Abstract
The concept of chimeric antigen receptor (CAR) T cell therapy emerged from cancer immunotherapy and has been rapidly adapted and developed for the treatment of autoimmune, especially B-cell-driven, diseases since the first publication of an article featuring a patient with systemic lupus erythematosus in 2021. Phase II studies are about to start, but up to now, only case reports and small series have been published. In contrast to hemato-oncological diseases, where an aggressive response to malignant cells and long-lasting persistence of CAR T cells has been aimed at and observed in many patients, this is not the case with autoimmune diseases but might not be necessary to control disease. Future studies will focus on the optimal target but also on the optimal level of immunogenicity. The latter can be influenced by numerous modulations that affect not only cytokine release but also regulation. In addition, there are potential applications in regulatory cells such as CAR regulatory T cells (Treg). The question of toxicity reduction must also be addressed, as long-term complications such as the potential development of malignant diseases, infections, or cytopenia must be considered even more critically in the area of autoimmune diseases than is the case for patients with oncologic diseases. Alternative antibody-based therapies using the same target (e.g., CD3/CD19 bispecific targeting antibodies) have not been used in these patients and might also be considered in the future. In conclusion, CAR T cell therapy represents a promising therapeutic approach for autoimmune diseases, offering a targeted strategy to modulate immune responses and restore immune tolerance.
Key Points
| CAR T cell therapy offers a promising therapy option in various autoimmune disease. However, the risk−benefit evaluation in the context of autoimmune disorders has not been established yet and must be reconsidered, as certain complications are not tolerable in patients with autoimmune diseases. |
| As in most autoimmune disorders there is no well described antigen, the broad B-cell targeting procedure seems to be the most sensible approach at this timepoint. However, it is probably worth pursuing other options, such as CAR regulatory T cells (Treg), or allogenous CAR T cells. |
History
The concept of CAR (chimeric antigen receptor) T cell therapy emerged from early research in cancer immunotherapy in the late 1980s [1, 2]. The idea was to engineer T cells to express synthetic receptors that could recognize and eliminate cancer cells expressing this antigen. However, it was not until the early 2000s that the first successful CAR T cell therapies for cancer were developed and tested in clinical trials, and anti-CD19 CAR T cells were approved for acute lymphoblastic leukaemia only in 2017 by the Food and Drug Administration [FDA; European Medicines Agency (EMA) approval in 2018]. Later on, the potential for the treatment of autoimmune disorders (AID) was recognized, and since 2021 when the first case of a patient with systemic lupus erythematosus (SLE) was published, the therapy has also spread quickly within the autoimmune field. However, knowledge is still limited, as cases with only around 20 patients with rheumatic diseases (treated with anti-CD19, and in one case with anti-CD19+ anti-BCMA), 2 patients with multiple sclerosis (anti-CD19), and 15 patients with myasthenia gravis (anti- B-cell maturation antigen [BCMA]) have been published so far [3–13]. This review focuses mainly on anti-CD19 CAR T cell therapy. However, other targets have been uses, i.e., in 14 patients with myasthenia gravis (MG) RNA-transfected CD8+ CAR T cells that express the CAR molecule over days (Descartes-08) and have to be applied repeatedly [8]. There are also published data for 12 patients with anti-aquaporin 4 (AQP4)-associated neuromyelitis optica spectrum disorders (NMOSD) who received anti-BCMA CAR T cell therapy [14].
Technology and Production
CAR T Cell Technology
CAR T cell technology involves the genetic modification of T cells to express chimeric antigen receptors, which are synthetic receptors that combine an extracellular antigen-binding domain with co-stimulatory molecule domains to stimulate T cell activation and proliferation. This enables T cell stimulation through antigen contact without the involvement of antigen-presenting cells and, theoretically, without the passing of other checkpoints. CAR molecules contain an extracellular region, transmembrane region, and intracellular region. The extracellular domain of the CAR typically consists of a single-chain variable fragment (scFv) derived from an antibody that recognizes the target antigen (surface peptide, but also carbohydrate and glycolipid recognition has been developed), the antigen recognition domain, and the hinge or spacer domain, which optimizes distance between the antigen-binding domain and cell surface to facilitate binding. Various B cell targets such as CD19, BMCA or CD22 are in therapeutic use or in trials. In the case of AID, mostly the scFv molecule derived from anti-human CD19 antibodies has been used. The receptor is anchored by the transmembrane domain, which derives from various surface proteins. Upon antigen binding, the intracellular signaling transduction domain (STD) of the CAR activates T cell effector functions, such as cytokine secretion and cytotoxicity, leading to targeted destruction of cells expressing the antigen. Different STDs are in use and can have an impact on CAR T reactivity, expansion, and memory formation.
