncertainties regarding the reasons that limit the use of GLP-1 RAs, or what could be done to close the gap with guideline recommendations. To investigate the potential barriers to and strategies for the optimal use of GLP-1 RAs in people with T2D with high CV risk or established CVD in the Spanish setting, a two-round Delphi consultation was conducted among primary care physicians, endocrinologists, cardiologists, and internal medicine specialists. The goal was to analyze the perspectives and perceptions of a multidisciplinary group of physicians currently treating people with T2D and CV risk or established CVD to reach a consensus that could help encourage wider application of current guidelines and improve patient care.
Methods
A national two-round Delphi study was conducted to reach a consensus on the barriers and strategies to prescribing GLP-1 RAs in patients with CV risk or established CVD [28–30]. The study was conducted over a 3-month period: the first Delphi round took place from March to April 2022, and the second Delphi round from April to May 2022.
A general scheme of the design and development of the Delphi consultation is shown in Fig. S1 in the electronic supplementary material. A multidisciplinary Scientific Committee, consisting of two endocrinologists, a family physician, an internist, and a cardiologist, was established. Each of the members of the Scientific Committee represented one of the national scientific societies endorsing the project: Spanish Society of Cardiology (SEC); Spanish Society of Diabetes (SED); Spanish Society of Endocrinology and Nutrition (SEEN); and Spanish Society of Internal Medicine (SEMI).
Ethical approval
The study was submitted to the Ethics Committee of 12 de Octubre Hospital (Madrid, Spain), which judged that, due to the nature of the study, further approval was not required. All participating physicians were aware of the objectives of the study, and that the results of this study would be published. Participants were identified and invited to participate through their respective scientific medical societies, and their views were independent and confidential. Participants were asked to complete the questionnaire according to their perceptions of their daily practice environment within their specialty, and not reflecting their own personal clinical practice. Participation was agreed in writing. Data were de-identified to protect the privacy of the participants.
Participants
Panelists eligible for participation in the study met the following selection criteria: they were specialists in endocrinology, family and community medicine, internal medicine, or cardiology, with at least 2 years of experience managing people with T2D and high CV risk or established CVD, and practicing in the public healthcare setting in Spain. Panelists were identified and invited to participate through their respective scientific societies.
Delphi Methodology
The Delphi questionnaire was designed by the Scientific Committee based on a targeted literature review. The final questionnaire consisted of 53 items (see Table S1 in the electronic supplementary material for details) and was comprised of two sections. Section A was only included in the first round and consisted of sociodemographic variables, information on the physician’s experience, and healthcare center characteristics. Section B included statements describing 24 potential barriers to the use of GLP-1 RAs in people with T2D and high CV risk or established CVD and 28 potential strategies to optimize the use of GLP-1 RAs and improve adherence to the recommendations of the clinical practice guidelines.
Panelists were asked to rate on a 7-point Likert scale (1 = strongly disagree; 2 = disagree; 3 = somewhat disagree; 4 = neither agree nor disagree; 5 = somewhat agree; 6 = agree; and 7 = strongly agree) their agreement or disagreement with the statements. Strategies were explored from two perspectives: appropriateness and feasibility. No free-text fields were included in the questionnaire. Physicians were asked to complete the questionnaire according to their perceptions of their daily practice environment within their specialty, and not reflecting their own personal clinical practice. All statements in the Delphi questionnaire were evaluated for consensus in a first round of consultation with the panelists. Consensus was reached when at least 70% of the panelists agreed (6–7 scores) or disagreed (1–2 scores) with the given statement. Those items for which consensus was not reached in the first round were sent for a second round of consultation. For statements reaching consensus, results correspond to those obtained in the round in which consensus was reached; for statements not reaching consensus, results from the second round are provided.
Statistical Methods
As Delphi surveys do not have a defined study size [31], the appropriate panel size was estimated at 100 panelists (to ensure a minimum of 20 per society or specialty). Considering an acceptance rate of 90%, it was estimated that 110 panelists should be invited to participate in the study. Absolute and relative frequencies were calculated to describe categorical variables. Means and standard deviations (SD), and medians and interquartile ranges (IQR), were calculated to express continuous variables. Sociodemographic characteristics (overall and for each panelist group) were described. Descriptive analyses were performed for the 7-point Likert scale items. First, the number and percentage of participants who selected each option were calculated for the two-round Delphi analysis. Next, the frequency and proportion for each response category (1–2: disagree; 3–5: neither agree nor disagree; and 6–7: agree) were calculated. The consensus was achieved based on the global sample and also described by the panelist group (endocrinologists, internists, cardiologists, family practitioners). The round in which consensus was reached was also recorded.
Results
A total of 108 panelists were invited to participate in the Delphi consultation. Of these, 97 completed the first round of the Delphi questionnaire (89.8% response rate) and 96 panelists completed the second round (99% response with respect to round one) (Fig. 1). Of the 97 panelists, 33 (34.0%) were endocrinologists, 22 (22.7%) were cardiologists, 22 (22.7%) were internal medicine specialists, and 20 (20.6%) were family and community medicine physicians. The respondents represented all the Spanish regions except Cantabria. The mean (± SD) age of the panelists was 52.3 ± 9.0 years and 59.8% were men (Table 1). Most of them (61%) worked in tertiary healthcare centers, 44% of which had a specialized diabetes unit. Participants had 22.4 ± 9.4 years of experience and attended 88.1 ± 63.6 patients weekly, 36.9 ± 20.4% of whom had a T2D diagnosis. Participants reported that 71.3 ± 28.1% of the people with T2D they attended weekly were considered of high CV risk or presented with established CVD, and declared that 38.7 ± 28.1% of them had prescriptions for GLP-1 RAs.
