Abstract
Palmitate rapidly and reversibly inhibits the uncoupled NADH oxidase activity catalysed by activated complex I in inside-out bovine heart submitochondrial particles (IC50 extrapolated to zero enzyme concentration is equal to 9 μM at 25 °C, pH 8.0). The NADH:hexa-ammineruthenium reductase activity of complex I is insensitive to palmitate. Partial (∼50%) inhibition of the NADH:external quinone reductase activity is seen at saturating palmitate concentration and the residual activity is fully sensitive to piericidin. The uncoupled succinate oxidase activity is considerably less sensitive to palmitate. Only a slight stimulation of tightly coupled respiration with NADH as the substrate is seen at optimal palmitate concentrations, whereas complete relief of the respiratory control is observed with succinate as the substrate. Palmitate prevents the turnover-induced activation of the de-activated complex I (IC50 extrapolated to zero enzyme concentration is equal to 3 μM at 25 °C, pH 8.0). The mode of action of palmitate on the NADH oxidase is qualitatively temperature-dependent. Rapid and reversible inhibition of the complex I catalytic activity and its de-active to active state transition are seen at 25 °C, whereas the time-dependent irreversible inactivation of the NADH oxidase proceeds at 37 °C. Palmitate drastically increases the rate of spontaneous de-activation of complex I in the absence of NADH. Taken together, these results suggest that free fatty acids act as specific complex I-directed inhibitors; at a physiologically relevant temperature (37 °C), their inhibitory effects on mitochondrial NADH oxidation is due to perturbation of the pseudo-reversible active–de-active complex I transition.
Keywords: energy transduction, fatty acid, mitochondria, NADH:ubiquinone oxidoreductase, palmitate, respiratory chain
Abbreviations: SMP, submitochondrial particles; SMPA, turnover-activated SMP; SMPD, thermally de-activated SMP; Q1, 2,3-dimethoxy-5-methyl-6-isoprenyl-1,4-benzoquinone
INTRODUCTION
Mitochondrial NADH:ubiquinone oxidoreductase (complex I) or its prokaryotic homologues (NDH-1) catalyse the oxidation of intramitochondrial or cytoplasmic NADH by ubiquinone coupled with vectorial translocation of protons across the inner mitochondrial (cytoplasmic) membrane. Mammalian complex I is the largest component of the mitochondrial respiratory chain and is composed of 46 different subunits [1] harbouring at least eight distinct redox components [2]. Bacterial operons encoding NDH-1 contain only 13–14 genes [3–5] and their transcription products are highly homologous with 14 core subunits of the mammalian complex I [6]. Since the catalytic properties of the mitochondrial enzyme and its prokaryotic homologues are very similar [7], it is safe to assume that only 14 out of 46 subunits of the mammalian enzyme are required to perform the catalytic capacity of complex I. The function of the more than 30 accessory subunits remains obscure. The homologous enzymes in lower eukaryotes (Neurospora crassa) [8] and plants [9] are also very complex, being composed of more than 30 individual subunits.
An obvious suggestion for the function(s) of the accessory subunits present in eukaryotic complex I is that they are somehow required for its stability and short- and long-term regulation. This is probably because the enzyme, being the major entry point for reducing equivalents into the respiratory chain, occupies a key position in general metabolism. The characteristic feature of the bovine mitochondrial complex I [10], and that of several other species [11,12], is the so-called active–de-active state transition (A→D transition). This phenomenon has been reviewed in [10] and is only briefly outlined in the present study as follows. Two catalytically and structurally distinct forms of the enzyme exist: the A-form, which is capable of full catalytic activity, and the catalytically inactive D-form. The slow A→D-form transition occurs when the enzyme resides under conditions where catalytic turnover is not permitted, i.e. in the absence of NADH and/or oxidized ubiquinone. The slow D→A-form transition takes place when the D-form is exposed to permissive conditions that allow turnover. The A→D-form transition is highly temperature-dependent, although significantly different activation barriers have been estimated for the enzyme from different species [11,12]. Thus pronounced de-activation of the mammalian complex I is seen only at a high temperature (>30 °C). The D-form is irreversibly modified by a number of SH reagents [13,14], which prevent the turnover-induced activation, whereas the A-form is insensitive to the SH reagents. The remarkable feature of the A→D transition, strongly suggestive of its physiological relevance, is that the phenomenon has been demonstrated for preparations of different complexity such as purified complex I [15], SMP (submitochondrial particles) [16], intact mitochondria [17], and most significantly, for mitochondria derived from intact beating hearts perfused under different oxygenation conditions [18]. The A→D transition is not found in prokaryotic NDH-1 [7,11], suggesting the involvement of the accessory subunits in the eukaryotic complex I.
It has become clear that two basically different mechanisms are possible for the action of any inhibitor (activator) on eukaryotic complex I. An effector can either decrease (or increase) the kinetic parameters of the active enzyme or it can perturb the equilibrium between the A- and D-forms as has been exemplified for the classical complex I inhibitor, rotenone [19].
