Abstract
The orexin-1 receptor interacts with β-arrestin-2 in an agonist-dependent manner. In HEK-293T cells, these two proteins became co-internalized into acidic endosomes. Truncations from the C-terminal tail did not prevent agonist-induced internalization of the orexin-1 receptor or alter the pathway of internalization, although such mutants failed to interact with β-arrestin-2 in a sustained manner or produce its co-internalization. Mutation of a cluster of three threonine and one serine residue at the extreme C-terminus of the receptor greatly reduced interaction and abolished co-internalization of β-arrestin-2–GFP (green fluorescent protein). Despite the weak interactions of this C-terminally mutated form of the receptor with β-arrestin-2, studies in wild-type and β-arrestin-deficient mouse embryo fibroblasts confirmed that agonist-induced internalization of this mutant required expression of a β-arrestin. Although without effect on agonist-mediated elevation of intracellular Ca2+ levels, the C-terminally mutated form of the orexin-1 receptor was unable to sustain phosphorylation of the MAPKs (mitogen-activated protein kinases) ERK1 and ERK2 (extracellular-signal-regulated kinases 1 and 2) to the same extent as the wild-type receptor. These studies indicate that a single cluster of hydroxy amino acids within the C-terminal seven amino acids of the orexin-1 receptor determine the sustainability of interaction with β-arrestin-2, and indicate an important role of β-arrestin scaffolding in defining the kinetics of orexin-1 receptor-mediated ERK MAPK activation.
Keywords: β-arrestin, extracellular-signal-regulated kinase (ERK), G-protein-coupled receptor, mitogen-activated protein kinase (MAPK), orexin, receptor internalization
Abbreviations: C1 and C2, cluster 1 and 2; DMEM, Dulbecco's modified Eagle's medium; ERK, extracellular-signal-regulated kinase; eYFP, enhanced yellow fluorescent protein; (e)GFP, (enhanced) green fluorescent protein; GPCR, G-protein-coupled receptor; HA, haemagglutinin; KRB, Krebs–Ringer buffer; MAPK, mitogen-activated protein kinase; MEF, mouse embryo fibroblast; RFP, red fluorescent protein; TAMRA, 5- and 6-carboxytetramethylrhodamine; VSV-G, vesicular-stomatitis virus G
INTRODUCTION
Efforts to identify a ligand able to activate a GPCR (G-protein-coupled receptor), initially denoted HFGAN72, led to the discovery and pairing [1] with hypothalamic peptides called orexin A and orexin B. Orexin A is a 33-amino-acid peptide and this is bound with high affinity by what is now called the orexin-1 receptor (reviewed in [2]). Stimulation of the orexin-1 receptor results in the elevation of intracellular Ca2+ levels [3,4]. Early studies clearly indicated the ability of orexin A to stimulate appetite and affect energy metabolism. Using a selective orexin-1 receptor antagonist, SB-334867, Haynes et al. [5] showed that the orexigenic effects of orexin A are, at least in part, mediated by the orexin-1 receptor, suggesting that orexin-1 receptor antagonists can be useful as medicines in anti-obesity programmes [6–8]. Despite this, there is currently little knowledge on the mechanisms of regulation of the orexin receptors.
Many GPCRs rapidly become internalized into the cell following agonist binding. In many cases the accepted scenario is that agonist binding leads to receptor phosphorylation, and this phosphorylation generates binding sites for a β-arrestin [9]. If the receptor and β-arrestin bind tightly, it can then result in their co-internalization into recycling endosomes via clathrin-coated pits as a result of the interaction of β-arrestin with clathrin adapter proteins [10–12]. Less effective interactions can also lead to internalization of the GPCR, but generally in this scenario the β-arrestin is rapidly segregated from the GPCR [13,14]. The human orexin-1 receptor appears to interact tightly with β-arrestins, as Evans et al. [15] reported the trafficking of both β-arrestin-1–GFP (green fluorescent protein) and β-arrestin-2–GFP into intracellular vesicles following the addition of orexin A to CHO (Chinesehamster ovary) cells stably expressing the receptor. The sites of interactions between β-arrestins and GPCRs have not been studied exhaustively, but appear to vary with the receptor. For example, whereas the third intracellular loop is important for the α2-adrenoceptor subtypes [16] and the M2 muscarinic receptor [17], the highly conserved Asp-Arg-Tyr domain at the bottom of transmembrane helix III plays a key role in the f-MLP (N-formyl peptide) receptor [18]. For a significant number of GPCRs, the C-terminal tail plays a key role. C-terminal truncation of a range of GPCRs, including the thyrotropin-releasing hormone receptor-1, both prevents interaction with β-arrestins and the capacity of the receptors to internalize in response to agonist [19,20]. The C-terminal tail of the orexin-1 receptor is predicted to comprise approx. 63 amino acids of which 16 are aliphatic hydroxy amino acids. It appears that key sites for interactions with β-arrestins in the C-terminal tail of GPCRs often comprise closely associated clusters of serine and threonine residues in which at least 3 out of 5 contiguous amino acids are candidate sites for phosphorylation [13,14].
A series of recent studies have indicated that β-arrestins not only participate in GPCR desensitization and internalization, but also in receptor signal transduction, as they can act as adaptor proteins for components of the ERK (extracellular-signal-regulated kinase) MAPK (mitogen-activated protein kinase) pathway (reviewed in [21,22]). Activation of the β2-adrenergic receptor results in rapid translocation of β-arrestin-1 together with c-Src to the activated receptor at the plasma membrane [23]. In addition to interacting with c-Src family tyrosine kinases, β-arrestins have recently been shown to directly interact with both MAPK and Raf-1 in response to the activation of the protease-activated receptor 2 [24]. GPCR mutants that interact differentially with β-arrestins might therefore be anticipated to show distinct effects on the extent or temporal kinetics of ERK MAPK signalling [25]. In the present study we have analysed this possibility using a detailed analysis of the sites of interaction of β-arrestin-2–GFP with the C-terminal tail of the orexin-1 receptor. We have identified a form of this receptor that interacts significantly less well with β-arrestin-2 than the wild-type orexin-1 receptor, but which still internalizes into cells in an agonist- and β-arrestin-dependent manner. We demonstrated that tight interactions are determined by a single cluster of three threonine and one serine residue at the extreme C-terminus of the receptor. Mutation of any two of these residues to alanine limits interactions with β-arrestin-2–GFP. The MAPKs ERK-1 and ERK-2 are maintained in the phosphorylated state for a significantly shorter period in response to stimulation of the mutant receptor, whereas the wild-type and mutant receptors are equally effective in causing elevation of intracellular [Ca2+].
EXPERIMENTAL
Materials
Filipin, nystatin and all materials for tissue culture were supplied by Sigma (Gillingham, Dorset, U.K.). Oligonucleotides were purchased from ThermoHybaid (Ulm, Germany). Orexin A and TAMRA (5- and 6-carboxytetramethylrhodamine)-labelled orexin A [15] were synthesized by GlaxoSmithKline (Harlow, Essex, U.K.). Antibodies recognizing ERK1/2 and their phosphorylated forms were from New England Biolabs (Hitchin, Herts., U.K.). The anti-GFP serum was raised in house against recombinantly expressed eGFP (enhanced GFP).
