Abstract
The effect of liver growth stimulation [using the rodent PXR (pregnane X receptor) activator PCN (pregnenolone-16α-carbonitrile)] in rats chronically treated with carbon tetrachloride to cause repeated hepatocyte necrosis and liver fibrogenesis was examined. PCN did not inhibit the hepatotoxicity of carbon tetrachloride. However, transdifferentiation of hepatic stellate cells and the extent of fibrosis caused by carbon tetrachloride treatment was significantly inhibited by PCN in vivo. In vitro, PCN directly inhibited hepatic stellate cell transdifferentiation to a profibrogenic phenotype, although the cells did not express the PXR (in contrast with hepatocytes), suggesting that PCN acts independently of the PXR. Mice with a functionally disrupted PXR gene (PXR−/−) did not respond to the antifibrogenic effects of PCN, in contrast with wild-type (PXR+/+) mice, demonstrating an antifibrogenic role for the PXR in vivo. However, PCN inhibited the transdifferentiation of PXR−/−-derived mouse hepatic stellate cells in vitro, confirming that there is also a PXR-independent antifibrogenic effect of PCN through a direct interaction with hepatic stellate cells. These data suggest that the PXR is antifibrogenic in rodents in vivo and that a PXR-independent target for PXR activators exists in hepatic stellate cells that also functions to inhibit fibrosis.
Keywords: collagen, fibrosis, liver, pregnane-X receptor, hepatic stellate cell, transdifferentiation
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CYP, cytochrome P450 (for gene nomenclature, see http://drnelson.utmem.edu/CytochromeP450.html); FCS, fetal-calf serum; GFAP, glial fibrillary acidic protein; HSC, hepatic stellate cell; PCN, pregnenolone-16α-carbonitrile; PPAR, peroxisome-proliferator-activated receptor; PXR, pregnane X receptor
INTRODUCTION
The liver performs many essential functions not performed by any other tissue within the body. Fulminant hepatic failure therefore results in death within days, with transplantation the only realistic option presently available. Human liver disease progression is mainly associated with chronic non-lethal damage. This causes a scarring fibrosis to accumulate in the liver that, with increasing severity, prevents effective liver regeneration and maintenance of liver function [1,2]. Liver damage of any aetiology causes fibrosis, and so, in the absence of an available prevention or cure for the primary injuring agent, a means of reducing fibrosis is likely to be the most effective therapeutic option for treating a variety of chronic liver diseases and avoiding the need for transplantation.
Liver fibrosis is dependent on HSCs (hepatic stellate cells) [1,2]. HSCs reside in the space of Disse between the endothelial cells lining the liver blood vessels and the main functional liver cell (hepatocyte). In response to hepatocyte necrosis, HSCs trans-differentiate from a quiescent to a myofibroblast-like phenotype that secretes the majority of the extracellular-matrix proteins that constitute (e.g. collagen type I) or contribute to (e.g. tissue inhibitors of metalloproteinases) scar formation [1,2]. The liver, as a metabolic sieve preventing ingested toxic chemicals from reaching the rest of the body, has evolved to respond to acute poisoning by regenerating itself through hepatocyte proliferation [3,4]. However, fibrosis in its most severe form (cirrhosis) inhibits effective regeneration and appears to be the cause of death in chronic liver diseases [5,6]. It might arguably be considered that liver fibrogenesis is a protective response to excessive hepatocyte necrosis. However, research over the last few years clearly indicates that stimulating the apoptosis of myofibroblast-like (profibrogenic) HSCs in the livers of rodents with fibrosis promotes liver recovery [7,8]. Fibrosis is therefore a deleterious response to liver damage and may be an inappropriate or misregulated response of the liver to damage (in contrast with regeneration).
Work by others has suggested that stimulating hepatocyte proliferation can protect the liver from otherwise fatal actute hepatic toxicity [9,10]. We therefore posed the hypothesis that promoting liver growth could also improve liver pathology in a model of chronic liver damage. Carbon tetrachloride administration was used as the liver-damaging agent [11] and PCN (pregnenolone-16α-carbonitrile) the liver-growth-promoting agent [12] [since the nuclear receptor mediating the growth effects of this compound, termed the PXR (pregnane X receptor) is restricted to expression in the liver and intestine] [13–15]. We show that PCN improved liver pathology independent of any effect on carbon tetrachloride-dependent damage. The effects were in part dependent on the presence of the PXR in the liver, but the data also suggest an additional PXR-independent antifibrogenic mechanism was operating within HSCs.
EXPERIMENTAL
Materials and animals
Carbon tetrachloride and PCN were obtained from Sigma Chemical Co. (Poole, Dorset, U.K.). RU486 (mifepristone) was generously provided by Roussel-Uclaf, Romainville, France. All other chemicals were of the highest purity available from local commercial sources. Male Sprague–Dawley rats were bred in-house at the Biological Services Unit, University of Aberdeen, Foresterhill, Aberdeen, Scotland, U.K. Heterozygous PXR−/+ mice originally derived by disruption of the PXR gene with a neomycin-resistance cassette and bred onto a C57Bl6 background [16] were crossed to generate wild-type (PXR+/+) and knockout (PXR−/−) mice and then back-crossed with PXR+/+ or PXR−/− mice respectively to generate sufficient animals of the required genotype. Mice were also bred and maintained at the Biological Services Unit, University of Aberdeen, Foresterhill, Aberdeen, Scotland, U.K. PXR genotype was determined by two separate PCR reactions to detect wild-type and knockout alleles in DNA derived from tail tips essentially as outlined in [17]. Mice used were back-crossed on to a C57Bl6 background for at least ten generations prior to their use in these studies.
