Abstract
Bulgecin A, a sulphonated N-acetyl-D-glucosamine unit linked to a 4-hydroxy-5-hydroxymethylproline ring by a β-glycosidic linkage, is a novel type of inhibitor for binuclear metallo-β-lactamases. Using steady-state kinetic analysis with nitrocefin as the β-lactam substrate, bulgecin A competitively inhibited the metallo-β-lactamase BceII from Bacillus cereus in its two-zinc form, but failed to inhibit when the enzyme was in the single-zinc form. The competitive inhibition was restored by restoring the second zinc ion. The single-zinc metallo-β-lactamase from Aeromonas veronii bv. sobria, ImiS, was not inhibited by bulgecin A. The tetrameric L1 metallo-β-lactamase from Stenotrophomonas maltophilia was subject to partial non-competitive inhibition, which is consistent with a kinetic model in which the enzyme bound to inhibitor retains catalytic activity. Docking experiments support the conclusion that bulgecin A co-ordinates to the zinc II site in metallo-β-lactamases via the terminal sulphonate group on the sugar moiety.
Keywords: antibiotic resistance, bulgecin A, inhibitor, metallo-β-lactamase, Stenotrophomonas, zinc co-ordination
Abbreviations: AAS, atomic absorption spectroscopy; SLT70, 70-kDa soluble lytic transglycosylase
INTRODUCTION
The metallo-β-lactamases are a family of clinically important zinc hydrolases. All possess a characteristic αββα fold and the active-site zinc-binding motif HXHXD (His-Xaa-His-Xaa-Asp), and most readily hydrolyse carbapenems, an important class of β-lactam antibiotics. Some metallo-β-lactamase genes, such as members of the IMP [1] and VIM [2] families, and SPM-1 [3], can be mobilized on mobile genetic elements, particularly Class 1 integrons [4].
There are currently no clinically useful inhibitors of metallo-β-lactamases; however, several studies have been undertaken with a variety of experimental inhibitors. These can be divided into (i) compounds which irreversibly covalently modify the enzyme, resulting in inhibition or inactivation of activity, (ii) compounds which chelate zinc from the active site, resulting in reversible inactivation of the enzymes, or (iii) compounds which competitively inhibit substrate binding, either by mimicking the structure of the β-lactam substrate or by co-ordinating to an active-site zinc ion, preventing binding of the β-lactam substrate or displacing a bound substrate. Inhibitors that covalently modify metallo-β-lactamases include small thiol-modifying reagents, such as mercuric(II) salts [5], p-chloromercuribenzoate [6], iodoacetic acid [7] and mercaptoacetic acid thiol esters [8]. Inhibitors that chelate the active-site zinc include EDTA, 1,10-phenanthroline, dipicolinic acid, two phenazines from Streptomyces spp. [9], bis(1N-tetrazol-5-yl)amine [10] and EGTA [11].
Although these two groups of compounds offer many useful experimental tools for probing reactive sites and mechanisms of β-lactam hydrolysis, neither covalent modifiers nor zinc chelators are likely to provide clinically useful inhibitors, because they are relatively non-specific and are likely to be inhibitory to physiologically important zinc hydrolases of the infected individual. More promising compounds are those which reversibly block the active site by competitive inhibition, since these offer the potential for modification of the structure to improve the specificity of the inhibitor for metallo-β-lactamases alone. Such compounds include biphenyl tetrazoles [10], mercaptophenyl acetic acid derivatives, which do not covalently modify the enzyme (probably because the phenyl ring sterically hinders the hydrolysis of the carbonyl-thiol bond) [8], trifluoromethyl alcohols and ketones [12], N-(2′-mercaptoethyl)-2-phenylacetamide [13], thiomandelic acid [14], D- and L-captopril inhibitors [15], inhibitors based on thiazolidinecarboxylic acid [16], 6-(mercaptomethyl) penicillinates [17] and thioxo-cephalosporin derivatives [18].
The majority of metallo-β-lactamases described are binuclear, with two zinc ions at their active sites. However, mononuclear (single-zinc) enzymes have been described. The metallo-β-lactamases of Aeromonas spp. exemplified by CphA from Aeromonas hydrophila are native mononuclear zinc enzymes, although they have a second, normally empty, zinc-binding site. When zinc is bound to this second site, it is inhibitory [19]. The metallo-β-lactamase from Bacillus cereus has two zinc sites with different affinities [20]. Accordingly, the enzyme can be manipulated to have one or two zinc ions. Both forms of the enzyme are hydrolytically active against β-lactams, although the binuclear form shows the greater activity. This ability to produce both mono- and bi-nuclear forms, based on the same metallo-β-lactamase, is exploited in the present study. Of particular relevance to the present work is the weak inhibition by Mes of the CcrA metallo-β-lactamase from Bacteriodes fragilis. A crystal structure of the enzyme inhibitor complex has been determined [21]. Mes co-ordinates to the zinc II site via an oxygen atom of its sulphonate group.