Plasmid construction
β-arrestin-2–GFP was obtained from Dr C. Krasel (Department of Pharmacology, University of Würzburg, Germany). To construct β-arrestin-2–RFP (red fluorescent protein), β-arrestin-2–GFP was digested with NheI and ApaI and the β-arrestin-2 fragment inserted in frame into the multiple cloning site of pAS Red NFP (Clontech, Palo Alto, CA, U.S.A.). HA (haemagglutinin)-tagged and VSV (vesicular-stomatitis virus)-G-tagged forms of the orexin-1 receptor were constructed by PCR amplification of the coding sequence of the human orexin-1 receptor [1] and inserted into the HindIII–XhoI site of pCDNA3 (Invitrogen, Paisley, Renfrewshire, Scotland, U.K.). To obtain orexin-1 receptor–eYFP (enhanced yellow fluoresecent protein), the pEYFP vector (PerkinElmer, Hounslow, Middx., U.K.) was digested with NotI/XhoI and the resulting eYFP fragment subcloned into the orexin-1 receptor in pCDNA3. The C-terminus truncation mutants of HA–orexin-1 receptor were engineered using standard PCR techniques by introducing a stop codon in the position immediately following the desired new C-terminus. The mutation of one or more serine/threonine residues to alanine, with the exception of the C2 (cluster 2) and C1C2 mutants, was also accomplished using standard PCR strategies by engineering primers carrying the appropriate base changes. The C2 and C1C2 mutants were generated employing overlap PCR as described by Fong [26].
Transient transfection of HEK-293T cells
HEK-293T cells were maintained in DMEM (Dulbecco's modified Eagle's medium) supplemented with 0.292 g/l L-glutamine and 10% (v/v) newborn calf serum at 37 °C in a 5% CO2 environment. Cells were grown to 60–80% confluency prior to transient transfection on poly(D-lysine)-coated coverslips. Transfection was performed using Lipofectamine™ reagent (Invitrogen, Paisley, Renfrewshire, Scotland, U.K.) according to the manufacturer's instructions.
Transient transfection of MEF (mouse embryonic fibroblast) cells
MEF wild-type, β-arrestin-1 and -2 [27,28] and Gq/G11 [29,30] knock-out cells were maintained in DMEM supplemented with 0.292 g/l L-glutamine and 10% (v/v) foetal calf serum at 37 °C in a 5% CO2 environment. Cells were grown to 60–80% confluency prior to transient transfection on poly(D-lysine)-coated coverslips. Transfection was carried out using the Nucleofector system (AMAXA, Cologne, Germany) according to the manufacturer's instructions.
Immunostaining for HA–orexin-1 receptor
Immunostaining was performed essentially according to the method of Cao et al. [31]. Cells were plated on to coverslips and transfected after 24 h with the appropriate constructs. After a further 24 h, the medium was changed for Hepes/DMEM containing 2.5 μg/ml of anti-HA antibody (Roche Molecular Biochemicals, Nutley, NJ, U.S.A.) and incubated for 40 min at 37 °C in 5% CO2. Where required, to give a final concentration of 0.5 μM agonist, Hepes/DMEM containing orexin A was added and incubated for 30 min at 37 °C in 5% CO2. Coverslips were washed three times with PBS and then cells fixed with 4% paraformaldehyde in PBS/5% sucrose for 10 min at room temperature followed by three more PBS washes. Cells were then permeabilized in 0.15% Triton X-100/3% non-fat milk/PBS (TM buffer) for 10 min at room temperature. The coverslips were subsequently incubated with an Alexa™ 594-labelled goat anti-mouse secondary antibody (Molecular Probes, Eugene, OR, U.S.A.) at a dilution of 1:400 (1–4 μg/ml), upside down on Nescofilm, for 1 h at room temperature, then washed twice in TM buffer and three times with PBS. Finally, coverslips were mounted on to microscope slides with 40% glycerol in PBS.
CypHer-5 detection of the VSV–orexin-1 receptor
Antibody labelling was carried out essentially as described in Adie et al. [32]. Cells were seeded on to coverslips the day before use and transfected with appropriate constructs. After 24 h the cells were washed twice with KRB (Krebs–Ringer buffer), pH 7.4, at room temperature and then incubated with CypHer-5-labelled anti-VSV-G antibody (Amersham Biosciences, Little Chalfont, Bucks., U.K.) at a concentration of 20 μg/ml for 30 min at room temperature in the presence of various inhibitors. After this time orexin A was added to the cells and incubated for 30 min at 37 °C in 5% CO2. Coverslips were washed three times with KRB and fixed with 4% paraformaldehyde in PBS/5% sucrose for 10 min at room temperature followed by three more washes with KRB. The coverslips were then mounted on to microscope slides with 40% glycerol in PBS.
Confocal laser-scanning microscopy
Cells were observed using a confocal laser-scanning microscope (Zeiss LSM 5 Pascal) using a Zeiss Plan-Apo 63×1.40 NA oil immersion objective, pinhole of 20 and electronic zoom 1 or 2.5. GFP and eYFP were excited using a 488 nm argon laser and detected with 505–530 nm band-pass filter. The Alexa™ 594 label, TAMRA–orexin A ligand and RFP were excited using a 543 nm helium/neon laser and detected with a 560 nm long-pass filter. To visualize the CypHer-5-labelled anti-VSV-G antibody, the cells were excited using a 633 nm helium/neon laser and detected with a 650 nm long-pass filter. The images were manipulated with MetaMorph software. For the receptor internalization studies only, fixed cells were used. Cells on glass coverslips were washed with PBS or KRB and fixed for 10 min at room temperature using 4% paraformaldehyde in PBS/5% sucrose, pH 7.4. After three washes with PBS or KRB, coverslips were mounted on to microscope slides with 40% glycerol in PBS.
Orexin-1 receptor–β-arrestin-2–GFP co-immunoprecipitation
Co-immunoprecipitation of receptor and β-arrestin-2 was essentially carried out as described previously [33]. HEK-293T cells were transiently transfected with VSV-G-tagged wild-type receptor or C1 mutant and β-arrestin-2–GFP. After transfection (24 h) the cells were incubated with vehicle or 0.5 μM orexin A for 15 min at 37 °C. The stimulations were stopped by addition of the membrane-permeant and reversible cross-linker dithiobis(succinimidyl propionate) (Sigma, Poole, Dorset, U.K) at a final concentration of 2 mM. The cells were then incubated under gentle agitation at room temperature, washed twice with 50 mM Tris/HCl, pH 7.4, in PBS to neutalize unreacted dithiobis(succinimidyl propionate), lysed in 0.5 ml of 50 mM Hepes, pH 7.4, 50 mM NaCl, 10% (v/v) glycerol, 0.5% (v/v) Nonidet P40, 2 mM EDTA, 100 μM Na3VO4, 1 mM PMSF, 10 μg/ml benzamidine, 5 μg/ml soya-bean trypsin inhibitor and 5 μg/ml leupeptin, and clarified by centrifugation (2 min at 153 g and 4 °C). Aliquots (25 μl) of whole cell lysates were removed and mixed with an equal volume of 2× reducing loading buffer. To isolate β-arrestin-2-bound orexin-1 receptor, BSA was added to a final concentration of 1% to 500 μg of each lysate. Immunoprecipitation was performed for 12–16 h at 4 °C using the anti-GFP serum and Protein G–Sepharose beads. Immune precipitates were washed 3 times with glycerol lysis buffer and eluted in 1× reducing loading buffer for 15 min at 45 °C. Proteins were resolved by SDS/PAGE and transferred on to PVDF membranes for detection of the protein. Immunodetection of VSV-G–orexin-1 receptor constructs was performed using the anti-VSV-G antibody and immunodetection of β-arrestin-2–GFP was performed using the anti-GFP serum. Immune complexes were then visualized by chemiluminescence detection using anti-mouse and anti-sheep horseradish-peroxidase-conjugated IgG, respectively.