A great variety of complex I-directed inhibitors have been described [20,21], with the vast majority of them acting at the enzyme–ubiquinone-junction site(s) without affecting activity with artificial electron acceptors such as NADH:ferricyanide [22] or NADH:hexa-ammineruthenium (III) [23] reductase activities. The only characteristic feature shared by these inhibitors is that they are either hydrophobic or amphiphilic and no clear structure–function correlation of their inhibitory capacity can be inferred from the available results. It has been proposed that a large hydrophobic cavity exists within the enzyme structure where the inhibitors can bind, thus preventing electron transfer from iron–sulphur centre N-2 to bulk ubiquinone [24,25]. The chemically simplest amphiphilic compounds that may serve as physiologically relevant inhibitors of complex I are free fatty acids (non-esterified fatty acids). It has been shown over the years by several independent groups that free fatty acids inhibit electron transfer activities and their inhibitory efficiency is substantially higher for NADH oxidase activity compared with that for respiration with succinate or for cytochrome c oxidase [26–32]. In pioneering studies by Rapoport and co-workers [30,33,34], it has been shown that fatty acids irreversibly inactivate the NADH-ubiquinone segment of the respiratory chain at a high temperature (37 °C). A selective denaturation of ‘an iron–sulphur protein’ of complex I induced by fatty acids was originally proposed to explain the strong temperature dependence of the irreversible inactivation [30], although no damage of any iron–sulphur cluster was found after treatment of the enzyme (SMP) with tetradecanoic acid for 2–6 h at 37 °C [34].
In the light of growing evidence for the involvement of complex I in a number of diseases and pathophysiological states and the importance of free fatty acids for metabolism under normal and pathophysiological conditions [35], it seemed worthwhile to get a closer insight into the nature of the complex I–free fatty acid interaction. Taking into account the results previously reported in the literature as briefly summarized above, we hypothesized that the active–de-active complex I transition plays an important role in this interaction. In this paper, the results supporting this hypothesis are presented. The preliminary results of this study have been published in abstract form [36].
EXPERIMENTAL
Bovine heart SMP and rat heart mitochondria were prepared and stored as described in [16] and [17] respectively. SMPA (turnover-activated SMP) was prepared as follows: SMP (5 mg/ml) were incubated in a mixture containing 0.25 M sucrose, 50 mM Tris/HCl (pH 8.0), 0.2 mM EDTA, 1 mM malonate (to activate succinate dehydrogenase) and 0.6 nmol/mg oligomycin (to block proton leakage) for 30 min at 30 °C. The suspension was diluted ten times into the same mixture containing 1 mM NADPH (to activate complex I) but no malonate and oligomycin and was further incubated for 45 min at 20 °C with continuous mixing to provide a free oxygen supply. The suspension was cooled on ice and centrifuged for 1 h at 0 °C at 30000 g. The precipitated particles were suspended in sucrose/Tris/EDTA mixture (see above) containing 0.2 mM malonate and kept on ice. SMPD (thermally de-activated SMP) were prepared by preincubation of SMPA at 37 °C for 15 min.
NADH, palmitic acid, EDTA, Tris, BSA, Q1 (2,3-dimethoxy-5-methyl-6-isoprenyl-1,4-benzoquinone), alamethicin, rotenone and N-ethylmaleimide were from Sigma. Piericidin A was a gift from Dr A. Kotlyar (Tel Aviv University, Tel Aviv, Israel). The stock of 10 mM solution of palmitic acid was prepared by dissolving the fatty acid in ethanol.
NADH oxidation was measured photometrically (ε340mM=6.22 or ε380mM=1.25) in the standard assay mixture comprising 0.25 M sucrose, 50 mM Tris/HCl (pH 8.0) and 0.2 mM EDTA. When NADH:Q1 reductase was assayed, 1 μg/ml antimycin A and 30 μM Q1 were added to the standard reaction mixture. NADH:hexa-ammineruthenium reductase [23] was assayed in the presence of 1 μg/ml antimycin A and 0.5 mM hexa-ammineruthenium. Succinate oxidase activity was measured photometrically by following fumarate formation (increase of absorption at 278 nm, ε278mM=0.3) in the standard assay mixture containing 10 mM potassium succinate. Protein content was determined by the Biuret assay. The experimental details are indicated in the legends to the Figures.
RESULTS
Effects of palmitate on the respiratory activities of SMP
A detailed study of structure–function relations such as the dependence of the inhibitory efficiency on the length of the alkyl chain and its unsaturation was not an immediate aim of this work. It should be noted, however, that all the effects reported in this paper for palmitate were also observed for other long-chain fatty acids, both saturated and unsaturated, and only quantitative differences in their efficiencies were observed.
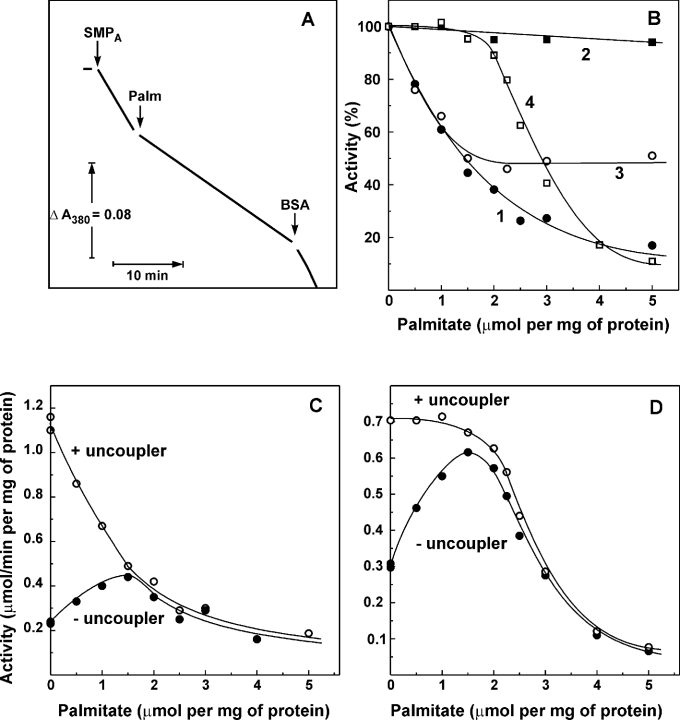
Figure 1 demonstrates the effects of palmitate on the respiratory activities of SMP at ambient temperature (25 °C). Instant inhibition of the uncoupled NADH oxidase activity by palmitate was rapidly abolished by BSA (Figure 1A). BSA also prevented the inhibition when added to the assay mixture before palmitate (results not shown). Rapidly reversible inhibition of the NADH oxidase activity was rather specific: no inhibition of succinate oxidase was seen up to the level of 2 μmol of palmitate added/mg of protein (Figure 1B, curve 4) and the NADH:hexa-ammineruthenium reductase activity was completely insensitive to palmitate (Figure 1B, curve 2). The titration pattern as depicted in Figure 1(B, curves 1, 2 and 4) shows that palmitate within the range of 0–2 μmol/mg of protein is acting as a specific inhibitor of the complex I–ubiquinone-junction site of the respiratory chain. When the inhibitory effect of palmitate on NADH oxidase as depicted in Figure 1(B, curve 1) was analysed as the 1/fractional inhibition versus palmitate concentration, a straight line plot was obtained (results not shown) suggesting a simple hyperbolic saturated inhibition. The NADH:quinone reductase activity measured with the externally added ubiquinone homologue Q1 was also sensitive to palmitate (Figure 1B, curve 3); however, in contrast with NADH oxidase, this activity was only partially (∼50%) sensitive to the inhibitor. It was conceivable that palmitate competes with Q1 for the specific binding site. However, the kinetic analysis revealed that palmitate acts on the NADH:Q1 reductase activity as a pure non-competitive (with Q1) inhibitor (results not shown). Interestingly, the residual activity of complex I in the presence of saturating palmitate was completely (>90%) inhibited by piericidin.