Carbon tetrachloride model of liver fibrosis
Liver damage was caused in rats (250–300 g body weight) and mice (25–30 g body weight) by administration of 2 ml of carbon tetrachloride/olive oil (1:1, v/v)/kg body weight by intraperitoneal injection twice weekly for up to 8 weeks. Control animals received 1 ml of olive oil/kg body weight. PCN was administered as a suspension in olive oil intraperitoneally at up to 100 mg/kg body weight; controls received olive-oil vehicle. At the end of studies, animals were killed by CO2 asphyxiation. Blood was collected from the inferior vena cava, allowed to clot, and serum was collected by centrifugation and analysed for ALT (alanine aminotransferase) and AST (aspartate aminotransferase) activities as previously outlined [7]. The liver was excised, divided, and fixed in formalin or snap-frozen in liquid nitrogen and stored at −80 °C for later analysis. Fixed tissue was embedded in wax and sections stained with haemotoxylin and eosin, Sirius Red or immunohistochemically stained for α-smooth muscle actin essentially as previously described [7]. Liver damage severity was assessed on a scale of 0–5 by a researcher blinded to the treatment groups by visual examination as outlined in Supplementary Table 1(A) (http://www.BiochemJ.org/bj/387/bj3870601add.htm). For the detection of α-smooth-muscle actin in mouse tissue, a modified procedure was used, since the primary antibody is a mouse monoclonal. Sections were stained using an FITC-conjugated anti-(α-smooth-muscle actin) mouse monoclonal antibody (Sigma Chemical Co.), followed by incubation with rabbit anti-FITC IgG (Dako, Ely, U.K.). Finally, sections were incubated with a biotin-conjugated anti-rabbit IgG and horseradish-peroxidase-conjugated streptavidin and stained as outlined in [7]. Slides were examined by a researcher blinded to the treatment groups and severities recorded on a scale of 0–5, as outlined in Supplementary Table 1 (http://www.BiochemJ.org/bj/387/bj3870601add.htm).
Hepatocyte and HSC isolation and culture
Rat hepatocytes were prepared by collagenase perfusion, essentially as previously described in [17], and cultured in William's Medium E supplemented with 1 μg/ml bovine insulin, 10% (v/v) FCS (fetal-calf serum), 80 units/ml penicillin and 80 μg/ml streptomycin on collagen type-I coated six-well plates (BD Biosciences). After 2 h, the medium was renewed without FCS and insulin supplementation and thereafter changed daily with renewed media additions where indicated. Rat and mouse HSCs were isolated by pronase/collagenase perfusion of the liver, followed by density-gradient centrifugation and elutriation as previously outlined [18]. HSCs typically displayed >95% purity at isolation (as determined by endogenous vitamin A fluorescence). HSCs were cultured in Dulbecco's modified Eagle's medium containing 4.5 g/l glucose and supplemented with 16% (v/v) FCS, 80 units/ml penicillin, 80 μg/ml streptomycin and 32 μg/ml gentamycin. Cells were seeded on to plastic culture dishes, conditions that result in transdifferentiation to the profibrogenic phenotype, as evidenced by the expression of α-smooth-muscle actin in 100% of cells (as determined by immunohistochemical staining). Only primary cultures of HSCs were used.
Microsomal CYP (cytochrome P450) enzyme assays
Liver microsomes were prepared essentially as previously outlined [19]. Testosterone hydroxylation was determined by HPLC analysis using authentic steroids as standards as previously outlined [17]. CYP2E activity and its inhibition by various compounds was determined using the probe substrate p-nitrophenol and by measuring the 4-nitrocatechol production spectrophotometrically at 511 nm, essentially as described in [20]. Total CYP was determined using the CO-reduced-versus-reduced spectrum in cell extracts as previously outlined [17].
Western blotting
Proteins extracts were subjected to SDS/PAGE under reducing conditions using a Bio-Rad MiniP2 electrophoresis apparatus. Protein was then transferred on to nitrocellulose and blocked overnight with 3% (w/v) dried milk/0.3% (w/v) Tween 20. Antibodies raised against the C-termini of CYP3A1/3A23 (IITGS) and CYP3A2 (VINGA) were used as described previously [21]. The anti-α-smooth-muscle actin, anti-desmin and anti-GFAP [anti-(glial fibrillary acidic protein)] antibodies were purchased from the Sigma Chemical Co. The anti-β-actin (cross-reacts with all actin isoforms) antibody was purchased from Chemicon (Chandlers Ford, Eastleigh, Hants., U.K.). The anti-CYP2E1 antibody was obtained from Professor Magnus Ingelman-Sundberg, Karolinska Institutet, Stockholm, Sweden. After incubation with primary antibodies, blots were incubated with the appropriate horseradish-peroxidase-conjugated anti-IgG antibody. Detection was accomplished using chemiluminescence with the Amersham ECL® (enhanced chemiluminescence) kit.
Reverse-transcription PCR for PXR mRNA expression
RNA was isolated from cells or whole tissue using RNeasy kits (Qiagen, Southampton, U.K.) or TRIzol (Invitrogen, Paisley, Renfrewshire, Scotland, U.K.) according to manufacturer's instructions and aliquots stored at −20 °C. For detection of receptor mRNA transcripts, the downstream (3′) primer employed in the PCR reaction was used to prime for first-strand cDNA essentially as described in [22]. PCR reaction mixtures were incubated at 95 °C for 1 min, followed by 1 min at the required annealing temperature (see Table 1), followed by 2 min at 73 °C for elongation for the required number of cycles (see Table 1). Amplified DNA was analysed on gels containing ethidium bromide.
Table 1. Primer sequences used in RT-PCR and PCR analysis.
| RT-PCR or PCR reaction | Upstream (5′) primer sequence | Downstream (3′) primer sequence | Annealing temperature (°C) | No. of PCR cycles | Size of band detected (bp) | Comments |
|---|---|---|---|---|---|---|
| rPXR | rPXRUS | rPXRDS | 62 | 30 | 475 | |
| ACCTGGCCGATGTGTCAACCTACA | ATCATGGCTGTCCTCACCGAGCT | |||||
| mPXR | Used rat 5′ upstream primer rPXRUS | mPXRDS | 60 | 35 | 477 | |
| GCAGCTCAGTGAGGACGGCCATGATC | ||||||
| PXR+/+ screen | PXR-ko+1 | PXR-ko-1 | 62 | 35 | 204 | |
| GCTGTACCACACCCCTCAACCC | AGACTCCAGTGGATCCCCCACACCTAT | |||||
| PXR−/− screen | PXR-F1 | PXR-R3 | 60 | 35 | 265 | PXR-R3 hybridizes to inserted neomicin gene |
| CTGGTCATCACTGTTGCTGTACCA | CTAAAGCGCATGCTCCAGACTGC |
Statistics
Student's t test was used to test data for statistical significance.