ERK1/2 phosphorylation and immunoblots
Cells were grown in 6-well plates and serum starved for 2 h prior to stimulation with 0.5 μM orexin A for the times indicated. Cells were then placed on ice, washed twice with cold PBS and lysed in RIPA buffer [25 mM Hepes, pH 7.5, 75 mM NaCl, 0.5% Triton X-100, 0.25 % sodium deoxycholate, 0.05 % SDS, 10 mM NaF, 5 mM EDTA, 10 mM Na2HPO4, 5 % (w/v) ethylene glycol]. After solubilizing the cells for 1 h at 4 °C, the lysates were centrifuged for 15 min at 20800 g at 4 °C to remove the insoluble material. The samples were mixed with 2× reducing loading buffer and heated for 3 min at 95 °C. ERK1/2 phosphorylation was detected by protein immunoblotting using phospho-ERK1/2-specific antibodies and anti-rabbit horseradish-peroxidase-conjugated IgG as secondary antibody for immunodetection. After visualizing the level of ERK1/2 phosphorylation, the PVDF membranes were stripped of Igs and reprobed using the anti-ERK1/2 antibody.
Calcium signalling studies
Single cell Ca2+ imaging studies were performed in either Gαq/Gα11 double-knock-out EF88 cells or HEK-293T cells, as described previously [34].
Miscellaneous
All experiments were performed on a minimum of three occasions.
RESULTS
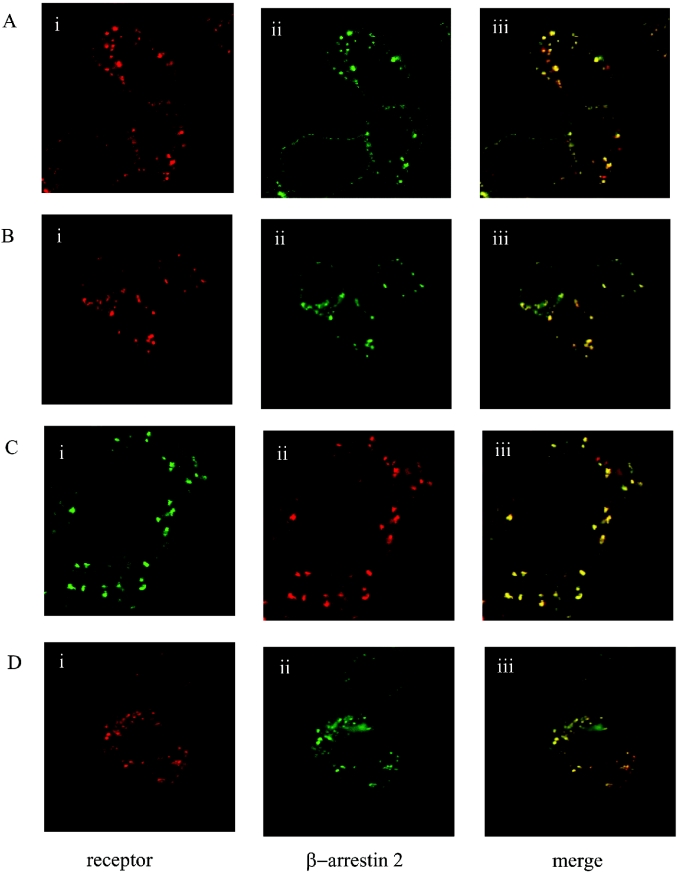
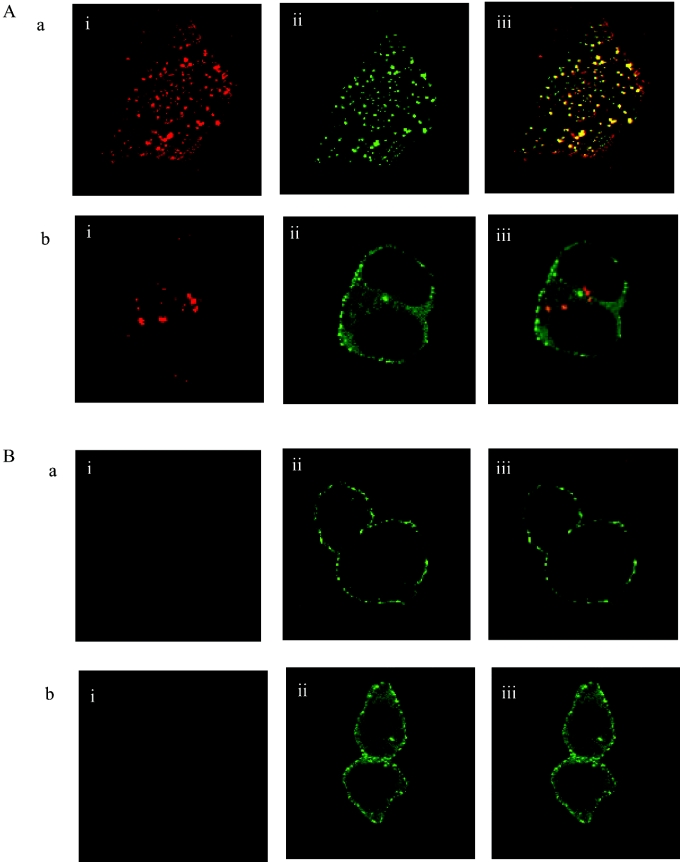
Following co-expression in HEK-293T cells of the human orexin-1 receptor and β-arrestin-2, the receptor was targeted predominantly to the cell surface, whereas β-arrestin-2 was distributed evenly throughout the cytoplasm (results not shown, but see [15]). Addition of orexin A (0.5 μM) as agonist for 30 min resulted in internalization of the receptor. This could be monitored by a number of distinct strategies. Firstly, addition of TAMRA-labelled orexin A as agonist allowed observation of internalization of this ligand, bound to the receptor, into punctate intracellular vesicles (Figure 1A). No specific binding or internalization of TAMRA–orexin A was observed in mock-transfected cells (results not shown). Interaction of β-arrestin-2–GFP with the TAMRA–orexin-A-occupied orexin-1 receptor was monitored by initial movement of β-arrestin-2–GFP to the plasma membrane [15] followed by its internalization into punctate vesicles. When the images corresponding to TAMRA–orexin A (red) and β-arrestin-2–GFP (green) were merged, it resulted in a yellow pattern of staining that indicates overlapping distribution of the two signals (Figure 1A). Secondly, a form of the orexin-1 receptor N-terminally tagged with the HA-epitope tag sequence was also internalized in response to addition of orexin A. The combined immunocytochemical detection of the receptor and image analysis again confirmed an overlap of its intracellular distribution with co-expressed β-arrestin-2–GFP (Figure 1B). Thirdly, orexin A caused internalization of a form of the orexin-1 receptor C-terminally tagged with eYFP (Figure 1C). The co-expression of this construct with a form of β-arrestin-2 C-terminally tagged with RFP from Anemonia sulcata again indicated the interaction and intracellular co-localization of these two proteins following agonist challenge (Figure 1C). Finally, a form of the receptor modified at the N-terminus to include the VSV-G-epitope tag was employed. The addition of a novel, pH-sensitive, cyanine dye termed CypHer-5 [32] linked to an anti-VSV-G antibody resulted in intracellular red fluorescence following stimulation with orexin A (Figure 1D). As this dye fluoresces only when in an acidic environment (pKa=6.1) [32,35], these results confirmed that the receptor and expressed β-arrestin-2–GFP were co-internalized into acidic endosomes.
Figure 1. Orexin-1 receptor co-internalizes with β-arrestin-2.