Fig. 1.
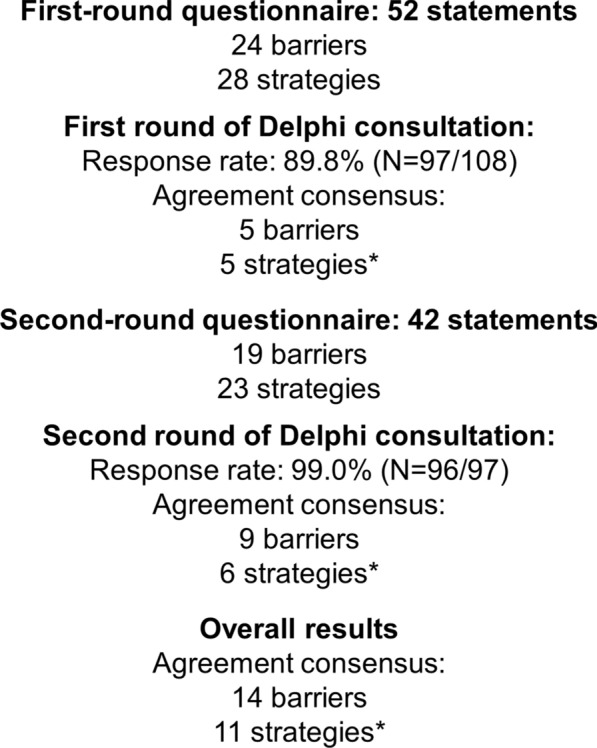
Development of the Delphi consensus. N number of physicians
Table 1.
Physicians’ sociodemographic characteristics, professional experience, and healthcare center characteristics (based on round one)
| Variable | Total (n = 97) | Endocrinology (n = 33) |
Cardiology (n = 22) |
Internal medicine (n = 22) |
Family and community medicine (n = 20) | |
|---|---|---|---|---|---|---|
| Age, years | Mean ± SD | 52.3 ± 9.0 | 49.6 ± 9.5 | 49.6 ± 8.9 | 53.6 ± 8.3 | 58.2 ± 5.1 |
| Median (IQR) | 53.0 (45.0–60.0) | 53.0 (41.0–57.0) | 49.0 (45.0–54.8) | 53.5 (47.0–60.8) | 59.0 (55.0–62.3) | |
| Sex, male | n (%) | 58 (59.8) | 18 (54.5) | 14 (63.6) | 12 (54.5) | 14 (70.0) |
| Years of experience | Mean ± SD | 22.4 ± 9.4 | 20.9 ± 9.8 | 17.6 ± 8.1 | 23.2 ± 9.0 | 29.5 ± 5.6 |
| Median (IQR) | 22.0 (16.0–30.0) | 22.0 (13.0–28.0) | 18 (12.5–20.0) | 20.0 (15.8–331.5) | 30.0 (25.0–32.8) | |
| Number of individuals attended weekly in consultation | Mean ± SD | 88.1 ± (63.6) | 93.3 ± 59.6 | 60.5 ± 39.0 | 37.5 ± 25.7 | 165.8 ± 40.6 |
| Median (IQR) | 75.0 (40.0–125.0) | 80.0 (60.0–100.0) | 50.0 (40.0–67.5) | 27.5 (16.3–60.0) | 150.0 (147.5–200.0) | |
| Percentage of people with a T2D diagnosis | Mean ± SD | 36.9 ± 20.4 | 41.4 ± 21.5 | 31.3 ± 9.1 | 52.4 ± 16.9 | 18.4 ± 13.3 |
| Percentage of people with T2D with high CV risk or established CVD | Mean ± SD | 71.3 ± 28.1 | 69.3 ± 26.4 | 89.7 ± 18.0 | 83.2 ± 15.6 | 41.1 ± 24.6 |
| Percentage of people with T2D with high CV risk or established CVD and a GLP-1 RA prescription (by the responder) | Mean ± SD | 38.7 ± 28.1 | 56.1 ± 23.1 | 20.2 ± 12.4 | 42.0 ± 27.1 | 26.6 ± 16.4 |
| Healthcare center level | ||||||
| Primary care | n (%) | 20 (20.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 20 (100) |
| Secondary care | n (%) | 18 (18.6) | 9 (27.3) | 3 (13.6) | 6 (27.3) | 0 (0.0) |
| Tertiary care | n (%) | 59 (60.8) | 24 (72.7) | 19 (86.4) | 16 (72.7) | 0 (0.0) |
| Diabetes unit in the work center | n (%) | 43 (44.3) | 20 (60.6) | 14 (63.6) | 7 (31.8) | 2 (10.0) |
CV cardiovascular, CVD cardiovascular disease, GLP-1 RA glucagon-like peptide 1 receptor agonist, n number, IQR interquartile range, SD standard deviation, T2D type 2 diabetes
Potential Barriers to GLP-1 RA Use
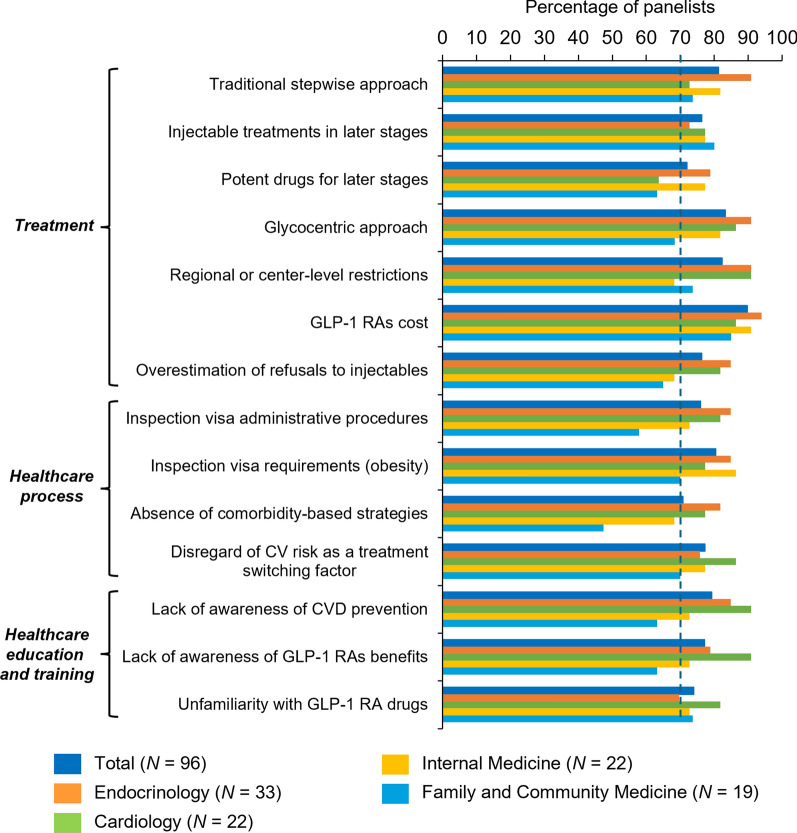
