Abstract
Mcl-1 (myeloid cell leukaemia-1) is a Bcl-2 family member with short-term pro-survival functions but whose other functions, demonstrated by embryonic lethality of knockout mice, do not involve apoptosis. In the present study, we show a cell-cycle-regulatory role of Mcl-1 involving a shortened form of the Mcl-1 polypeptide, primarily localized to the nucleus, which we call snMcl-1. snMcl-1 interacts with the cell-cycle-regulatory protein Cdk1 (cyclin-dependent kinase 1; also known as cdc2) in the nucleus, and Cdk1 bound to snMcl-1 was found to have a lower kinase activity. The interaction with Cdk1 occurs in the absence of its cyclin partners and is enhanced on treatment of cells with G2/M blocking agents, but not by G1/S blocking. The snMcl-1 polypeptide is present during S and G2 phases and is negligible in G1. Overexpression of human Mcl-1 in a murine myeloid progenitor cell line resulted in a lower rate of proliferation. Furthermore, Mcl-1-overexpressing cells had lower total Cdk1 kinase activity compared with parental cells, in both anti-Cdk1 and anti-cyclin B1 immunoprecipitates. The latter results suggest that binding to snMcl-1 alters the ability of Cdk1 to bind its conventional partner, cyclin B1. Given the important role of Cdk1 in progression through G2 and M phases, it is probable that the inhibition of Cdk1 activity accounts for the inhibitory effect of Mcl-1 on cell growth.
Keywords: Cdk, cell cycle, kinase, myeloid cell leukaemia-1 (Mcl-1), nuclear localization, proteolysis
Abbreviations: Cdk, cyclin-dependent kinase; GM-CSF, granulocyte/macrophage colony-stimulating factor; Mcl-1, myeloid cell leukaemia-1; NLS, nuclear localization signal; PCNA, proliferating-cell nuclear antigen; PI, propidium iodide; RT, reverse transcriptase
INTRODUCTION
The Bcl-2 family of proteins are known regulators of cell death and survival, acting as regulators of membrane integrity at both the mitochondria and the endoplasmic reticulum [1–5]. They are characterized by the presence of one to four BH (Bcl-2 homology) domains that are known to be important in their function by regulating associations with other family members. Mcl-1 (myeloid cell leukaemia-1) is a pro-survival Bcl-2 family member that was originally identified as a result of its up-regulation in a human myeloblastic leukaemia cell line that was induced to differentiate along the monocyte lineage [6,7]. Interestingly, rapid up-regulation of Mcl-1 was observed when ML-1 cells differentiated along the monocyte, but not the granulocyte lineage [7]. Mcl-1 has been shown to have only moderate pro-survival activity compared with the significant anti-apoptotic effects of Bcl-2 or Bcl-XL [8,9]. When the Mcl-1 gene was knocked out in mice, Mcl-1–/– embryos failed to implant, thus showing peri-implantation lethality, and perhaps most significantly, these embryos displayed no evidence of altered apoptosis [10]. Thus it is clear that Mcl-1 must serve other functions in addition to its activity as an anti-apoptotic Bcl-2 family member. One study has suggested a role for Mcl-1 in blocking progression through S-phase of the cell cycle [11], by an association with PCNA (proliferating-cell nuclear antigen). These intriguing characteristics of Mcl-1 led us to examine its potential role in cell-cycle regulation.
Among the many key players in cell-cycle regulation are the Cdks (cyclin-dependent kinases) and their specialized partner proteins called the cyclins. Cdks catalyse phosphorylation of various downstream targets, while the cyclins control the ability of Cdks to phosphorylate the appropriate target proteins. The activity of Cdks during cell cycle is regulated by four molecular mechanisms [12,13]: association with a specific cyclin, activation by phosphorylation on Thr-160 by Cdk activating kinase, inhibitory phosphorylation on Tyr-15 by Wee1 and on adjacent residue Thr-14 catalysed by MytI and lastly, dephosphorylation of the latter residues by the Cdc25 phosphatase.
On the basis of co-immunoprecipitation experiments with both the anti-Mcl-1 and anti-Cdk1 antibodies, we show that endogenous Mcl-1 interacts with the cell-cycle-dependent kinase, Cdk1 (also known as cdc2) in the human promyelocytic leukaemia cell line, HL-60. Most interestingly, the Mcl-1–Cdk1 interaction primarily involves a truncated form of Mcl-1, distinct from the reported splice variant, which appears as a result of proteolysis at the C-terminus. The shortened form of Mcl-1 is found associated with Cdk1 in the nucleus, and its presence is dependent on the stage of the cell cycle, being found in S and G2, but not in G1. Endogenous Cdk1 associated with Mcl-1 in HL-60 cells was found to have lower activity, as measured by histone H1 kinase assays, and the Cdk1 was not associated with its conventional partner cyclins. In addition, Cdk1 activity is greatly decreased in Mcl-1-overexpressing cells, which exhibit a delay in cell-cycle progression. Together, our results suggest that a fragment of Mcl-1, which we termed snMcl-1, acts as a key regulator of cell-cycle progression by an inhibitory effect on Cdk1 activity. We believe that this novel function of Mcl-1 may explain its critical role in mammalian development, and defines a new mode of cell-cycle regulation by a member of the Bcl-2 family.
EXPERIMENTAL
Antibodies and reagents
Antibodies to human Mcl-1 (S-19), human Mcl-1CT (K-20), Cdc2 p34 (Sc-54), cyclin B1 (H 433) and actin (I-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Antibody detecting phospho-Tyr-15 of Cdk1 and anti-histone H3 were from Upstate Biotechnology, Lake Placid, NY, U.S.A.) and anti-murine Mcl-1 was from BD Biosciences (Mississauga, ON, Canada). Aphidicolin, nocodazole and Paclitaxel (Taxol) were from Calbiochem (La Jolla, CA, U.S.A.). [γ-32P]ATP was purchased from ICN Biomedicals (Costa Mesa, CA, U.S.A.). RPMI 1640, fetal bovine serum and Protein G–agarose beads were purchased from Canadian Life Technologies (Burlington, ON, Canada).
Cell-culture conditions and synchronization
Human promyelocytic HL-60, human progenitor myeloid TF-1 and human T-cell Jurkat and murine progenitor myeloid FDC-P1 cell lines (A.T.C.C., Manassas, VA, U.S.A.) were cultured in RPMI 1640 supplemented with 10% (v/v) fetal bovine serum, 1 mM sodium pyruvate, 2 mM L-glutamine and 10 μM 2-mercaptoethanol. Since TF-1 and FDC-P1 are cytokine-dependent cell lines, the growth media were supplemented with conditioned media containing human GM-CSF (granulocyte/macrophage colony-stimulating factor; 1% CGMI) and murine IL-3 [2.5% (v/v) WEHI-3] respectively. For synchronization, cells were blocked at G1/S boundary by overnight treatment with 1 μg/ml of Aphidicolin. The cells were washed three times with 1× PBS and normal growth medium was added. For studying the kinetics of Mcl-1–Cdk1 complex formation the time point corresponding to G1/S boundary was designated as zero time. Similarly, blocking of cells at the G2/M boundary was accomplished by overnight treatment with either 0.3 μg/ml nocodazole or 0.1 μM of Taxol.