Production of CAR T Cells
The production of CAR T cells for AID follows a similar process to that used for cancer therapy. So far, only autologous CAR T cells have been used for AIDs. For manufacturing autologous T cells, leukapheresis is the first step in therapy, and the discontinuation of (T cell-toxic) immunosuppression before apheresis has to be considered. A minimum of > 1–4 × 109 T cells should be harvested [15]. Peripheral blood mononuclear cells (PBMCs) are isolated from the patient’s blood and activated ex vivo using specific cytokines such as interleukin-2 (IL-2), IL-7, and IL-15. These activated T cells are then genetically transduced with a lentiviral or retroviral vector. This can be done either by commercially used products or in some hospitals inside good manufacturing practice (GMP) facilities using semi-automated production. The engineered T cells are expanded in culture before being re-infused back into the patient. The optimal number of re-infused CAR T cells has yet to be determined, mostly about 1 × 106 anti-CD19 CAR T cells per kg bodyweight were applied. Conditioning chemotherapy [fludarabine and cyclophosphamide(Flu/Cy)], which is administered shortly before re-infusion, enables those few CAR T cells to expand in the patient.
Different Generations of CAR
Different generations of CAR molecules are available, with the main difference concerning the STD, mostly the co-stimulatory domain; however, the second-generation CAR, which uses the co-stimulatory domain either from CD28 or 4-1BB, was primarily used in the published cases of AID so far. In one patient with systemic sclerosis (SSc), a third-generation CAR (which combines both, i.e., CD28 and 4-1BB, co-stimulatory domains) was used. Nevertheless, the field is constantly evolving to further improve the CAR T construct or development of “armoured” CAR T cells, i.e., co-targeting of several antigens, improved expansion and survival, combination with immunomodulators, improvement of tumor microenvironment, and so on [16, 17].
Complications
The currently used conditioning treatment in AID is Flu/Cy, which involves Flu 25 mg/m2 for three doses (days − 5 to − 3) and one dose of Cy 500 or 1000 mg/m2 (day − 3). It can cause cytopenia and other typical side effects of a classical chemotherapy but is usually well tolerated in these patients. So far, no serious infections or cytopenia regarding conditioning chemotherapy have been reported in the field of AID. However, Merkt et al. [12] brought the possibility of fludarabine-associated pneumonitis to attention, which might be a point to be considered in the treatment of patients with interstitial lung disease (ILD). The role of the conditioning chemotherapy is still a matter of debate, but has been deemed beneficial in increasing the proliferation and persistence of CAR T cells, and especially Flu seems essential [18].
The immune suppression after CAR T cell treatment, however, goes beyond the initial cytopenia after conditioning chemotherapy: hypogammaglobulinemia associated with CD19+ B cell depletion has been observed in patients with AID and might predispose patients to various infectious complications. The incidence of hypogammaglobulinemia in patients with AID has to be determined, but it varies between 20 and 90% for patients with malignant diseases who have received anti-CD/anti-BCMA CAR T cell treatment [19]. So far, no long lasting hypogammaglobulinemia or severe infections have been reported for the field of AID, but we probably can expect similar complications for all B cell-depleting strategies as kind of a class effect: serious infectious events after rituximab (RTX) have been observed with an incidence about 5–10 per 100 person-years, and RTX-associated hypogammaglobulinemia affects about 15% of patients with AID [20–23]. However, risk factors such as low baseline immunoglobulin levels, vasculitis, and repetitive RTX cycles cannot be extrapolated, and further clinical trials are necessary to determine the amount of hypogammaglobulinemia in this patient group.
Furthermore, long-lasting cytopenia that goes beyond the effect of chemotherapy is described. Clinicians observe a typical biphasic course, showing a renewed decline after the initial regeneration. The mechanism, however, has not been elucidated so far. Certain patient-related factors are associated with prolonged cytopenia, occurring in hematologic patients and generally not applying to patients with AID: severe cytokine release syndrome (CRS) and immune-effector-cell-associated neurotoxicity syndrome (ICANS), or intensive (antineoplastic) pre-treatment.