Of 24 items describing potential barriers to GLP-1 RA use, which included treatment-related barriers and barriers associated with the healthcare process, healthcare organization and resources, and healthcare education and training, the participants reached a consensus in 14 (all results below are given considering all specialties together, unless otherwise indicated) (Fig. 2; see Table S2 in the electronic supplementary material for final results). Of the 14 barriers which achieved consensus, 5 (36%) were agreed on by all specialties: the traditional stepwise approach that contributes to later use of GLP-1 RAs (#2 in Table S2), the use of injectable traditionally used in later stages of the disease (#3), costs for patients not meeting the specific medical criteria established by the health authorities in order to be able to be prescribed GLP1-RAs under reimbursed conditions (visa requirements) (#10), the administrative procedures for obtaining prescription authorization (#13), and not considering CV risk as a switching treatment factor (#17). In contrast, for the remaining ten barriers that did not obtain consensus, individual agreement was not reached in any subgroup.
Fig. 2.
Barriers to the use of GLP-1 RAs: percentage of agreement (6–7 agree) by subgroup. CV cardiovascular, CVD cardiovascular disease, GLP-1 RAs glucagon-like peptide 1 receptor agonists, N number of physicians
Seven of the 11 treatment-related barriers (64%) reached consensus among all participants. The perceived barriers associated with the use of GLP-1 RAs that achieved consensus were related to therapeutic inertia [the traditional stepwise approach (#2), the use of injectable treatments in later stages of the disease (#3), using the most potent drugs for later stages of the disease (#4), and the glycocentric approach (#5)], regional- or center-level restrictions (#6), GLP-1 RA cost for patients who do not meet the obesity requirement to be prescribed GLP1-RAs under funded conditions (#10), and the overestimation of patients' refusal of injectable drugs (#11).
Regarding barriers related to the healthcare process, consensus was achieved in 4 of 10 barrier statements (40%). The main limitations of the use of GLP-1 RAs were related to administrative requirements (#12 and #13), the absence of comprehensive and individualized treatment strategies based on comorbidities of people with T2D (#16), and not considering CV risk as a switching treatment factor (#17). None of the barriers related to healthcare organization and resources (lack of time in consultation, lack of support staff, or the absence of a shared electronic medical record history) achieved consensus. In contrast, the three proposed barriers to healthcare education and training level reached consensus. Participants agreed that the lack of awareness of the importance of CV prevention (#22), the lack of awareness of GLP-1 RA benefits in the management of people with T2D (#23), and the unfamiliarity of healthcare professionals using GLP-1 RAs for CV risk control (#24), hinder the use of GLP-1 RAs in routine clinical practice.
