Abstract
In the present study, we have investigated the role of cholesterol in maintaining the barrier properties of the model intestinal cell line Caco-2. We have extracted membrane cholesterol using methyl-β-cyclodextrin and demonstrated that maximally, methyl-β-cyclodextrin lowered cell cholesterol levels by 40–45%. Depletion of cell cholesterol was accompanied by an 80–90% decrease in monolayer transepithelial electrical resistance and a significant increase in the paracellular permeability of dextrans of 4, 10 and 40 kDa. The increase in dextran permeability was most pronounced for the two lower molecular mass species. In addition to the decline in the barrier properties of the monolayers, extraction of cell cholesterol produced an increase in the Triton X-100 solubility of claudin 3, claudin 4 and occludin, and the loss of all three proteins from the plasma membrane (tight junctions). In contrast, removal of cholesterol had no detectable influence on the detergent solubility or morphological distribution of claudin 1. These results indicate that membrane cholesterol is a critical factor in maintaining the barrier property of epithelial monolayers. More specifically, cholesterol appears to stabilize the association of certain proteins with the tight junctions.
Keywords: Caco-2 cell, cholesterol, claudin, occludin, tight junction
Abbreviations: MCD, methyl-β-cyclodextrin; MDCK, Madin–Darby canine kidney; MEM, modified Eagle's medium; PIP2, phosphatidylinositol 4,5-bisphosphate; TEER, transepithelial electrical resistance; TX100, Triton X-100
INTRODUCTION
Cholesterol is an important structural lipid that is primarily located in the plasma membrane of cells. Past research has indicated a link between membrane cholesterol and barrier function in epithelial monolayers; specifically cholesterol appears to modulate tight-junction assembly/integrity. Tight junctions consist of an array of multimeric protein complexes that form a network of continuous anastomosing intramembranous strands, which constitute the permeability barrier between adjacent cells in epithelial or endothelial sheets [1–3]. The first indication of a link between cholesterol and tight junctions came from a series of morphological studies using electron microscopy to detect filipin– cholesterol aggregates in the plasma membrane of hepatic epithelial cells [4]. This work suggested that high concentrations of cholesterol are present in and around the tight-junction strands. Further evidence was obtained using MCD (methyl-β-cyclodextrin) to deplete membrane cholesterol from MDCK (Madin–Darby canine kidney) cells. These studies demonstrated that depleting membrane cholesterol decreased the TEER (transepithelial electrical resistance), increased the mannitol flux and produced a morphological redistribution of the tight-junction protein occludin. This suggests that cholesterol is required for the maintenance of tight-junction integrity [5]. In contrast, a previous study using various pharmacological approaches including MCD to extract membrane cholesterol indicated that depleting cholesterol has a positive influence on tight-junction assembly and development of TEER [6]. These studies demonstrate a link between membrane cholesterol and tight-junction integrity, however, the nature of this association is not clear.
A significant proportion of membrane cholesterol is clustered with sphingomyelin and glycosphingolipids forming dynamic microdomains called lipid rafts. Interaction between the hydrocarbon chains of the sphingolipids and cholesterol promotes the condensation of the lipids within the rafts, resulting in the generation of a domain that has a liquid-ordered structure [7,8]. The raft domains are, therefore, significantly less fluid than the bulk of the plasma membrane and, unlike the rest of the membrane, are resistant to extraction with non-ionic detergents such as Triton X-100 (referred to as TX100) and Lubrol WX. Owing to the unique biophysical characteristics of the lipid rafts, these domains attract and sequester a variety of membrane proteins [9]. Such proteins include GPI (glycosylphosphatidylinositol)-anchored membrane proteins, the src family of tyrosine kinases, both low-molecular-mass and classical heterotrimeric G-proteins, mitogen receptors, various serine/threonine kinases and transmembrane proteins like occludin and connexins. Rafts can therefore be viewed as platforms for the sequestration of specific cassettes of proteins thereby facilitating their interaction. This can result in the initiation of a signalling pathway or assembly of structural multiprotein assemblies [9–11].
The hyperphosphorylated forms of the tight-junction proteins occludin and ZO-1 are associated with Triton-insoluble lipid rafts isolated from T84 [12]. This has led to the suggestion that lipid rafts may play a role in promoting tight-junction assembly/integrity. However, ZO-1, being a cytosolic protein, should not be located in lipid rafts. Its presence within the rafts therefore suggests that TX100 did not disrupt its interaction with tight-junction proteins such as claudin and/or occludin and may not have fully solubilized the cell membranes. Disruption of lipid rafts using various approaches has produced contradictory evidence regarding the role played by lipid rafts in maintaining tight-junction integrity [12,13]. Thus the relationship between lipid rafts and the fidelity of tight junctions is at best unclear.
In the present study, we have re-examined the relationship between membrane cholesterol, barrier function and tight junctions. Using Caco-2 cells as a model intestinal epithelial cell line, partial depletion of membrane cholesterol with MCD produced a nearly complete collapse of the TEER but only a moderate increase in paracellular permeability. The loss of TEER and increase in permeability were accompanied by a change in the TX100 solubility and morphological redistribution of the tightjunction proteins claudin 3, claudin 4 and occludin. However, cholesterol depletion had no effect on the detergent solubility or distribution of claudin 1. These results demonstrate that membrane cholesterol is critical for maintaining the barrier function by stabilizing the association of specific proteins with the tight junctions.
MATERIALS AND METHODS
Materials
Tissue culture media and supplements were purchased from Invitrogen (Paisley, U.K.). Aprotinin, pepstatin and leupeptin were obtained from Calbiochem (Nottingham, U.K.). The tightjunction protein antibodies were purchased from Zymed (San Francisco, CA, U.S.A.), whereas the horseradish peroxidase-conjugated secondary antibodies were from Bio-Rad (Hemel Hempstead, U.K.). All other reagents were of the highest possible quality and were obtained from Sigma-Aldrich (Poole, Dorset, U.K.) unless stated otherwise.