Immunoblotting
Total cell lysates were obtained by lysing cells in ice-cold solubilization buffer A [50 mM Tris/HCl, pH 7.7, 1% Triton X-100, 10% (v/v) glycerol, 100 mM NaCl, 2.5 mM EDTA and 10 mM NaF]. For extraction of cytosolic proteins, cells were lysed in buffer A containing low (0.25%) Triton X-100, followed by centrifugation at 600 g for 5 min to pellet primarily the nuclei. The supernatants were stored as cytosolic proteins and pellets were used for extraction of nuclear proteins. The pellets were washed twice before resuspension in buffer A followed by sonication using a Sonic Dismembrator 550 (Fisher Scientific, Nepean, ON, Canada) at setting 3 for 10 s. The extracted proteins were centrifuged at 23000 g for 5 min. The supernatants contained the nuclear proteins. The extracted proteins were boiled in SDS sample buffer for 5 min and resolved using standard SDS/PAGE. Transfers were made by semi-dry blotting on to nitrocellulose membranes. The membranes were blocked for 1 h in 5% (w/v) low-fat dry milk in Tris-buffered saline with 0.05% Tween 20 followed by overnight incubation at 4 °C with appropriate antibody. Anti-rabbit, anti-mouse or anti-goat antibodies conjugated to horseradish peroxidase were used to detect the immunocomplexes by enhanced chemiluminescence (Amersham International, Oakville, ON, Canada).
Co-immunoprecipitation assay
Typically, 2–4 mg of protein extracts were used for immunoprecipitations. Extracted proteins were incubated for 2 h to overnight with either 2 μg of anti-Mcl-1 or 1 μg each of cyclin B1 or Cdk1 antibody. The antibody-bound proteins were precipitated with Protein G–agarose beads. The protein complexes were eluted in SDS sample buffer.
Flow cytometry
Cells for flow cytometric analysis were fixed in 70% (v/v) ethanol. The cells were subsequently stained in PBS containing 50 μg/ml of PI (propidium iodide), 100 μg of RNase A and 0.1% glucose. Cells were analysed using an Epics XL Flow Cytometer (Coulter, Miami, FL, U.S.A.). Sorting of HL-60 cells was performed on a BD FACS Vantage SE Turbo cell sorter at the University of British Columbia Flow Cytometry facility.
Northern-blot and RT (reverse transcriptase)–PCR analyses
HL-60, TF-1 and FDC-P1 cells were grown to confluence. Total RNA was isolated from these cells using single-step acid guanidinium thiocyanate method. RNA was separated at 20 μg/lane on a formaldehyde 1% agarose gel. The RNA was transferred on to a nylon membrane (Hybond-N; Amersham International) and hybridized with Mcl-1 or 18 S probes labelled with [α-32P]dCTP (Amersham International) by random prime labelling. The Mcl-1 and 18 S probes were obtained using a PCR on cDNA from TF-1 cells. For Mcl-1, primers specific to the complete coding region were used; forward primer: ATGTTTGGCCTCAAAAGA; reverse primer: CTATCTTATTAGATATGC. The same primers were used for RT–PCR analysis.
Characterization of snMcl-1 by MS
Immunoprecipitations were performed with 2 μg of anti-Mcl-1 incubated with 2.5 mg of protein extracts for 2 h with end-to-end rotation at 4 °C. The immunocomplexes were magnetically labelled by adding 50 μl of Protein G microbeads (μMACS microbeads; Miltenyi Biotec, Auburn, CA, U.S.A.) for 1 h. The magnetic separation of the beads was performed according to the manufacturer's instructions. The protein band was excised from the gel, reduced, alkylated and digested overnight with trypsin as described in [14]. Extracted peptides were characterized by LC-MS/MS (liquid chromatography-tandem MS) on a hybrid linear ion-trap instrument (Q-Trap; Applied Biosystems, Framingham, MA, U.S.A.) [15] coupled with a nanoflow HPLC system (LC Packings, San Francisco, CA, U.S.A.). The resulting peptide fragment spectra were searched against a comprehensive non-redundant protein database (NCBI 20031016) using Mascot [16]. Detection of the following tryptic peptides was used to identify Mcl-1 with a total score of 133: LLFFAPTR (residues 96–103, score 38), QSLEIISR (residues 177–184, score 42), and IVTLISFGAFVAK (residues 264–276, score 53). Correct fragment ion assignment was guaranteed by manual inspection in each case.
In vitro kinase assay
Total cell lysates were incubated with either anti-Mcl-1, -Cdk1 or -cyclin B1 antibody for 2 h with end-to-end rotation at 4 °C. This was followed by the addition of Protein G–agarose beads for 1 h. After extensive washing, beads were resuspended in kinase assay dilution buffer (25 mM β-glycerophosphate, 20 mM Mops, 5 mM EGTA, 2 mM EDTA, 20 mM MgCl2, 250 μM dithiothreitol and 5 μM β-methyl aspartic acid, pH 7.2). Finally, 1 μg/ml histone H1 and 2 μCi of [γ-32P]ATP with unlabelled 50 μM ATP were added to the beads and the reaction allowed to proceed for 20 min at 30 °C.
Generation of stable Mcl-1 transfectants
Mcl-1 blunt end cloned into pLNCX was a gift from Dr A. Karsan (UBC, Vancouver, BC, Canada). Mcl-1:pLNCX was retrovirally introduced into murine FDC-P1 cells as described elsewhere [17]. The retrovirus-infected cells were selected in 800 μg/ml G418 (Invitrogen).
RESULTS
Mcl-1 expression is regulated during cell-cycle progression
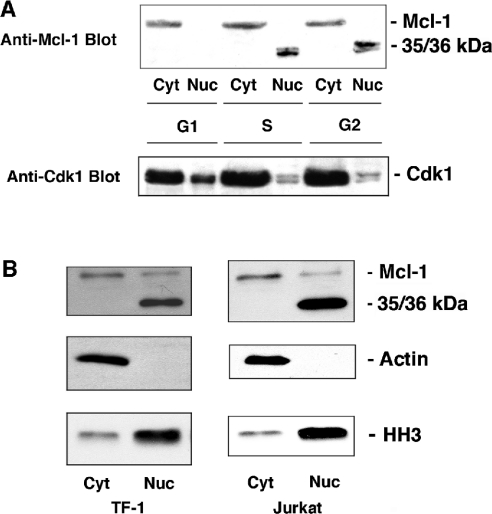
Since it has been shown previously that Mcl-1 expression in HeLa cells slows progression through the cell cycle and that Mcl-1 can be localized to the nucleus [11], we investigated its potential role in the regulation of the cell cycle. To determine whether levels of Mcl-1 expression are altered by progression through the cell cycle, normally growing HL-60 cells were sorted into G1, S and G2 populations. Cytosolic and nuclear preparations were made from each of these cell populations and blotted for the presence of Mcl-1. The full-length Mcl-1 (at 41 kDa) was present only in cytosolic extracts at all cell-cycle stages; however, two closely spaced bands at a lower apparent molecular mass (35–36 kDa) were observed only in the nuclear extracts from S and G2 cells (Figure 1A).
Figure 1. A short form of Mcl-1 is detected in the S and G2 phases of the cell cycle.
(A) Cytosolic and nuclear proteins were extracted from HL-60 cells that were sorted into G1, S and G2 stages of the cell cycle. Expression of Mcl-1 and Cdk1 was determined by immunoblotting. (B) Cytosolic and nuclear fractions from TF-1 and Jurkat cells were blotted for Mcl-1 as well as for actin, or histone H3 to define the purity of fractions. Results are representative of three independent experiments.