RESULTS
Rodent PXR activator PCN inhibits fibrogenesis in rats without modulating carbon tetrachloride hepatotoxicity
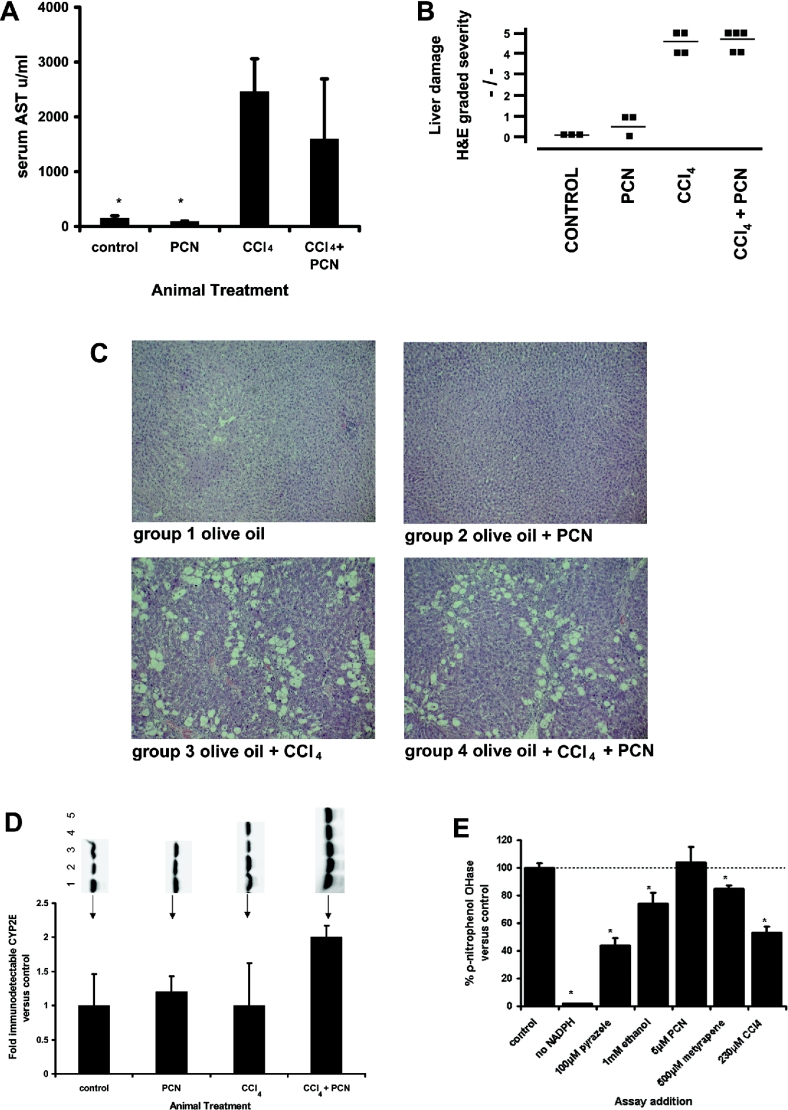
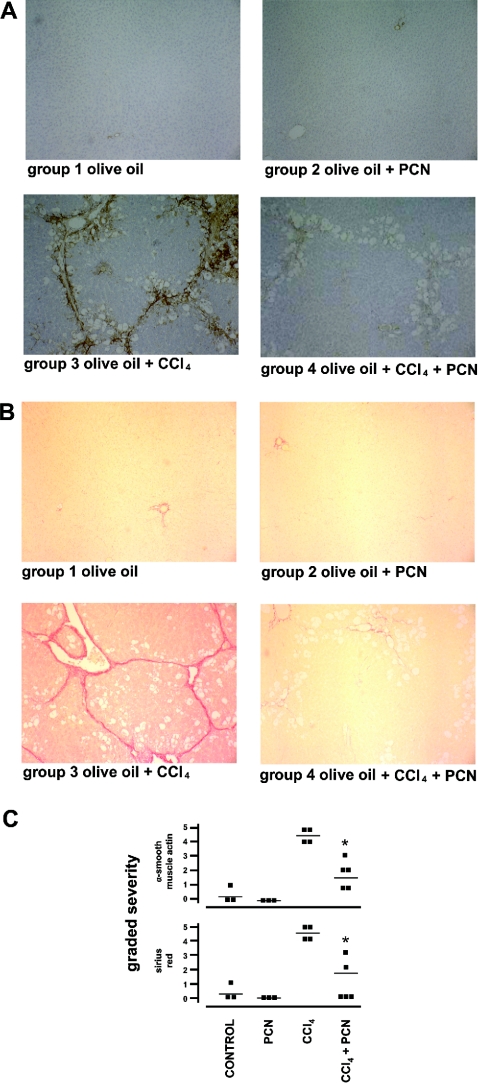
PCN administration to rodents causes liver hypertrophy [12] and, in the rats used in these studies, PCN alone caused a statistically significant increase in relative liver weight (results not shown). Carbon tetrachloride is a hepatotoxin through its metabolism by cytochrome P450 2E (CYP2E) to a trichloromethyl free radical. Reaction with oxygen produces a highly reactive peroxytrichloromethyl radical that initiates a damaging cycle of lipid membrane peroxidation and cell death [23]. Twice weekly administration of carbon tetrachloride for 6 weeks caused sustained hepatocyte damage, as evidenced by significant increases in serum AST levels and marked histological changes in liver sections stained with haematoxylin and eosin (Figures 1a–1c). Administration of PCN to rats did not result in liver damage or significantly affect the level of liver damage caused by carbon tetrachloride (Figure 1c). PCN did not therefore interfere significantly with the metabolism of carbon tetrachloride to toxic metabolites (supported by an absence of effect on CYP2E expression or CYP2E-mediated enzyme activity; Figures 1d and 1e). Administration of carbon tetrachloride over 6 weeks resulted in the development of a fibrosis within the liver, as judged by extensive intralobular α-smooth-muscle actin immunostaining (detecting trans-differentiated profibrogenic HSCs) and intense Sirius Red-stained bands (detecting collagens) (Figures 2a–2c). Despite lacking a significant effect on carbon tetrachloride hepatoxicity, Figure 2 demonstrates that PCN administration to carbon tetrachloride-treated rats significantly decreased both intralobular α-smooth-muscle-actin immunostaining and intense Sirius Red staining in liver sections. Thus PCN was antifibrogenic in rat liver through (a) mechanism(s) independent of any effect on the liver damaging event.