HEK-293T cells were transfected with various forms of the human orexin-1 receptor and β-arrestin-2. Post-transfection (24 h) cells were challenged with orexin-A (0.5 μM) for 30 min. Cells were then fixed and visualized. The distribution of the orexin-1 receptor (i), β-arrestin-2 (ii) and a composite of these images (iii) are shown. (A) Wild-type orexin-1 receptor+β-arrestin-2–GFP, stimulated with TAMRA-orexin-A. (B) N-terminally HA-tagged orexin-1 receptor+β-arrestin-2–GFP, stimulated with orexin-A in the presence of anti-HA antibody. (C) Orexin-1 receptor–eYFP+β-arrestin-2–RFP, stimulated with orexin-A. (D) N-terminally VSV-G-tagged orexin-1 receptor+β-arrestin-2–GFP, stimulated with orexin-A in the presence of CypHer-5-labelled anti-VSV-G antibody.
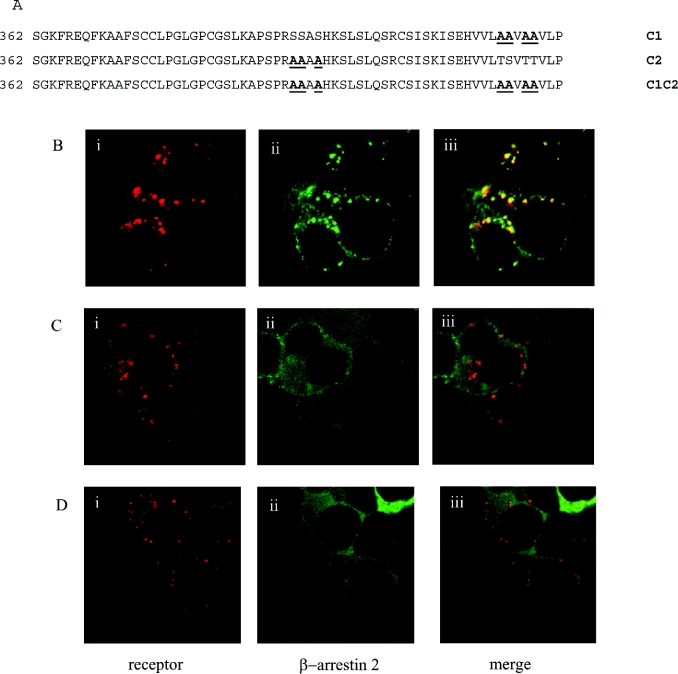
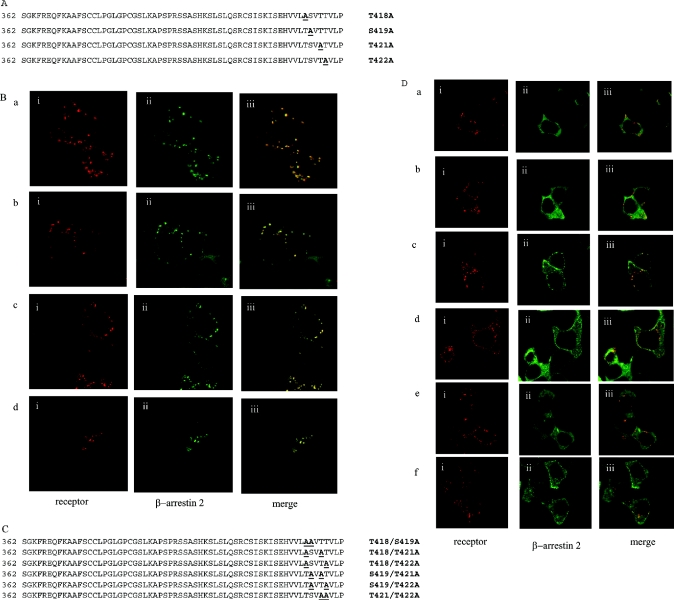
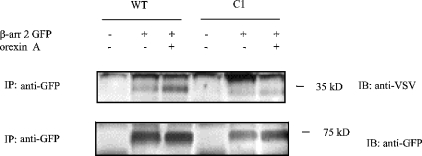
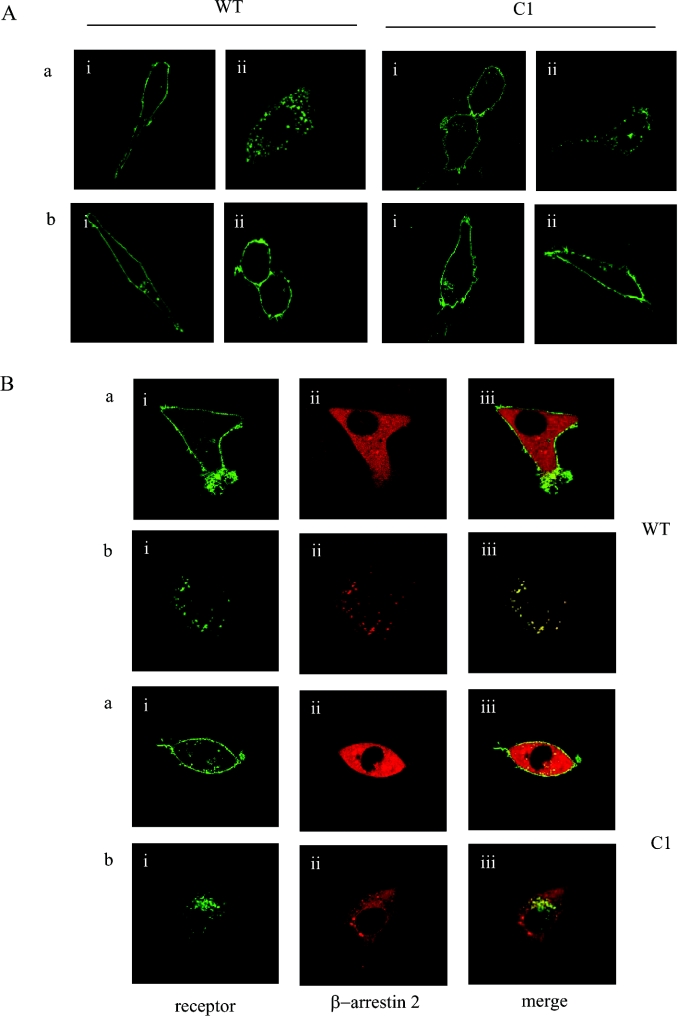
For many GPCRs the C-terminal tail plays an important role in agonist-mediated internalization. The predicted C-terminal region of the orexin-1 receptor is some 63 amino acids in length and contains 16 aliphatic hydroxy amino acids. To ascertain the importance of the C-terminal tail of the orexin-1 receptor in agonist-induced internalization and in interaction with β-arrestin-2, a series of C-terminally truncated forms of the receptor was produced that each contained the N-terminal HA-epitope tag (Figure 2A). Each of these truncated forms was internalized in response to addition of orexin A when expressed in HEK-293T cells (Figures 2B and 2C, and results not shown). However, when co-expressed with β-arrestin-2–GFP there was no indication of strong, maintained interactions of the truncated forms of the receptor with the β-arrestin, because co-internalization of the two proteins was not observed in response to addition of orexin A (Figures 2B and 2C, and results not shown). Whereas co-expression of β-arrestin-2–GFP with the full-length orexin-1 receptor resulted in the appearance of yellow intracellular vesicles, reflecting overlapping distribution of the receptor and β-arrestin-2 (Figure 1B), for each of the truncation mutants the vesicles containing the internalized receptors appeared red on a background of green that reflects non-overlapping, cytoplasmic β-arrestin-2–GFP (Figures 2B and 2C). The truncation mutants eliminated two clusters of hydroxy amino acids located at positions 393–396 and 418–422. Such clusters of hydroxy amino acids have been suggested to be important binding sites for β-arrestins [13–14]. We thus converted the serine and threonine residues of these clusters into alanine (Figure 3A). Mutation of all three serines in the cluster located between amino acids 393–396 (C2) did not modify interaction of the receptor with β-arrestin-2–GFP. This mutated receptor behaved essentially as the wild-type in that its internalization into punctate vesicles in response to orexin A was accompanied by co-internalization of β-arrestin-2–GFP (Figure 3B). By contrast, alteration of all four hydroxy amino acids in the cluster between amino acids 418–422 to alanines (C1) greatly reduced translocation of β-arrestin-2–GFP from the cytoplasm to the plasma membrane and resulted in internalization of the receptor without co-internalization of β-arrestin-2–GFP (Figure 3C). Combination of the two sets of mutants (C1C2 mutant) also produced a form of the receptor that was unable to interact with β-arrestin-2–GFP with high affinity. Internalization of this form of the receptor in response to orexin A also occurred without co-internalization of β-arrestin-2–GFP (Figure 3D). To investigate the contribution of specific hydroxy amino acids in C1, each of these four amino acids was individually modified to alanine (Figure 4A). Sustained agonist-mediated interaction with β-arrestin-2–GFP was not abolished by any of these single point mutations, because addition of orexin A resulted in the co-localization of the receptor and β-arrestin-2–GFP in punctate intracellular vesicles (Figure 4B). By contrast, further mutation to produce all of the possible combinations in which two of the four hydroxy amino acids in this cluster were mutated to alanines (Figure 4C) eliminated any detectable co-internalization of β-arrestin-2–GFP along with these forms of the receptor (Figure 4D). For all the double mutants, cells in the field were observed in which there was a degree of movement to the plasma membrane of β-arrestin-2–GFP (Figure 4D). However, unlike in equivalent experiments with the wild-type orexin-1 receptor, a substantial fraction of the β-arrestin-2–GFP remained in the cytoplasm. To provide a biochemical correlate for the above studies, β-arrestin-2–GFP was co-expressed in HEK-293T cells with VSV-G-tagged forms of either the wild-type or the C1 mutation of the orexin-1 receptor. β-arrestin-2–GFP was immunoprecipitated with an anti-GFP serum, samples resolved by SDS/PAGE and probed for the presence of either the wild-type or C1 forms of the receptor by immunoblotting to detect the VSV-G epitope. Even without prior stimulation with orexin A, some wild-type orexin-1 receptor was present in the anti-GFP immunoprecipitates (Figure 5). Levels were increased substantially by treatment of the cells with orexin A (0.5 μM, 15 min) (Figure 5). Lower amounts of the C1 mutant were present in GFP immunoprecipitates from unstimulated cells and this was not increased by treatment with orexin A (Figure 5).