Caco-2 cell culture
Caco-2 cells were maintained in MEM (modified Eagle's medium) supplemented with 10% (v/v) foetal calf serum, 2 mM L-glutamine, 50 units/ml penicillin G, 50 μg/ml streptomycin and 1× non-essential amino acids at 37 °C, 5% CO2. Cells were fed every 3 or 4 days and subcultured every 7 days using 0.25% trypsin/0.2% EDTA in PBS. For experimentation, cells (passages 41–60) were seeded at a density of 3×105 cells/cm2 on Transwell (Corning, Corning, NY, U.S.A.) permeable polyester filters (0.4 μm pore size) with surface areas of either 1.00 or 4.7 cm2. The filters were fed every 3 or 4 days. Cells reached confluence 4–6 days after seeding with a steady TEER being observed 10–12 days after that. Monolayers were taken for experimentation 3 or 4 days after the TEER had attained a steady value, typically 14–21 days after seeding.
Treatment with MCD
To extract membrane cholesterol from the Caco-2 monolayers, the standard MEM tissue culture media in both the apical and basolateral chambers were carefully replaced with MEM containing the desired concentration of MCD. The monolayers were then incubated at 37 °C in 5% CO2 for a set period before extraction or measurement of either TEER or paracellular permeability. In all the experiments, employing cells grown on Transwells, MCD was added to both the apical and basolateral chambers of the Transwell plates.
Measurement of Caco-2 cell cholesterol
After treatment with MCD, the Caco-2 monolayers were washed twice with ice-cold PBS. The cells were then scrapped into 200 μl of lysis buffer [150 mM NaCl, 25 mM Hepes (pH 7.4), 1% (v/v) TX100, 2 mM EDTA, 10 mM NaF, 2 mM sodium orthovanadate and 10 μg/ml aprotinin, leupeptin and pepstatin]. The cell suspension was incubated on ice for 30 min before being sonicated (three 3 s bursts at half power from an MSE sonicator). Finally, the cell lysates were assayed for cholesterol using the Infinity cholesterol assay system (Sigma-Aldrich) according to the manufacturer's instructions. The amount of cellular cholesterol was initially normalized to total cellular protein and then expressed as a percentage of the amount of cholesterol present in untreated, control cells. Cholesterol was also measured in the gradient fractions from the lipid raft isolations.
Preparation of cholesterol–MCD complexes
The cholesterol–MCD complexes were produced as described by Pike and Miller [14]. In brief, 6 mg of cholesterol was dissolved in 80 μl of a propan-2-ol/chloroform (2:1, v/v) mixture and MCD (200 mg) was dissolved in 2.2 ml of water. The MCD solution was then heated to 80 °C and the cholesterol added in 20 μl aliquots with stirring. The solution was kept at 80 °C and continuously stirred until the solution was clear.
Measurement of transepithelial resistance
Transepithelial resistance of the Caco-2 monolayers was measured using an EVOM epithelial voltohmmeter (World Precision Instruments, Hamden, CT, U.S.A.) with a pair of chopstick electrodes.
Measurement of paracellular permeability
Paracellular permeability was quantified by measuring the transepithelial flux of FITC-labelled dextrans of different molecular masses (4, 10 or 40 kDa). In brief, Caco-2 monolayers were treated with 5 mM MCD for 2 h; FITC-labelled dextran was then added to the apical side of the monolayers to a final concentration of either 200 μg/ml (4.7 cm2 Transwells) or 700 μg/ml (0.33 cm2 Transwells). After incubation for a further 3 h, two 200 μl aliquots of the medium were removed from the basolateral chambers, and FITC-dextran fluorescence measured using a Victor 1420 plate reader (Wallac, Gaithersburg, MD, U.S.A.).
Detergent extraction of Caco-2 cell monolayers
After treatment with MCD, the Caco-2 monolayers were washed twice with ice-cold PBS. Ice-cold TX100 buffer [100 μl; 120 mM NaCl, 25 mM Hepes (pH 7.5), 1% TX100, 2 mM EDTA, 25 mM NaF, 1 mM sodium orthovanadate and 10 μg/ml aprotinin, leupeptin and pepstatin] was then added to each filter and the monolayers scraped with a small cell scraper to detach the cells. The TX100 buffer containing the Caco-2 cells was then carefully removed and the filters washed with 100 μl of TX100 buffer to remove all the residual cells. The two aliquots of buffer containing the cells released from the filter were then pooled and incubated on ice for 30 min. The detergent-insoluble material was then pelleted by centrifugation in an Eppendorf microfuge (30 min, 13000 g at 4 °C). After centrifugation, the supernatant (detergent-soluble fraction) was carefully removed and stored frozen at −20 °C. Modified RIPA buffer [100 μl; 25 mM Hepes (pH 7.5), 1% SDS, 2 mM EDTA, 25 mM NaF, 1 mM sodium orthovanadate and 10 μg/ml aprotinin, leupeptin and pepstatin] was then added to the pellet and sonicated (three 3 s bursts) to resuspend the detergent-insoluble material. The resulting suspension (detergent-insoluble fraction) was then stored frozen at −20 °C.