The same membrane was probed for Cdk1 expression as a control, and Figure 1(A) (lower panel) shows that the expression of Cdk1 was fairly constant, particularly in the cytosol, and the protein was present in both the cytosol and nucleus during all stages of cell cycle. In S and G2 phases, the nuclear Cdk1 consisted of two distinct bands, which is reflective of the dephosphorylated and phosphorylated states of Cdk1.
As shown in Figure 1(B), the band at 35/36 kDa could be detected with anti-Mcl-1 antibody in Jurkat cells, as well as TF-1 cells, but only in nuclear extracts. The detection of histone H3 was used as a control for nuclear preparations, showing that there was some nuclear contamination of cytosolic fractions. Furthermore, probing for actin showed that the nuclear fractions were free of cytosolic contamination. However, there was some full-length Mcl-1 that was present in nuclear fractions from these cells, which might be attributed to association with either endoplasmic reticulum or nuclear membranes. The most striking result is that the 35/36 kDa bands are exclusively localized in the nuclear fractions. In the present study, we further characterize this short nuclear form of Mcl-1 that we have called snMcl-1.
Short nuclear Mcl-1 in not the alternatively spliced variant
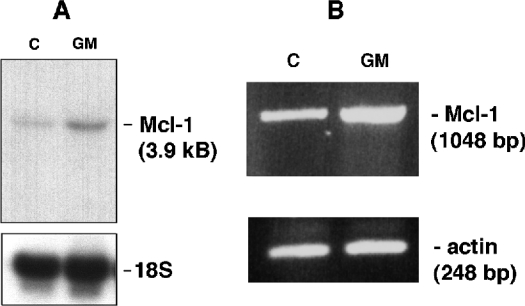
Since we consistently observed the band at 35/36 kDa corresponding to snMcl-1 in many experiments, therefore we performed investigations into the nature of this fragment. A short alternatively spliced variant of Mcl-1 (Mcl-1S) has already been reported in the literature [18,19]; we therefore investigated the possibility of the snMcl-1 protein being the spliced variant. Northern-blot analysis was used to examine mRNA from HL-60, TF-1 and murine FDC-P1 cells. Results in Figure 2 show that the only detectable band in TF-1 cells was the one corresponding to the full-length Mcl-1 message. As we have shown previously, treatment of these cells with GM-CSF resulted in an increase in Mcl-1 message levels [20]. Similar analyses were repeated multiple times with TF-1 cells, as well as HL-60 and FDC-P1 cells (results not shown), and there was no evidence of a smaller mRNA species. Figure 2(B) shows that by RT–PCR analysis of cDNA from TF-1 cells, there was also an increase in Mcl-1 message in response to GM-CSF treatment, but no evidence of a shorter product. The reported splice variant would have been amplified with the primers used here. This same result was also obtained from an analysis of HL-60 cells under conditions that normally showed the presence of snMcl-1 (results not shown).
Figure 2. (A) Northern blotting and (B) RT–PCR results for a single mRNA corresponding to Mcl-1.
Polyadenylated RNA extracted from TF-1 cells that were unstimulated (C) or treated with GM-CSF (GM) were probed for the presence of Mcl-1 message using a 32P-labelled probe encompassing the complete coding region of human Mcl-1. The only detectable band corresponded to the reported size of Mcl-1 message (3.9 kb). (B) RT-PCR was done using samples from control (C) and GM-CSF-treated (GM) TF-1 cells. Only a band corresponding to the complete coding region of Mcl-1 was detected.
Mcl-1 associates with the cell-cycle regulatory protein Cdk1
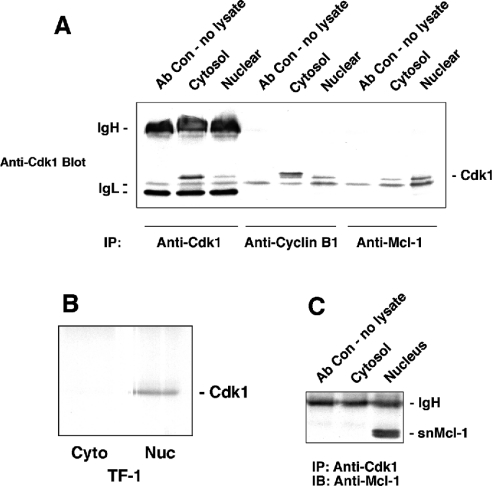
In an attempt to show association between Mcl-1 and proteins involved in cell-cycle regulation, we attempted to determine whether any of the Cdks might be associated with Mcl-1. Cytosolic and nuclear proteins from normally proliferating HL-60 cells were immunoprecipitated with anti-Mcl-1 and probed for cell-cycle regulatory proteins Cdk1 and Cdk2. Immunoblotting for Cdk1 showed the presence of specific 34 kDa bands in both cytosolic and nuclear extracts (Figure 3A). However, most of the Mcl-1-associated Cdk1 was present in the nuclear fraction. Figure 3(A) also shows the results of immunoprecipitating Cdk1 with antibodies to cyclin B1 or Cdk1. As expected, association of cyclin B1 and Cdk1 was observed in both the cytosol and nucleus [21]. There is clearly a higher level of Cdk1 present in cytosolic, compared with nuclear extracts, as we have shown above, and the amount of Cdk1 co-immunoprecipitated with anti-cyclin B1 antibodies is also greater in the cytosol. Co-immunoprecipitation of Mcl-1 and Cdk2 was never detected under similar conditions (results not shown).
Figure 3. Co-immunoprecipitation results for Cdk1, Mcl-1 and snMcl-1.
(A) Cdk1 co-immunoprecipitates with Mcl-1. Cytosolic and nuclear proteins were extracted from asynchronous HL-60 cells and immunoprecipitated with antibody to Cdk-1, cyclin B1 or Mcl-1. Cdk1 (34 kDa) was detected by immunoblotting. IgH and IgL refer to bands detected due to immunoglobulin heavy and light chains respectively. (B) Co-immunoprecipitation of Mcl-1 and Cdk1 can be detected in TF-1 cells. Cytosolic (Cyto) and nuclear (Nuc) extracts from TF-1 cells were immunoprecipitated with anti-Mcl-1 and probed for the presence of Cdk1. The band below Cdk1 in (A) that was attributed to IgL was not seen in (B) due to the use of different batches of antibody. (C) snMcl-1 co-immunoprecipitates with anti-Cdk1. Cytosolic and nuclear proteins were extracted from asynchronous HL-60 cells and immunoprecipitated with anti-Cdk1. Immunoblotting with anti-Mcl-1 revealed a doublet at 35/36 kDa in the nuclear extract only that corresponds to snMcl-1. Each panel is representative of at least four independent experiments.
The co-immunoprecipitation of Mcl-1 with Cdk1 was also observed in nuclear extracts from TF-1 (Figure 3B) and Jurkat (results not shown) cell lines. Thus we have observed this association, based on the co-immunoprecipitation, in at least three different haemopoietic cell lines, showing that it is not a phenomenon observed in a single cell type, nor is it the result of overexpression of either of these proteins.