Figure 1. Lack of impact of PCN administration on carbon tetrachloride (‘CCl4’)-mediated hepatotoxicity.
Rats were administered carbon tetrachloride and/or PCN (25 mg/kg body weight; one injection/week) as outlined in the Experimental section for 6 weeks. (A) AST levels in serum from the indicated treatment groups; results are the means±S.D. for three to five animals/treatment group. (B) Severity scores for liver damage as determined from examination of haematoxylin-and-eosin-stained liver sections (independently scored for severity on a scale of 0–5 (normal–extensive damage) by an examiner blinded to the treatment groups. Each point represents the score for a section from a single animal; the number of individual animals is equal to the number of points per treatment group. The mean score is indicated by the line. (C) Representative liver sections from the indicated treatment groups stained with haematoxylin and eosin. (D) Western-blot immunoquantification of microsomal CYP2E levels from the indicated treatment groups; results are means±S.D. for three to five animals/treatment group. (E) Effect of PCN and CYP inhibitors (final concentrations in assay as indicated) on microsomal p-nitrophenol hydroxylase (CYP2E) activities (control levels 450±42 pmol of 4-nitrocatechol produced/min per mg of microsomal protein; means±S.D. of three separate determinations). Note the vehicle solvent DMSO is a substrate (not shown) for CYP2E and therefore markedly inhibits p-nitrophenol hydroxylase activities. Accordingly, all potential inhibitors of this assay were dissolved directly into the assay buffer. PCN was used from a saturated stock (actual concentration determined through UV–visible absorbance spectroscopy at 248 nm). * Indicates significantly different activity versus control (P>95%) using Student's t test.
Figure 2. PCN inhibits the carbon tetrachloride (‘CCl4’)-dependent liver fibrosis.
Rats were administered carbon tetrachloride and/or PCN (25 mg/kg body weight; one injection/week) as outlined in the Experimental section for 6 weeks. (A) Representative liver sections from the indicated treatment groups immunostained for α-smooth-muscle actin. (B) Representative liver sections from the indicated treatment groups and stained with Sirius Red. (C) Severity scores for α-smooth-muscle actin and Sirius Red. Liver sections were independently scored for severity on a scale of 0–5 (normal–extensive staining/fibrosis) by an examiner blinded to the treatment groups. Each point represents the score for a section from a single animal, the number of individual animals being equal to the number of points per treatment group. The mean score is indicated by the line. * Indicates significantly lower damage versus carbon tetrachloride-treated group, P>95% using Student's t test.
PCN inhibits the transdifferentiation of rat HSCs in vitro despite an absence of PXR expression in rat HSCs
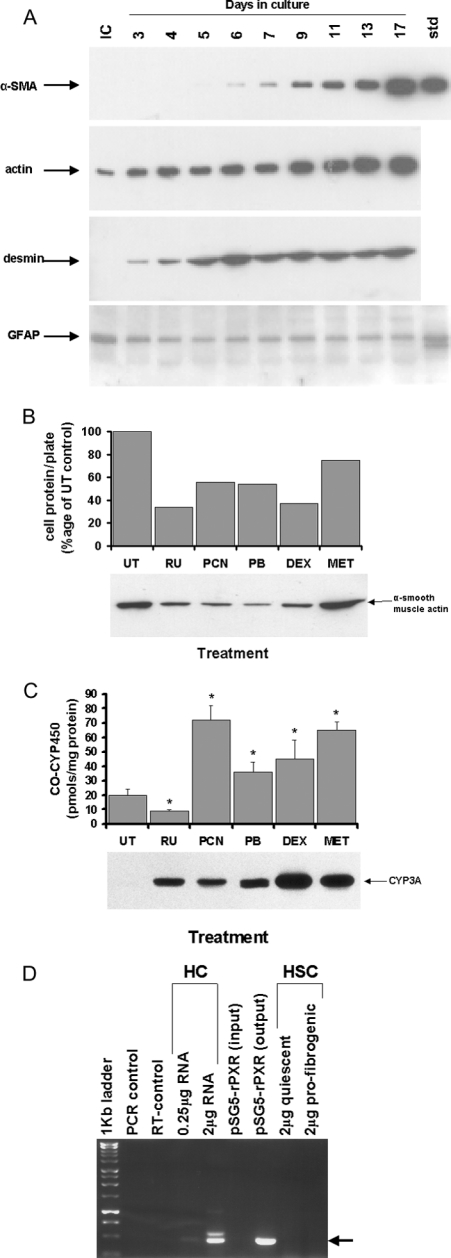
Proteolytic digestion of the liver was used to isolate and culture hepatocytes and quiescent HSCs from rat livers by standard protocols. Under the culture conditions employed, quiescent HSCs underwent a spontaneous transdifferentiation to the profibrogenic myofibroblast expressing desmin and α-smooth-muscle actin (Figure 3a). The effects of PCN and other known PXR activators on this process of transdifferentiation were examined using α-smooth-muscle-actin levels as a measure of fibrogenic potential. It can be seen that PCN and other PXR activators inhibited transdifferentiation, indicating that these compounds interacted, at least in part, directly on the cells (Figure 3b). Figure 3(c) confirms the functional PXR-activating effects of PCN and other known PXR activators, since the concentrations employed also induced the expression of the PXR-dependent gene CYP3A23 [13–15] and total CYP in cultured hepatocytes. However, rat HSCs did not contain detectable levels of PXR mRNA and protein, in contrast with hepatocytes (Figure 3d), suggesting that the effects of the PXR activators on HSCs in vitro were independent of the PXR.