One of the primary concerns is the risk of cytokine release syndrome (CRS) and immune-effector-cell-associated neurotoxicity syndrome (ICANS), which can occur due to the rapid proliferation and activation of CAR T cells in response to antigen stimulation [24]. CRS is characterized by systemic inflammation and can manifest as fever, hypotension, and organ dysfunction, while ICANS presents as neurological symptoms ranging from mild confusion to severe encephalopathy. Management of CRS and ICANS typically involves supportive care and the administration of immunosuppressive agents, such as corticosteroids or IL-6 receptor antagonists, i.e., tocilizumab. So far, only low-grade CRS and only one case of ICANS have been described in the treatment of AID. The low frequency and intensity of CRS and ICANS are probably associated with the much lower numbers of targeted cells (i.e., CD19-positive B cells) compared with B malignancies.
Additionally, the long-term persistence of CAR T cells raises concerns regarding the development of malignant complications. But again, these data derive from patients with malignant diseases, and other risk factors might apply: recently, the development of (CAR) T cell lymphoma in the context of CAR T cell therapy has been described [25]. Also, subsequent malignancies such as non-melanoma skin cancer and myelodysplastic syndrome have been reported [26]. Strategies to mitigate these risks include the use of transiently active CAR T cells or the incorporation of regulatory mechanisms to limit CAR T cell function once the autoimmune response has been suppressed. In AID, however, the evidence of CAR T cells in the peripheral blood is mostly limited, and in most cases (Merkt et al. [12] reported a long-lasting CAR T persistence in one patient with SSc), CAR T positive cells are no longer detectable after about 100 days after infusion.
Another complication associated with CAR T cell therapy is the potential for off-target toxicity, where CAR T cells recognize and attack healthy tissues expressing low levels of the target antigen, leading to unintended tissue damage and adverse events. This risk highlights the importance of selecting target antigens with restricted expression patterns or employing strategies to enhance the specificity of CAR T cells, such as the use of dual-targeting CARs or combinatorial antigen recognition.
Taken together, the risk–benefit evaluation in the context of autoimmune disorders has not been establishes yet and must be reconsidered, as certain complications are not tolerable in patients with mortality and morbidity even higher than the general population, but have a considerably better prognosis than patients with (relapsed) malignant diseases. Therefore, the European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party has recently published recommendations on how to proceed with this treatment option in AID [27].
Therapeutic Effect
AID arise from dysregulated immune responses against self-antigens, leading to tissue damage and dysfunction. Traditional treatments for autoimmune diseases often involve immunosuppressive drugs that broadly dampen immune activity. Thus, immunotherapy, particularly anti-CD19 CAR T cell therapy, might minimize some off-target effects by focusing their target on one pillar of the immune system, in this case CD19+ B cells. It has been hypothesized that, by targeting a broad range of B cells via CD19, the self-sustaining circle of autoimmunity might be disrupted, thereby achieving a reset of the immune system. This might explain why, even though B cells reoccur, the remission sustains. It will be interesting to see whether these individuals experience a flare up due to their genetic background, which might support the development of autoimmunity. Furthermore, their ability to migrate into tissue and bind to target structures might enable a deep B cell depletion beyond the reach of therapeutic monoclonal antibodies such as RTX and other B-cell targeting biological disease-modifying drugs. Notably, only RTX has been adequately examined in different AID and is approved for, e.g., rheumatoid arthritis (RA) and antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) [28, 29]. Newer and maybe more effective anti-CD20 antibodies are just under clinical examination (e.g., obinutuzumab for the treatment of lupus nephritis [30]).
The following cases and case series have been published, illustrating the beneficial effect of anti-CD19/anti-BCMA targeting CAR T cell therapy in AID. Reported cases of CRS were common, however mild (all grade < 2). The medians were calculated using the available data.