To validate further the association of Mcl-1 and Cdk1, we performed immunoprecipitation with anti-Cdk1 antibody and probed for associated Mcl-1. Immunoblotting with anti Mcl-1 antibody exhibited a doublet of two closely spaced bands at 35–36 kDa only in the nuclear extracts (Figure 3C), which corresponds to the snMcl-1 polypeptide. With anti-Cdk1 immunoprecipitates, we have always seen this polypeptide migrating as a doublet on gels, and phosphatase treatment showed that this band-shift resulted from phosphorylation of the protein (results not shown). No fulllength Mcl-1 protein was detectable in anti-Cdk1 immunoprecipitates from either cytosolic or nuclear extracts.
Cdk1 associates with Mcl-1 in the absence of conventional cyclin partners
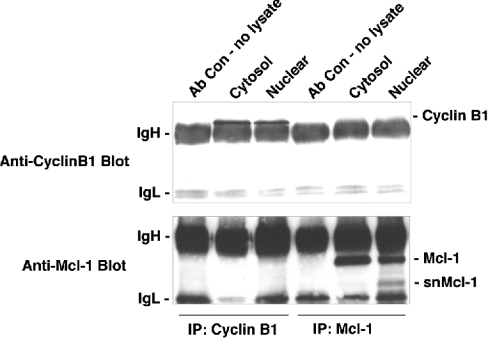
We tested for the presence of cyclin B1 in the anti-Mcl-1 immunoprecipitates and found that it was undetectable (Figure 4). Similarly, in anti-cyclin B1 immunoprecipitates, which have associated Cdk1 (as shown in Figure 3A), there was no evidence of Mcl-1. Similarly, we were not able to detect the presence of cyclin A1 or A2 in anti-Mcl-1 immunoprecipitates (results not shown). These results suggested that the association between Mcl-1 and Cdk1 occurs in the absence of the conventional binding partners for Cdk1. It should be noted that in the anti-Mcl-1 immunoprecipitates from nuclear extracts (Figure 4, lower panel), the presence of full-length Mcl-1 was detected but we cannot be sure how much of this is due to cytosolic contamination. However, we have consistently observed the 35–36 kDa snMcl-1 polypeptide only in nuclear extracts.
Figure 4. Association of cyclin B1 and Mcl-1 with Cdk1 are independent of each other.
Cytoplasmic and nuclear extracts from HL-60 cells were immunoprecipitated with antibodies to cyclin B1 or Mcl-1. The same blot was probed for the presence of cyclin B1 (upper panel) or Mcl-1 (lower panel). ‘Ab Con – no lysate’ represents a sample of beads incubated with the respective antibodies, in the absence of cell lysate. Each of the co-immunoprecipitations was repeated in at least six independent experiments with similar results.
Association of snMcl-1 and Cdk1 depends on the stage of the cell cycle
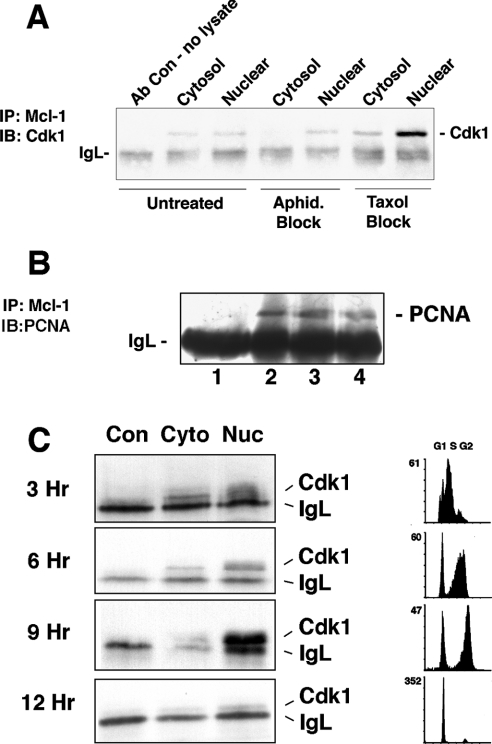
Our next objective was to characterize further the formation of the Cdk1–Mcl-1 complex, and its dependence on the stage of the cell cycle. HL-60 cells were synchronized at various stages of the cell cycle by blocking at G1/S using Aphidicolin and near G2/M, or more precisely at prometaphase, using Taxol. As shown in Figure 5(A), immunoprecipitation with anti-Mcl-1, followed by immunoblotting with antibody against Cdk1, showed that blocking the cell cycle at G1/S with Aphidicolin treatment had no significant effect on the formation of the Cdk1–Mcl-1 complex. On the other hand, treatment of cells with Taxol always resulted in a significant increase in the presence of the complex in the nuclear extracts. Treatment with nocodazole, which also blocks at the same point as Taxol, resulted in a similar increase in the Cdk1/Mcl-1 association as that observed with Taxol (results not shown). Hence maximal formation of the Cdk1–Mcl-1 complex appears to be at the G2/M or early mitosis stage of the cell cycle.
Figure 5. Co-immunoprecipitation of Mcl-1 and Cdk1 is maximal near G2/M.
(A) Cytosolic and nuclear proteins were extracted from HL-60 cells that were untreated, or treated with aphidicolin (Aphid.) to block cells at G1/S, or Taxol to block cells at G2/M. Presence of Cdk1 associated with Mcl-1 was determined. (B) HL-60 cells were either untreated (lane 2) or treated with aphidicolin (lane 3) or Taxol (lane 4). Lane 1 represents a mock immunoprecipitation with no cell lysate to show immunoglobulin light chain (IgL) and the other samples were immunoprecipitated with anti-Mcl-1. Immunoblotting was with antibody directed to PCNA. (C) HL-60 cells blocked with aphidicolin were washed and released for indicated times, at which point cytosolic (Cyto) and nuclear (Nuc) extracts were immunoprecipitated with anti-Mcl-1. Immunoblotting was done to detect Cdk1. Con indicates samples of a mock immunoprecipitation with the same antibody but no cell proteins. Flow cytometry was performed after PI staining to determine the cell-cycle stage at each of the time points. Results are representative of at least three independent experiments.
Previous studies by Fujise et al. [11] showed that Mcl-1 associated with PCNA. We were able to confirm that PCNA was co-immunoprecipitated with Mcl-1 in our experiments (Figure 5B). However, unlike the Mcl-1/Cdk1 association, there was no difference in the amount of PCNA detected when Mcl-1 was immunoprecipitated from cells that were blocked using Aphidicolin or Taxol. We have not yet determined how the interaction of Mcl-1 with PCNA may affect its association with Cdk1.
In other experiments, cells were synchronized at G1/S by treatment with Aphidicolin and then released for 3, 6, 9 and up to 12 h. Association between Mcl-1 and Cdk1 was maximal after 9 h following the release from G1/S block (Figure 5C), coinciding with the point when most cells were passing through G2 and M phases, as indicated in the representative flow cytometry tracings. Together, these results suggest that the association of snMcl-1/Cdk1 may be greatest at G2/M.