Lipid raft isolation
For each raft isolation, cells were harvested from a single 4.7 cm2 Transwell filter. In brief, cells were scraped into 800 μl of ice-cold isolation buffer [150 mM NaCl, 25 mM Tris (pH 7.4), 2 mM EGTA, 2 mM NaF, 10 mM NaVO4 and 10 mM sodium pyrophosphate]. To this solution, 800 μl of 2.0% TX100 was added and the cell suspension was then left on ice for 30 min. The resulting cell extract was then brought to 40% (w/v) sucrose by the addition of an equal volume of 80% sucrose and 3 ml of this was placed at the bottom of an ultracentrifuge tube. This was over-layered with 6 ml of 30% sucrose and 3 ml of 5% sucrose. Both sucrose solutions were made in isolation buffer and contained 0.1% TX100. The gradients were then centrifuged in a SW41 rotor at 200000 g for 20 h. After centrifugation, the gradient was divided into 12 fractions of 1 ml each and they were subsequently analysed by Western blot.
Western-blot analysis
SDS/PAGE was performed as described previously [15]. For Western blotting, Caco-2 cell extracts were separated on either 12 or 10% polyacrylamide gel and electrophoretically transferred on to PVDF membranes, which were then incubated with the appropriate primary antibody. The bound primary antibodies were then detected using a horseradish peroxidase-conjugated secondary antibody in conjunction with the ECL® detection system (Amersham Biosciences, Piscataway, NJ, U.S.A.). Typical exposure times for the blots were 1–5 min.
Immunocytochemistry
Caco-2 cells were seeded on 8-well chamber slides (Lab-tek, Elkhart, IN, U.S.A.) and fed every 3–4 and 10–14 days after seeding, and the confluent Caco-2 monolayers were exposed to 5 mM MCD for 3 h. MCD treatment of cells on the Lab-tek chamber slides resulted in an identical level of cholesterol depletion (∼45%) as observed for cells grown on the Transwell inserts. After MCD treatment, the cell monolayers were fixed for 10 min at room temperature (22 °C) with 4% (w/v) paraformaldehyde (claudin 1 and occludin) or 20 min at −20 °C with methanol (claudins 3 and 4). After fixation, the monolayers were washed with PBS and solubilized with 0.5% TX100. The monolayers were then blocked for 1 h and incubated overnight with the appropriate primary antibody. Finally, bound primary antibody was detected using species-specific secondary antibodies conjugated to either FITC (claudin 1 and occludin) or rhodamine (claudins 3 and 4). Images were captured using confocal laser scanning microscopy (Biorad MRC 1024MP confocal scanning system mounted on a Nikon Eclipse TE300 Fluorescence microscope). A gallery of 30–40 optical sections (0.5 μm) through the z-plane was obtained and the composite images were processed using confocal assistant V4.02.
Statistical analysis
Results are expressed as means±S.E.M., except where stated to the contrary. Statistical analysis was performed by Student's t test or one-way ANOVA followed by the Turkey pairwise multiple-comparison test with P<0.05 considered statistically significant.
RESULTS
Depletion of Caco-2 cell cholesterol by MCD
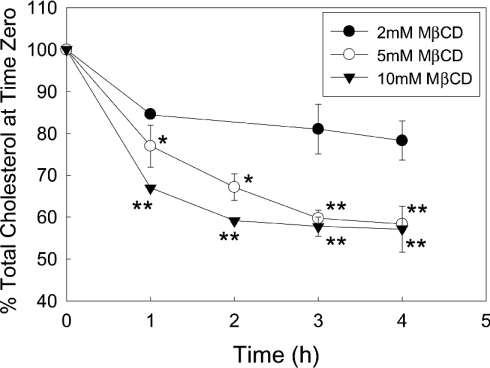
MCD produced a concentration-dependent decrease in Caco-2 cell cholesterol (Figure 1). Maximally, MCD removed ∼40% of the cell cholesterol; the average cellular concentration of cholesterol in untreated cells was measured at 43.50±2.96 (S.D.) μg/mg of protein. Examination of the time course of MCD-induced cholesterol depletion (Figure 2) showed that both 5 and 10 mM MCD ultimately extracted 40–45% of the cell cholesterol. However, the time courses of cholesterol extraction for 5 and 10 mM MCD were different, the higher concentration of MCD producing a more rapid depletion of the lipid. The lowest concentration of MCD tested (2 mM) produced a 15% decrease in cholesterol, which was observed after the first hour of incubation. To determine whether prolonged exposure to MCD would produce any further decrease in cholesterol level, Caco-2 cells were incubated without (control) or with MCD (5 or 10 mM) for 2.5 and 24 h. After 2.5 h, 5 and 10 mM MCD had decreased cholesterol levels to 66.10±3.03 and 58.47±4.71% of control (means±S.E.M. for three independent experiments) respectively. In comparison, after 24 h, 5 and 10 mM MCD had decreased cholesterol levels to 63.87±2.41 and 63.65±1.70% of control (means±S.E.M. for three independent experiments) respectively. There is no significant difference between any of these values, demonstrating that prolonged incubation with MCD did not decrease the cell cholesterol level below that achieved after 2.5 h. To ensure that MCD does not get saturated with cholesterol, the MCD/media was replaced with fresh MCD/media after 8 h. Replacing the MCD had no significant influence on the level of cholesterol extraction obtained with either 5 or 10 mM MCD. We also examined whether adding the MCD in serum-free media would increase the level of cholesterol depletion. No detectable difference was observed between the level of extraction produced in serum-free versus serum-enriched media (results not shown).
Figure 1. Depletion of Caco-2 cell cholesterol by MCD.
Caco-2 cells were grown to confluence on Transwell filters. The monolayers were treated with increasing concentrations of MCD (added to both the apical and basolateral chambers of the Transwell) for 1 h before the cells were harvested and lysed. The cell lysates were subsequently assayed for cholesterol and protein content. The results are expressed as percentage of control and are the means±S.E.M. for three independent experiments, *P<0.01 and **P<0.001.