Muller et al. [9] summarized the cases of 15 patients, of which five with SLE, two with idiopathic inflammatory myositis (IIM), and one with SSc had been already published before [3–5, 7, 11] and are therefore only mentioned once in this summary. Of the 15 patients, 8 were diagnosed with SLE, 4 with SSc, and 3 with IIM. All demonstrated active disease despite prior therapy with at least two standard treatments. Anti-CD19 CAR T cells were used with a second generation CAR. The eight SLE patients (median age, 24 years; median disease duration, 5 years; median follow-up, 17 months) showed signs of active disease with high activity in disease-specific scores [median Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score 13 of (score ranges 0–150) and at least one category A case per the British Isles Lupus Assessment Group (BILAG) Index, i.e., “severe flare”], and all of them had proven renal involvement at baseline. After anti-CD19 CAR T cell therapy, all of them reached treatment-free remission after 6 months (for one patient only 3-month follow-up has been shown), and the disease-associated antibodies against double-stranded DNA (dsDNA) were no longer detectable, suggesting a profound elimination of autoreactive clones.
From the published cases, four patients with IIM [6, 9] (median age, 42 years; median disease duration, 2 years; median follow-up, 12 months) also showed normalization of their creatine kinase (CK) levels and muscle strength [normalization on manual muscle test (MMT-8)]. Autoantibodies declined but never normalized, and one patient [6] achieved remission only after adding mycophenolate mofetil (MMF), potentially due to an induced cytokine storm that might have further stimulated autoreactive cytotoxic T cells.
All of the five SSc patients [9, 12] (median age, 38 years; median disease duration, 2 years; median follow-up, 10 months) had severe skin involvement, demonstrated by modified Rodnan skin score (mRSS; median, 24). Also, all of them were diagnosed with lung involvement (data on lung function during follow up were not published in all cases), three were diagnosed with heart involvement, and one was diagnosed with renal involvement. After anti-CD19 CAR T cell therapy, mRSS decreased in all patients (median decrease, 9 points). In one of the two separately published patients with SSc, lung involvement remained stable [7], whereas in the other patient an improvement of lung function and fibroblast activation confirmed by 68Ga-fibroblast activation protein-inhibitor positron emission tomography was reported [12]. This last patient received the third-generation anti-CD19 CAR and was additionally treated with mycophenolate/nintedanib after CAR T cell therapy. In all published SSc patients, autoantibodies declined but only disappeared in the one patient treated with the third-generation CAR [31].
In the two, very recently published cases of multiple sclerosis (MS [13]), the authors described CAR-T cell presence and expansion in the cerebrospinal fluid (CSF) without clinical signs of neurotoxicity and, in particular, no case of ICANS. In the one patient with a longer observational period (patient 1, approximately 100 days; patient 2, approximately 20 days), a decrease of antibody production in the CFS was reported as a possible sign of response to treatment, clinical parameters were improved or stable, and one new T2 lesion without contrast enhancement was detected; however, it remained stable on subsequent magnetic resonance imaging (MRI).
For MG, the treatment with anti-BMA rCAR-T (14 patients [8]) and anti-CD19 CAR T (1 patient [10]) cell therapy showed promising results with clinically meaningful improvement at up to 9 months of follow-up [mean improvement of −7 on the Quantitative Myasthenia Gravis score (QMG)] in the anti-BCMA and 70% decrease of the autoantibody, decrease of QMG, and improvement of muscle strength in the 1 patient who received anti-CD19 CAR T cells.
Similar results were obtained for the 12 patients with AQP4 neuromyelitis optica spectrum disorders (NMSOD), who were treated with second-generation anti-BCMA CAR T cells: during the follow-up of a median 6 months, 11 patients had no relapse, and also 11 patients’ AQP-4 antibodies decreased [14].
Future Perspectives
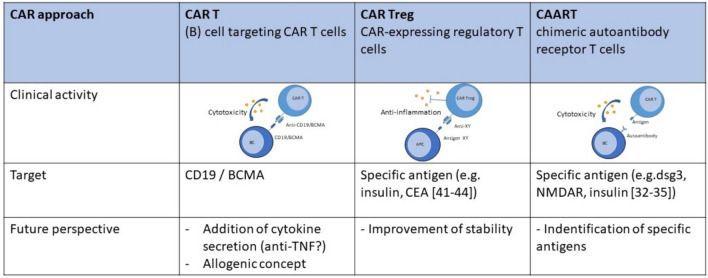
Several factors need to be considered in the design of CAR T cells for autoimmune diseases (Fig. 1), including the choice of target antigen, the specificity and affinity of the CAR, and strategies to minimize off-target effects and promote regulatory T cell function. Additionally, the use of inducible CARs or suicide genes can provide an added layer of control over CAR T cell activity, allowing for the modulation or elimination of CAR T cells if adverse events occur, thus increasing safety.
Fig. 1.