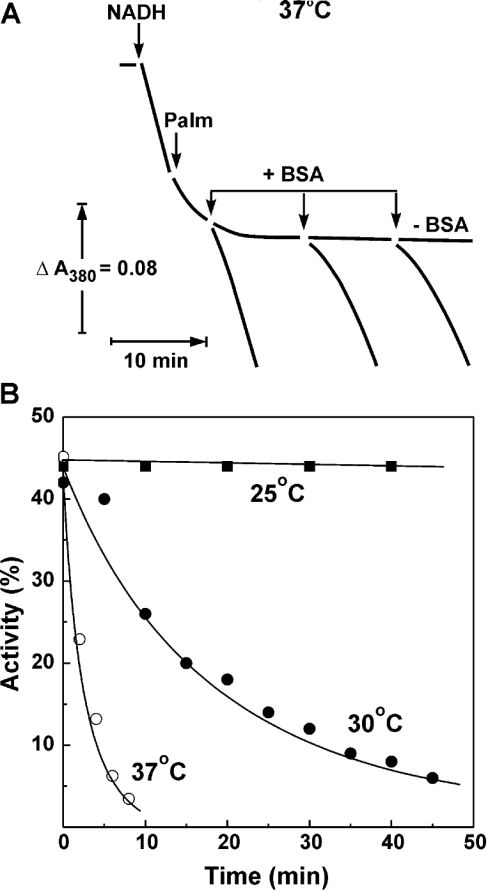
Figure 1. Effect of palmitate on the catalytic activities of SMP.
(A) Actual tracing of the NADH oxidase activity catalysed by pre-activated SMP (SMPA, 10 μg/ml) at 25 °C. Gramicidin D (0.05 μg/ml) was present in the standard assay mixture containing 0.5 mM NADH. Palmitate (15 μM) and BSA (2 mg/ml) were added where indicated. (B) Inhibition of the NADH oxidase (curve 1, ●), NADH:hexa-ammineruthenium reductase (curve 2, ■), NADH:Q1 reductase (curve 3, ○) and succinate oxidase (curve 4, □) activities by palmitate. Gramicidin (0.05 μg/ml) was present in all the assays. Specific activity of 100% corresponds to 1.0, 1.1, 0.33 and 0.65 μmol of the substrate oxidized·min−1·(mg of protein)−1 for NADH oxidase, NADH:hexa-ammineruthenium reductase, NADH:Q1 reductase and succinate oxidase respectively. The residual, palmitate-insensitive NADH:Q1 reductase activity [0.16 μmol·min−1·(mg of protein)−1] was decreased down to <5% by piericidin (10 nmol/mg of protein). Coupled (●) and uncoupled (○, 0.05 μg/ml gramicidin was present) NADH (C) and succinate oxidase activities (D) were measured.
It can be concluded that uncoupled NADH oxidase activity is much more sensitive to palmitate compared with succinate oxidase activity. Since NADH is the major substrate of mitochondrial respiration, and free fatty acids are considered as natural uncouplers, we decided to compare the uncoupling efficiency of palmitate with NADH and succinate as the respiratory substrates using tightly coupled SMP as the model system for physiological state 4 or state 3 respiration. The results presented in Figures 1(C) and 1(D) show that, due to its inhibitory effect on complex I, palmitate only slightly stimulates coupled NADH oxidase. The respiratory activity with NADH as the substrate in the presence of ‘optimal’ palmitate reached only approx. 30% of the fully uncoupled NADH oxidase activity, whereas almost 100% of the succinate oxidase capacity was revealed at the same ‘optimal’ palmitate concentration.