The snMcl-1 polypeptide is probably generated as a result of C-terminal proteolysis
Since Cdk1 specifically associates with what appears to be a shortened form of Mcl-1, we attempted to characterize further the nature of this fragment by MS analysis of the polypeptide. HL-60 cells were blocked at G1/S with aphidicolin and then released to allow them to approach G2/M, resulting in appearance of the greatest amount of snMcl-1. The anti-Mcl-1 immunoprecipitates from cells treated in this manner were subjected to SDS/PAGE and the band corresponding to snMcl-1 was cut from the gel, trypsindigested, and peptides were analysed by MS. Three peptides were detected that matched human Mcl-1. These were at residues 96–103, 177–184 and 264–276, which are underlined in the hMcl-1 sequence in Figure 6 (also see the Experimental section). Of greatest significance was the presence of the peptide corresponding to residues 264–276. The reported alternatively spliced version of Mcl-1 [18,19] has an altered C-terminus, underlined in the hMcl-1s sequence in Figure 6, that would not include this peptide.
Figure 6. Sequence of human Mcl-1 (hMcl-1) and the Mcl-1 splice variant (hMcl-1s).
In hMcl-1, peptides underlined were identified by MS. The last 19 out of 20 amino acids are hydrophobic and make up the membrane insertion domain. In hMcl-1s, alternative splicing results in an alternative C-terminus, indicated by the underlined region. Note that the IVTLISFGAFVAK peptide identified in the band corresponding to snMcl-1 (see text) does not appear in the splice variant.
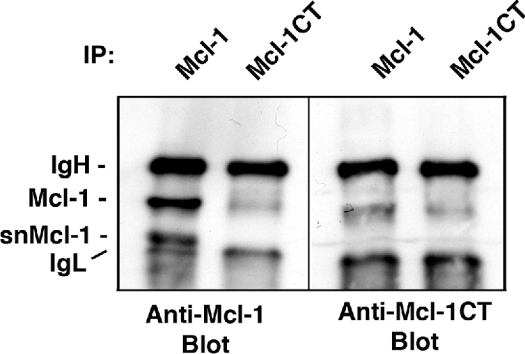
Mcl-1 has a C-terminal membrane insertion domain that probably targets the protein to mitochondrial or other membranes in the cell, similar to Bcl-2 and Bcl-XL [3,22,23]. This would suggest that the generation of snMcl-1 could result from the loss of the C-terminus. Thus additional characterization was performed using an antibody raised against a C-terminal epitope of Mcl-1. According to the manufacturer's instructions, the antibody was raised against a 20 amino acid peptide that did not include the last 20 hydrophobic amino acids of Mcl-1 (the precise location was not disclosed). Results in Figure 7 show that the anti-C-terminal antibody immunoprecipitated only full-length Mcl-1 and not snMcl-1, and it only detected full-length Mcl-1 on an immunoblot of anti-Mcl-1 immunoprecipitates. Taking all of these results together, we have provided solid evidence that snMcl-1 may result from a proteolytic cleavage near the C-terminus of Mcl-1, yielding a shortened form that has lost its C-terminal transmembrane domain.
Figure 7. An antibody detecting the C-terminus of Mcl-1 does not detect snMcl-1.
HL-60 cells were blocked at G1/S with Aphidicolin, washed and released for 9 h, then cell extracts were immunoprecipitated (IP) and immunoblotted with antibody directed against the N-terminal half of Mcl-1 (Mcl-1) or an antibody against the C-terminus (Mcl-1CT), as indicated. Similar results were obtained in three independent experiments.
Cdk1 associated with snMcl-1 has lower kinase activity
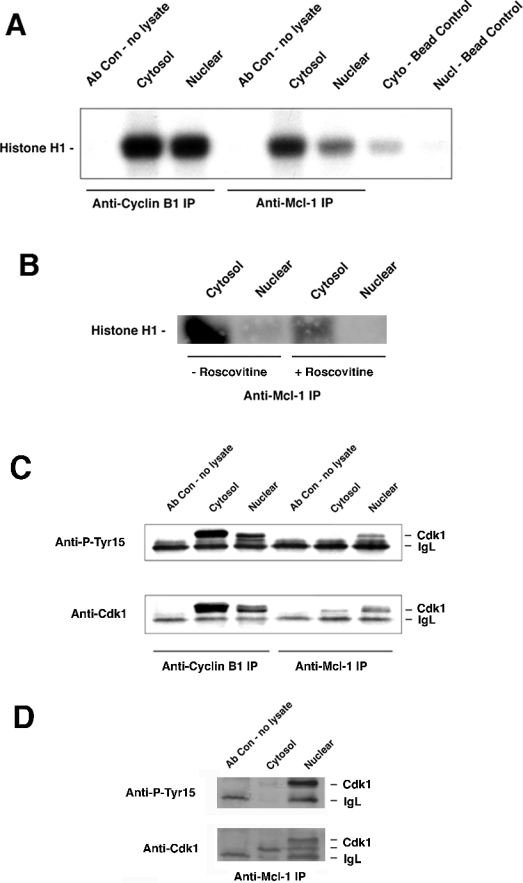
To determine the possible effect of Mcl-1 on Cdk1 kinase activity, we measured the histone H1 kinase activity associated with Mcl-1 using an in vitro kinase assay. As seen in Figure 8(A), cyclin B1-associated Cdk1, from both cytosolic as well as nuclear extracts, phosphorylated histone H1. Mcl-1-associated Cdk1 from cytosolic extracts phosphorylated histone H1 to a lesser extent, corresponding to the lesser amount of Cdk1 in those immunoprecipitates (Figure 8C). However, there was even lower activity in the Mcl-1-associated Cdk1 from nuclear extracts, despite a relatively greater amount of Cdk1 in those immunoprecipitates (Figure 8C). As noted above, we cannot be certain about the nature of the Mcl-1 and Cdk1 association in cytosolic extracts, but it may represent association with full-length Mcl-1. The Cdk1 assay of anti-Mcl-1 immunoprecipitates was repeated in the presence or absence of 25 μM roscovitine, a potent Cdk inhibitor, which decreased the activity significantly (Figure 8B). Higher concentrations of roscovitine could completely inhibit the kinase activity (results not shown).
Figure 8. Assay of Cdk1 kinase activity associated with Mcl-1.
(A) Histone H1 in vitro kinase assay was used to determine Cdk1 kinase activity in anti-cyclin B1 or anti-Mcl-1 immunoprecipitates from nuclear or cytosol extracts of HL-60 cells 9 h after release from a G1/S block. (B) Assay of anti-Mcl-1 immunoprecipitates from cytosol or nuclear extracts were performed in the absence or presence of 25 μM roscovitine, which was added to the in vitro kinase assay. (C) Cdk1 and phospho-Tyr-15 Cdk1 of samples corresponding to (A) were detected by immunoblotting. (D) Twice as much total protein as was used for (C) was immunoprecipitated with anti-Mcl-1 and blotted for Cdk1 or phospho-Tyr-15 Cdk1.
The amount of Cdk1 activity, as in Figure 8(A), was normalized based on the amount of Cdk1 protein present in the immunoprecipitates, as in Figure 8(C). When results from three different experiments were averaged, comparison of activity in anti-cyclin B1 immunoprecipitates from cytosolic or nuclear extracts showed no significant difference. However, the anti-Mcl-1 immunoprecipitates showed 9 (±6)-fold greater relative activity in the cytosolic samples.