Different concepts using chimeric antigen receptors (CAR) or chimeric autoantibody receptors (CAAR) for the treatment of autoimmune diseases
What Defines a Good Target?
Previous experience of CAR T cell therapy in humans mainly focuses on the effector immune cells, i.e., CD19+ B cells. By targeting CD19, the idea is to reach profound B cell depletion, as this surface marker is present on a broad spectrum of B cells, including plasmablasts. In theory, the self-sustaining system of autoimmunity that leads to further organ damage and antigen exposure is interrupted, therefore resulting in a “reset” of the immune system and a sustained remission without therapy even when CAR T cells are no longer detectable in the patient. On the downside, immunosuppression and a consecutive higher risk of infections have been observed. Targeting BCMA, a marker primarily expressed on plasma cells and mature B cells, with CAR T cells showed similar complications. In the context of autoimmune diseases, another more specific approach might be available, if the autoantigen is well described: CAR T cells can be designed to target either autoantigens directly or immune cells involved in the autoimmune response, such as autoreactive T cells or B cells. For example, in type 1 diabetes, CAR T cells targeting the antigen insulin peptide presented by major histocompatibility complex (MHC) II could be used to selectively eliminate these “responsible” autoimmune cells to prevent further destruction of pancreatic tissue [32, 33]. In myasthenia gravis, chimeric autoantibody receptor (CAAR) T cells showed promising in vitro results [34]. Similar results were shown for N-methyl-d-aspartate receptor (NMDAR) encephalitis [35]. Other authors could demonstrate in a murine model of cardiac disease that CAR T cells targeting fibroblast activation protein (FAP) resulted in a reduction of the development of fibrosis [36–38]. This approach is fascinating not only because of the possibility to directly target fibrosis, which is of major importance in various connective tissue diseases such as SSc, but also because the authors were able to generate CAR T cells in vivo using nanoparticles to deliver the mRNA into T cells. Unfortunately, although typical autoantibodies in various rheumatic diseases are known, there is not one specific antigen for many AID. Therefore, the general targeting of B cells seems to be the right approach for most autoimmune diseases for now, but a better understanding of the pathogenesis of various autoimmune diseases in the future may help to design more specific concepts in the long term.
Beyond Antigen Targeting
Alternatively, CAR T cells targeting autoreactive T cells or B cells could be employed to suppress aberrant immune responses and restore immune tolerance. One approach to re-establish tolerance is via regulatory T cells (Tregs). Tregs have demonstrated a therapeutic suppressive potential in autoimmune diseases; however, the nonspecific antigen recognition results in a general immunosuppression, thereby limiting their feasibility. CAR Treg might either derive from autologous Tregs or FoxP3-transduced CAR T cells and can be engineered for specific antigens through immune suppressive cytokines or direct cellular crosstalk [39, 40]. Publications so far describe preclinical data in mural models of multiple sclerosis, type 1 diabetes, and autoimmune colitis [41–44], thereby demonstrating the feasibility of the concept and also reduction of inflammation.
Allogenic CAR T Cells
Even though in most cases patients with AID are not as heavily pre-treated as patients with cancer, severe cases potentially require frequent changes in therapy, and it is not surprising that, in the examples mentioned above, in the field of rheumatic diseases there was an average of six previous immunosuppressive therapies. RTX and Cy are commonly used to treat refractory cases and might influence the “quality” of immune effector cells [45]. Another point that needs consideration is the problem of time from the decision on therapy to the subsequent application, as an organizational effort is necessary (leukapheresis, transduction, expansion, and lymphodepletion). Allogeneic “off-the-shelf” CAR T cells offer certain advantages: standardization, immediate availability, and potential cost reduction [46, 47]. However, the potential allogenic effects, i.e., graft-versus-host disease (GvHD) and fratricide, have to be considered. Hu et al. [48] successfully used genetic engineering to generate CD7-targeting CAR-T cells (CAR-NKi-tKO) that resist fratricide (CD7-KO), GvHD (TCR-KO), and allo-rejections from both CD4+ T cells (HLA-II-KO) and NK cells (NKi).