Figure 3. Effect of PCN on the transdifferentiation of Rat HSCs in vitro.
(A) Time course for the expression of α-smooth muscle actin (‘α-SMA’) and desmin in rat HSC primary culture. Freshly isolated (IC) rat HSCs and cells cultured for the indicated time were harvested and cell extracts (10 μg/lane) probed for the indicated protein by Western blotting. Std, standard. (B) Effect of PXR activator addition to rat HSC primary culture on the transdifferentiation of HSCs in vitro. Abbreviations: UT, vehicle control only (<0.5%, v/v, DMSO); RU, 10 μM RU486; PCN, 20 μM PCN; PB, 1 mM phenobarbital; DEX, 1 μM dexamethasone; MET, 500 μM metyrapone). The levels of α-smooth muscle actin were determined by Western blotting (5 μg of protein/lane) after 9 days of continuous treatment. (C) Effect of PXR activator treatment on the levels of total spectrophotometrically detectable cytochrome P450 and CYP3A1/3A23 levels in primary cultures of rat hepatocytes. The concentration of PXR activators was as given in (B), except that treatment time was 48 h. (D) Reverse-transcription PCR analysis of RNA from rat hepatocytes and HSCs. Total RNA was subjected to reverse-transcription PCR analysis for PXR expression as outlined in the Experimental section. PCR control, no 1st-strand cDNA control; RT-control, no RNA control; HC, freshly isolated hepatocyte RNA (template levels as indicated); pSG5-rPXR, a plasmid encoding the rat PXR cDNA as template (input: template plasmid in PCR reaction; output: amplified fragment after PCR reaction); HSC, 2-day quiescent HSC or profibrogenic 14-day transdifferentiated HSC RNA (template levels as indicated). The lower band corresponds to a fragment of amplified PXR mRNA (sequenced confirmed; results not shown); the upper band amplified from rat hepatocytes was cloned and sequenced and was found to be unrelated to the PXR.
PCN-dependent antifibrogenesis requires a functional PXR in vivo
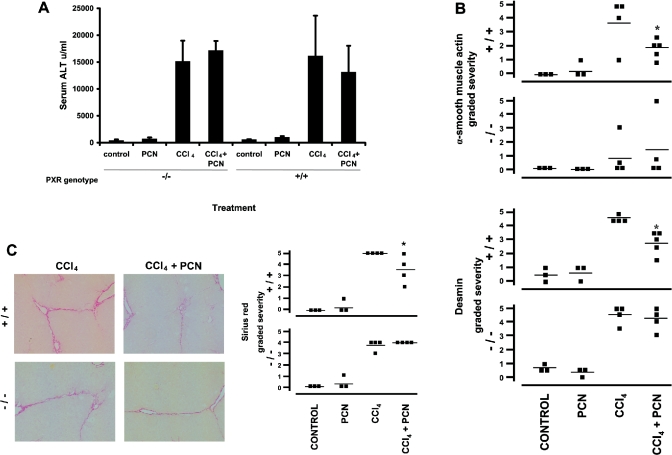
To determine if the antifibrogenic effects of PCN in vivo are dependent on the expression of a functional PXR in vivo, the effect of PCN on carbon tetrachloride-dependent liver fibrosis in PXR+/+ mice and mice with a disruption to the transcriptional function of the PXR protein (PXR−/−) was examined. The PXR is an orphan nuclear receptor that is known to regulate the expression of hepatocyte CYP3A genes in response to a diverse range of xenobiotics [15]. Mice genotype was routinely monitored by PCR (Supplementary Figure 1a; http://www.BiochemJ.org/bj/387/bj3870601add.htm) and PXR−/− mice failed to respond to PCN administration with respect to CYP3A induction at the level of microsomal protein and testosterone 6β hydroxylase activity – in contrast with PXR+/+ mice (Supplementary Figures 1b and 1c; http://www.BiochemJ.org/bj/387/bj3870601add.htm). In addition to confirming the absence of a functional PXR transcriptional response in the PXR−/− mice used in these studies, PCN administration to either genotype did not affect the levels of expression of the carbon tetrachloride-activating CYP2E (Supplementary Figure 1b; http://www.BiochemJ.org/bj/387/bj3870601add.htm). As observed in rats, PXR+/+ and PXR−/− mice sustained similar levels of liver damage between the two genotypes (Figure 4a). Figure 4(b) demonstrates that PCN-dependent reductions in intralobular α-smooth-muscle actin or desmin immunostaining were lost in PXR−/− mice treated with carbon tetrachloride. Figure 4(c) shows that PCN-dependent decreases in fibrosis were lost in PXR−/− mice treated with carbon tetrachloride, as judged by Sirius Red staining of liver sections. These experiments suggest that the antifibrogenic effects of PCN in the mouse were dependent – in part – on the PXR in vivo. However, for the same amount of liver damage, the extent of intralobular (α-smooth-muscle-actin-positive) staining HSCs was markedly decreased in PXR−/− compared with PXR+/+ mice (Figure 4b), although this was not mirrored by a significant decrease in fibrosis (Figure 4c).
Figure 4. Antifibrogenic effects of PCN in the liver are lost in PXR−/− mice.
Mice were administered carbon tetrachloride (‘CCl4’) for 8 weeks and/or PCN (100 mg/kg body weight; one injection/week for the last 4 weeks of treatment) as outlined in the Experimental section for 8 weeks. (A) ALT levels in serum from the indicated treatment groups; results are means±S.D. for three to five animals/treatment group. (B) Liver sections stained for either α-smooth-muscle actin or desmin were independently scored for severity on a scale of 0–5 (normal–extensive staining/fibrosis) by an examiner blinded to the treatment groups. Each point represents the score for a section from a single animal, and the number of individual animals is equal to the number of points per treatment group. The mean score is indicated by the horizontal line. * Indicates significantly lower transdifferentiation versus carbon tetrachloride-treated group; P>95% using Student's t test. (C) Representative liver sections from the indicated treatment groups and stained with Sirius Red. Severity scores are given in the right-hand panel. * Indicates significantly lower fibrosis versus carbon tetrachloride-treated group; P>95% using Student's t test.