Potential Strategies to Optimize the Use of GLP-1 RAs
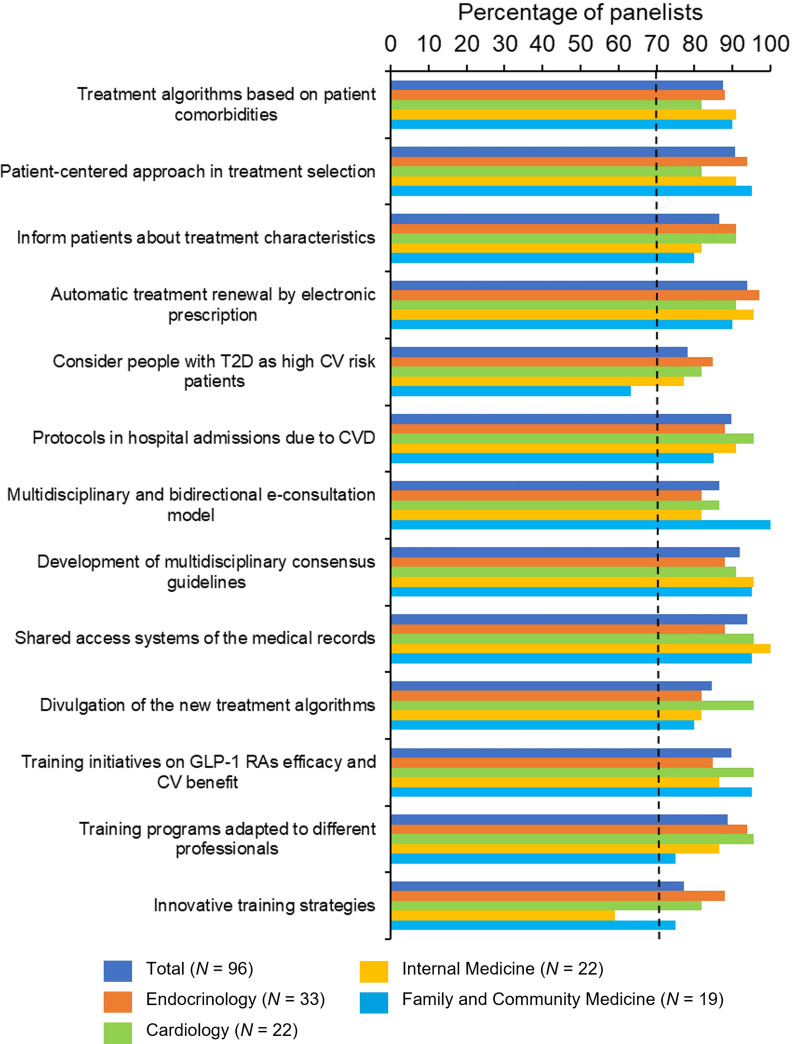
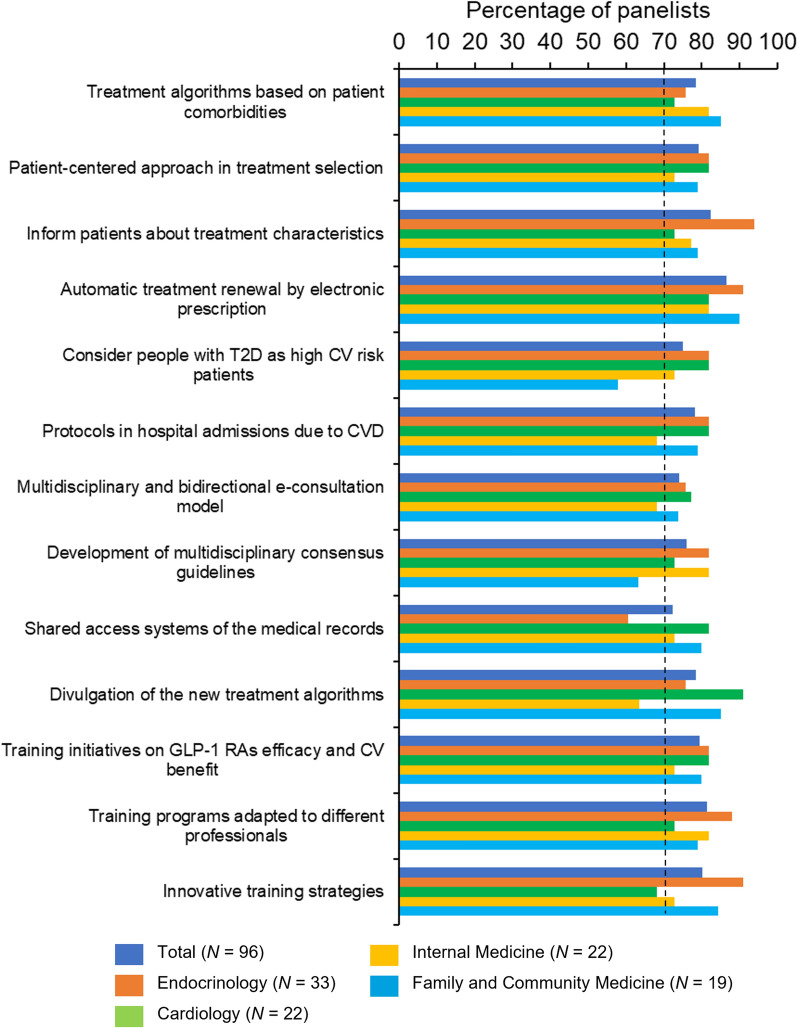
Of 28 items describing potential solutions to optimize the use of GLP-1 RAs according to clinical practice guidelines, global consensus on both suitability and feasibility was reached on 13 statements (46%). Of these, only six achieved consensus across all specialties. Figures 3 and 4 show the results for suitability and feasibility, respectively, of each proposed strategy reaching consensus after the two rounds of Delphi consultation (see Table S3 in the electronic supplementary material for the final results).
Fig. 3.
Potential solutions that reached a consensus (suitability), by subgroup. CV cardiovascular, CVD cardiovascular disease, GLP-1 RAs glucagon-like peptide 1 receptor agonists, N number of physicians, T2D type 2 diabetes
Fig. 4.
Potential solutions that reached a consensus (feasibility), by subgroup. CV cardiovascular, CVD cardiovascular disease, GLP-1 RAs glucagon-like peptide 1 receptor agonists, N number of physicians, T2D type 2 diabetes
Regarding potential solutions related to the treatment, panelists reached consensus on the suitability and feasibility of the promotion of a more patient-centered approach to treatment selection (#3 in Table S3), and the implementation of treatment optimization protocols in people with T2D with recent hospital admissions due to CVD (#11). At the healthcare process level, physicians agreed that all people with T2D should be considered high CV risk patients (#10), that the cooperation between scientific societies to develop updated multidisciplinary consensus guidelines should be promoted (#17), and that simple treatment algorithms based on patient comorbidities should be developed and implemented (#1).
Concerning organizational and resource-related potential solutions, the panelists agreed on the need to establish a multidisciplinary and bidirectional e-consultation model (#16), the implementation of shared access systems of medical records to facilitate communication between specialties (#18), and the implementation of automatic electronic treatment renewal (#7). Finally, regarding healthcare education and training solutions, consensus was obtained on the need for informing patients about the essential characteristics of their treatment (#6), the dissemination of the new treatment algorithms by the scientific societies and/or the industry (#22), the promotion and development of training initiatives on GLP-1 RA efficacy and CV benefit (#25), the promotion of training programs adapted to different professional profiles (#26), and the definition of innovative training strategies (#27).