Conclusion
In summary, CAR T cell therapy represents a promising approach for the treatment of autoimmune diseases from various origins by selectively targeting pathogenic immune cells or autoantigens involved in the autoimmune process. However, other B cell targeting strategies offer advantageous in terms of availability and controllability [49, 50]. Further research is needed to optimize CAR T cell design, improve safety profiles, and assess long-term outcomes in clinical trials. By addressing these challenges, CAR T cell therapy has the potential to revolutionize the management of autoimmune diseases and provide patients with safe, long-lasting, and effective treatment. Nevertheless, at this point clinical trials are mandatory for the confirmation of the efficacy and safety of the available anti-CD19 CAR T cell in prospective trials.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Funding
Open Access funding was enabled and organized by Projekt DEAL. This work was supported by the Medical Faculty University Hospital Tuebingen’s Clinician Scientist program Grant no. 477-0-0 awarded to Ann-Christin Pecher.
Conflicts of interest
The authors A.C.P., L.H., C.L., and J.H. declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent (participation and publication)
Not applicable.
Authors’ contributions
A.C.P., L.H., C.L., and J.H. contributed to the implementation of the existing research data and to the writing of the manuscript.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Code availability
Not applicable.
References
- 1.Gross G, Gorochov G, Waks T, Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc. 1989;21(1 Pt 1):127–30. [PubMed] [Google Scholar]
- 2.Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149(3):960–8. 10.1016/0006-291x(87)90502-x. 10.1016/0006-291x(87)90502-x [DOI] [PubMed] [Google Scholar]
- 3.Mougiakakos D, Kronke G, Volkl S, Kretschmann S, Aigner M, Kharboutli S, et al. CD19-Targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385(6):567–9. 10.1056/NEJMc2107725. 10.1056/NEJMc2107725 [DOI] [PubMed] [Google Scholar]
- 4.Mackensen A, Muller F, Mougiakakos D, Boltz S, Wilhelm A, Aigner M, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022;28(10):2124–32. 10.1038/s41591-022-02017-5. 10.1038/s41591-022-02017-5 [DOI] [PubMed] [Google Scholar]
- 5.Muller F, Boeltz S, Knitza J, Aigner M, Volkl S, Kharboutli S, et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet. 2023;401(10379):815–8. 10.1016/S0140-6736(23)00023-5. 10.1016/S0140-6736(23)00023-5 [DOI] [PubMed] [Google Scholar]
- 6.Pecher AC, Hensen L, Klein R, Schairer R, Lutz K, Atar D, et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA. 2023;329(24):2154–62. 10.1001/jama.2023.8753. 10.1001/jama.2023.8753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann C, Muller F, Distler JHW, Gyorfi AH, Volkl S, Aigner M, et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann Rheum Dis. 2023;82(8):1117–20. 10.1136/ard-2023-223952. 10.1136/ard-2023-223952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granit V, Benatar M, Kurtoglu M, Miljkovic MD, Chahin N, Sahagian G, et al. Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study. Lancet Neurol. 2023;22(7):578–90. 10.1016/S1474-4422(23)00194-1. 10.1016/S1474-4422(23)00194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Volkl S, et al. CD19 CAR T-cell therapy in autoimmune disease—a case series with follow-up. N Engl J Med. 2024;390(8):687–700. 10.1056/NEJMoa2308917. 10.1056/NEJMoa2308917 [DOI] [PubMed] [Google Scholar]
- 10.Haghikia A, Hegelmaier T, Wolleschak D, Bottcher M, Desel C, Borie D, et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 2023;22(12):1104–5. 10.1016/S1474-4422(23)00375-7. 10.1016/S1474-4422(23)00375-7 [DOI] [PubMed] [Google Scholar]
- 11.Taubmann J, Knitza J, Muller F, Volkl S, Aigner M, Kleyer A, et al. Rescue therapy of antisynthetase syndrome with CD19-targeted CAR-T cells after failure of several B-cell depleting antibodies. Rheumatology (Oxford). 2024;63(1):e12–4. 10.1093/rheumatology/kead330. 10.1093/rheumatology/kead330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merkt W, Freitag M, Claus M, Kolb P, Falcone V, Rohrich M, et al. Third-generation CD19. CAR-T cell-containing combination therapy in Scl70+ systemic sclerosis. Ann Rheum Dis. 2024;83(4):543–6. 10.1136/ard-2023-225174. 10.1136/ard-2023-225174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischbach F, Richter J, Pfeffer LK, Fehse B, Berger SC, Reinhardt S, et al. CD19-targeted chimeric antigen receptor T cell therapy in two patients with multiple sclerosis. Med. 2024. 10.1016/j.medj.2024.03.002. 10.1016/j.medj.2024.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Qin C, Tian DS, Zhou LQ, Shang K, Huang L, Dong MH, et al. Anti-BCMA CAR T-cell therapy CT103A in relapsed or refractory AQP4-IgG seropositive neuromyelitis optica spectrum disorders: phase 1 trial interim results. Signal Transduct Target Ther. 2023;8(1):5. 10.1038/s41392-022-01278-3. 10.1038/s41392-022-01278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worel N, Holbro A, Vrielink H, Ootjers C, Le Poole K, Beer-Wekking I, et al. A guide to the collection of T-cells by apheresis for ATMP manufacturing-recommendations of the GoCART coalition apheresis working group. Bone Marrow Transplant. 2023;58(7):742–8. 10.1038/s41409-023-01957-x. 10.1038/s41409-023-01957-x [DOI] [PubMed] [Google Scholar]