Figure 2. C-terminal truncation of the orexin-1 receptor prevents sustained interactions with β-arrestin-2 but not agonist-induced internalization.
A series of C-terminal truncation mutants of the HA-tagged orexin-1 receptor was generated (A). These were transfected into HEK-293T cells along with β-arrestin-2–GFP. The cells were then stimulated with orexin A (0.5 μM, 30 min) in the presence of anti-HA antibody and visualized following permeabilization and addition of secondary antibody. Representative data are shown for the 394-Stop (B) and 374-Stop (C) mutants. The distribution of the orexin-1 receptor (i), β-arrestin-2 (ii) and a composite of these images (iii) are shown.
Figure 3. A single cluster of hydroxy amino acids is responsible for sustained interactions between the orexin-1 receptor and β-arrestin-2.
The cluster of four hydroxy amino acids between residues 418–422 (C1) and a second cluster of three hydroxy amino acids between residues 393–396 (C2) of the orexin-1 receptor were mutated to alanine residues. These two sets of mutations were also combined (C1,C2) (A). These were transfected into HEK-293T cells along with β-arrestin-2–GFP and treated as described in Figure 2. Representative data are shown for the C2 (B), C1 (C) and C1,C2 (D) mutants. The distribution of the orexin-1 receptor (i), β-arrestin-2 (ii) and a composite of these images (iii) are shown.
Figure 4. Double, but not single, point mutations in C1 of the orexin-1 receptor disrupt sustained interactions with β-arrestin-2.
The cluster of four hydroxy amino acids between residues 418–422 of the orexin-1 receptor were mutated either singly (A, B) or in pairs (C, D). These were transfected into HEK-293T cells along with β-arrestin-2–GFP and treated as described in Figure 3. Representative data are shown for T418A (B, a), S419A (B, b), T421A (B, c), T422A (B, d), T418A/S419A (D, a), T418A/T421A (D, b), T418A/T422A (D, c), S419A/T421A (D, d), S419A/T422A (D, e) and T421A/T422A (D, f). The distribution of the orexin-1 receptor (i), β-arrestin-2 (ii) and a composite of these images (iii) are shown.
Figure 5. C1 mutant binds β-arrestin-2 less well than the wild-type orexin 1 receptor.
VSV-G-tagged forms of the wild-type (WT) and C1 orexin-1 receptor were transiently co-expressed with β-arrestin-2–GFP (β-arr 2 GFP) in HEK-293T cells. Post-transfection (24 h) the cells were stimulated with vehicle or 0.5 μM orexin A for 15 min. Following addition of 2 mM dithiobis(succinimidyl propionate), β-arrestin-2–GFP was immunoprecipitated (IP) using an anti-GFP serum and the samples resolved on SDS/PAGE. β-Arrestin-2–GFP and forms of the receptor bound were monitored by immunoblotting (IB) with the anti-GFP and anti-VSV-G sera respectively. Two further experiments produced similar results.
To further explore the nature of the intracellular vesicles into which the wild-type and C1 mutant of the orexin-1 receptor were trafficked, we used the forms of these receptors N-terminally modified to incorporate the VSV-G-epitope tag. After co-expression of β-arrestin-2–GFP and addition of CypHer-5-tagged anti-VSV-G antibody followed by addition of orexin A for 30 min, many of the intracellular vesicles containing the wild-type orexin-1 receptor appeared yellow due to the presence of both the receptor and β-arrestin-2–GFP (Figure 6A). By contrast, in cells expressing the C1 mutant and β-arrestin-2–GFP, the intracellular vesicles appeared red (Figure 6A). This reflects the fact that these vesicles are indeed acidic, but that β-arrestin-2–GFP did not co-internalize with the C1 mutant. Despite this variation in interaction with β-arrestin-2–GFP, the pathways of internalization were similar. Elevated osmolality provided by addition of 0.4 M sucrose blocked internalization of the wild-type orexin-1 receptor in response to agonist (Figure 6B). However, the presence of sucrose did not prevent translocation of β-arrestin-2–GFP to the plasma membrane or its appearance in punctate structures at or close to the cell surface. These punctate structures were not acidic. Red fluorescence corresponding to the protonated form of CypHer-5 was not detected, and following merging of the signals from the red and green channels, no yellow signal was produced close to the plasma membrane (Figure 6B). Equally, in cells co-expressing the C1 receptor mutant, no red signal was generated following addition of orexin-A in the presence of sucrose, and thus no internalization of the receptor into an acidic environment could be observed. Clear translocation of β-arrestin-2–GFP to the plasma membrane was observed (Figure 6B). However, compared with the wild-type receptor much of the cellular β-arrestin-2–GFP was not translocated, further indicating the interactions of β-arrestin-2–GFP with the C1 mutant to be much reduced compared with the wild-type receptor. Pre-treatment of cells with either nystatin or filipin did not alter the patterns of interaction between the receptor and β-arrestin-2–GFP or internalization of either the wild-type or the C1 mutant (results not shown). These results suggest that neither form of this receptor internalizes to any significant extent in response to orexin A via cholesterol-rich detergent-insensitive domains.