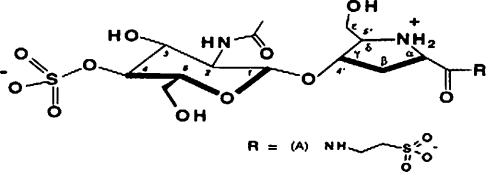
Bulgecins are O-sulphonated glycopeptides produced by Pseudomonas acidophila and Pseudomonas mesoacidophila [22] that potentiate the antibacterial activity of β-lactam antibiotics and produce characteristic bulges when added to bacteria such as Escherichia coli in association with β-lactams. Ps. mesoacidophila contains three such glycopeptide components, A, B and C, which make up 88%, 2% and 10% of the total bulgecin content respectively [23]. These compounds specifically target the 70-kDa soluble lytic transglycosylase (SLT70) from E. coli [24,25]. The SLT70 transglycosylase catalyses an intramolecular glycosyltransferase reaction, resulting in the formation of 1,6-anhydromuropeptides, which are believed to function as a signal for the induction of some inducible β-lactamase expression in Gram-negative bacteria [26,27]. The structure of bulgecin A is shown in Figure 1. In the present paper, we report that bulgecin A also inhibits binuclear zinc-dependent metallo-β-lactamases. It interacts specifically with the zinc II ion, probably as a consequence of co-ordination between one of the sulphonate groups and the zinc ions. The characteristics of inhibition for the tetrameric L1 metallo-β-lactamase from Stenotrophomonas maltophilia, monomeric BceII enzyme from B. cereus (in both its mononuclear and binuclear forms) and the monomeric, mononuclear ImiS, have been determined, and a model of inhibitor binding to the L1 metallo-β-lactamase is presented and used to identify those amino acid residues that are potentially involved in inhibitor binding. These enzymes have been chosen as representative types of a family of enzymes which also includes clinically relevant metallo-β-lactamases.
Figure 1. Structure of bulgecin A.
Reprinted with permission from [25]. Copyright (1995) American Chemical Society.
EXPERIMENTAL
Preparation of metallo-β-lactamases
Preparation of L1 from S. maltophilia
The L1 gene [28] was amplified by PCR. PCR products were TA-cloned into plasmid vector pTrcHis2-TOPO (Invitrogen, Paisley, U.K.). Following induction of high-level L1 production with IPTG (isopropyl β-D-thiogalactoside), a periplasmic fraction was prepared by lysozyme treatment. L1 was purified from this crude preparation by using ion-exchange and gel filtration in 50 mM cacodylate buffer containing 10 μM ZnCl2, as described by Avison et al. [29].
Preparation of ImiS from Aeromonas veronii bv. sobria
An overnight culture of the high-level β-lactamase-expressing mutant, 163a-M [30], was used as the source of ImiS. Cells were harvested, and ImiS was purified from a periplasmic preparation by a two-stage FPLC process, as described by Walsh et al. [31].
Preparation of BceII from B. cereus
The BceII enzyme of B. cereus 569/H was obtained commercially from Sigma–Aldrich, Poole, U.K. As supplied, the enzyme is binuclear [two zinc atoms per subunit, confirmed by AAS (atomic absorption spectroscopy) in the present study, Table 1] and the preparation contained BSA as a stabilizer. The metallo-β-lactamase was separated from the BSA by gel filtration through a Superdex 200 column (Amersham Biosciences, Little Chalfont, Bucks., U.K.) using 50 mM cacodylate buffer, pH 7.0. The mononuclear form (one zinc atom per subunit) was prepared by dialysing the purified enzyme against 50 mM cacodylate buffer containing 1 μM ZnCl2 at pH 6.0 for 2 days. The binuclear form was restored by re-dialysing against 50 mM cacodylate buffer containing 100 μM ZnCl2 at pH 7.0 for a further 1 day.
Table 1. Zinc content and type of inhibition seen with the enzymes used in the present study.
Results are means±S.D. for three measurements.
| Enzyme | Zn content (mol of Zn2+/mol of enzyme) | Type of inhibition | Ki (μM) |
|---|---|---|---|
| L1 | 1.9±0.3 | Partial non-competitive | 2.5 |
| ImiS | 0.8±0.2 | No inhibition | >900 |
| BceII | 2.1±0.2 | Competitive | 230 |
| BceII (mononuclear) | 0.9±0.3 | No inhibition | >900 |
Zinc assay
Assays to determine the zinc content per monomer of each enzyme were performed using AAS in a Unicam 919 Atomic Absorption Spectrometer at 213.9 nm. Protein was prepared for AAS as described by Simm et al. [32].