As mentioned earlier, the ability of the Cdk1–cyclin B1 complex to drive the cell into mitosis is regulated by inhibitory phosphorylation of Cdk1 on the Tyr-15 residue. Hence we determined whether or not Mcl-1-associated Cdk1 was subject to a similar type of regulation. Our results showed that while cyclin B1-associated Cdk1 was phosphorylated in both the cytosolic as well as nuclear compartments, Tyr-15 phosphorylation on Cdk1 that was present in anti-Mcl-1 immunoprecipitates was only detectable from the nuclear extracts (Figure 8C). The higher level of Tyr-15 phosphorylation in the Mcl-1 immunoprecipitates from nuclear extracts is consistent with the decreased kinase activity observed in the in vitro kinase assay. The level of phospho-Tyr-15 in the anticyclin B1 immunoprecipitates is consistent with other studies, which have shown that only a fraction of the total cellular Cdk1 is dephosphorylated at Tyr-15 and that the fraction provides sufficient Cdk1 activity. To show that the much lower detection of phosphorylated Tyr-15 was not due to the relatively lower amount of Cdk1 in the cytosolic immunoprecipitates, more total protein was used and samples were allowed to separate further by SDS/PAGE. The results in Figure 8(D) show that although total Cdk1 was easily detected in cytosolic extracts, hardly any protein was phosphorylated at Tyr-15, based on anti-phospho-Tyr-15 detection, as well as the slower migration of Cdk1 on the gel. The slower migrating form of Cdk1 was observed in the nuclear fractions in both anti-Ptyr-15 and anti-Cdk1 blots. Results in Figure 8 were obtained using cells that were synchronized at G1/S and released for 9 h, corresponding to the time of maximal Cdk1 detected in anti-Mcl-1 immunoprecipitates. However, at all times tested after the release from G1/S block, there was no detectable phospho-Tyr-15 in cytosolic Mcl-1-associated Cdk1 (results not shown).
Overexpression of Mcl-1 suppresses cell proliferation
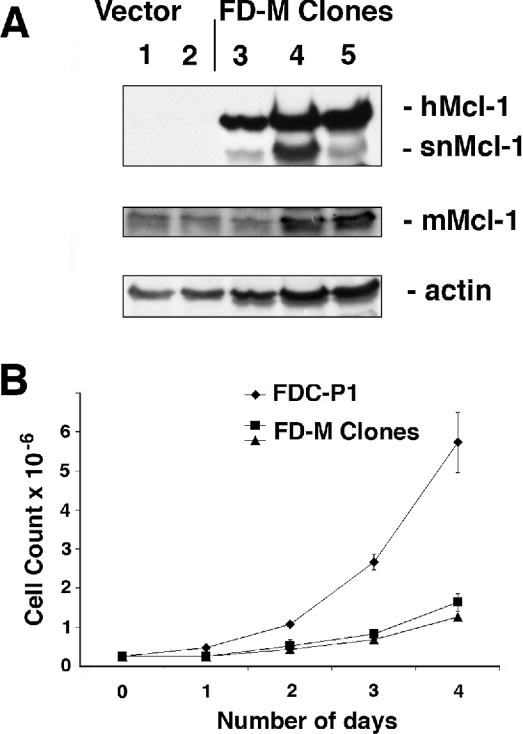
Human Mcl-1 was expressed in a murine cell line, FDC-P1, which is a haemopoietic progenitor cell line dependent on IL-3 or GM-CSF for growth. Similar to the report of Fujise et al. [11], we found that the stable expression of Mcl-1 by retroviral infection in this haemopoietic cell line caused an inhibitory effect on the proliferation of the cells. We selected several clones that expressed Mcl-1, designated FD-M clones (Figure 9A). When the FD-M cells were tested for the presence of human Mcl-1, the antibody detected two bands, one that corresponded to the size of full-length Mcl-1 at 41 kDa and the other that was approx. 5 kDa smaller, corresponding to the snMcl-1 we have characterized above (Figure 9A). Detection of murine Mcl-1 in the vector alone and FD-M clones (the antibodies are species-specific) showed that endogenous Mcl-1 expression was not significantly altered in the FD-M clones. When cell growth was assessed by determining cell numbers over several days, we found that there was an inhibitory effect of Mcl-1 expression on growth of the cells (Figure 9B). We tested whether the longer doubling time of the FD-M clones could be attributed to any change in apoptosis. Cells were starved of cytokine by withdrawal of IL-3 from the growth medium and subsequently analysed for subdiploid DNA content by PI staining. In several independent experiments, we found that after 24 h of cytokine withdrawal, 100% of parental FDC-P1 cells had undergone apoptosis, whereas only 50% of the Mcl-1 overexpressing cells had undergone apoptosis. This is consistent with the reported capability of Mcl-1 to prolong cell survival.
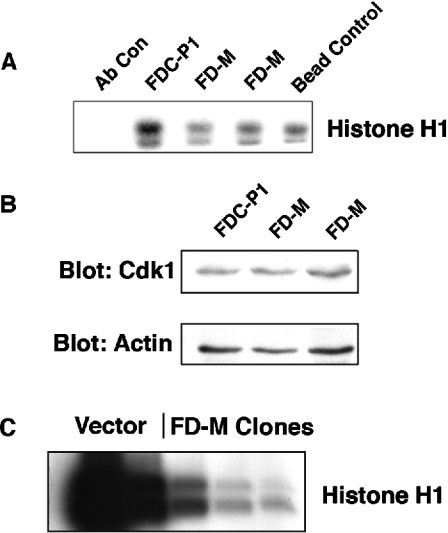
Figure 9. Overexpression of human Mcl-1 in murine FDC-P1 cells.
(A) Levels of human Mcl-1 protein were analysed in whole cell lysates of two clones of FDC-P1 cells with vector alone and three clones expressing Mcl-1 (FD-M). The middle panel was re-probed using anti-murine Mcl-1 (mMcl-1), which migrates at 39 kDa, and the lower panel shows the actin loading control. (B) Overexpression of Mcl-1 slows cell growth. FDC-P1 or two of the FD-M clones were seeded at 50000 cells/ml and the cell counts were measured every 24 h.
Mcl-1 overexpression results in lower Cdk1 activity and cyclin B1/Cdk1 association
We performed an in vitro kinase assay to determine the activity of Cdk1 in FDC-P1 containing the retroviral vector alone, compared with two of the Mcl-1-expressing clones. Figure 10(A) shows that compared with the vector alone, the two FD-M clones had significantly decreased Cdk1 activity. Immunoblotting of total cell lysates with anti-Cdk1 showed a similar amount of Cdk 1 in the Mcl-1-expressing clones as in parental cells (Figure 10B), based on normalization with an anti-actin antibody. Thus the decreased level of Cdk1 activity caused by expression of Mcl-1 was not due to any significant change in Cdk1 protein expression.
Figure 10. Assay of Cdk1 kinase activity in FD-M clones.
(A) Histone H1 in vitro kinase assay was used to determine Cdk1 kinase activity in anti-Cdk1 immunoprecipitates from parental cells and Mcl-1 overexpressing clones. Results are representative of four separate experiments. (B) Whole cell extracts from FDC-P1 or FD-M clones were prepared and blots were probed with antibodies to either Cdk1 or actin as a loading control. Results are representative of at least three independent experiments. (C) Histone H1 in vitro kinase assay was used to determine Cdk1 kinase activity in anti-cyclin B1 immunoprecipitates from vector alone and Mcl-1 overexpressing clones. Results are representative of three experiments.