- 16.Labanieh L, Mackall CL. CAR immune cells: design principles, resistance and the next generation. Nature. 2023;614(7949):635–48. 10.1038/s41586-023-05707-3. 10.1038/s41586-023-05707-3 [DOI] [PubMed] [Google Scholar]
- 17.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–67. 10.1038/s41571-019-0297-y. 10.1038/s41571-019-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohty M, Minnema MC. Lymphodepleting conditioning regimens. In: Kroger N, Gribben J, Chabannon C, Yakoub-Agha I, Einsele H, editors. The EBMT/EHA CAR-T cell handbook. Springer Cham; 2022. p. 131–3. [PubMed]
- 19.Wudhikarn K, Perales MA. Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy. Bone Marrow Transplant. 2022;57(10):1477–88. 10.1038/s41409-022-01756-w. 10.1038/s41409-022-01756-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stabler S, Giovannelli J, Launay D, Cotteau-Leroy A, Heusele M, Lefevre G, et al. Serious infectious events and immunoglobulin replacement therapy in patients with autoimmune disease receiving rituximab: a retrospective cohort study. Clin Infect Dis. 2021;72(5):727–37. 10.1093/cid/ciaa127. 10.1093/cid/ciaa127 [DOI] [PubMed] [Google Scholar]
- 21.Md Yusof MY, Vital EM, McElvenny DM, Hensor EMA, Das S, Dass S, et al. Predicting severe infection and effects of hypogammaglobulinemia during therapy with rituximab in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. 2019;71(11):1812–23. 10.1002/art.40937. 10.1002/art.40937 [DOI] [PubMed] [Google Scholar]
- 22.Gottenberg JE, Ravaud P, Bardin T, Cacoub P, Cantagrel A, Combe B, et al. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum. 2010;62(9):2625–32. 10.1002/art.27555. 10.1002/art.27555 [DOI] [PubMed] [Google Scholar]
- 23.Tony HP, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kotter I, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res Ther. 2011;13(3):R75. 10.1186/ar3337. 10.1186/ar3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. 10.1016/j.bbmt.2018.12.758. 10.1016/j.bbmt.2018.12.758 [DOI] [PubMed] [Google Scholar]
- 25.Prasad V. T-cell lymphoma from CAR T-cell therapy—a new safety notice. JAMA. 2024;331(5):389–90. 10.1001/jama.2023.27885. 10.1001/jama.2023.27885 [DOI] [PubMed] [Google Scholar]
- 26.Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26(1):26–33. 10.1016/j.bbmt.2019.08.003. 10.1016/j.bbmt.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco R, Alexander T, Del Papa N, Muller F, Saccardi R, Sanchez-Guijo F, et al. Innovative cellular therapies for autoimmune diseases: expert-based position statement and clinical practice recommendations from the EBMT practice harmonization and guidelines committee. EClinicalMedicine. 2024;69: 102476. 10.1016/j.eclinm.2024.102476. 10.1016/j.eclinm.2024.102476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buch MH, Smolen JS, Betteridge N, Breedveld FC, Burmester G, Dorner T, et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(6):909–20. 10.1136/ard.2010.144998. 10.1136/ard.2010.144998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellmich B, Sanchez-Alamo B, Schirmer JH, Berti A, Blockmans D, Cid MC, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. 2024;83(1):30–47. 10.1136/ard-2022-223764. 10.1136/ard-2022-223764 [DOI] [PubMed] [Google Scholar]
- 30.Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81(1):100–7. 10.1136/annrheumdis-2021-220920. 10.1136/annrheumdis-2021-220920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkt W, Lorenz HM, Schmitt M. CAR T-cell therapy in autoimmune disease. N Engl J Med. 2024;390(17):1628–9. 10.1056/NEJMc2403705. 10.1056/NEJMc2403705 [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi S, Thelin MA, Parrish HL, Deshpande NR, Lee MS, Karimzadeh A, et al. A biomimetic five-module chimeric antigen receptor ((5M)CAR) designed to target and eliminate antigen-specific T cells. Proc Natl Acad Sci USA. 2020;117(46):28950–9. 10.1073/pnas.2012495117. 10.1073/pnas.2012495117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Sosinowski T, Cox AR, Cepeda JR, Sekhar NS, Hartig SM, et al. Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II: peptide complex modulate the progression of autoimmune diabetes. J Autoimmun. 2019;96:50–8. 10.1016/j.jaut.2018.08.004. 10.1016/j.jaut.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh S, O'Connor K, Payne A. MuSK chimeric autoantibody receptor (CAAR) T cells for antigen-specific cellular immunotherapy of myasthenia gravis. Neurology. 2020;94(15_supplement).