Discussion
The objective of the two-round Delphi consultation described here was to identify the main perceived barriers to GLP-1 RA use in people with T2D and high CV risk or established CVD, as well as potential solutions in the Spanish setting. Our study achieved a high rate of response among the invited participants, as well as a high level of consensus among clinical specialties. This Delphi consultation showed that the main barriers identified were therapeutic inertia, a lack of a comprehensive approach to CV risk, lack of knowledge on the usefulness of GLP-1 RAs in CV prevention and treatment, and economic/administrative barriers. The potential strategies with the highest consensus included the need to establish simple protocols that integrate awareness of CV risk monitoring, improved training of professionals and patients, and the use of new technologies. However, responses by specialties were heterogeneous and there was a lack of consensus across all specialties for many of the statements analyzed.
Therapeutic inertia is one of the well-known causes delaying the implementation of clinical practice guideline recommendations into routine clinical practice in people with T2D [16, 32]. Improved training of health professionals, coupled with the implementation of simpler protocols that integrate awareness of CV risk monitoring, could help in solving the problem. For example, GLP-1 RAs have been associated for some years with weight reduction, and this could have restricted their preferential prescription to people with obesity [23–25]. To overcome these barriers, promoting a patient-centered approach in therapeutic decisions and the patient's involvement in managing the disease were identified as potential solutions. Innovative training initiatives on GLP-1 RA efficacy and CV benefit adapted to different professional profiles also appeared to be a necessary solution to overcoming therapeutic inertia. In line with this, several publications have previously encouraged training in optimizing the use of available therapies and cooperation in local training activities to ensure that the CV benefit of SGLT-2is and GLP-1 RAs is more widely acknowledged [16, 33, 34]. As diabetes therapies are constantly evolving, systematic medical education of all healthcare providers involved in the treatment of people with T2D should be a priority to overcome clinical inertia.
A significant barrier agreed by the panelists was the absence of comprehensive and individualized treatment strategies based on comorbidities of people with T2D in the Spanish setting, as well as neglecting CV risk as a factor for switching treatment. As a possible solution, panelists agreed to consider all people with T2D as high CV risk patients. This approach had been suggested before as a possible solution to the general low use of GLP-1 RAs and SGLT-2is [33]. In this regard, a recent survey suggested that one of the most influential clinical factors in the choice of glucose-lowering treatment in Spain was the presence of CVD (the other being high HbA1c) [25]. However, family physicians found this approach neither suitable nor feasible. This could be a reflection of the low perception of CV risk in people with T2D observed among this group of physicians [3].
The high number of pharmacologic agents available to treat T2D and the complexity of treatment algorithms were not identified as key barriers for the prescription of GLP-1 RAs, as proposed by other authors [35, 36]. Nonetheless, participants agreed on the need to develop and implement simple treatment algorithms based on patient comorbidities and their dissemination by the different scientific societies and/or the industry. Also, they agreed that treatment optimization protocols in people with T2D with recent hospital admissions due to CVD should be implemented. It has been suggested that the simplification of treatment algorithms that may be too complicated for primary care physicians, who often have very limited time to treat their patients, is necessary [33]. Further, panelists agreed on the use of a multidisciplinary approach of collaboration between specialties in the treatment of people with T2D through the implementation of bidirectional e-consultation models and shared access systems of medical records. Several studies have already pointed out the need to provide more opportunities for interdisciplinary collaboration and increase the number of healthcare professionals in multidisciplinary teams to provide optimal patient care and promote adherence to clinical practice guidelines [7, 16, 37].
Panelists agreed that the overestimation of patients’ refusal of injectable drugs limits the early use of GLP-1 RAs, which are recommended before insulin initiation as the first injectable therapy [6, 8]. However, some studies have identified a patient preference for oral medications [24]. Dose frequency and specific details of the treatment administration process, such as the type of injection device or needle size, also impact patient preference regarding GLP-1 RAs, and a better knowledge of these will be important to help overcome possible barriers [38–40]. Also, it is possible that some specialties (e.g., internal medicine physicians) are less familiar with injectable drugs and that this results in reduced prescription by this group.
In Spain, administrative requirements for GLP-1 RA prescription, such as the renewal of treatment or the administrative burden associated with the procedure physicians must follow to obtain prescription authorization, were also identified as potential barriers. As a potential solution to this, panelists agreed on allowing automatic electronic renewal of GLP-1 RA prescriptions. Minimization of the administrative burden has been proposed as an incentive to increase the use of GLP-1 RAs [17]. However, the review of the medical criteria required to receive a GLP-1 RA prescription did not reach a consensus between specialists as a potential solution. Currently, in Spain, a high BMI (≥ 30 kg/m2) is an essential factor for reimbursement. The cost of the treatment with GLP-1 RAs for individuals who do not meet the obesity requirement to access GLP-1 RAs via funded prescriptions was considered a barrier to early access. However, for those at risk, treatment at early stages of the disease is beneficial and should be encouraged, irrespective of BMI [33].
In Spain and other European countries, the high cost gap between old and new glucose-lowering medicines such as GLP-1 RAs could affect market uptake and consequently patient access [41]. However, other countries with no specific BMI requirement also register low use of GLP-1 RAs, suggesting that the cost limitation could not be a major obstacle in its wider uptake [18, 42]. Finally, panelists also considered that many healthcare professionals are unfamiliar with GLP-1 RA use for CV risk control, probably due to a lack of knowledge about the importance of CV prevention and the CV benefit of GLP-1 RAs (also considered a relevant barrier). The results of our study showed a lower percentage of patients with high CV risk or established CVD with GLP-1 RA prescriptions treated by cardiologists compared with other specialties. This could reflect a preferential use of SGLT-2is by cardiologists in Spain. In this regard, a recent study showed that the use of SGLT-2is was highest in patients treated by cardiologists versus primary care physicians, endocrinologists, and other specialists [19].