Figure 2. Time courses of cholesterol depletion by increasing concentrations of MCD.
Caco-2 cells were grown to confluence on Transwell filters. The monolayers were treated with 2, 5 or 10 mM MCD (MCD was added to both the apical and basolateral chambers of the Transwells) for increasing times before the cells were extracted. The cell lysates were subsequently assayed for cholesterol and protein content. The results are expressed as percentage of the control and are the means±range for two independent experiments for 2 mM MCD and means±S.E.M. for three independent experiments for 5 and 10 mM MCD; *P<0.01 and **P<0.001.
Influence of MCD on barrier function of Caco-2 monolayers
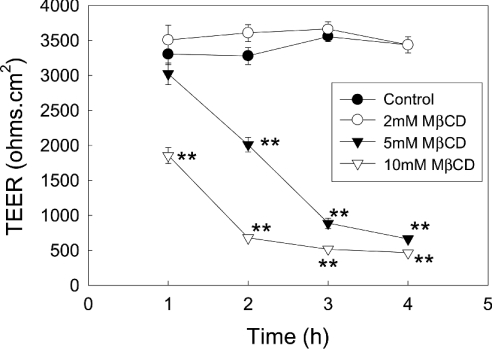
To determine the influence of depleting cholesterol on TEER of Caco-2 cell monolayers, MCD was added to both the apical and basolateral chambers of Transwell filter plates (Figure 3). The lowest concentration of MCD (2 mM) tested did not produce any significant change in the monolayer TEER. In contrast, both 5 and 10 mM MCD produced a time-dependent decline in TEER. Maximally, both 5 and 10 mM MCD produced an 80–90% decrease in TEER, although the lower concentration of MCD did take approx. 1 h longer to attain this level of reduction.
Figure 3. Influence of increasing concentrations of MCD on monolayer TEER.
Caco-2 cells were grown to confluence on Transwell filters. The monolayers were then treated with increasing concentrations of MCD (MCD was added to both the apical and basolateral chambers of the Transwells) and TEER measured every hour for 5 h. The results shown are the means±S.E.M. for four independent experiments; *P<0.01 and **P<0.001.
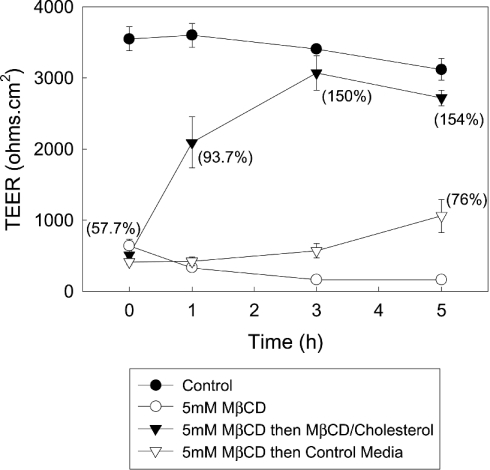
As a control, to determine whether the influence of MCD on TEER is due to removal of cholesterol, we next determined whether it is possible to reverse the collapse of the TEER by replenishing cell cholesterol (Figure 4). Addition of MCD–cholesterol to the monolayers produced a rapid recovery in TEER. Monolayer resistance had recovered by over 50% after 1 h and had totally recovered by 3 h. Similarly, the level of cell cholesterol rapidly increased after the addition of the MCD–cholesterol complexes. By 1 h after initiation of repletion, cholesterol had returned to near-control values and by 3 h the level of cell cholesterol had stabilized at a level above that seen in control cells. In comparison, when MCD was removed and replaced with control media we only observed a partial recovery in TEER and cell cholesterol over the time period examined.
Figure 4. Recovery of TEER after repletion of cell cholesterol.
Caco-2 cell monolayers were treated with media that did or did not contain 5 mM MCD (MCD was added to both the apical and basolateral chambers of the Transwells). After 2.5 h, the media in both the apical and basolateral chambers was replaced with fresh control media or media that contained either 5 mM MCD or MCD–cholesterol complexes. TEER was then measured every hour for 5 h. The results shown are the means±S.E.M. for three independent experiments. The numbers in brackets represent the level of total cell cholesterol expressed as a percentage of the cholesterol concentration measured in control cells and are the means for three independent experiments.
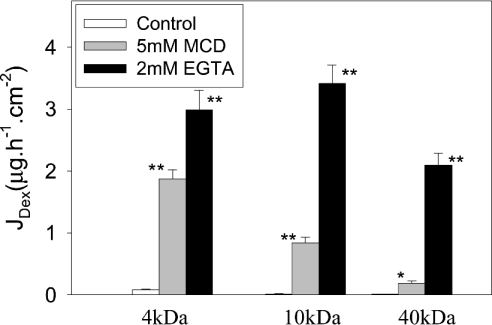
To determine whether MCD altered the permeability of the paracellular pathway, we measured the flux of FITC-labelled dextrans of defined size (4, 10 and 40 kDa) across control and MCD-treated Caco-2 cell monolayers grown on Transwell filters (Figure 5). As a positive control for these flux studies, we used monolayers exposed to 2 mM EGTA, which results in the loss of tight-junction integrity and opening of the paracellular pathway. MCD (5 mM) produced a significant increase in the flux of all three sizes of dextran, although the smaller the size of the dextran the more pronounced the increase in flux. This is most clearly illustrated if the flux values obtained using the MCD-treated cells are compared with those obtained from the monolayers exposed to 2 mM EGTA. If the flux values obtained with EGTA represent the maximum possible, then the flux of the 2, 10 and 40 kDa dextrans after MCD treatment was 62.8, 24.5 and 8.8% of maximum respectively. This clearly indicates that MCD was preferentially increasing the permeability of the smaller sized dextrans. As previously observed for TEER, repletion of cellular cholesterol after MCD treatment did produce a recovery in barrier function and a decrease in paracellular permeability. As per the TEER experiment, cell monolayers were exposed to MCD (5 mM) for 2.5 h before the MCD was replaced with MCD–cholesterol complexes. Paracellular permeability was measured using 10 kDa FITC-labelled dextran 3 h after the addition of the cholesterol complexes. Dextran flux across control monolayers that had never been exposed to MCD was 0.057±0.003 (S.D.) μg·h−1·cm−2 compared with 0.065±0.002 (S.D.) μg·h−1·cm−2 for monolayers that had been exposed to MCD but had had their cholesterol replenished by incubation with MCD–cholesterol complexes. In contrast, dextran flux across monolayers that had been exposed to MCD for the entire experiment was 0.68±0.04 (S.D.) μg· h−1·cm−2.