Effect of palmitate on D→A transition
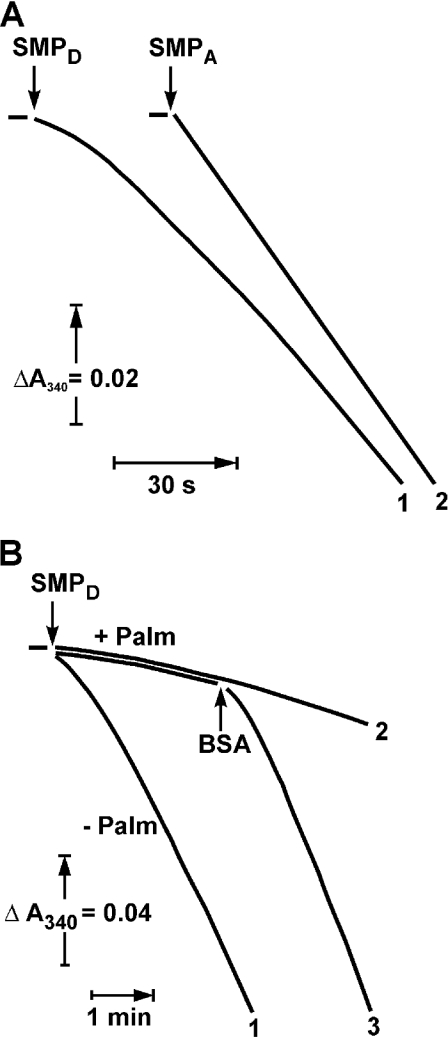
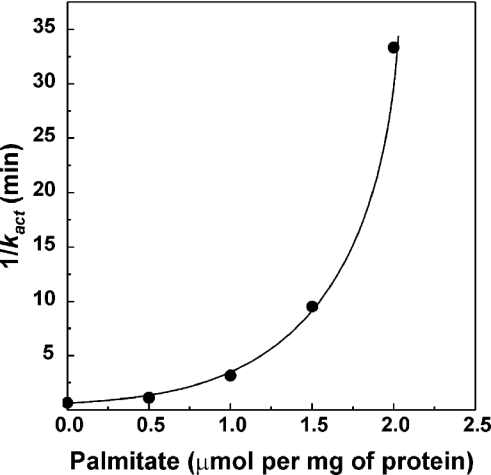
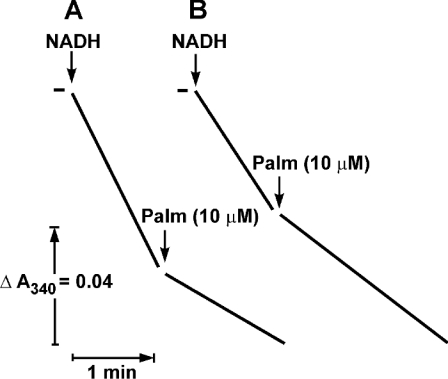
It has been demonstrated that a de-activated enzyme shows a prominent lag in the onset of the NADH oxidase reaction [16]. The first-order rate constant for the turnover-dependent D→A-form transition is 1.2 min−1 at 25 °C, pH 8.0 [13], and the lag-phase is clearly seen when NADH oxidation is followed at relatively high time-sensitivity resolution, as it is shown in Figure 2(A, curve 1). The effect of palmitate on the NADH oxidase activity of SMP pretreated to convert complex I into its D-form as it is seen at lower time-sensitivity resolution is depicted in Figure 2(B). Under these assay conditions, the turnover-dependent D→A transition was seen as a short lag-phase (curve 1), which was significantly prolonged in the presence of palmitate (curve 2). To quantify the inhibitory effect of palmitate on the D→A-form transition, the rate of activation was measured as a function of palmitate concentration assuming that the time course of the transition obeys first-order kinetics. Strong co-operativity with respect to the concentration of added palmitate was evident (Figure 3).
Figure 2. Effect of palmitate on the de-activated NADH oxidase at 25 °C.
(A) Time course of NADH oxidation catalysed by de-activated and activated particles [SMPD (curve 1) and SMPA (curve 2) respectively]. The reaction was started by the addition of SMP (10 μg/ml) to the standard mixture containing 0.1 mM NADH and gramicidin D (0.05 μg/ml). BSA did not influence the lag-phase in NADH oxidation catalysed by SMPD (results not shown). (B) Effect of palmitate on time course of NADH oxidation catalysed by de-activated particles. The experimental conditions were the same as in (A) except for a different time-sensitivity resolution. Palmitate (15 μM) was present (curves 2 and 3) and BSA (2 mg/ml) was added (curve 3) where indicated.
Figure 3. Effect of palmitate on the turnover-dependent activation of NADH oxidase.
The time course of NADH oxidation catalysed by de-activated particles was followed as shown in Figure 2 in the presence of different palmitate concentrations. The apparent first-order rate constants for the time-dependent increase of the catalytic activity traced at better time resolution were calculated from the linear log[vt→∞/(vt→∞−vt)]−t dependence, where vt and vt→∞ are the reaction rates measured at time t and those measured for SMPA in the presence of a given palmitate concentration respectively.
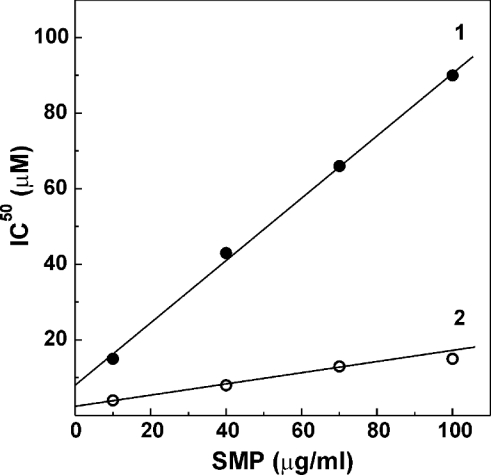
The solubility of long-chain fatty acids in the aqueous phase is very low [37,38] and it is thus expected that a complex equilibrium exists between the inhibitor bound to the lipid phase and palmitate that is present as a monomer and its associates in solution. Since the lipid/water partition coefficient for long-chain fatty acids is of the order of 104, any inhibitory (or activating) effect of palmitate on the membrane-bound enzymes should be quantitatively treated in terms of ‘tight binding’ inhibition (activation) in spite of the fact that the total concentration of palmitate is much higher compared with that of the enzyme (see [7] and references cited therein). Indeed, the apparent half-maximal concentrations of palmitate necessary to inhibit either the catalytic centre activity of the A-form or to prevent the D→A-form transition were linearly dependent on the concentration of SMP in the assay system (Figure 4). At any given concentration of SMP, the efficiency of palmitate in the inhibition of the D→A-form transition was considerably higher than that for the inhibition of the catalytic capacity of the active enzyme.