This study has several limitations. Since highly expert participants were selected for the consultation, the sample may not fully represent the medical population under investigation. However, to identify barriers and, in particular, solutions, a high level of expertise in the field is more important. In this sense, panelists had extensive experience managing people with T2D with high CV risk or established CVD. In addition, almost all participants were members of at least one diabetes and/or CV risk-related working group. Likewise, the present results should be interpreted considering the observational and exploratory nature of the survey based on self-reporting, reflecting the participants’ subjective perceptions. In this regard, physicians were asked to rate the statements according to their perceptions of their daily practice environment within their specific specialty, and not reflecting their own personal clinical practice. Also, when completing the Delphi questionnaire, physicians were asked to consider only outpatients with T2D and not those who were hospitalized. Another possible limitation was the use of a structured questionnaire without free-text fields where qualitative insights from participants could be obtained. In this respect, all statements were designed to be clear and understandable to participants to ensure the correct and consistent interpretation of the questionnaire. Additionally, at the time this study was conducted, global and temporary supply problems affecting availability of injectable GLP-1 RAs did not exist as they do at the time of writing this article, so participants could not evaluate this issue during the Delphi consultation [43]. Finally, the study captured the perceptions of healthcare providers at a single point in time, but clinical guidelines and medical practices are constantly evolving, and this, in turn, could affect the study results. Future studies should address if changes in perceptions occur after the release of new guidelines.
Conclusion
This study highlights the importance of integrating the use of therapies with CV benefit into the treatment of people with T2D in accordance with clinical practice guidelines. In the Spanish setting, this is the first multidisciplinary consensus to identify barriers and strategies to optimize the use of GLP-1 RAs in people with T2D with high CV risk or with established CVD. Physicians identified clinical, healthcare, and economic/administrative barriers that limit the use of GLP-1 RAs and agreed on potential solutions. While the study was conducted in Spain, the underutilization of GLP-1 RAs in people with high CV risk or with established CVD has been observed in many countries and represents a global concern. These results could be helpful in assisting the implementation of the use of therapies with CV benefit in the treatment of people with T2D, according to the clinical practice guidelines.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of this study.
Medical Writing/Editorial Assistance
Francisco López de Saro and Sheridan Henness (Rx Communications, Mold, UK) provided medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
Author Contribution
All authors contributed substantially to this study and are in agreement with the content of the manuscript. Conception/design: Esther Artime, Héctor David de Paz, Miriam Rubio de Santos, and Silvia Díaz-Cerezo; Collection and/or assembly of data: Héctor David de Paz; Data analysis and interpretation: Manuel Botana López, Miguel Camafort Babkowski, Raquel Campuzano Ruiz, Ana Cebrián Cuenca, Manuel Gargallo Fernández, Héctor David de Paz, Jennifer Redondo-Antón, Esther Artime, Silvia Díaz-Cerezo, Miriam Rubio de Santos; Manuscript writing: Manuel Botana López, Miguel Camafort Babkowski, Raquel Campuzano Ruiz, Ana Cebrián Cuenca, Manuel Gargallo Fernández, Héctor David de Paz, Jennifer Redondo-Antón, Esther Artime, Silvia Díaz-Cerezo, Miriam Rubio de Santos; Final approval of manuscript: all authors.
Funding
This study, the journal’s rapid service fee, and the open access fee were funded by Eli Lilly and Company.
Data Availability
All data generated or analysed during this study are included in this published article/as supplementary information files.
Declarations
Conflict of Interest
Jennifer Redondo-Antón, Esther Artime, Silvia Díaz-Cerezo, and Miriam Rubio de Santos are employees and minor shareholders of Eli Lilly and Company. Manuel Botana López, Miguel Camafort Babkowski, Raquel Campuzano Ruiz, Ana Cebrián Cuenca, Manuel Gargallo Fernández, and Héctor David de Paz declare no conflict of interest.
Ethical Approval
The study was submitted to the Ethics Committee of 12 de Octubre Hospital (Madrid, Spain), which judged that, due to the nature of the study, further approval was not required. All participating physicians were aware of the objectives of the study, and that the results of this study would be published. Participants were identified and invited to participate through their respective scientific medical societies, and their views were independent and confidential. Participants were asked to complete the questionnaire according to their perceptions of their daily practice environment within their specialty, and not reflecting their own personal clinical practice. Participation was agreed in writing. Data were de-identified to protect the privacy of the participants.
Footnotes
Prior presentation: This work was previously presented at the 63rd Congress of the Spanish Society for Endocrinology and Nutrition (Las Palmas de Gran Canaria, Spain, 26–28 October 2022).
Manuel Botana López, Miguel Camafort Babkowski, Raquel Campuzano Ruiz, Ana Cebrián Cuenca and Manuel Gargallo Fernández have contributed equally and on behalf of their medical societies.