Figure 5. MCD increases the permeability of Caco-2 monolayer to FITC-dextrans.
Caco-2 cell monolayers were treated with either 5 mM MCD or 2 mM EGTA (MCD or EGTA was added to both the apical and basolateral chambers of the Transwells). After 2 h, either 4, 10 or 40 kDa FITC-dextran was added to the apical side of the monolayer. After an additional 3 h incubation, aliquots of the basolateral media were removed and the amount of FITC-dextran fluorescence present was determined. The results shown are the means±S.E.M. for four independent experiments. *P<0.01 and **P<0.001.
Influence of MCD on the detergent solubility of tight-junction proteins
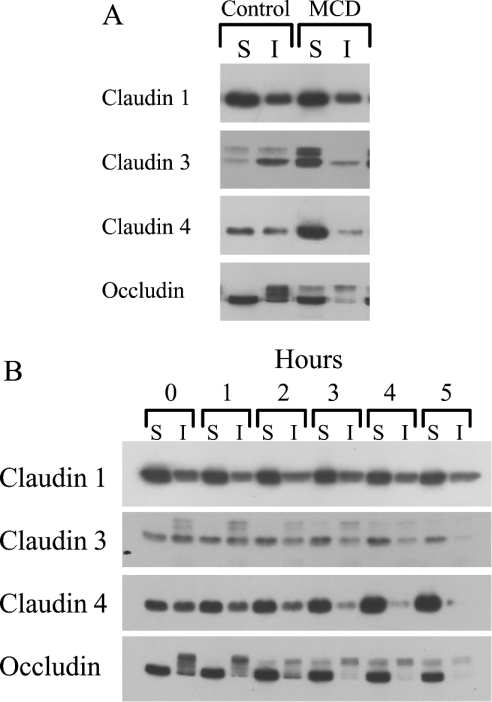
The permeability of the paracellular pathway is primarily dependent on the composition and organization of the tight-junction strands that encircle apical domain of epithelial cells. To examine if partial depletion of cell cholesterol had an impact on the tight junctions to increase permeability, we determined whether MCD treatment altered the TX100 solubility of the integral membrane proteins that are known to be the main components of tight junctions in Caco-2 cells, namely occludin and claudins 1, 3 and 4 (Figure 6). We found that incubation of confluent Caco-2 monolayers with 5 mM MCD produced a significant change in the Triton solubility of claudins 3, 4, and occludin but not claudin 1 (Figure 6A). Under control conditions, the bulk of claudin 3 was found in the TX100-insoluble fraction, while claudin 4 was approximately equally distributed between the two soluble and insoluble fractions. However, after MCD treatment, the bulk of each claudin was found in the TX100-soluble fraction. MCD induced a similar redistribution in occludin, and this shift is accompanied by the partial loss of the phosphorylated forms of the protein having a higher molecular mass.
Figure 6. Influence of MCD on the TX100 solubility of tight-junction proteins.
(A) Caco-2 cell monolayers were treated with 5 mM MCD for 5 h and then scraped into lysis buffer (MCD was added to both the apical and basolateral chambers of the Transwell). The cell lysates were then extracted as described in the Materials and methods section, and the TX100 soluble (S) and insoluble (I) fractions isolated and subjected to Western-blot analysis using antibodies for claudins 1, 3, 4 and occludin. The blot shown is representative of two independent experiments. (B) Caco-2 cell monolayers were treated with 5 mM MCD. At set times the cells were scrapped into lysis buffer and TX100 soluble (S) and insoluble (I) fractions isolated as described in the Materials and methods section. The fractions were then analysed for occludin and claudins 1, 3 and 4 by Western-blot analysis. The blot shown is representative of three independent experiments.
To establish a time course for the redistribution of claudins 3, 4 and occludin, Caco-2 cells were incubated with MCD and sample monolayers extracted with Triton every hour up to 5 h post-addition of MCD (Figure 6B). Changes in the distribution of claudins 3 and 4 were apparent after 2 h and appear near-complete between 3 and 4 h. In comparison, a partial loss in the higher molecular mass, phosphorylated, forms of occludin is observed after 1 h with full redistribution/dephosphorylation being seen between 3 and 4 h post-MCD. As seen previously there was no detectable shift in the distribution of claudin 1. In addition to the change in detergent solubility, there was also an indication that at later time periods (after 4 h) there may have been a reduction in claudin 3 levels.