Figure 4. Relative inhibitory efficiency of palmitate on the catalytic activity (line 1) and on the activation rate (line 2) of NADH oxidase at 25 °C.
Line 1, half-maximal inhibitory concentrations of palmitate on the rate of NADH oxidation were determined as depicted in Figure 1(B, curve 1) at different protein concentrations in the assay mixture. Line 2, the concentrations of palmitate required to decrease the rate constant ka by 50% as described in Figure 2 were determined at different protein concentrations. The values of IC50 extrapolated to zero enzyme concentration were 9 and 3 μM for lines 1 and 2 respectively.
Effect of palmitate on NADH oxidation as a function of temperature
The A→D-form transition is extremely temperature-dependent [16]. Since palmitate was shown to inhibit the activity of complex I (Figure 1) and to prevent the D→A transition, we decided to see how the overall effect of palmitate on NADH oxidase activity depends on temperature. The results depicted in Figure 5 show qualitative difference as seen for different temperatures. In contrast with what has been observed at 25 °C (Figure 1A), an instant partial inhibition of NADH oxidase activity by palmitate at 37 °C was followed by a further slow decrease of activity, down to almost zero levels. NADH oxidase activity was completely re-activated by BSA at the early phase of the palmitate-induced time-dependent inhibition and only partial re-activation was seen at a later time. The time-dependent inhibition by palmitate was extremely temperature-dependent as it is shown in Figure 5(B), very similar to what has been shown for the spontaneous de-activation of complex I [16].
Figure 5. Time-dependent inactivation of NADH oxidase by palmitate at different temperatures.
(A) Actual tracing of NADH oxidation at 37 °C. The reaction was initiated by the addition of NADH (0.5 mM) and gramicidin D (0.05 μg/ml) to SMP (10 μg/ml) in the standard reaction mixture. Palmitate (15 μM) and BSA (2 mg/ml) were added at the time indicated by arrows. (B) Time course of the palmitate-induced inhibition at different temperatures measured as shown in (A). The activity at zero time (∼45% of that measured in the absence of palmitate) corresponds to instant inhibition caused by the addition of fatty acid. No albumin was present in the assay mixture.
The final level of palmitate-induced inhibition of NADH oxidase at 37 °C was both concentration- and time-dependent, and it was very difficult, if not impossible, to obtain reliable equilibrium titration results, as it has been performed at 25 °C (Figure 1B). However, the inhibitory effect of palmitate as it occurred at 37 °C was also specific for complex I: 2 min incubation of SMP in the presence of palmitate (2 μmol/mg of protein) resulted in more than 90% inhibition of NADH oxidase, whereas the succinate oxidase activity was decreased by not more than 20%.
Although the results shown in Figure 5 strongly suggest that the A→D transition of complex I is involved in the inhibitory effect of palmitate, another interpretation seemed possible. It could be envisaged that the slow temperature-dependent inhibition of complex I is due to a slow flip-flopping of palmitate across the membrane and to its tight binding to an inhibitory site located on (or exposed to) the cytoplasmic side of the inner mitochondrial membrane. This possibility was ruled out in experiments with right-side intact mitochondria permeabilized by alamethicin [39] to make complex I accessible to externally added NADH (Figure 6). Only instant inhibition of the NADH oxidase activity in both the mitochondria and inside-out SMP by palmitate was seen at 25 °C.
Figure 6. Inhibitory effect of palmitate on inside-out SMP and right-side-out permeabilized mitochondria.
(A) Rat heart mitochondria (10 μg/ml) were added to the standard assay mixture at 25 °C containing alamethicin (20 μg/ml) and 1 mM MgCl2. After 1 min preincubation, 2 mM EDTA was added and the reaction was initiated by the addition of 0.1 mM NADH. (B) SMPA (10 μg/ml) were added to the standard assay mixture at 25 °C and the reaction was started by the addition of 0.1 mM NADH and 0.05 μg/ml gramicidin D.
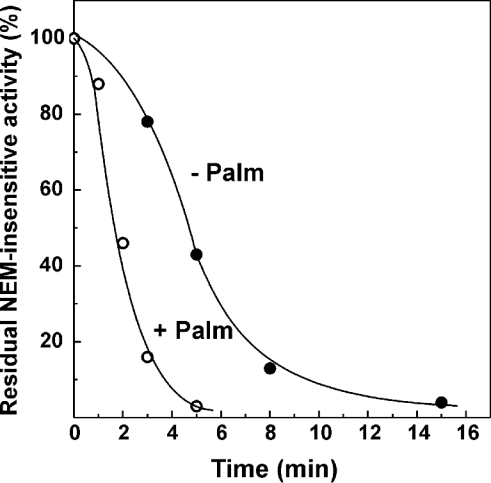
If the de-activation of complex I is involved in the slow temperature-dependent inhibition of NADH oxidase (Figure 5), the acceleration of the spontaneous A→D transition by palmitate could explain why enzyme activity remains constant at 37 °C in the absence of the inhibitor. The results presented in Figure 7 show that, indeed, palmitate considerably increased the spontaneous de-activation of complex I. Taken together, the results shown in Figures 2, 5 and 7 suggest the following explanation for the time- and temperature-dependent inactivation of complex I by palmitate. When NADH is oxidized at 37 °C, the de-activation of complex I does not significantly contribute to the overall enzyme activity measured because any relatively slow de-activation act is immediately compensated for by a relatively rapid turnover-dependent re-activation and the reaction proceeds at a constant rate. In the presence of palmitate, the de-activation rate is increased, and the turnover-dependent re-activation is prevented. These two effects result in a strong time-dependent inhibition of NADH oxidase as is seen at 37 °C.