References
- 1.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5:444–70. 10.4239/wjd.v5.i4.444. 10.4239/wjd.v5.i4.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83. 10.1186/s12933-018-0728-6. 10.1186/s12933-018-0728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cebrián-Cuenca AM, Mata-Cases M, Franch-Nadal J, Mauricio D, Orozco-Beltrán D, Consuegra-Sánchez L. Half of patients with type 2 diabetes mellitus are at very high cardiovascular risk according to the ESC/EASD: data from a large Mediterranean population. Eur J Prev Cardiol. 2022;28:e32–4. 10.1093/eurjpc/zwaa073. 10.1093/eurjpc/zwaa073 [DOI] [PubMed] [Google Scholar]
- 4.Artime E, Romera I, Díaz-Cerezo S, Delgado E. Epidemiology and economic burden of cardiovascular disease in patients with type 2 diabetes mellitus in Spain: a systematic review. Diabetes Ther. 2021;12:1631–59. 10.1007/s13300-021-01060-8. 10.1007/s13300-021-01060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reyes-García R, Moreno-Pérez O, Bellido-Castañeda V, Botana López M, Rodríguez-Hervada AD, Fernández-García D. Abordaje integral de las personas con diabetes tipo 2. Área de Conocimiento de Diabetes de la Sociedad Española de Endocrinología y Nutrición. https://www.seen.es; 2023. Accessed 28 Mar 2023.
- 6.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. 10.1093/eurheartj/ehz486. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 7.Marx N, Davies MJ, Grant PJ, Mathieu C, Petrie JR, Cosentino F, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9:46–52. 10.1016/S2213-8587(20)30343-0. 10.1016/S2213-8587(20)30343-0 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Professional Practice Committee, American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, et al. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2022;45:S125–43. 10.2337/dc22-S009. [DOI] [PubMed]
- 9.Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;2022(45):2753–86. 10.2337/dci22-0034. 10.2337/dci22-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol. 2019;74:e177–232. 10.1016/j.jacc.2019.03.010. 10.1016/j.jacc.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sociedad Española de Medicina Interna (SEMI). Actualización 2022 para el tratamiento de la DM2 del Grupo de Diabetes, Obesidad y Nutrición, https://www.fesemi.org/sites/default/files/documentos/grupos/noticias/algoritmo_semi_de_diabetes_2022_0.pdf; 2022. Accessed 13 Apr 2023.
- 12.Fernández Olmo MR, Cordero Fort A, Torres Llergo J, Marzal Martín D, Baquero Alonso M, Martínez Quesada M, et al. Selección de lo mejor del año en 2022 en riesgo vascular y rehabilitación cardiaca [Selection of the best of 2022 in vascular risk and cardiac rehabilitation]. REC: CardioClinics. 2023;58:S21–7. 10.1016/j.rccl.2022.10.002. 10.1016/j.rccl.2022.10.002 [DOI] [Google Scholar]
- 13.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85. 10.1016/S2213-8587(19)30249-9. 10.1016/S2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- 14.Marsico F, Paolillo S, Gargiulo P, Bruzzese D, Dell’Aversana S, Esposito I, et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2020;41:3346–58. 10.1093/eurheartj/ehaa082. 10.1093/eurheartj/ehaa082 [DOI] [PubMed] [Google Scholar]
- 15.Romera I, Rubio-de Santos M, Artola S, Suárez Fernández C, Conget I. GLP-1 RAs in Spain: a short narrative review of their use in real clinical practice. Adv Ther. 2023;40:1418–29. 10.1007/s12325-023-02442-z. 10.1007/s12325-023-02442-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schernthaner G, Shehadeh N, Ametov AS, Bazarova AV, Ebrahimi F, Fasching P, et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19:185. 10.1186/s12933-020-01154-w. 10.1186/s12933-020-01154-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Draznin B, Hirsch IB. Time to follow the evidence: glycemic control and cardiovascular benefits of new diabetes medications. Am J Med. 2021;134:420–2. 10.1016/j.amjmed.2020.12.012. 10.1016/j.amjmed.2020.12.012 [DOI] [PubMed] [Google Scholar]
- 18.Nelson AJ, Ardissino M, Haynes K, Shambhu S, Eapen ZJ, McGuire DK, et al. Gaps in evidence-based therapy use in insured patients in the United States with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. J Am Heart Assoc. 2021;10: e016835. 10.1161/JAHA.120.016835. 10.1161/JAHA.120.016835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold SV, Tang F, Cooper A, Chen H, Gomes MB, Rathmann W, et al. Global use of SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes. Results from DISCOVER. BMC Endocr Disord. 2022;22:111. 10.1186/s12902-022-01026-2. 10.1186/s12902-022-01026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministerio de Sanidad, Gobierno de España. Nomenclátor. https://www.sanidad.gob.es/profesionales/medicamentos.do; 2022. Accessed 22 Dec 2022.