Lack of association of the tight-junction proteins with TX100-insoluble raft domains
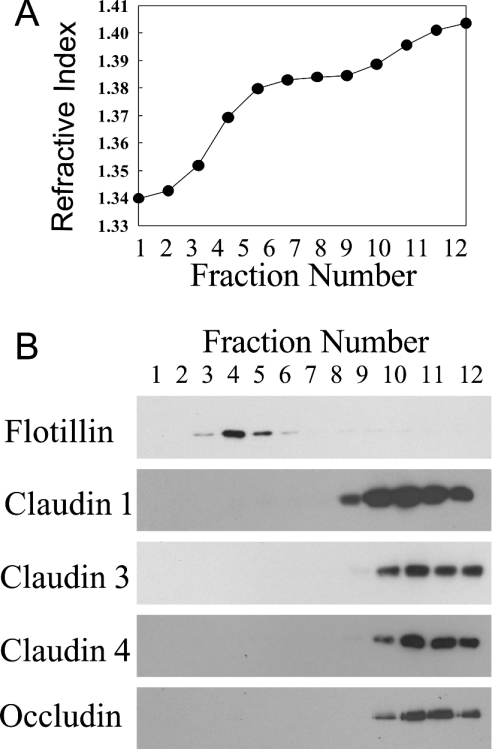
Recent research has shown that the higher molecular mass phosphorylated forms of occludin are associated with TX100-insoluble lipid rafts [12]. Therefore the alteration in occludin solubility observed with MCD might reflect a change in the lipid raft association of this protein. To examine this possibility we isolated TX100-insoluble lipid rafts from Caco-2 cells (Figure 7). To fix the location of lipid rafts within the sucrose gradient we employed flotillin, a known raft-associated protein, as a marker. As expected, flotillin was found to float near the top of the gradient in fractions 3–5. Flotillin fractions also contained more than 70% of the total amount of cholesterol present in the entire gradient, further supporting the idea that fractions 3–5 contain the extracted lipid rafts. In contrast, all the four tight-junction proteins were located at the bottom of the gradient in fractions 9–12. Therefore, in Caco-2 cells, occludin and the three isoforms of claudin do not appear to be associated with TX100-insoluble lipid rafts.
Figure 7. Tight-junction proteins are not associated with TX100 insoluble domain in Caco-2 cells.
Caco-2 cell monolayers were lysed in 1% TX100 and then centrifuged on a three-stage discontinuous sucrose gradient to separate the TX100-insoluble raft fractions. After centrifugation, the gradients were divided into 12 fractions of 1 ml each. The refractive index of each fraction was determined before (A) it was analysed by Western blotting (B). The blots shown are representative of two independent experiments.
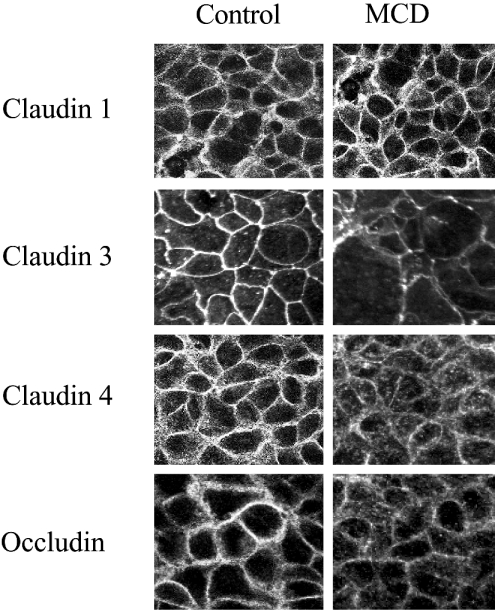
Effect of MCD on the morphological distribution of tight-junction proteins
To examine how cholesterol depeletion influenced the morphological distribution of the tight-junctions proteins, confluent monolayers of Caco-2 cells were incubated in the presence or absence of 5 mM MCD for 3 h, the cells were then fixed and the distribution of the tight-junctions proteins examined by immunofluorescent confocal microscopy (Figure 8). In control, untreated monolayers, occludin and claudins 1, 3 and 4 were predominantly localized to the cell plasma membrane producing a characteristic ‘chicken wire’ pattern of staining consistent with their distribution in tight junctions. Depletion of membrane cholesterol with MCD had no apparent influence on the distribution of claudin 1 but did have a marked effect on the localization of claudins 3, 4 and occludin. MCD treatment produced a decrease in staining at the cell plasma membrane of all three proteins. With claudin 4 and occludin, the loss of plasma membrane staining was accompanied by an apparent increase in punctate cytosolic staining.
Figure 8. Influence of MCD on the morphological distribution of tight-junction proteins in Caco-2 cell monolayers.
Caco-2 cell monolayers were treated with media that did or did not contain 5 mM MCD. After 3 h, the monolayers were fixed and stained for claudins 1, 3, 4 and occludin. The stained monolayers were then observed using a confocal laser scanning microscope. The images shown were produced by merging a series of 30–40 optical sections (0.5 μM) through the z-plane of the specimen.
DISCUSSION
In the present study, we have examined the relationship between membrane cholesterol and barrier function in Caco-2 cells, a model intestinal epithelial cell line. Cell cholesterol was extracted using MCD, an approach that is both rapid and highly efficient. Furthermore, MCD is entirely surface acting, so, apart from removing cholesterol, it has a minimal effect on cell membranes [16]. Maximally, MCD produced a 40–45% decrease in cell cholesterol. This level of depletion is comparable with that previously obtained in freshly isolated porcine enterocytes [17], but is significantly less than the 70–75% removal found in MDCK cells [5]. There are two possible explanations for the relative inefficiency of MCD in extracting cholesterol from intestinal epithelial cells. First, high concentrations of glycosphingolipids can retard or even inhibit the extraction of membrane cholesterol by MCD [18]. Given that the glycolipid content of the brush border membrane of intestinal epithelial cells is extraordinarily high (>30% of total lipid compared with <10% for renal epithelial cells) [19], it may be particularly hard for MCD to remove cholesterol from the intestinal cells including the Caco-2 cell line used in the present study. Secondly, Caco-2 cells may have a large intracellular pool(s) of cholesterol that is not accessible to MCD. Intestinal epithelial cells, unlike epithelial cells derived from other tissues, do have the capacity to absorb, package and then secrete cholesterol [20]; therefore the presence of a significant pool of intracellular cholesterol can be expected. Further reduction in cholesterol levels can be achieved by combining MCD treatment with a 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor such as lovastatin [17]. However, our concern in using such a compound is that it would inhibit the synthesis of not only cholesterol but also non-sterol isoprenoid products necessary for the post-translational modification and subsequent activity of numerous critical intracellular proteins. Consequently, using a 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor in conjunction with MCD could have triggered responses that reflect isoprenoid rather than cholesterol depletion.