Enzyme kinetics
Steady-state enzyme kinetics were performed using, as substrates, nitrocefin (Becton-Dickinson, Cockeysville, MD, U.S.A.), with product formation monitored at 482 nm (L1 and BceII), and Imipenem (Merck Sharpe & Dohme, Hoddesdon, Herts., U.K.), with substrate hydrolysis monitored at 299 nm (ImiS). Initial rate absorbance data at various substrate and inhibitor concentrations (10–150 μM) were collected using a Lambda 35 spectrophotometer (PerkinElmer, Cambridge, U.K.) and analysed using Eadie–Hofstee plots (v against v/[S]) [33]. All assays were performed in 50 mM cacodylate buffer, pH 7.0, containing 100 μM Zn2+ as ZnCl2 at 25 °C, with the exception of assays on the single-zinc form of BceII, which contained 1 μM ZnCl2. The appropriate concentration of inhibitor dissolved in 18.2 MΩ water was added to the substrate immediately before the addition of enzyme.
N-Acetylglucosamine and hydroxyproline, the substituent components of bulgecin A, were also tested for inhibitory activity up to maximum concentrations of 200 μM.
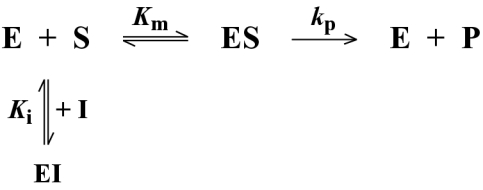
Data for BceII were fitted to Scheme 1, using eqn 1:
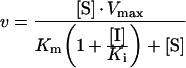 |
(1) |
where v is the rate of hydrolysis (in μM·s−1), Vmax is the maximal velocity in the absence of inhibitor (=[E]kp), [S] is the substrate concentration (in μM) and [I] is the concentration of inhibitor. Fitting was achieved with the Ledveburg–Marquadt non-linear least-squares algorithm using the program MATLAB [17].
Scheme 1. Reaction flow scheme for the BceII metallo-β-lactamase inhibition.
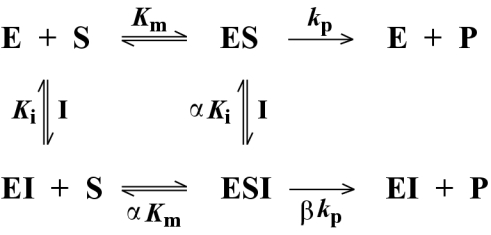
Data for L1 were fitted to Scheme 2 using eqn 2:
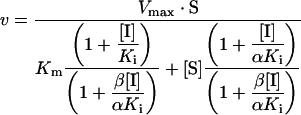 |
(2) |
where α is a factor representing the affinity of enzyme bound to inhibitor for substrate and β is a factor representing the ability of the ESI intermediate to produce product. The different kinetic profiles fitted were the simplest reaction schemes that could account for the different Eadie–Hofstee plots seen for BceII and L1 (Figures 2 and 3), taking account of previously reported negatively co-operative effects in the L1 enzyme [32] and the molecular architecture of the L1 active site (see discussion below).
Scheme 2. Reaction flow scheme for the L1 metallo-β-lactamase inhibition.
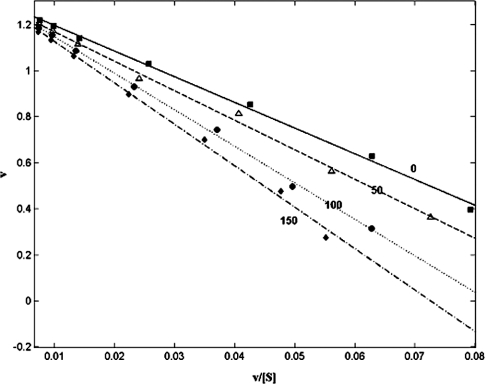
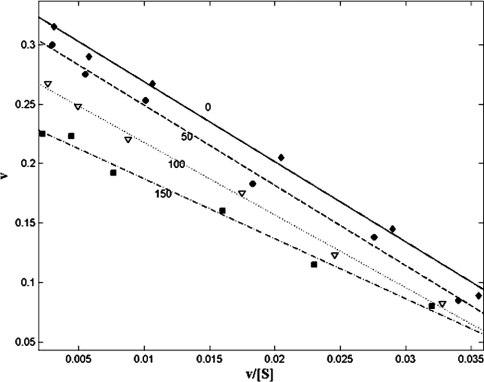
Figure 2. Eadie–Hofstee plot of the effect of bulgecin A on the BceII 569/H metallo-β-lactamase from B. cereus with nitrocefin as substrate.