Figure 7. Effect of palmitate on the spontaneous thermal de-activation of NADH oxidase.
SMPA (100 μg/ml) were incubated at 37 °C in 0.25 mM sucrose, 50 mM Tris/HCl (pH 8.0) and 0.2 mM EDTA in the presence or absence of 65 μM palmitate (the concentration which inhibits NADH oxidase activity by 70% at 25 °C at this protein content). The samples were diluted 10 times in the standard assay mixture (25 °C) containing BSA (2 mg/ml) to remove palmitate (see Figure 1A), 1 mM N-ethylmaleimide was added to inhibit the de-activated enzyme irreversibly [14,17] and after 1 min incubation the residual activity initiated by the addition of 0.1 mM NADH was determined.
DISCUSSION
Besides their metabolic role in the provision of energy, long-chain free fatty acids exert diverse effects on cellular membranes and on the catalytic activities of many enzymes. In particular, various functions of mitochondria are strongly affected by free fatty acids (see reviews [40–42]). Most of the recent studies on free fatty acid–mitochondrial interaction are focused on the direct or indirect energy dissipation effects caused by their selective protonophoric activity mediated by a number of inner membrane carriers such as the adenine nucleotide translocase, uncoupling proteins and other anion-specific carriers, and on the fatty acid-induced unselective permeability of the inner mitochondrial membrane [42]. Previously published results [26–34] and results of the present study (Figure 1) show that free long-chain fatty acids (as exemplified by palmitate) selectively inhibit the active form of mitochondrial complex I. It has been proposed that mild uncoupling of mitochondria may be physiologically advantageous because it would prevent the ΔμH+-dependent generation of a superoxide radical [43] and cause relief of respiratory control, thus maintaining a favourable intramitochondrial NAD+/NADH ratio needed for a high rate of ATP production [44,45]. The results shown in Figure 1(C) hardly support these proposals: only a weak relief of respiratory control with NADH (the major source of mitochondrial respiration under physiological conditions) as the substrate was induced by palmitate. It is also expected that the inhibition of complex I at the quinone-junction site would increase rather than decrease the rate of superoxide production. It should be noted, however, that the nature of the artificially induced respiratory control in inside-out SMP may differ from that in intact isolated mitochondria where the rate of respiration (coupled or uncoupled) depends, besides the respiratory chain activity, on co-ordinated operation of the dicarboxylate-, nucleotide- and Pi-translocases and the intramitochondrial-specific NAD+-dependent dehydrogenases.
In contrast with simple hyperbolic-like inhibition of NADH-oxidase activity by palmitate, only partial inhibition of the NADH:quinone reductase activity was observed (Figure 1B), and the residual palmitate-insensitive NADH oxidation was fully sensitive to piericidin. To our knowledge, free fatty acids as exemplified by palmitate are the only complex I–ubiquinone-junction site inhibitors demonstrating this titration pattern, which is fairly in line with the presence of two piericidin-binding sites ([7] and references cited therein). Whatever be the precise explanation for the titration pattern shown in Figure 1(B), it deserves further study aimed to dissect the electron transfer pathway within complex I.
Palmitate not only prevents the turnover-induced activation of complex I but it also increases the rate of de-activation (Figure 7), thus resulting in partially irreversible inactivation of complex I at physiological temperature (37 °C). The mechanistic nature of the apparent irreversibility of inactivation remains obscure. Formally, the inactivation of complex I at a high temperature can be visualized as the two-step process where the catalytically inactive enzyme–fatty acid complex is formed in a rapidly reversible temperature-independent (or only slightly dependent) reaction followed by a highly temperature-dependent transformation into a very tight enzyme–inhibitor complex. The latter step is accompanied by structural rearrangements similar to that taking place during the spontaneous de-activation process. The ‘catalytic’ effects of fatty acids such as facilitated dissociation of some tightly bound component (subunit, annular phospholipid or metal cation) also cannot be excluded.
In addition to the inhibitory effect on the active form of complex I, palmitate is shown to prevent the turnover-induced activation of the D-form of the enzyme (Figure 2). This effect is also reversible under conditions where the de-activation rate is negligible (25 °C). The titration pattern is clearly indicative of strong co-operativity in the inhibition. Both inhibition of the active enzyme and effect on the activation can be considered if palmitate acts either at the hydrophobic membranous phase (or at the membrane–aqueous interphase) or at the aqueous phase-exposed part of the enzyme. An equilibrium between the membrane-bound and aqueous species is expected at any given concentration of SMP and added fatty acid due to the strong amphiphilic nature of long-chain fatty acids and their very high hydrophobic–hydrophilic phase partition coefficients [37]. The co-operative nature of the inhibitory effect as depicted in Figure 3 may correspond to a model where the aqueous phase tail-to-tail dimers is the species interacting with the enzyme. If correct, this model predicts that long-chain α,ω-dicarboxylic acids should be potent inhibitors of the D→A-form transition. Following the same arguments, the hydrogen bond-stabilized membrane-bound protonated–de-protonated fatty acid dimer can be considered as the actual inhibitor species.