Finally, immunoprecipitation of cyclin B1, followed by a Cdk1 kinase assay, demonstrated that the FD-M clones also had lower Cdk1 activity in this assay (Figure 10C). The latter result supports the possibility that increased levels of snMcl-1 may cause a reduction in Cdk1 activity by altering the ability of the kinase to bind at least one of its partner cyclins.
DISCUSSION
There is growing evidence that molecules that regulate apoptosis may also be involved in cell-cycle regulation and vice versa. For example, it is known that Bcl-2 and Bcl-XL cause a block in cell-cycle re-entry from G0 in fibroblasts, and their effect on cell cycle appears to be indistinguishable from the pro-survival effect [24], although an earlier study had dissociated the cell survival and cell-cycle effects of Bcl-2 [25]. The study by Janumyan et al. [24] showed that the cell-cycle inhibitory effect of Bcl-XL was overcome by expression of Bad, suggesting that the cell-cycle block was integral to the survival-promoting effect of Bcl-XL [24]. We and others have also reported an effect of Bad on the promotion of cell-cycle progression [17,26,27].
The ability of Mcl-1 to inhibit cell-cycle progression has been reported previously [11], and was attributed to an effect on S-phase, based on the decreased ability of Mcl-1-expressing cells to incorporate bromodeoxyuridine. However, based on the studies we report in this paper, Mcl-1 appears to be having a more general cell-cycle inhibitory effect. In the FDC-P1 cells expressing Mcl-1, there was no apparent accumulation of cells at any particular stage of the cell cycle, despite the significant inhibitory effect on cell proliferation. The effect of Mcl-1 on the cell cycle is probably the result of a shortened form that we have termed snMcl-1. We have presented several lines of evidence that this represents a fragment of Mcl-1 that probably results from proteolysis, rather than a splice variant. There was no evidence of a shorter mRNA species corresponding to a splice variant, nor an RT–PCR fragment of smaller size, and the snMcl-1 polypeptide contained a peptide corresponding to human Mcl-1 that could not be found in a reported Mcl-1 splice variant [18,19]. Finally, in the murine FDC-P1 cells expressing human Mcl-1, both full-length human Mcl-1 and snMcl-1 were observed. Furthermore, immunoblotting with an antibody raised against the C-terminus of Mcl-1 did not detect snMcl-1, supporting our prediction that the C-terminal membrane-anchoring domain of Mcl-1 is not present in snMcl-1. In contrast with full-length Mcl-1, which is known to be membrane anchored, most probably at the mitochondria, the lack of a transmembrane domain would allow the snMcl-1 to migrate into the nucleus.
A recent study has reported the proteolytic cleavage of Mcl-1 by caspase 3 [28]. We believe that snMcl-1 is not a product of caspase activity, since the size of the fragments reported in these studies was smaller than snMcl-1. Although we cannot completely rule out a role for caspases at this point, we have observed snMcl-1 in proliferating cells, and have seen no correlation between its presence and apoptosis of cells.
A question that remains unanswered about snMcl-1 is the reason for its nuclear localization and the nature of its potential regulatory effect on Cdk1 activity. The activity of cyclin B1/Cdk1 has been shown to be influenced by their cellular localization [21,29,30]. Whether Mcl-1/Cdk1 complexes are affected similarly is an area for future investigation. Interestingly, we observed some activity of Mcl-1-associated Cdk1 from cytosolic extracts, but very little from nuclear extracts. Since we were not able to detect cyclin A1 or B1 in the same immunoprecipitates, the question remains as to whether Mcl-1 and snMcl-1 have unique functions in regulating Cdk1 activity.
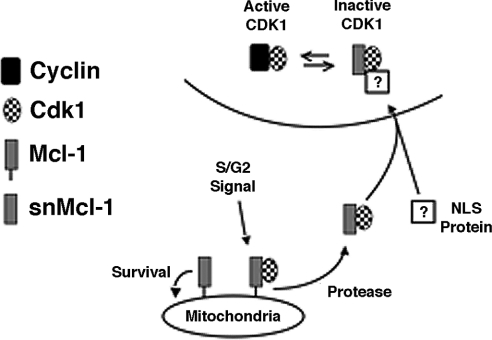
The sequence of Mcl-1 does not suggest the presence of an NLS (nuclear localization signal). It is therefore possible that interaction with some other protein provides the necessary NLS function for a Mcl-1/Cdk1-containing complex (Figure 11). As suggested in our model, it is also possible that snMcl-1 somehow decreases cyclin binding to Cdk1, consistent with the results of the cyclin B1/Cdk1 kinase activity assay. Of course, we have not yet proven that the association of Mcl-1 and Cdk1 is direct, and thus one or more other proteins may be mediating the association. Another question regarding the Mcl-1/Cdk1 association in the cytoplasm is whether this may first occur with full-length Mcl-1. Then, cleavage to form snMcl-1 may result in their rapid translocation into the nucleus, explaining why we have seen snMcl-1 exclusively in the nuclear fractions. Further work will be required to determine the exact mechanisms involved.
Figure 11. Model depicting possible dual functions of Mcl-1.
In this proposed model, full-length Mcl-1 at the mitochondria fulfils a Bcl-2-like function, but a signal arising in S/G2 leads to its proteolysis, releasing snMcl-1, which can bind to Cdk1. We propose association with a protein containing an NLS that mediates rapid translocation to the nucleus. snMcl-1 may act to sequester Cdk1 from its active complex in association with its partner cyclins.
The association of Mcl-1 with Cdk1 has been observed to be independent of the kinase's association with either of its partner cyclins. However, it is noteworthy that the Cdk1 associated with snMcl-l is phosphorylated at Tyr-15, the inhibitory phosphorylation site. Normally, this phosphorylation occurs only once the cyclin is associated, as the cyclin–Cdk association is supposed to allow access of either MytI or Wee1 to the Tyr-15 site. Perhaps, the conformational changes induced in Cdk1 by the presence of snMcl-1 are distinct from those induced by cyclin B1, as Cdk1 associated with Mcl-1 undergoes Tyr-15 phosphorylation primarily in the nucleus (Figure 8B). The fact that snMcl-1-associated Cdk1 is phosphorylated in the nuclear extracts may be consistent with the kinase being Wee1, which has been shown to localize to the nucleus [31,32].
The fact that Mcl-1/Cdk1 association is observed in cells with endogenous levels of these proteins suggests that generation of snMcl-1 may represent an additional level of regulation in normal cell-cycle progression. While the exact mechanisms regulating formation of snMcl-1 are currently not clear, our results suggest a cell cycle stage-dependent proteolysis of full-length Mcl-1, probably during S and G2 phases, since snMcl-1 is not observed in the G1 phase of the cell cycle. Furthermore, snMcl-1 must be further degraded, probably by proteasome-mediated events, as cells exit mitosis. Since the half-life of Mcl-1 is known to be very short [20], there must be dynamic changes in the levels of both Mcl-1 and snMcl-1 throughout the cell cycle.
Perhaps, the most important aspect of our finding is that it provides a possible explanation for the critical importance of Mcl-1 in development. We have demonstrated here that Mcl-1 expression can enhance cell survival, and we would suggest that this may be performed by full-length Mcl-1 functioning like Bcl-2 and Bcl-XL. Perhaps the more important function of Mcl-1 in regulating cell-cycle events appears to be mediated by the shortened snMcl-1, which is found to be associated with Cdk1 in the nucleus. However, in light of our results, one must consider whether it is this function of Mcl-1 that also contributes to its anti-apoptotic effect, or whether the dual functions of Mcl-1 occur by completely separate events. It is certain that the intriguing characteristics of this Bcl-2 family protein will lead to many new avenues of investigation.