The rate of hydrolysis in μmol/min per 10 nmol of enzyme, v, is shown on the y-axis. The rate/substrate concentration (μmol/min per μM of nitrocefin), v/[S], is shown on the x-axis. ■, Linear regression fit of uninhibited BceII activity. △, Linear regression fit of activity in the presence of 50 μM bulgecin A. ●, Linear regression fit of activity in the presence of 100 μM bulgecin A. ◆, Linear regression fit of activity in the presence of 150 μM bulgecin A. All data points are the average of three separate experiments. The S.E.M. was <0.05 for all data points, and regression coefficients of fitted lines was >0.99.
Figure 3. Eadie–Hofstee plot of the effect of bulgecin A on the L1 metallo-β-lactamase from S. maltophilia with nitrocefin as substrate.
The rate of hydrolysis in μmol/min per 10 nmol of enzyme, v, is shown on the y-axis. The rate/substrate concentration (μmol/min per μM of nitrocefin), v/[S], is shown on the x-axis. ■, Linear regression fit of uninhibited L1 activity. △, Linear regression fit of activity in the presence of 50 μM bulgecin A. ●, Linear regression fit of activity in the presence of 100 μM bulgecin A. ◆, Linear regression fit of activity in the presence of 150 μM bulgecin A. All data points are the average of three separate experiments. The S.E.M. was <0.05 for all data points, and regression coefficients of fitted lines was >0.99.
Protein–inhibitor docking
The molecular structure of bulgecin A was extracted from the Protein Data Bank (code 1LMC), which is a 2.0 Å (1 Å=0.1 nm) resolution structure of bulgecin A with E. coli lysozyme. A solvent-accessible Connolly surface [34] of the L1 metallo-β-lactamase crystal structure (Protein Data Bank code 1SML) was created with Quantum Chemistry Program Exchange program 429 (Indiana University, Bloomington, IN, U.S.A.) using a 1.4 Å solvent probe. The extracted bulgecin A structure was docked into this surface using the FlexX docking algorithm (Tripos, Cambridge, U.K.) with a 130 Å×130 Å×130 Å grid centred on the atomic co-ordinates of zinc I and a grid spacing of 0.375 Å. All rings were treated as rigid, and all other bonds were allowed to rotate freely. The 30 lowest-energy docked conformers were retained for analysis.
RESULTS
Preparation of metallo-β-lactamases
Each of the metallo-β-lactamase preparations used contained a single protein, demonstrated by a single band on SDS/PAGE gels stained with Coomassie Blue G250. The molecular mass of each protein was consistent with the predicted molecular mass for the particular metallo-β-lactamase. The zinc contents per subunit for the various enzymes are shown in Table 1.
Inhibition of metallo-β-lactamases by bulgecin A
ImiS and the mononuclear zinc form of the BceII 569/H metallo-β-lactamase were not inhibited by bulgecin A at any concentration within the range tested (50–150 μM). In contrast, the binuclear zinc form of the monomeric enzyme from B. cereus was competitively inhibited by bulgecin A (Figure 2). Fitting of these data to eqn 1 gave a Ki of 230±10 μM. When the mononuclear zinc form of BceII was dialysed against 100 μM Zn2+, to restore the bi-nuclear zinc state, competitive inhibition with bulgecin A was restored. Inhibition of the L1 metallo-β-lactamase by bulgecin A showed partial non-competitive kinetics (Figure 3), consistent with the enzyme–inhibitor complex retaining some catalytic activity (see enzyme Scheme 2 above). Fitting these data to eqn 2 gave estimates of Ki=2.5±0.3 μM with α=0.65±0.15 and β=0.9±0.1. Regression coefficients of fitted curves were always >0.985. The types of inhibition seen for the different enzymes investigated are noted in Table 1. The inhibition was not time-dependent for 2<t<30 min, where t is the time of prior incubation with inhibitor (results not shown). Neither N-acetylglucosamine nor hydroxyproline inhibited any of the enzymes at concentrations up to 200 μM.