PCN-dependent antifibrogenesis in mice is also dependent on a PXR-independent direct effect on HSCs
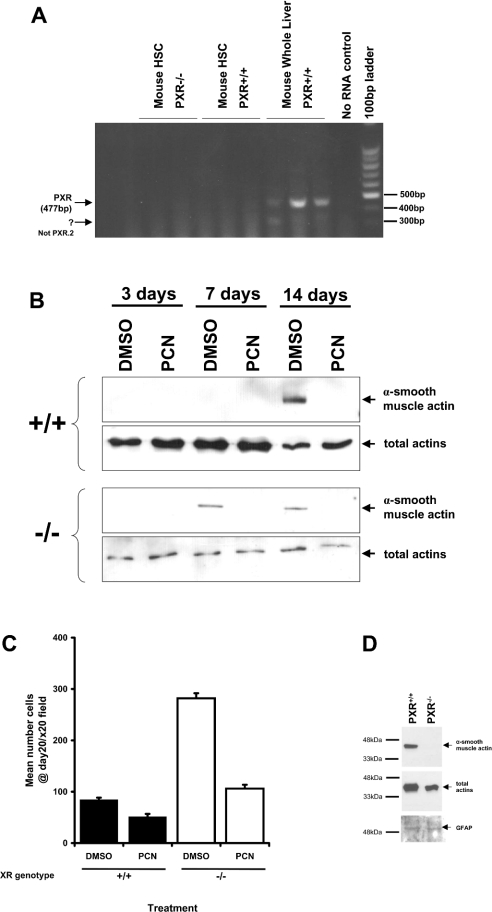
As observed with rat HSCs, mouse HSCs did not express detectable levels of PXR mRNA, as determined by reverse-transcription PCR (Figure 5a). To test the hypothesis that PCN acts directly on HSCs independently of the PXR, HSCs derived from PXR+/+ and PXR−/− animals were isolated and cultured, with or without PCN treatment. It can be seen that HSCs isolated from PXR+/+ mice took longer to proliferate and transdifferentiate compared with HSCs isolated from PXR−/− mice. However, PCN inhibited both the proliferation and transdifferentiation of mouse HSCs irrespective of the PXR genotype (Figures 5b and 5c). Interestingly, HSCs isolated from PXR−/− mice became, or were outgrown by, α-smooth-muscle-actin-negative cells with passaging. This is consistent with the apparent low number of α-smooth-muscle-actin-positive cells in liver sections from carbon tetrachloride-treated PXR−/− mice, despite high levels of fibrosis (Figure 4b and 4c) and suggests that, under certain conditions, it may be possible to generate α-smooth-muscle-actin-negative profibrogenic HSCs.
Figure 5. PXR genotype and mouse HSC transdifferentiation: effect of PCN.
(A) RT-PCR analysis for PXR mRNA expression. Primers used are given in Table 1. (B) Western blot for α-smooth-muscle actin or total actin levels in extracts from transdifferentiating mouse HSCs. (C) HSCs were isolated from four PXR+/+ or PXR−/− mice, pooled, counted and seeded at approx. 50 cells/field of view. The number of attached cells per ×20 field were then counted in eight randomly selected fields to quantify the proliferation of the HSCs. (D) Expression of α-smooth-muscle actin, total actin and GFAP in extracts from passage-2 HSCs derived from PXR+/+ or PXR−/− mice.
DISCUSSION
PCN is an antagonist of the glucocorticoid receptor [24,25] and agonist of rat and mouse PXR [13,14,26] (note that there are species differences in the ligand selectivity of the PXR; notably PCN does not activate the human PXR [13]). An antagonism of the glucocorticoid receptor by PCN is unlikely to play a role in inhibiting fibrosis. Glucocorticoids inhibit the inflammatory response [27], which plays a role in promoting fibrosis progression in general and in the carbon tetrachloride model [1,2,28]. Therefore PCN might be expected to have either no effect or to exacerbate fibrogenesis through an inhibition of glucocorticoid signalling. The decrease in fibrogenesis observed in this study with PCN and the loss of this effect in mice with a disrupted PXR gene indicates that PCN was acting – at least in part – through an activation of the PXR to achieve an inhibition of fibrogenesis.
The PXR is an orphan nuclear receptor that has been shown to mediate the inducible expression of CYP3A enzymes in liver and intestine [13–15]. However, the antifibrogenic effect of PCN is likely to be dependent on its liver-growth-promoting properties [12]. The liver contains four subfamilies of xenobiotic-metabolizing enzymes (CYP1A, CYP2B, CYP3A and CYP4A), and expression of genes within each subfamily is controlled by a xenobiotic receptor transcription factor [the aryl hydrocarbon receptor, the constitutive androstane receptor, the PXR and the PPAR (peroxisome-proliferator-activated-receptor)-α respectively] [29]. In addition to modulating the expression of their respective CYP genes in response to receptor–agonist interaction, potent activators of xenobiotic receptor transcription factors (e.g. dioxin, phenobarbital, PCN and peroxisomal proliferators respectively) also promote a marked increase in liver size. Gene knockout studies demonstrate that the xenobiotic receptors mediate both CYP induction and an increase in liver size [28,30–32]. Specifically, the growth-promoting effect of PCN has been shown to be dependent on a functional PXR gene [12] and we observed a similar effect with PCN and other PXR activators (i.e. a loss of liver growth response in PXR−/− mice) in our studies (results not shown). The precise mechanisms that result in liver growth via the PXR have not been elucidated. There is good evidence to suggest that growth is associated with an increase in hepatocyte proliferation and a decrease in apoptosis when rodents are treated with peroxisome proliferators [33]. However, the mechanisms controlling this altered tissue homoeostasis have not been fully described, although it could be speculated that many of the factors which control liver regeneration in response to hepatocyte necrosis or surgical ablation of the liver may be regulated directly or indirectly by the xenobiotic receptor transcription factors. Liver regeneration occurs through existing hepatocyte proliferation and is controlled by a number of cytokines released from several of the cell types within the liver [34]. Likely candidates are cytokines such as tumour necrosis factor-α and interleukin-6 [3,4]. To date, the role – if any – of the PXR in liver cytokine gene expression is unknown, although this is under active investigation in our laboratory.