Acknowledgments
We thank S. Lin for technical assistance with the MS analysis, A. Johnson for help with cell sorting, J. Chen for help with preparation of Figures and Dr M. Germain for helpful discussions. This work was supported by the Canadian Cancer Society. V.D. is the recipient of a CIHR/BCLA Senior Scientist and a Michael Smith Foundation for Health Research Senior Scholar award.
References
- 1.Chao D. T., Korsmeyer S. J. BCL-2 family: regulators of cell death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 2.Adams J. M., Cory S. The Bcl-2 protein family – arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 3.Reed J. C., Jurgensmeier J. M., Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim. Biophys. Acta. 1998;1366:127–137. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 4.Germain M., Mathai J. P., Shore G. C. BH-3-only BIK functions at the endoplasmic reticulum to stimulate cytochrome c release from mitochondria. J. Biol. Chem. 2002;277:18053–18060. doi: 10.1074/jbc.M201235200. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari D., Pinton P., Szabadkai G., Chami M., Campanella M., Pozzan T., Rizzuto R. Endoplasmic reticulum, Bcl-2 and Ca2+ handling in apoptosis. Cell Calcium. 2002;32:413–420. doi: 10.1016/s0143416002002014. [DOI] [PubMed] [Google Scholar]
- 6.Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang T., Buchan H. L., Townsend K. J., Craig R. W. Mcl-1, a member of the Bcl-2 family, is induced rapidly in response to signals for cell differentiation or death, but not to signals for cell proliferation. J. Cell. Physiol. 1996;166:523–536. doi: 10.1002/(SICI)1097-4652(199603)166:3<523::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Zhou P., Qian L. P., Bieszczad C. K., Noelle R., Binder M., Levy N. B., Craig R. W. Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood. 1998;92:3226–3239. [PubMed] [Google Scholar]
- 9.Zhou P., Qian L. P., Kozopas K. M., Craig R. W. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997;89:630–643. [PubMed] [Google Scholar]
- 10.Rinkenberger J. L., Horning S., Klocke B., Roth K., Korsmeyer S. J. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Fujise K., Zhang D., Liu J., Yeh E. T. Regulation of apoptosis and cell cycle progression by MCL1. Differential role of proliferating cell nuclear antigen. J. Biol. Chem. 2000;275:39458–39465. doi: 10.1074/jbc.M006626200. [DOI] [PubMed] [Google Scholar]
- 12.Morgan D. O. Principles of CDK regulation. Nature (London) 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 13.Obaya A. J., Sedivy J. M. Regulation of cyclin-Cdk activity in mammalian cells. Cell. Mol. Life Sci. 2002;59:126–142. doi: 10.1007/s00018-002-8410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc J. C., Hager J. W., Ilisiu A. M., Hunter C., Zhong F., Chu I. Unique scanning capabilities of a new hybrid linear ion trap mass spectrometer (Q TRAP) used for high sensitivity proteomics applications. Proteomics. 2003;3:859–869. doi: 10.1002/pmic.200300415. [DOI] [PubMed] [Google Scholar]
- 16.Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Dramsi S., Scheid M. P., Maiti A., Hojabrpour P., Chen X., Schubert K., Goodlett D. R., Aebersold R., Duronio V. Identification of a novel phosphorylation site, Ser-170, as a regulator of bad pro-apoptotic activity. J. Biol. Chem. 2002;277:6399–6405. doi: 10.1074/jbc.M109990200. [DOI] [PubMed] [Google Scholar]
- 18.Bae J., Leo C. P., Hsu S. Y., Hsueh A. J. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J. Biol. Chem. 2000;275:25255–25261. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- 19.Bingle C. D., Craig R. W., Swales B. M., Singleton V., Zhou P., Whyte M. K. Exon skipping in Mcl-1 results in a bcl-2 homology domain 3 only gene product that promotes cell death. J. Biol. Chem. 2000;275:22136–22146. doi: 10.1074/jbc.M909572199. [DOI] [PubMed] [Google Scholar]
- 20.Schubert K. M., Duronio V. Distinct roles for extracellular-signal-regulated protein kinase (ERK) mitogen-activated protein kinases and phosphatidylinositol 3-kinase in the regulation of Mcl-1 synthesis. Biochem. J. 2001;356:473–480. doi: 10.1042/0264-6021:3560473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J., Song H., Walsh S., Bardes E. S., Kornbluth S. Combinatorial control of cyclin B1 nuclear trafficking through phosphorylation at multiple sites. J. Biol. Chem. 2001;276:3604–3609. doi: 10.1074/jbc.M008151200. [DOI] [PubMed] [Google Scholar]
- 22.Yang T., Kozopas K. M., Craig R. W. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J. Cell Biol. 1995;128:1173–1184. doi: 10.1083/jcb.128.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudner J., Jendrossek V., Belka C. New insights in the role of Bcl-2 and the endoplasmic reticulum. Apoptosis. 2002;7:441–447. doi: 10.1023/a:1020087108926. [DOI] [PubMed] [Google Scholar]
- 24.Janumyan Y. M., Sansam C. G., Chattopadhyay A., Cheng N., Soucie E. L., Penn L. Z., Andrews D., Knudson C. M., Yang E. Bcl-x(L)/Bcl-2 coordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J. 2003;22:5459–5470. doi: 10.1093/emboj/cdg533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang D. C., O'Reilly L. A., Strasser A., Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maslyar D. J., Aoki M., Vogt P. K. The growth-promoting activity of the Bad protein in chicken embryo fibroblasts requires binding to protein 14-3-3. Oncogene. 2001;20:5087–5092. doi: 10.1038/sj.onc.1204662. [DOI] [PubMed] [Google Scholar]
- 27.Chattopadhyay A., Chiang C. W., Yang E. BAD/BCL-(xL) heterodimerization leads to bypass of G0/G1 arrest. Oncogene. 2001;20:4507–4518. doi: 10.1038/sj.onc.1204584. [DOI] [PubMed] [Google Scholar]
- 28.Snowden R. T., Sun X. M., Dyer M. J., Cohen G. M. Bisindolylmaleimide IX is a potent inducer of apoptosis in chronic lymphocytic leukaemic cells and activates cleavage of Mcl-1. Leukemia. 2003;17:1981–1989. doi: 10.1038/sj.leu.2403088. [DOI] [PubMed] [Google Scholar]
- 29.Hagting A., Karlsson C., Clute P., Jackman M., Pines J. MPF localization is controlled by nuclear export. EMBO J. 1998;17:4127–4138. doi: 10.1093/emboj/17.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore J. D., Yang J., Truant R., Kornbluth S. Nuclear import of Cdk/cyclin complexes: identification of distinct mechanisms for import of Cdk2/cyclin E and Cdc2/cyclin B1. J. Cell Biol. 1999;144:213–224. doi: 10.1083/jcb.144.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldin V., Ducommun B. Subcellular localisation of human wee1 kinase is regulated during the cell cycle. J. Cell Sci. 1995;108:2425–2432. doi: 10.1242/jcs.108.6.2425. [DOI] [PubMed] [Google Scholar]
- 32.McGowan C. H., Russell P. Cell cycle regulation of human WEE1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]