Docking of bulgecin into the L1 crystal structure
Docking simulations of bulgecin A with the L1 metallo-β-lactamase using the FlexX docking algorithm showed bulgecin as interacting with the zinc site via the sulphonate group of the sugar moiety. The 30 lowest-energy structures show little movement of the sugar ring and a degree of flexibility in the positioning of the proline ring, which appears to form an interaction with Asp14 of the protein. On closer inspection, it was found that this docking was critically dependent on the state of protonation of the bulgecin molecule. In the present study, the bulgecin A model used was generally fully deprotonated; although both of the sulphonate groups of bulgecin A would be deprotonated under physiological conditions, it is not known whether the molecule becomes protonated in the active site of metallo-β-lactamases. The effect of protonation was therefore also investigated by computer simulation. When both sulphonate groups are protonated, the bulgecin molecule is modelled as binding to the zinc site of L1 via its taurine moiety. Protonation at the sugar sulphonate, but not the taurine sulphonate, resulted in two thirds of the lowest-energy structures showing bulgecin binding via its taurine sulphonate and the remaining third showing binding via the sulphonate group of the sugar (results not shown). Conversely, when the taurine sulphonate is protonated, but the sugar sulphonate is not, all of the lowestenergy structures show the bulgecin A molecule binding via the sulphonate group of the sugar (results not shown) in a manner virtually identical with that of the fully deprotonated form. Deprotonation of the proline nitrogen had no effect on binding, giving only minor changes in the position of the proline ring. The structure of the lowest-energy conformer (fully deprotonated bulgecin A) together with associated L1 residues are shown in Figure 4. The Protein Data Bank file of the lowest-energy conformer docked into the L1 crystal structure (code 1SML) is available at http://www.BiochemJ.org/bj/387/bj3870585add.htm.
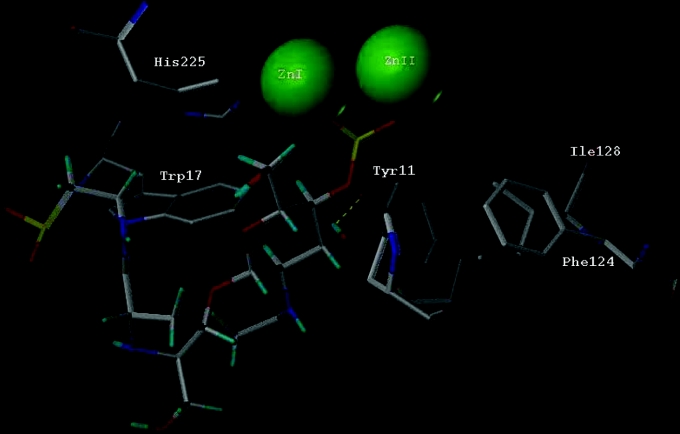
Figure 4. Interaction of bulgecin A with the metallo-β-lactamase from S. maltophilia.
The bulgecin A molecule (Protein Data Bank code 1LMC; bulgecin A in complex with lysozyme) has been docked into the crystal structure of L1 metallo-β-lactamase (Protein Data Bank code 1SML) using the program FlexX. Amino acid residues implicated in stabilizing and/or binding the molecule are labelled in accordance with 1SML. Phe124 and Ile128 are the beginning and the end respectively of the active-site loop implicated in catalysis.
DISCUSSION
The proliferation of zinc-dependent metallo-β-lactamases poses a significant threat to the future efficacy of treatment of many severe bacterial infections that currently are treatable with the latest β-lactams, e.g. carbapenems. The threat could be substantially reduced if effective inhibitors of these enzymes were available. In the present paper, we have reported inhibition of two binuclear zinc metallo-β-lactamases, L1 from S. maltophilia and BceII from B. cereus, by the O-sulphonated glycopeptide, bulgecin A. We have shown that such inhibition is a consequence of the binuclear state, and predict that this will be extendable to other more clinically relevant binuclear metallo-β-lactamases, such as the IMP and VIM groups.
Bulgecin A inhibited the binuclear zinc β-lactamases tested, but neither the mononuclear zinc enzyme, ImiS, from A. veronii bv. sobria nor the mononuclear form of the B. cereus enzyme BceII was inhibited. It is known from the crystal structure that the mononuclear zinc form of BceII retains zinc I [35–37]. Although there is currently no crystal structure for ImiS, biochemical and molecular genetic studies of the closely related enzyme CphA from A. hydrophila AE036 [19] indicate that the single zinc atom associated with each enzyme molecule is equivalent to zinc I in bi-nuclear zinc enzymes. These observations suggest that the specific interactions of bulgecin A with some zinc-dependent β-lactamases is mediated via the zinc II ion, a view that is strongly supported by the differential inhibition of the binuclear zinc form of BceII. The results of docking studies with bulgecin A and the L1 metallo-β-lactamase support this interpretation and suggest further that the interaction involves co-ordination of the sulphonate group of the inhibitor to the second zinc ion. This conclusion is in accord with the crystal structure of the complex of the inhibitor Mes and the metallo-β-lactamase from B. fragilis [21] in which the sulphonate group of the inhibitor is co-ordinated to zinc II. The time-independence of this inhibition suggests that bulgecin is not acting as a zinc chelator in the manner of EDTA, EGTA or 1,10-dipicolinic acid [11]. The fact that neither N-acetylglucosamine nor hydroxyproline, constituent parts of bulgecin A, inhibit any of the metallo-β-lactamases tested indicates that the inhibition exhibited by bulgecin A cannot be explained solely by interactions between the enzymes and the sugar or peptide constituents themselves.