- 21.Conget I, Mauricio D, Ortega R, Detournay B, CHADIG study investigators. Characteristics of patients with type 2 diabetes mellitus newly treated with GLP-1 receptor agonists (CHADIG Study): a cross-sectional multicentre study in Spain. BMJ Open. 2016;6:e010197. 10.1136/bmjopen-2015-010197. 10.1136/bmjopen-2015-010197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mata-Cases M, Franch-Nadal J, Ortega E, Real J, Gratacòs M, Vlacho B, et al. Glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: real-world evidence from a Mediterranean area. Curr Med Res Opin. 2019;35:1735–44. 10.1080/03007995.2019.1618806. 10.1080/03007995.2019.1618806 [DOI] [PubMed] [Google Scholar]
- 23.Tofé S, Argüelles I, Mena E, Serra G, Codina M, Urgeles JR, et al. Real-world GLP-1 RA therapy in type 2 diabetes: a long-term effectiveness observational study. Endocrinol Diabetes Metab. 2019;2:e00051. 10.1002/edm2.51. 10.1002/edm2.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norrbacka K, Sicras-Mainar A, Lebrec J, Artime E, Díaz S, Tofé-Povedano S, et al. Glucagon-like peptide 1 receptor agonists in type 2 diabetes mellitus: data from a real-world study in Spain. Diabetes Ther. 2021;12:1535–51. 10.1007/s13300-021-01039-5. 10.1007/s13300-021-01039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obaya Rebollar JC, Miravet Jiménez S, Aranbarri Osoro I, Carramiñana Barrera FC, García Soidán FJ, Cebrián Cuenca AM. Management of patient profiles with type 2 diabetes mellitus in primary care in Spain: CONTROVERTI2 Program. SEMERGEN. 2022;48:23–37. 10.1016/j.semerg.2021.07.009. 10.1016/j.semerg.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 26.Morillas C, Escalada J, Palomares R, Bellido D, Gómez-Peralta F, Pérez A. Treatment of type 2 diabetes by patient profile in the clinical practice of endocrinology in Spain: Delphi study results from the Think Twice Program. Diabetes Ther. 2019;10:1893–907. 10.1007/s13300-019-0671-x. 10.1007/s13300-019-0671-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López de la Torre M, Botana López M, Rozas Moreno P, Merino Torres J, Escalada San Martín J, Pérez Maraver M. Abordaje en la práctica clínica de endocrinólogos españoles de los aspectos cardiovasculares y renales del paciente con diabetes mellitus tipo 2: resultados de un estudio Delphi. Endocrinología Diabetes y Nutrición. 2021;68:111–2. [Google Scholar]
- 28.Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8:e1000393. 10.1371/journal.pmed.1000393. 10.1371/journal.pmed.1000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67:401–9. 10.1016/j.jclinepi.2013.12.002. 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Birko S, Dove ES, Özdemir V. Evaluation of nine consensus indices in Delphi foresight research and their dependency on Delphi survey characteristics: a simulation study and debate on Delphi design and interpretation. PLoS One. 2015;10: e0135162. 10.1371/journal.pone.0135162. 10.1371/journal.pone.0135162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf & Manage. 2004;42:15–29. 10.1016/j.im.2003.11.002. 10.1016/j.im.2003.11.002 [DOI] [Google Scholar]
- 32.Khunti K, Gomes MB, Pocock S, Shestakova MV, Pintat S, Fenici P, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427–37. 10.1111/dom.13088. 10.1111/dom.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosenzon O, Del Prato S, Schechter M, Leiter LA, Ceriello A, DeFronzo RA, et al. From glucose lowering agents to disease/diabetes modifying drugs: a «SIMPLE» approach for the treatment of type 2 diabetes. Cardiovasc Diabetol. 2021;20:92. 10.1186/s12933-021-01281-y. 10.1186/s12933-021-01281-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seidu S, Cos X, Brunton S, Harris SB, Jansson SPO, Mata-Cases M, et al. A disease state approach to the pharmacological management of type 2 diabetes in primary care: a position statement by Primary Care Diabetes Europe. Prim Care Diabetes. 2021;15:31–51. 10.1016/j.pcd.2020.05.004. 10.1016/j.pcd.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 35.O’Keefe JH, Nassif ME, Magwire ML, O’Keefe EL, Lavie CJ. The elephant in the room: why cardiologists should stop ignoring type 2 diabetes. Prog Cardiovasc Dis. 2019;62:364–9. 10.1016/j.pcad.2019.08.001. 10.1016/j.pcad.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 36.Cos X, Seidu S, Brunton S, Harris SB, Jansson SPO, Mata-Cases M, et al. Impact on guidelines: the general practitioner point of view. Diabetes Res Clin Pract. 2020;166:108091. 10.1016/j.diabres.2020.108091. 10.1016/j.diabres.2020.108091 [DOI] [PubMed] [Google Scholar]
- 37.Gimeno JA, Cánovas G, Durán A. Factors associated with adherence to clinical practice guidelines for patients with type 2 diabetes mellitus: results of a Spanish Delphi consensus. J Diabetes Res. 2021;2021:9970859. 10.1155/2021/9970859. 10.1155/2021/9970859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thieu VT, Robinson S, Kennedy-Martin T, Boye KS, Garcia-Perez L-E. Patient preferences for glucagon-like peptide-1 receptor-agonist treatment attributes. Pateint Prefer Adherence. 2019;13:561–76. 10.2147/PPA.S187907. 10.2147/PPA.S187907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boye K, Ross M, Mody R, Konig M, Gelhorn H. Patients’ preferences for once-daily oral versus once-weekly injectable diabetes medications: the REVISE study. Diabetes Obes Metab. 2021;23:508–19. 10.1111/dom.14244. 10.1111/dom.14244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matza LS, Cutts KN, Stewart KD, Norrbacka K, García-Pérez L-E, Boye KS. Health state utilities associated with treatment process for oral and injectable GLP-1 receptor agonists for type 2 diabetes. Qual Life Res. 2021;30:2033–43. 10.1007/s11136-021-02808-2. 10.1007/s11136-021-02808-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mardetko N, Nabergoj Makovec U, Locatelli I, Janez A, Kos M. Uptake of new antidiabetic medicines in 11 European countries. BMC Endocr Disord. 2021;21:127. 10.1186/s12902-021-00798-3. 10.1186/s12902-021-00798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernando K, Bain SC, Holmes P, Jones PN, Patel DC. Glucagon-like peptide 1 receptor agonist usage in type 2 diabetes in primary care for the UK and beyond: a narrative review. Diabetes Ther. 2021;12:2267–88. 10.1007/s13300-021-01116-9. 10.1007/s13300-021-01116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agencia Española del Medicamento. La AEMPS emite recomendaciones para evitar o paliar problemas de suministro con los medicamentos análogos del GLP-1; 2022. https://www.aemps.gob.es/informa/la-aemps-emite-recomendaciones-para-evitar-o-paliar-problemas-de-suministro-con-los-medicamentos-analogos-del-glp-1/. Accessed 13 Apr 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article/as supplementary information files.