The results of the present study also indicate that there is a PXR-independent mode of action of PCN, since the PXR was not detected in rodent HSCs and yet PCN treatment in an in vitro model of fibrosis inhibited HSC transdifferentiation to a profibrogenic phenotype. It is known that rat and human HSCs express the PPARγ nuclear receptor and that ligands for this receptor inhibit transdifferentiation of HSCs [35–37]. However, PCN has not been reported to interact with the PPARγ. Limiting the numbers of HSCs in the liver is being recognized as an effective means of combating liver fibrosis. The identification of a protein that functions to inhibit HSC transdifferentiation/proliferation and whose ligands also activate hepatocyte growth via the PXR may be of fortuitous clinical value. This protein remains to be identified.
There is some debate about the origin of the cells that give rise to fibrosis in the liver. Two populations of cells have recently been proposed by one group, namely the HSCs and liver myofibroblasts [38,39]. Liver myofibroblasts constitutively express α-smooth-muscle actin and are located at the central and the portal vein in normal liver [38,39]. The relative absence of an α-smooth-muscle-actin-positive population of cells in carbon tetrachloride-treated PXR−/− mice may be associated with a loss of liver myofibroblast proliferation and/or a failure of HSCs to completely transdifferentiate to cells that express α-smooth-muscle actin. The absence of α-smooth-muscle actin, but presence of GFAP in passaged HSCs from PXR−/− mice, support both of these possibilities (in that it has been suggested that, in vitro, passaged HSCs are outgrown by liver myofibroblasts) [38].
Clinically, the avoidance or treatment of the primary hepatotoxic insult is the first approach to liver-disease management. Fibrosis occurs when the primary hepatotoxic insult cannot be modulated, and so there is a need to treat the fibrosis as a way of preventing end-stage chronic liver disease. To clearly test for a target protein's antifibrogenic effect, an interaction between the target protein's experimental ligand and the primary hepatotoxic insult must be avoided. We demonstrate that PCN is antifibrogenic and does not mediate its antifibrogenic effect through a decrease in the toxicity of carbon tetrachloride (since a decrease in fibrosis would likely be the outcome of a treatment that protected against the primary hepatotoxic agent). The present results clearly indicate that the administration of PCN inhibits fibrogenesis and that the target protein (the PXR) is in part responsible for this effect.
Online data
Acknowledgments
This work was supported by grants from the Wellcome Trust to S.J.T., from the Primary Biliary Cirrhosis Foundation to D.K.K., from the BBSRC (Biotechnology and Biological Sciences Research Council) to C.J.M. and from the Scottish Hospitals Research Endowment Trust to L.J.E. The assistance of Professor Steven Kliewer (Department of Molecular Biology, University of Texas Southwestern Medical Center, Dallas, TX, U.S.A.) is gratefully acknowledged.
References
- 1.Friedman S. L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R., Brenner D. A. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin. Liver Dis. 2001;21:437–451. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- 3.Fausto N., Campbell J. S. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 4.Costa R. H., Kalinichenko V. V., Holterman A. X., Wang X. Transcription factors in liver development, differentiation, and regeneration. Hepatology. 2003;38:1331–1347. doi: 10.1016/j.hep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Gines P., Cardenas A., Arroyo V., Rodes J. Management of cirrhosis and ascites. N. Engl. J. Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 6.Gabele E., Brenner D. A., Rippe R. A. Liver fibrosis: signals leading to the amplification of the fibrogenic hepatic stellate cell. Front. Biosci. 2003;8:d69–d77. doi: 10.2741/887. [DOI] [PubMed] [Google Scholar]
- 7.Wright M. C., Issa R., Smart D. E., Trim N., Murray G. I., Primrose J. N., Arthur M. J., Iredale J. P., Mann D. A. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–698. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- 8.Dekel R., Zvibel I., Brill S., Brazovsky E., Halpern Z., Oren R. Gliotoxin ameliorates development of fibrosis and cirrhosis in a thioacetamide rat model. Dig. Dis. Sci. 2003;48:1642–1647. doi: 10.1023/a:1024792529601. [DOI] [PubMed] [Google Scholar]
- 9.Hogaboam C. M., Simpson K. J., Chensue S. W., Steinhauser M. L., Lukacs N. W., Gauldie J., Strieter R. M., Kunkel S. L. Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther. 1999;6:573–584. doi: 10.1038/sj.gt.3300858. [DOI] [PubMed] [Google Scholar]
- 10.Simpson K., Hogaboam C. M., Kunkel S. L., Harrison D. J., Bone-Larson C., Lukacs N. W. Stem cell factor attenuates liver damage in a murine model of acetaminophen-induced hepatic injury. Lab Invest. 2003;83:199–206. doi: 10.1097/01.lab.0000057002.16935.84. [DOI] [PubMed] [Google Scholar]
- 11.Lee J. I., Lee K. S., Paik Y. H., Nyun Park Y., Han K. H., Chon C. Y., Moon Y. M. Apoptosis of hepatic stellate cells in carbon tetrachloride induced acute liver injury of the rat: analysis of isolated hepatic stellate cells. J. Hepatol. 2003;39:960–966. doi: 10.1016/s0168-8278(03)00411-2. [DOI] [PubMed] [Google Scholar]
- 12.Staudinger J., Liu Y., Madan A., Habeebu S., Klaassen C. D. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab. Dispos. 2001;29:1467–1472. [PubMed] [Google Scholar]