Figure 6. Disruption of sustained interactions with β-arrestin-2 does not alter the pathways of agonist-induced internalization of the orexin-1 receptor.
N-terminally VSV-G-tagged forms of the wild-type (a) or C1 mutant (b) of the orexin-1 receptor were transfected into HEK-293T cells along with β-arrestin-2–GFP. Cells were untreated (A) or treated with sucrose (0.4 M) (B). Subsequent to addition of anti-VSV-G antibody labelled with CypHer-5, orexin A was added (0.5 μM, 30 min) and the cells visualized. The distribution of the orexin-1 receptor (i), β-arrestin-2 (ii) and a composite of these images (iii) are shown.
To define unequivocally the role of β-arrestins in the internalization of the orexin-1 receptor, we employed MEF cells derived from a β-arrestin-1 and β-arrestin-2 double knock-out mouse [27]. Both these cells and equivalent wild-type MEF cells were transiently transfected with C-terminally eYFP-tagged forms of the wild-type receptor or the C1 mutant. Orexin A produced internalization of both receptor constructs in the wild-type MEF cells, but was unable to cause internalization of either the wild-type or the C1 mutant in the β-arrestin-negative cells (Figure 7A). Orexin A-mediated internalization of both forms of the orexin-1 receptor was reconstituted in the β-arrestin knock-out cells by co-expression of β-arrestin-2–RFP (Figure 7B). These studies re-capitulated those performed in HEK-293T cells in that co-internalization of β-arrestin-2-RFP was observed with the wild-type orexin-1 receptor, but β-arrestin-2–RFP did not co-internalize with the C1 mutant (Figure 7B).
Figure 7. Internalization of both the wild-type orexin-1 receptor and the C1 mutant is β-arrestin-dependent.
(A) Wild-type (a) or β-arrestin-1 and -2 double knock-out (b) MEF cells were transiently transfected with C-terminally eYFP-tagged forms of the wild-type (WT) orexin-1 receptor or the C1 mutant (C1). Post-transfection (24 h) the cells were treated with vehicle (i) or agonist (0.5 μM orexin A, 30 min; ii), fixed and visualized. (B) β-Arrestin-2–RFP was co-transfected with eYFP-tagged forms of the wild-type or the C1 mutant of orexin-1 receptor mutant. The cells were challenged with vehicle (a) or 0.5 μM orexin A for 30 min (b). The distribution of the orexin-1 receptor (i), β-arrestin-2 (ii) and a composite of these images (iii) are shown.
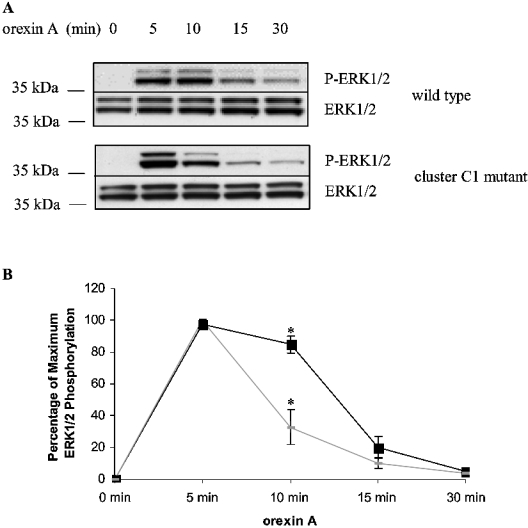
It has been suggested that scaffolding of GPCRs with β-arrestins can determine the effectiveness and implications of agonist-mediated activation of MAPK cascades [24–25]. We tested this following expression of either the wild-type or C1 mutation of the orexin-1 receptor in HEK-293T cells. In both cases 0.5 μM orexin A was able to stimulate the production of phosphorylated forms of ERK1 and ERK2 (Figure 8). Despite this, clear differences were noted. Phosphorylation of ERK1 and ERK2 was maintained for a significantly longer period in cells expressing the wild-type orexin-1 receptor compared with those expressing the C1 mutation (Figure 8). The extent of ERK1 and ERK2 phosphorylation produced by the C1 mutant, as measured by the area under the curve in Figure 8, was only 65% of that produced by the wild-type receptor. The C2 mutant produced orexin-A-mediated phosphorylation of ERK1 and ERK2 that was similar in extent and duration to the wild-type receptor (results not shown). The differences in the kinetics and extent of ERK phosphorylation did not reflect general poor signal transduction capacity of the C1 mutant. The wild-type and C1 forms of the receptor displayed similar capacities and characteristics in their ability to mediate elevation of intracellular [Ca2+] in response to orexin A when expressed in HEK-293T cells (Figure 9A). The C-terminally eYFP-tagged forms of each of the wild-type, C1 and C2 mutants of the orexin-1 receptor also produced similar elevations of intracellular [Ca2+] when co-expressed with Gα11 in Gq/G11 knock-out MEF cells (Figure 9B).
Figure 8. Sustained interactions between β-arrestin-2 and the orexin-1 receptor prolong ERK1/2 activation.
HEK-293T cells transiently expressing the wild-type orexin-1 receptor or the C1 mutant for 24 h were serum starved for 2 h and then stimulated with 0.5 μM orexin A for the times indicated. (A) The activation of ERK1 and 2 was assessed by immunoblotting with a anti-phospho-ERK1/2 antibody (P-ERK1/2). Expression levels of ERK1 and ERK2 were monitored using antibodies directed against total population of ERK1 and ERK2. (B) Quantitation of densitometric analysis of four similar experiments. The results are represented as the means±S.E.M. Orexin-1 receptor (■) or the C1 mutant (◆).
Figure 9. Wild-type and C1 mutant forms of the orexin-1 receptor have similar effects on intracellular [Ca2+].
(A) N-terminally VSV-G-tagged forms of the wild-type (WT) and C1 forms of the orexin-1 receptor (Orexin1R) were expressed in HEK-293T cells. (B) C-terminally eYFP-tagged forms of the wild-type, C1 and C2 mutants of the orexin-1 receptor were transfected along with Gα11 into Gαq/Gα11 knock-out MEF cells. The effect of 0.5 μM orexin A on intracellular [Ca2+] was then recorded in individual cells. The results are pooled from 6 cells expressing each construct.
DISCUSSION
There is great interest in the orexin receptor system and its usefulness in therapeutic intervention in both appetite control and sleep regulation [6]. It is thus surprising that virtually nothing is known about the regulation of the orexin receptors following stimulation. As with many members of the rhodopsin family of GPCRs, the C-terminal tail of the human orexin-1 receptor contains a substantial number of aliphatic hydroxy amino acids. In many cases, such amino acids can contribute to or provide docking sites for β-arrestins subsequent to the binding of agonist to the receptor. Interactions of the orexin-1 receptor with both β-arrestin-1 and β-arrestin-2 have previously been observed by monitoring the co-internalization of the receptor and GFP-tagged forms of the β-arrestins in response to the natural ligand orexin A [15]. In the present studies we have investigated the molecular details of this interaction and the effects upon signal transduction of modifying it. Although the C-terminal tail of the orexin-1 receptor contains 16 hydroxy amino acids, key interactions are provided by a single cluster of four amino acids at the extreme C-terminal tail. A series of studies have indicated that such clusters of serine and threonine residues can provide an effective binding site for β-arrestins [13,14]. However, despite the presence of a second such cluster in the C-terminal tail of the orexin-1 receptor between amino acids 393–396, concerted mutation of these residues did not alter interaction with β-arrestin-2 significantly. Although these studies employed the human orexin-1 receptor, sequence comparisons show that the rat and mouse forms of this receptor also have a hydroxy amino acid cluster comparable with the C1 region of the human sequence, whereas the C2 sequence in the human receptor is limited to only a pair of adjacent serine residues in rat and mouse. Furthermore, although not examined in the present study, the human, rat and mouse forms of the orexin-2 receptor also have a C-terminal hydroxy amino acid cluster that is likely to define sustained interactions with β-arrestins.