Treatment of Caco-2 cell monolayers with MCD produced a dramatic decrease in TEER coupled with an increase in the permeability of the paracellular pathway. Comparable changes in barrier properties have previously been observed with MDCK cell monolayers after partial depletion of cellular cholesterol [5]. Therefore a requirement for membrane cholesterol to maintain barrier integrity is not unique to Caco-2 cells, and is quite probably a general phenomenon for all epithelial cells and possibly endothelial cells. Our finding that MCD (2 mM) extracted between 10 and 15% of cell cholesterol without producing detectable changes in paracellular permeability demonstrates that there is no direct relationship between total cell cholesterol and barrier function. This suggests that the cells can either tolerate removal of a certain amount of membrane cholesterol or can replenish what has been removed from intracellular stores. Alternatively, there could be two pools of extractable membrane cholesterol, one of which is more readily accessible to MCD but is not required to maintain barrier function. Previous studies have demonstrated in a variety of cell types that there are two, possibly, three pools of cholesterol present in the plasma membrane that are extracted at different rates by MCD [16,21].
Although MCD did trigger a near-collapse in TEER, its influence on paracellular permeability was far less profound. Results from the dextran flux studies indicate that removal of cholesterol produced only a partial opening of the paracellular pathway. Whether the changes in the permeability of the paracellular pathway are totally responsible for the decrease in the TEER is not clear. However, as membrane cholesterol does control the activity of many of the ion channels and transporters [22–24], the collapse in the TEER probably reflects how depletion of cholesterol influences the permeability of both the paracellular and transcellular pathways producing a decline in barrier function of the monolayer. Our laboratory is currently examining this question, specifically how cholesterol extraction influences transcellular ion-flux.
The MCD-triggered decline in the barrier properties of the Caco-2 cell monolayers was accompanied by biochemical and morphological redistribution of claudins 3, 4 and occludin, three key components of tight junctions in Caco-2 cells. Specifically, MCD produced an increase in the TX100 solubility and a decrease in the plasma membrane (tight junction) localization of all three proteins. Curiously, MCD had no influence on the TX100 solubility or distribution of a fourth tight-junction protein, namely claudin 1. These findings suggest that membrane cholesterol somehow stabilizes the association of certain proteins with tight junctions to maintain the barrier properties of the cell monolayers.
How can membrane cholesterol promote or stabilize the association of proteins with tight junctions? Cholesterol is an important structural lipid that is a key component of detergent-insoluble membrane domains commonly called lipid rafts. These domains are enriched in cholesterol and glycosphingolipids and due to their distinctive lipid composition have unique biophysical characteristics that promote the sequestration and subsequent association of a range of integral membrane proteins. These domains can therefore facilitate the assembly of multiprotein assemblies. Given this property it is not hard to imagine how rafts could function to promote or stabilize the assembly of tight junctions. Although previous studies have demonstrated that occludin, and possibly claudin 1, do associate with TX100-insoluble raft domains [12,13], we found no evidence for such an interaction in Caco-2 cells. Furthermore, none of the claudin isoforms examined appeared to associate with rafts either. The reason for the disparity between our findings and those in the literature is not clear. It is true that there is some cell-to-cell variation in raft association that has been observed for certain proteins; however, an alternative explanation relates to the conditions employed to isolate the raft fractions. Several recent studies have clearly demonstrated that it is possible to generate ‘artifactually’ raft-like fractions due to incomplete solubilzation of the cell membranes [18]. To eliminate this possibility, we used very stringent raft isolation conditions (protein/detergent ratio of 1:10) that might have resulted in the solubilization of occludin. If true, then one has to question whether occludin is truly a raft-associated protein. However, our results clearly demonstrate that MCD cannot be adversely influencing barrier function by decreasing the association of any of the tight-junction proteins examined with TX100-insoluble raft domains as no such interaction appears to exist in Caco-2 cells. It should be noted that TX100-insoluble raft domains are only one of the many detergent-insoluble microdomains present in the plasma membrane. These other domains are either partially or wholly soluble in TX100 and hence cannot be isolated using this particular detergent. Therefore we must add the caveat that MCD may be influencing barrier function/protein distribution by decreasing the association of the proteins with a raft domain(s) that is soluble in TX100. This possibility is currently being examined in the laboratory.
Although the findings indicate that the change in detergent solubility and morphological distribution of the tight-junction proteins does not directly reflect disassociation of the proteins from lipid rafts, it is possible that these changes, albeit indirectly, are linked to the destabilization of raft domains. Lipid rafts have been shown to sequester significant amounts of PIP2 (phosphatidylinositol 4,5-bisphosphate), which in turn modulates the activity of an assortment of PIP2-binding proteins to regulate the local dynamics of the actin cytoskeleton. Depletion of membrane cholesterol results in the destabilization of the rafts and a redistribution of PIP2, leading to changes in the subplasmalemmal actin cytoskeleton [25]. It is not hard to envisage how such alteration in cytoskeletal architecture could result in the release of the tight-junction proteins leading to an increase in TX100 solubility and a morphological redistribution.