We have confirmed and extended the pioneering observation of Rapoport and co-workers [30,31,34] on the strong temperature dependence of the inhibitory effect of fatty acids (Figure 5). There is an approx. 10-fold increase in the rate of the palmitate-induced inactivation when the temperature of the assay was increased from 30 to 37 °C, closely corresponding to the previously reported extremely high activation energy for the A→D-form transition (240 kJ/mol [16]).
A final note concerns the physiological relevance of inhibitory palmitate (and other free long-chain fatty acids) effects on complex I. Owing to their extremely low solubility [37,38], the presence of substantial amounts of free, protein-unbound fatty acids in the cytosol or mitochondrial matrix seems unlikely under physiological or pathophysiological conditions. However, substantial accumulation of free fatty acids in mitochondrial membranes has been detected during ischaemic exposure of the rat liver [46] or during storage of isolated mitochondria [47]. Interestingly, progressive damage of complex I during global ischaemia in Langendorff-perfused rat hearts, a condition where accumulation of free fatty acids in the mitochondrial membrane is expected, has been reported in [48]. To relate the phenomena described in this report to physiologically relevant conditions, an extensive study on the interaction between mitochondrial membranes and fatty acids bound to fatty acid carrier proteins [49,50] is evidently needed. The present study suggests that, under physiological conditions, complex I is the primary target for the deteriorating effects of long-chain free fatty acids on mitochondria.
Acknowledgments
This study was supported by the Russian Foundation for Fundamental Research (grant 02-04-48679 to A.D.V. and grant 03-04-48202 to V.G.G.), Program Leading Schools in Science (grant 00-15-97798 to A.D.V.), NIH Fogarty International Center (grant 1R03TW006041 to G.C. and A.D.V.) and the Department of Veterans Affairs (G.C.).
References
- 1.Carroll J., Shannon R. J., Fearnley I. M., Walker J. E., Hirst J. Definition of the nuclear encoded protein composition of bovine heart mitochondrial Complex I: identification of two new subunits. J. Biol. Chem. 2002;277:50311–50317. doi: 10.1074/jbc.M209166200. [DOI] [PubMed] [Google Scholar]
- 2.Ohnishi T., Magnitsky S., Toulokhonova L., Yano T., Yagi T., Burbaev D. S., Vinogradov A. D. EPR studies of the possible binding sites of the cluster N2, semiquinones, and specific inhibitors of the NADH:quinone oxidoreductase (complex I) Biochem. Soc. Trans. 1999;27:586–591. doi: 10.1042/bst0270586. [DOI] [PubMed] [Google Scholar]
- 3.Weidner U., Geiger S., Ptock A., Friedrich T., Leif H., Weiss H. The gene locus of the proton-translocating NADH:ubiquinone oxidoreductase in Escherichia coli: organization of the 14 genes and relationship between the derived proteins and subunits of mitochondrial complex I. J. Mol. Biol. 1993;233:109–122. doi: 10.1006/jmbi.1993.1488. [DOI] [PubMed] [Google Scholar]
- 4.Xu X., Matsuno-Yagi A., Yagi T. DNA sequencing of the remaining genes of the gene cluster encoding the energy-transducing NADH-quinone oxidoreductase of Paracoccus denitrificans. Biochemistry. 1993;32:968–981. doi: 10.1021/bi00054a030. [DOI] [PubMed] [Google Scholar]
- 5.Dupuis A., Chevallet M., Darrouzet E., Duborjal H., Lunardi J., Issartel J. P. The Complex I from Rhodobacter capsulatus. Biochim. Biophys. Acta. 1998;1364:147–165. doi: 10.1016/s0005-2728(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 6.Fearnley I. M., Walker J. E. Conservation of sequences of subunits of mitochondrial complex I and their relationships with other proteins. Biochim. Biophys. Acta. 1992;1140:105–134. doi: 10.1016/0005-2728(92)90001-i. [DOI] [PubMed] [Google Scholar]
- 7.Grivennikova V. G., Roth R., Zakharova N. V., Hägerhäll C., Vinogradov A. D. The mitochondrial and prokaryotic proton-translocating NADH:ubiquinone oxidoreductases: similarities and dissimilarities of the quinone-junction sites. Biochim. Biophys. Acta. 2003;1607:79–90. doi: 10.1016/j.bbabio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Videira A., Duarte M. From NADH to ubiquinone in Neurospora mitochondria. Biochim. Biophys. Acta. 2002;1555:187–191. doi: 10.1016/s0005-2728(02)00276-1. [DOI] [PubMed] [Google Scholar]
- 9.Rasmusson A. G., Heiser V., Zabaleta E., Brennicke A., Grohmann L. Physiological, biochemical and molecular aspects of mitochondrial complex I in plants. Biochim. Biophys. Acta. 1998;1364:101–111. doi: 10.1016/s0005-2728(98)00021-8. [DOI] [PubMed] [Google Scholar]