- 13.Lehmann J. M., McKee D. D., Watson M. A., Willson T. M., Moore J. T., Kliewer S. A. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kliewer S. A., Moore J. T., Wade L., Staudinger J. L., Watson M. A., Jones S. A., McKee D. D., Oliver B. B., Willson T. M., Zetterstrom R. H., et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 15.Wright M. C. The cytochrome P450 3A4 inducer metyrapone is an activator of the human pregnane X receptor. Biochem. Soc. Trans. 1999;27:387–391. doi: 10.1042/bst0270387. [DOI] [PubMed] [Google Scholar]
- 16.Staudinger J. L., Goodwin B., Jones S. A., Hawkins-Brown D., MacKenzie K. I., LaTour A., Liu Y., Klaassen C. D., Brown K. K., Reinhard J., et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marek C. J., Cameron G. A., Elrick L. J., Hawksworth G. M., Wright M. C. Generation of hepatocytes expressing functional cytochromes P450 from a pancreatic progenitor cell line in vitro. Biochem. J. 2003;370:763–769. doi: 10.1042/BJ20021545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orr J. G., Leel V., Cameron G. A., Marek C. J., Haughton E. L., Elrick L. J., Trim J. E., Hawksworth G. M., Halestrap A. P., Wright M. C. Mechanism of action of the antifibrogenic compound gliotoxin in rat liver cells. Hepatology. 2004;40:232–242. doi: 10.1002/hep.20254. [DOI] [PubMed] [Google Scholar]
- 19.Wright M. C., Wang X. J., Pimenta M., Ribeiro V., Paine A. J., Lechner M. C. Glucocorticoid receptor-independent transcriptional induction of cytochrome P450 3A1 by metyrapone and its potentiation by glucocorticoid. Mol. Pharmacol. 1996;50:856–863. [PubMed] [Google Scholar]
- 20.Fang C., Lindros K. O., Badger T. M., Ronis M. J., Ingelman-Sundberg M. Zonated expression of cytokines in rat liver: effect of chronic ethanol and the cytochrome P450 2E1 inhibitor, chlormethiazole. Hepatology. 1998;27:1304–1310. doi: 10.1002/hep.510270516. [DOI] [PubMed] [Google Scholar]
- 21.Wright M. C., Edwards R. J., Pimenta M., Ribeiro V., Ratra G. S., Lechner M. C., Paine A. J. Developmental changes in the constitutive and inducible expression of cytochrome P450 3A2. Biochem. Pharmacol. 1997;54:841–846. doi: 10.1016/s0006-2952(97)00264-5. [DOI] [PubMed] [Google Scholar]
- 22.Leel V., Elrick L. J., Solares J., Ingram N., Charlton K. A., Porter A. J., Wright M. C. Identification of a truncated rat p28-related protein expressed in kidney. Biochem. Biophys. Res. Commun. 2004;316:872–877. doi: 10.1016/j.bbrc.2004.02.137. [DOI] [PubMed] [Google Scholar]
- 23.Weber L. W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 24.Schuetz E. G., Guzelian P. S. Induction of cytochrome P-450 by glucocorticoids in rat liver. II. Evidence that glucocorticoids regulate induction of cytochrome P-450 by a nonclassical receptor mechanism. J. Biol. Chem. 1984;259:2007–2012. [PubMed] [Google Scholar]
- 25.Wright M. C., Paine A. J. Induction of the cytochrome P450 3A subfamily in rat liver correlates with the binding of inducers to a microsomal protein. Biochem. Biophys. Res. Commun. 1994;201:973–979. doi: 10.1006/bbrc.1994.1797. [DOI] [PubMed] [Google Scholar]
- 26.Moore L. B., Parks D. J., Jones S. A., Bledsoe R. K., Consler T. G., Stimmel J. B., Goodwin B., Liddle C., Blanchard S. G., Willson T. M., et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J. Biol. Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 27.Schleimer R. P. An overview of glucocorticoid anti-inflammatory actions. Eur. J. Clin. Pharmacol. 1993;45:S3–S7. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- 28.Badger D. A., Sauer J. M., Hoglen N. C., Jolley C. S., Sipes I. G. The role of inflammatory cells and cytochrome P450 in the potentiation of carbon tetrachloride-induced liver injury by a single dose of retinol. Toxicol. Appl. Pharmacol. 1996;141:507–519. doi: 10.1006/taap.1996.0316. [DOI] [PubMed] [Google Scholar]
- 29.Waxman D. J. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch. Biochem. Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Salguero P., Pineau T., Hilbert D. M., McPhail T., Lee S. S., Kimura S., Nebert D. W., Rudikoff S., Ward J. M., Gonzalez F. J. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 31.Wei P., Zhang J., Egan-Hafley M., Liang S., Moore D. D. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature (London) 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 32.Lee S. S., Pineau T., Drago J., Lee E. J., Owens J. W., Kroetz D. L., Fernandez-Salguero P. M., Westphal H., Gonzalez F. J. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts R. A., Chevalier S., Hasmall S. C., James N. H., Cosulich S. C., Macdonald N. PPAR alpha and the regulation of cell division and apoptosis. Toxicology. 2002;181–182:167–170. doi: 10.1016/s0300-483x(02)00275-5. [DOI] [PubMed] [Google Scholar]
- 34.Fausto N. Liver regeneration. J. Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 35.Galli A., Crabb D., Price D., Ceni E., Salzano R., Surrenti C., Casini A. Peroxisome proliferator-activated receptor gamma transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology. 2000;31:101–108. doi: 10.1002/hep.510310117. [DOI] [PubMed] [Google Scholar]
- 36.Marra F., Efsen E., Romanelli R. G., Caligiuri A., Pastacaldi S., Batignani G., Bonacchi A., Caporale R., Laffi G., Pinzani M., Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 37.Miyahara T., Schrum L., Rippe R., Xiong S., Yee H. F., Motomura K., Anania F. A., Willson T. M., Tsukamoto H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J. Biol. Chem. 2000;275:35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 38.Knittel T., Kobold D., Saile B., Grundmann A., Neubauer K., Piscaglia F., Ramadori G. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205–1221. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- 39.Ramadori G., Saile B. Mesenchymal cells in the liver – one cell type or two? Liver. 2002;22:283–294. doi: 10.1034/j.1600-0676.2002.01726.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.