In conclusion, our results demonstrate that depletion of membrane cholesterol in epithelial cells causes a change in the detergent solubility and morphological distribution of certain tight-junction proteins. This reorganization of tight-junction architecture results in a collapse of TEER and a ‘loosening’ of the paracellular permeability barrier. These observations provide additional evidence for the important role membrane lipids, and in particular cholesterol, play in stabilizing the tight-junction network and further demonstrates the importance of the claudin family of proteins in the maintenance of epithelial barrier properties. In addition, they demonstrate that there are significant differences in the interaction of claudins 1, 3 and 4 with tight junctions.
Acknowledgments
We thank Mr R. Fernandez for his excellent technical assistance. This work was supported by a grant D17799 from the Biotechnology and Biological Sciences Research Council.
References
- 1.Mitic L. L., Anderson J. M. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 2.Tsukita S., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–296. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 3.Schneeberger E. E., Lynch R. D. The tight junction: a multifunctional complex. Am. J. Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 4.Feltkamp C. A., Van der Waerden A. W. Junction formation between cultured normal rat hepatocytes. An ultrastructural study on the presence of cholesterol and the structure of developing tight-junction strands. J. Cell Sci. 1983;63:271–286. doi: 10.1242/jcs.63.1.271. [DOI] [PubMed] [Google Scholar]
- 5.Francis S. A., Kelly J. M., McCormack J., Rogers R. A., Lai J., Schneeberger E. E., Lynch R. D. Rapid reduction of MDCK cell cholesterol by methyl-β-cyclodextrin alters steady state transepithelial electrical resistance. Eur. J. Cell Biol. 1999;78:473–484. doi: 10.1016/s0171-9335(99)80074-0. [DOI] [PubMed] [Google Scholar]
- 6.Stankewich M. C., Francis S. A., Vu Q. U., Schneeberger E. E., Lynch R. D. Alterations in cell cholesterol content modulate calcium induced tight junction assembly by MDCK cells. Lipids. 1996;31:817–828. doi: 10.1007/BF02522977. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed S. N., Brown D. A., London E. On the origin of sphingolipid-cholesterol rich detergent domains in cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 8.Hooper N. M. Detergent-insoluble glycosphingolipid/cholesterol membrane domains, lipid rafts and caveolae. Mol. Membr. Biol. 1999;16:145–156. doi: 10.1080/096876899294607. [DOI] [PubMed] [Google Scholar]
- 9.Pike L. J. Lipid rafts: bringing order to chaos. J. Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Simons K., Toorme D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 11.Ikonen E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 2001;13:470–477. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 12.Nusrat A., Parkos C. A., Verkade P., Foley C. S., Liang T. W., Innis-Whitehouse W., Eastburn K. K., Madara J. L. Tight junctions are membrane microdomains. J. Cell Sci. 2000;113:1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 13.Leung L. W., Contrareras R. G., Flores-Maldonado C., Cereijido M., Rodriguez-Boulan E. Inhibitors of glycosphingolipid biosynthesis reduce transepithelial electrical resistance in MDCK1 and FRT cells. Am. J. Physiol. 2003;284:C1021–C1030. doi: 10.1152/ajpcell.00149.2002. [DOI] [PubMed] [Google Scholar]
- 14.Pike L. J., Miller J. M. Cholesterol depletion delocalises phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J. Biol. Chem. 1998;273:22298–22304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 15.Padfield P. J., Panesar N. Calcium-dependent secretion from SLO-permeabilized rat pancreatic acini requires diffusible cytosolic proteins. Am. J. Physiol. 1995;269:G647–G652. doi: 10.1152/ajpgi.1995.269.5.G647. [DOI] [PubMed] [Google Scholar]
- 16.Ilangumaran S., Hoessli D. C. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 1998;335:433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen G. H., Niels-Christiansen L. L., Thorsen E., Immerdahl L., Danielsen E. M. Cholesterol depletion of enterocytes: effect on the golgi complex and apical membrane trafficking. J. Biol. Chem. 2000;275:5136–5142. doi: 10.1074/jbc.275.7.5136. [DOI] [PubMed] [Google Scholar]
- 18.Schuck S., Honsho M., Ekroos K., Shevchenko A., Simons K. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danielsen E. M., Hansen G. H. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim. Biophys. Acta. 2003;1617:1–9. doi: 10.1016/j.bbamem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal J., Anwar K., Hussain M. M. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cell. J. Biol. Chem. 2003;278:31610–31620. doi: 10.1074/jbc.M301177200. [DOI] [PubMed] [Google Scholar]
- 21.Haynes M. P., Phillips M. C., Rothblat G. H. Efflux of cholesterol from different cellular pools. Biochemistry. 2000;39:4508–4517. doi: 10.1021/bi992125q. [DOI] [PubMed] [Google Scholar]
- 22.Brady J. D., Rich T. C., Le X., Stafford K., Fowler C. J., Lynch L., Karpen J. W., Brown R. L., Martens J. R. Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol. Pharmacol. 2004;65:503–511. doi: 10.1124/mol.65.3.503. [DOI] [PubMed] [Google Scholar]
- 23.Martens J. R., O'Connell K., Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol. Sci. 2004;25:16–21. doi: 10.1016/j.tips.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 24.O'Connell K. M., Martens J. R., Tamkun M. M. Localisation of ion channels to lipid raft domains within the cardiovascular system. Trends Cardiovasc. Med. 2004;14:37–42. doi: 10.1016/j.tcm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Kwik J., Boyle S., Fooksman D., Margolis L., Scheetz M. P., Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]