The type of inhibition observed for the L1 and BceII β-lactamases differs, being competitive for the latter and partial hyperbolic non-competitive for L1. Bearing in mind the conclusions drawn from the docking studies, competitive inhibition of BceII by bulgecin A is expected. For L1, the form of the Eadie–Hofstee plot (Figure 3) is that normally associated with uncompetitive inhibition, i.e. that observed when an inhibitor binds to the enzyme–substrate complex, but not the free enzyme. However, an examination of the L1 active site reveals that if a β-lactam substrate were bound to the zinc I atom (as occurs in hydrolysis), bulgecin A binding would be sterically impeded.
This inhibition profile can be explained by the tetrameric nature of L1, a structure in which the subunits interact via their N-termini and in which negative co-operativity is seen [32,38]. Hence, binding of bulgecin A to one subunit might be expected to influence the binding kinetics of the inhibitor to adjacent subunits, a typical example of non-competitive inhibition. Under conditions in which α is approximately equal to β (eqn 2) the partial non-co-operative system outlined in Scheme 2 will yield kinetics with a form similar to uncompetitive kinetics [33]. The fitted values of α and β are consistent with this interpretation. This is the minimum kinetic model which will explain these results. It is possible that this is an oversimplification, in that there may be multiple ESI complexes, corresponding to one, two and three molecules of bulgecin A bound to one or more of the subunits of the enzyme complex. Unfortunately, bulgecin A is currently not produced commercially, and more detailed kinetic analyses than those reported in the present paper must await further synthesis of the compound.
The results of the docking study (Figure 4) fully support the hypothesis that bulgecin A co-ordinates with the zinc II ion of binuclear zinc-dependent β-lactamases. The predicted structure also allows us to draw some conclusions as to which amino acid groups at or near the active site of the L1 metallo-β-lactamase are likely to be involved in binding bulgecin A. In particular, amino acid residues that have already been implicated in substrate binding are predicted to be close to the bulgecin A molecule, e.g. the amino acids comprising the short Phe124–Ile128 loop [38] and Trp17 [32]. In addition, Ser185, which co-ordinates to zinc II via a water molecule in the native enzyme [38], is displaced by the sulphonate group of bulgecin A in the docked structure. Other amino acids, at positions that have not been specifically identified with the active site, may also contribute to the association with bulgecin A, specifically Ala228, Tyr249 and Gln260.
Although bulgecin A itself is not a particularly potent inhibitor of the binuclear zinc-dependent β-lactamases examined at concentrations that would be clinically meaningful, nonetheless the molecule represents a new class of inhibitor of these enzymes and a valuable tool with which to probe mechanistic aspects of the activities of these clinically important enzymes.
Multimedia adjunct
Acknowledgments
We thank Dr J. Spencer and Professor A.R. Clarke, Department of Biochemistry, University of Bristol, Bristol, U.K., for helpful discussions on enzyme kinetics, and Professor Dr B. Weidemann, Institute for Microbiology and Biotechnology, University of Bonn, Bonn, Germany, for his generous donation of bulgecin A. A.M.S. is funded by the Biotechnology and Biological Sciences Research Council, grant number 7/C15746. M.B.A. is funded by the Wellcome Trust.