In the present studies, mutation of the C-terminal cluster of hydroxy amino acids or truncation of much of the C-terminal tail did not prevent orexin A-mediated internalization of the orexin-1 receptor. Furthermore, the pathway of internalization of the orexin-1 receptor in HEK-293T cells seemed unaffected by the effectiveness of interaction with β-arrestin-2. This is interesting in that alternative pathways for internalization in HEK-293 cells, for at least the 5HT2A (5-hydroxytryptamine 2A) receptor, have been demonstrated [36], but this is clearly not a general default pathway for GPCR entry in these cells when interactions with β-arrestins are either diminished or lacking. Despite no longer interacting as robustly with β-arrestin-2 as the wild-type orexin-1 receptor, studies using β-arrestin knock-out MEF cells clearly demonstrated that internalization of the C1 cluster mutation remained β-arrestin dependent. Although not explored in these studies, there is increasing evidence that the β-arrestin-1 and β-arrestin-2 isoforms may play different roles in receptor function [37,38], and it will be interesting, in time, to evaluate the effects of each β-arrestin isoform as the orexin-1 receptor is clearly able to interact with both [15].
There has been great interest recently in the suggestion that not all aspects of GPCR-mediated signalling require activation of the G protein. For example, activation of c-Src by the angiotensin II AT1 receptor is still detected with a form of the receptor unable to couple to G proteins [39]. Related studies using the same receptor have shown that over-expression of β-arrestins facilitates angiotensin II-stimulated activation of ERK MAPK activity [40], whereas RNAi (RNA interference)-mediated depletion of β-arrestins impairs activation [41]. These studies implicate β-arrestins as being a major component in G-protein-independent ERK MAPK activation. Furthermore, the combined use of a mutant form of the angiotensin II AT1 receptor, an analogue of angiotensin II that is able to induce β-arrestin recruitment, but not G-protein activation, and a RNAi strategy has demonstrated unequivocally the co-existence of G-protein- and β-arrestin-mediated pathways of ERK activation in HEK-293 cells [42].
Direct binding of β-arrestins to ERK1/2 inhibits ERK-dependent transcription as ERK coupled to β-arrestins is retained within the cytosol [40]. Moreover, the activation of ERK bound to β-arrestins is favoured in a setting of a stable receptor–β-arrestin interaction, which is determined by the C-terminus of the receptor [43]. However, the situation may be yet more complex, because as noted above, β-arrestin-1 and β-arrestin-2 play very different roles in these processes [37]. As noted above, considerable attention is being paid to the roles of β-arrestins in providing scaffolds for alternative signalling by GPCRs ([44] and reviewed in [45,46]). Due to the poor interaction properties of the C1 orexin-1 receptor mutant with β-arrestin-2 we compared the ability of the wild-type orexin-1 receptor and the C1 mutant to regulate both Ca2+ elevation and ERK MAPK phosphorylation. There was little difference noted in orexin A-mediated elevation of intracellular [Ca2+] and this may reflect that this is a rapid G-protein-mediated response. In contrast, phosphorylation of ERK1 and ERK2 was significantly more prolonged when the wild-type orexin-1 receptor was activated than when studies were performed with the C1 mutation. These results are contrary to what might be expected if high-affinity binding of β-arrestin-2 resulted in desensitization of all responses and provide further weight to the concept that GPCR–β-arrestin interactions can generate or modulate a distinct panoply of cellular responses from those mediated via G-protein interaction.
The sustainability of interactions between GPCRs and β-arrestins can also control the speed of recycling of the GPCR back to the plasma membrane, and thus the re-sensitization of functional responses of the GPCR (reviewed in [9]). Although studies in β-arrestin knock-out cells clearly indicate that this is not to be a universal feature [28], it is frequently the case that sustained interaction with a β-arrestin slows the rate of GPCR recycling. It might thus be anticipated that the C-terminal mutants of the orexin-1 receptor will recycle more rapidly and re-sensitize more quickly than the wild-type receptor. This will require direct analysis, but the availability of the pH-sensitive cyanine dye CypHer-5 [32,35] is likely to be valuable in this regard. The rate of clearance of red fluorescence from the cells following challenge with orexin-A and its removal may provide a real-time monitor of the rate of exit from the acidic environment of the recycling endosomes. The C-terminal mutants of the orexin-1 receptor generated and characterized in the present study should thus be valuable reagents to examine a range of issues of general interest, including the importance of phosphorylation for internalization. In combination with cell lines lacking expression of various G proteins [34] and/or the use of siRNAs (small interfering RNAs) against specific G proteins [38], they should also be valuable to further delineate signals that reflect β-arrestin rather than G-protein function.
Acknowledgments
We thank the Wellcome Trust for support of part of these studies.
References
- 1.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., Williams S. C., Richardson J. A., Kozlowski G. P., Wilson S., et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 2.Samson W. K., Resch Z. T. The hypocretin/orexin story. Trends Endocrinol. Metab. 2000;11:257–262. doi: 10.1016/s1043-2760(00)00273-3. [DOI] [PubMed] [Google Scholar]
- 3.Smart D., Jerman J. C., Brough S. J., Rushton S. L., Murdock P. R., Jewitt F., Elshourbagy N. A., Ellis C. E., Middlemiss D. N., Brown F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br. J. Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lund P. E., Shariatmadari R., Uustare A., Detheux M., Parmentier M., Kukkonen J. P., Akerman K. E. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J. Biol. Chem. 2000;275:30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- 5.Haynes A. C., Jackson B., Chapman H., Tadayyon M., Johns A., Porter R. A., Arch J. R. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul. Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 6.Smart D., Sabido-David C., Brough S. J., Jewitt F., Johns A., Porter R. A., Jerman J. C. SB-334867-A: the first selective orexin-1 receptor antagonist. Br. J. Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiesi M., Huppertz C., Hofbauer K. G. Pharmacotherapy of obesity: targets and perspectives. Trends Pharmacol. Sci. 2001;22:247–254. doi: 10.1016/s0165-6147(00)01664-3. [DOI] [PubMed] [Google Scholar]
- 8.Smart D., Haynes A. C., Williams G., Arch J. R. Orexins and the treatment of obesity. Eur. J. Pharmacol. 2002;440:199–212. doi: 10.1016/s0014-2999(02)01429-2. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson S. S. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 10.Goodman O. B., Jr, Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., Benovic J. L. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 11.Laporte S. A., Oakley R. H., Holt J. A., Barak L. S., Caron M. G. The interaction of β-arrestin with the AP-2 adaptor is required for the clustering of β 2-adrenergic receptor into clathrin-coated pits. J. Biol. Chem. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- 12.Goodman O. B., Jr, Krupnick J. G., Gurevich V. V., Benovic J. L., Keen J. H. Arrestin/clathrin interaction: localization of the arrestin binding locus to the clathrin terminal domain. J. Biol. Chem. 1997;272:15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- 13.Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor–β-arrestin complexes after receptor endocytosis. J. Biol. Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 14.Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G. Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 15.Evans N. A., Groarke D. A., Warrack J., Greenwood C. J., Dodgson K., Milligan G., Wilson S. Visualizing differences in ligand-induced β-arrestin–GFP interactions and trafficking between three recently characterized G protein-coupled receptors. J. Neurochem. 2001;77:476–485. doi: 10.1046/j.1471-4159.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 16.DeGraff J. L., Gurevich V. V., Benovic J. L. The third intracellular loop of α2-adrenergic receptors determines subtype specificity of arrestin interaction. J. Biol. Chem. 2002;277:43247–43252. doi: 10.1074/jbc.M207495200. [DOI] [PubMed] [Google Scholar]