- 35.Reincke SM, von Wardenburg N, Homeyer MA, Kornau HC, Spagni G, Li LY, et al. Chimeric autoantibody receptor T cells deplete NMDA receptor-specific B cells. Cell. 2023;186(23):5084–97. 10.1016/j.cell.2023.10.001. 10.1016/j.cell.2023.10.001 [DOI] [PubMed] [Google Scholar]
- 36.Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573(7774):430–3. 10.1038/s41586-019-1546-z. 10.1038/s41586-019-1546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rurik JG, Tombacz I, Yadegari A, Mendez Fernandez PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375(6576):91–6. 10.1126/science.abm0594. 10.1126/science.abm0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583(7814):127–32. 10.1038/s41586-020-2403-9. 10.1038/s41586-020-2403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arjomandnejad M, Kopec AL, Keeler AM. CAR-T regulatory (CAR-Treg) cells: engineering and applications. Biomedicines. 2022. 10.3390/biomedicines10020287. 10.3390/biomedicines10020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riet T, Chmielewski M. Regulatory CAR-T cells in autoimmune diseases: progress and current challenges. Front Immunol. 2022;13: 934343. 10.3389/fimmu.2022.934343. 10.3389/fimmu.2022.934343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation. 2012;9:112. 10.1186/1742-2094-9-112. 10.1186/1742-2094-9-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elinav E, Adam N, Waks T, Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology. 2009;136(5):1721–31. 10.1053/j.gastro.2009.01.049. 10.1053/j.gastro.2009.01.049 [DOI] [PubMed] [Google Scholar]
- 43.Tenspolde M, Zimmermann K, Weber LC, Hapke M, Lieber M, Dywicki J, et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J Autoimmun. 2019;103: 102289. 10.1016/j.jaut.2019.05.017. 10.1016/j.jaut.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 44.Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther. 2014;22(5):1018–28. 10.1038/mt.2014.41. 10.1038/mt.2014.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das RK, Vernau L, Grupp SA, Barrett DM. Naive T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov. 2019;9(4):492–9. 10.1158/2159-8290.CD-18-1314. 10.1158/2159-8290.CD-18-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–99. 10.1038/s41573-019-0051-2. 10.1038/s41573-019-0051-2 [DOI] [PubMed] [Google Scholar]
- 47.Castelli S, Young RM, June CH. Off-the-shelf CAR T cells to treat cancer. Cell Res. 2022;32(12):1036–7. 10.1038/s41422-022-00745-4. 10.1038/s41422-022-00745-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge W, et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022;32(11):995–1007. 10.1038/s41422-022-00721-y. 10.1038/s41422-022-00721-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holzer MT, Ruffer N, Huber TB, Kotter I, Ostendorf L, Krusche M. Daratumumab for autoimmune diseases: a systematic review. RMD Open. 2023. 10.1136/rmdopen-2023-003604. 10.1136/rmdopen-2023-003604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kvacskay P, Merkt W. CD19.CAR-T cells versus obinutuzumab—who will win the race on deep B cell depletion in systemic autoimmunity? Rheumatology (Oxford). 2024. 10.1093/rheumatology/keae144. 10.1093/rheumatology/keae144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.