References
- 1.Laraki N., Galleni M., Thamm I., Riccio M. L., Amicosante G., Frère J.-M., Rossolini G. M. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob. Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauretti L., Riccio M. L., Mazzariol A., Cornaglia G., Amicosante G., Fontana R., Rossolini G. M. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 1999;43:1584–1590. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toleman M. A., Simm A. M., Murphy T. A., Gales A. C., Biedenbach D. J., Jones R. N., Walsh T. R. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: report from the SENTRY antimicrobial surveillance programme. J. Antimicrob. Chemother. 2002;50:673–694. doi: 10.1093/jac/dkf210. [DOI] [PubMed] [Google Scholar]
- 4.Bennett P. M. Integrons and gene cassettes: a genetic construction kit for bacteria. J. Antimicrob. Chemother. 1999;43:1–4. [PubMed] [Google Scholar]
- 5.Bush K., Jacoby G. A., Medeiros A. A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush K. Characterization of β-lactamases. Antimicrob. Agents Chemother. 1989;33:259–263. doi: 10.1128/aac.33.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne D. J. Metallo-β-lactamases: a new therapeutic challenge. J. Med. Microbiol. 1993;39:93–99. doi: 10.1099/00222615-39-2-93. [DOI] [PubMed] [Google Scholar]
- 8.Payne D. J., Bateson J. H., Gasson B. C., Proctor D., Khushi T., Farmer T. H., Tolson D. A., Bell D., Skett P. W., Marshall A. C., et al. Inhibition of metallo-β-lactamases by a series of mercaptoacetic acid thiol ester derivatives. Antimicrob. Agents Chemother. 1997;41:135–140. doi: 10.1128/aac.41.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilpin M. L., Fulston M., Payne D., Cramp R., Hood I. Isolation and structure determination of two novel phenazines from a Streptomyces with inhibitory activity against metallo-enzymes, including metallo-β-lactamases. J. Antibiot. 1995;48:1081–1085. doi: 10.7164/antibiotics.48.1081. [DOI] [PubMed] [Google Scholar]
- 10.Toney J. H., Fitzgerald P. M., Grover-Sharma N., Olson S. H., May W. J., Sundelof J. G., Vanderwall D. E., Cleary K. A., Grant S. K., Wu J. K., et al. Antibiotic sensitization using biphenyl tetrazoles as potent inhibitors of Bacteroides fragilis metallo-β-lactamase. Chem. Biol. 1998;5:185–196. doi: 10.1016/s1074-5521(98)90632-9. [DOI] [PubMed] [Google Scholar]
- 11.Laraki N., Franceschini N., Rossolini G. M., Santucci P., Meunier C., de Pauw E., Amicosante G., Frère J.-M., Galleni M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter M. W., Felici A., Galleni M., Paul-Soto R., Adlington R. M., Baldwin J. E., Frère J.-M., Globov M., Schofield C. J. Trifluoromethyl alcohol and ketone inhibitors of metallo-β-lactamases. Bioorg. Med. Chem. Lett. 1996;6:2455–2458. [Google Scholar]
- 13.Bounaga S., Laws A. P., Galleni M., Page M. I. The mechanism of catalysis and the inhibition of the Bacillus cereus zinc-dependent β-lactamase. Biochem. J. 1998;331:703–711. doi: 10.1042/bj3310703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollard C., Moali C., Papamicael C., Damblon C., Vessilier S., Amicosante G., Schofield C. J., Galleni M., Frère J.-M., Roberts G. C. Thiomandelic acid, a broad spectrum inhibitor of zinc β-lactamases: kinetic and spectroscopic studies. J. Biol. Chem. 2001;276:45015–45023. doi: 10.1074/jbc.M107054200. [DOI] [PubMed] [Google Scholar]
- 15.Antony J., Gresh N., Olsen L., Hemmingsen L., Schofield C. J., Bauer R. Binding of D- and L-captopril inhibitors to metallo-β-lactamase studied by polarizable molecular mechanics and quantum mechanics. J. Comput. Chem. 2002;23:1281–1296. doi: 10.1002/jcc.10111. [DOI] [PubMed] [Google Scholar]
- 16.Salsbury F. R., Jr, Crowley M. F., Brooks C. L., 3rd Modeling of the metallo-β-lactamase from B. fragilis: structural and dynamic effects of inhibitor binding. Proteins. 2001;44:448–459. doi: 10.1002/prot.1110. [DOI] [PubMed] [Google Scholar]
- 17.Buynak J. D., Chen H., Vogeti L., Gadhachanda V. R., Buchanan C. A., Palzkill T., Shaw R. W., Spencer J., Walsh T. W. Penicillin-derived inhibitors that simultaneously target both metallo- and serine-β-lactamases. Bioorg. Med. Chem. Lett. 2004;14:1299–1304. doi: 10.1016/j.bmcl.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Tsang W. Y., Dhanda A., Schofield C. J., Frère J.-M., Galleni M., Page M. I. The inhibition of metallo-β-lactamase by thioxo-cephalosporin derivatives. Bioorg. Med. Chem. Lett. 2004;14:1737–1739. doi: 10.1016/j.bmcl.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez Valladares M., Felici A., Weber G., Adolph H. W., Zeppezauer M., Rossolini G. M., Amicosante G., Frère J.-M., Galleni M. Zn(II) dependence of the Aeromonas hydrophila AE036 metallo-β-lactamase activity and stability. Biochemistry. 1997;36:11534–11541. doi: 10.1021/bi971056h. [DOI] [PubMed] [Google Scholar]