- 17.Lee K. B., Ptasienski J. A., Pals-Rylaarsdam R., Gurevich V. V., Hosey M. M. Arrestin binding to the M2 muscarinic acetylcholine receptor is precluded by an inhibitory element in the third intracellular loop of the receptor. J. Biol. Chem. 2000;275:9284–9289. doi: 10.1074/jbc.275.13.9284. [DOI] [PubMed] [Google Scholar]
- 18.Bennett T. A., Maestas D. C., Prossnitz E. R. Arrestin binding to the G protein-coupled n-formyl peptide receptor is regulated by the conserved ‘DRY’ sequence. J. Biol. Chem. 2000;275:24590–24594. doi: 10.1074/jbc.C000314200. [DOI] [PubMed] [Google Scholar]
- 19.Groarke D. A., Drmota T., Bahia D. S., Evans N. A., Wilson S., Milligan G. Analysis of the C-terminal tail of the rat thyrotropin-releasing hormone receptor-1 in interactions and cointernalization with β-arrestin 1–green fluorescent protein. Mol. Pharmacol. 2001;59:375–385. doi: 10.1124/mol.59.2.375. [DOI] [PubMed] [Google Scholar]
- 20.Hanyaloglu A. C., Vrecl M., Kroeger K. M., Miles L. E., Qian H., Thomas W. G., Eidne K. A. Casein kinase II sites in the intracellular C-terminal domain of the thyrotropin-releasing hormone receptor and chimeric gonadotropin-releasing hormone receptors contribute to β-arrestin-dependent internalization. J. Biol. Chem. 2001;276:18066–18074. doi: 10.1074/jbc.M009275200. [DOI] [PubMed] [Google Scholar]
- 21.Shenoy S. K., Lefkowitz R. J. Multifaceted roles of β-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem. J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall R. A., Lefkowitz R. J. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ. Res. 2002;91:672–680. doi: 10.1161/01.res.0000037000.74258.03. [DOI] [PubMed] [Google Scholar]
- 23.Luttrell L. M., Ferguson S. S., Daaka Y., Miller W. E., Maudsley S., Della Rocca G. J., Lin F., Kawakatsu H., Owada K., Luttrell D. K., et al. β-Arrestin-dependent formation of β2 adrenergic receptor-src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 24.DeFea K. A., Zalevsky J., Thoma M. S., Dery O., Mullins R. D., Bunnett N. W. β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tohgo A., Choy E. W., Gesty-Palmer D., Pierce K. L., Laporte S., Oakley R. H., Caron M. G., Lefkowitz R. J., Luttrell L. M. The stability of the G protein-coupled receptor–β-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 26.Fong T. M. Overview of mutagenesis techniques. In: Wess J., editor. Structure–Function Analysis of G Protein-Coupled Receptors. New York: Wiley–Liss; 1999. pp. 12–20. [Google Scholar]
- 27.Kohout T. A., Lin F. S., Perry S. J., Conner D. A., Lefkowitz R. J. β-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vines C. M., Revankar C. M., Maestas D. C., LaRusch L. L., Cimino D. F., Kohout T. A., Lefkowitz R. J., Prossnitz E. R. N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J. Biol. Chem. 2003;278:41581–41584. doi: 10.1074/jbc.C300291200. [DOI] [PubMed] [Google Scholar]
- 29.Offermanns S., Zhao L. P., Gohla A., Sarosi I., Simon M. I., Wilkie T. M. Embryonic cardiomyocyte hypoplasia and craniofacial defects in Gαq/Gα11-mutant mice. EMBO J. 1998;17:4304–4312. doi: 10.1093/emboj/17.15.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao J., Yuan H., Xie W., Simon M. I., Wu D. Specific Involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors. J. Biol. Chem. 1998;273:27118–27123. doi: 10.1074/jbc.273.42.27118. [DOI] [PubMed] [Google Scholar]
- 31.Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature (London) 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 32.Adie E. J., Kalinka S., Smith L., Francis M. J., Marenghi A., Cooper M. E., Briggs M., Michael N. P., Milligan G., Game S. A pH-sensitive fluor, CypHer 5, used to monitor agonist-induced G protein-coupled receptor internalization in live cells. Biotechniques. 2002;33:1152–1157. doi: 10.2144/02335dd10. [DOI] [PubMed] [Google Scholar]
- 33.Charest P. G., Bouvier M. Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances β-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J. Biol. Chem. 2003;278:41541–41551. doi: 10.1074/jbc.M306589200. [DOI] [PubMed] [Google Scholar]
- 34.Liu S., Carrillo J. J., Pediani J. D., Milligan G. Effective information transfer from the α1b-adrenoceptor to Gα 11 requires both β/γ interactions and an aromatic group four amino acids from the C terminus of the G protein. J. Biol. Chem. 2002;277:25707–25714. doi: 10.1074/jbc.M201015200. [DOI] [PubMed] [Google Scholar]
- 35.Milligan G. High-content assays for ligand regulation of G-protein-coupled receptors. Drug Discov. Today. 2003;8:579–585. doi: 10.1016/s1359-6446(03)02738-7. [DOI] [PubMed] [Google Scholar]
- 36.Gray J. A., Sheffler D. J., Bhatnagar A., Woods J. A., Hufeisen S. J., Benovic J. L., Roth B. L. Cell-type specific effects of endocytosis inhibitors on 5-hydroxytryptamine2A receptor desensitization and resensitization reveal an arrestin-, GRK2-, and GRK5-independent mode of regulation in human embryonic kidney 293 cells. Mol. Pharmacol. 2001;60:1020–1030. doi: 10.1124/mol.60.5.1020. [DOI] [PubMed] [Google Scholar]
- 37.Ahn S., Wei H., Garrison T. R., Lefkowitz R. J. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by β-arrestins 1 and 2. J. Biol. Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 38.Barnes W. G., Reiter E., Violin J. D., Ren X.-R., Milligan G., Lefkowitz R. J. β-arrestin 1 and Gαq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 2004 doi: 10.1074/jbc.M412924200. DOI 10.1074/jbc.M412924200. [DOI] [PubMed]
- 39.Seta K., Nanamori M., Modrall J. G., Neubig R. R., Sadoshima J. AT1 receptor mutant lacking heterotrimeric G protein coupling activates the Src–Ras–ERK pathway without nuclear translocation of ERKs. J. Biol. Chem. 2002;277:9268–9277. doi: 10.1074/jbc.M109221200. [DOI] [PubMed] [Google Scholar]
- 40.Tohgo A., Pierce K. L., Choy E. W., Lefkowitz R. J., Luttrell L. M. β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 41.Ahn S., Nelson C. D., Garrison T. R., Miller W. E., Lefkowitz R. J. Desensitization, internalization, and signaling functions of β-arrestins demonstrated by RNA interference. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei H., Ahn S., Shenoy S. K., Karnik S. S., Hunyady L., Luttrell L. M., Lefkowitz R. J. Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn S., Maudsley S., Luttrell L. M., Lefkowitz R. J., Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for β2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 1999;274:1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 44.McDonald P. H., Chow C. W., Miller W. E., Laporte S. A., Field M. E., Lin F. T., Davis R. J., Lefkowitz R. J. β-Arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 45.Miller W. E., Lefkowitz R. J. Expanding roles for β-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr. Opin. Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 46.Luttrell L. M., Lefkowitz R. J. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]