- 20.Paul-Soto R., Bauer R., Frère J.-M., Galleni M., Meyer-Klaucke W., Nolting H., Rossolini G. M., de Seny D., Hernandez-Valladares M., Zeppezauer M., Adolph H. W. Mono- and binuclear Zn2+-β-lactamase: role of the conserved cysteine in the catalytic mechanism. J. Biol. Chem. 1999;274:13242–13249. doi: 10.1074/jbc.274.19.13242. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald P. M., Wu J. K., Toney J. H. Unanticipated inhibition of the metallo-β-lactamase from Bacteroides fragilis by 4-morpholineethanesulfonic acid (MES): a crystallographic study at 1.85-Å resolution. Biochemistry. 1998;37:6791–6800. doi: 10.1021/bi9730339. [DOI] [PubMed] [Google Scholar]
- 22.Imada A., Kintaka K., Nakao M., Shinagawa S. Bulgecin, a bacterial metabolite which in concert with β-lactam antibiotics causes bulge formation. J. Antibiot. 1982;35:1400–1403. doi: 10.7164/antibiotics.35.1400. [DOI] [PubMed] [Google Scholar]
- 23.Shinagawa S., Maki M., Kintaka K., Imada A., Asai M. Isolation and characterization of bulgecins, new bacterial metabolites with bulge-inducing activity. J. Antibiot. 1985;38:17–23. doi: 10.7164/antibiotics.38.17. [DOI] [PubMed] [Google Scholar]
- 24.Templin M. F., Edwards D. H., Holtje J.-V. A murein hydrolase is the specific target of bulgecin in Escherichia coli. J. Biol. Chem. 1992;267:20039–20043. [PubMed] [Google Scholar]
- 25.Thunnissen A.-M. W. H., Rozeboom H. J., Kalk K. H., Dijkstra B. W. Structure of the 70-kDa soluble lytic transglycosylase complexed with bulgecin A: implications for the enzymatic mechanism. Biochemistry. 1995;34:12729–12737. doi: 10.1021/bi00039a032. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs C., Frère J.-M., Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in Gram-negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs C., Huang L. J., Bartowsky E., Normark S., Park J. T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh T. R., Hall L., Assinder S. J., Nichols W. W., Cartwright S. J., MacGowan A. P., Bennett P. M. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim. Biophys. Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 29.Avison M. B., Higgins C. S., von Heldreich C. J., Bennett P. M., Walsh T. R. Plasmid location and molecular heterogeneity of the L1 and L2 β-lactamase genes of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2001;45:413–419. doi: 10.1128/AAC.45.2.413-419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh T. R., Payne D. J., MacGowan A. P., Bennett P. M. A clinical isolate of Aeromonas sobria with three chromosomally mediated inducible β-lactamases: a cephalosporinase, a penicillinase and a third enzyme, displaying carbapenemase activity. J. Antimicrob. Chemother. 1995;35:271–279. doi: 10.1093/jac/35.2.271. [DOI] [PubMed] [Google Scholar]
- 31.Walsh T. R., Gamblin S., Emery D. C., MacGowan A. P., Bennett P. M. Enzyme kinetics and biochemical analysis of ImiS, the metallo-β-lactamase from Aeromonas sobria 163a. J. Antimicrob. Chemother. 1996;37:423–431. doi: 10.1093/jac/37.3.423. [DOI] [PubMed] [Google Scholar]
- 32.Simm A. M., Higgins C. S., Carenbauer A. L., Crowder M. W., Bateson J. H., Bennett P. M., Clarke A. R., Halford S. E., Walsh T. R. Characterization of monomeric L1 metallo-β-lactamase and the role of the N-terminal extension in negative cooperativity and antibiotic hydrolysis. J. Biol. Chem. 2002;277:24744–24752. doi: 10.1074/jbc.M201524200. [DOI] [PubMed] [Google Scholar]
- 33.Seigel I. H. New York: John Wiley & Sons; 1975. Enzyme Kinetics Behaviour and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. [Google Scholar]
- 34.Connolly M. L. Analytical molecular surface calculation. J. Appl. Crystallogr. 1983;16:548–558. [Google Scholar]
- 35.Carfi A., Pares S., Duee E., Galleni M., Duez C., Frère J.-M., Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carfi A., Duee E., Galleni M., Frère J.-M., Dideberg O. 1.85 Å resolution structure of the zinc (II) β-lactamase from Bacillus cereus. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998;54:313–323. doi: 10.1107/s0907444997010627. [DOI] [PubMed] [Google Scholar]
- 37.Fabiane S. M., Sohi M. K., Wan T., Payne D. J., Bateson J. H., Mitchell T., Sutton B. J. Crystal structure of the zinc-dependent β-lactamase from Bacillus cereus at 1.9 Å resolution: binuclear active site with features of a mononuclear enzyme. Biochemistry. 1998;37:12404–12411. doi: 10.1021/bi980506i. [DOI] [PubMed] [Google Scholar]
- 38.Ullah J. H., Walsh T. R., Taylor I. A., Emery D. C., Verma C. S., Gamblin S. J., Spencer J. The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophilia at 1.7 Å resolution. J. Mol. Biol. 1998;284:125–136. doi: 10.1006/jmbi.1998.2148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.