- 10.Vinogradov A. D., Grivennikova V. G. The mitochondrial Complex I: progress in understanding of catalytic properties. IUBMB Life. 2001;52:129–134. doi: 10.1080/15216540152845920. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikova V. G., Serebryanaya D. V., Isakova E. P., Belozerskaya T. A., Vinogradov A. D. The transition between active and de-activated forms of NADH:ubiquinone oxidoreductase (Complex I) in the mitochondrial membrane of Neurospora crassa. Biochem. J. 2003;369:619–626. doi: 10.1042/BJ20021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maklashina E., Kotlyar A. B., Cecchini G. Active/de-active transition of respiratory Complex I in bacteria, fungi and animals. Biochim. Biophys. Acta. 2003;1606:95–103. doi: 10.1016/s0005-2728(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 13.Kotlyar A. B., Sled V. D., Vinogradov A. D. Effect of Ca2+ ions on the slow active/inactive transition of the mitochondrial NADH:ubiquinone reductase. Biochim. Biophys. Acta. 1992;1098:144–150. doi: 10.1016/s0005-2728(05)80329-9. [DOI] [PubMed] [Google Scholar]
- 14.Gavrikova E. V., Vinogradov A. D. Active/de-active transition of the mitochondrial complex I as revealed by specific sulfhydryl group labeling. FEBS Lett. 1999;455:36–40. doi: 10.1016/s0014-5793(99)00850-9. [DOI] [PubMed] [Google Scholar]
- 15.Maklashina E. O., Sled V. D., Vinogradov A. D. Hysteresis in the behavior of bovine heart mitochondrial Complex I: kinetic and thermodynamic parameters of the slow reversible active/inactive transition. Biochemistry (Moscow) 1994;59:707–714. [PubMed] [Google Scholar]
- 16.Kotlyar A. B., Vinogradov A. D. Slow active/inactive transition of the NADH:ubiquinone reductase. Biochim. Biophys. Acta. 1990;1019:151–158. doi: 10.1016/0005-2728(90)90137-s. [DOI] [PubMed] [Google Scholar]
- 17.Grivennikova V. G., Kapustin A. N., Vinogradov A. D. Catalytic activity of NADH-ubiquinone oxidoreductase (Complex I) in intact mitochondria. Evidence for the slow active/inactive transition. J. Biol. Chem. 2001;276:9038–9044. doi: 10.1074/jbc.M009661200. [DOI] [PubMed] [Google Scholar]
- 18.Maklashina E., Sher Y., Zong-Zhou H., Gray M. O., Karliner J. S., Cecchini G. Effect of anoxia/reperfusion on the reversible active/deactive transition of NADH-ubiquinone oxidoreductase (Complex I) in rat heart. Biochim. Biophys. Acta. 2002;1556:6–12. doi: 10.1016/s0005-2728(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 19.Grivennikova V. G., Maklashina E. O., Gavrikova E. V., Vinogradov A. D. Interaction of the mitochondrial NADH-ubiquinone reductase with rotenone as related to the enzyme active/inactive transition. Biochim. Biophys. Acta. 1997;1319:223–232. doi: 10.1016/s0005-2728(96)00163-6. [DOI] [PubMed] [Google Scholar]
- 20.Degli Esposti M. Inhibitors of NADH-ubiquinone reductase: an overview. Biochim. Biophys. Acta. 1998;1364:222–235. doi: 10.1016/s0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi H. Structure-activity relationships of some Complex I inhibitors. Biochim. Biophys. Acta. 1998;1364:236–244. doi: 10.1016/s0005-2728(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 22.Singer T. P. Determination of the activity of succinate, NADH, choline, and α-glycerophosphate dehydrogenase. Methods Biochem. Anal. 1974;22:123–175. doi: 10.1002/9780470110423.ch3. [DOI] [PubMed] [Google Scholar]
- 23.Sled V. D., Vinogradov A. D. Kinetics of the mitochondrial NADH-ubiquinone oxidoreductase interaction with hexaammineruthenium (III) Biochim. Biophys. Acta. 1993;1141:262–268. doi: 10.1016/0005-2728(93)90051-g. [DOI] [PubMed] [Google Scholar]
- 24.Degli Esposti M., Crimi M., Ghelli A. Natural variation in the potency and binding sites of mitochondrial quinone-like inhibitors. Biochem. Soc. Trans. 1994;22:209–213. doi: 10.1042/bst0220209. [DOI] [PubMed] [Google Scholar]
- 25.Okun J. G., Lűmmen P., Brandt U. Three classes of inhibitors share a common binding domain in mitochondrial Complex I (NADH:ubiquinone oxidoreductase) J. Biol. Chem. 1999;274:2625–2630. doi: 10.1074/jbc.274.5.2625. [DOI] [PubMed] [Google Scholar]
- 26.Luzikov V. N., Saks V. A., Berezin I. V. Comparative study of thermal degradation of electron transfer particle and reconstituted respiratory chain. Relation of electron transfer to reactivation of submitochondrial particles. Biochim. Biophys. Acta. 1970;223:16–30. doi: 10.1016/0005-2728(70)90127-1. [DOI] [PubMed] [Google Scholar]
- 27.Schewe T., Coutelle Ch., Rapoport S. Über die Beziehunģ zwischen Molekülstructur und Hemmwirkunģ von Fettsäuren und Monoģlyzeriden auf die Atmunģskette von Mitochondrien-Partikeln. Acta Biol. Med. Ger. 1971;27:13–28. [PubMed] [Google Scholar]
- 28.Hillered L., Chan P. H. Effects of arachidonic acid on respiratory activities in isolated brain mitochondria. J. Neurosci. Res. 1998;19:94–100. doi: 10.1002/jnr.490190113. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi Y., Morii H., Tamura M., Hayaishi O., Watanabe Y. A possible mechanism of mitochondrial dysfunction during cerebral ischemia: inhibition of mitochondrial respiration by arachidonic acid. Arch. Biochem. Biophys. 1991;289:33–38. doi: 10.1016/0003-9861(91)90438-o. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig P., Schewe T., Rapoport S. Lanģsame irreversible Hemmunģ der Atmunģskette nichtphosphorylierender submitochondrialer Partikel durch freie Fettsäuren und deren Monoģlyzeride und Methylester. Acta Biol. Med. Ger. 1977;36:981–998. [PubMed] [Google Scholar]
- 31.Ludwig P., Bartels M., Schewe T., Rapoport S. Selective inactivation of the NADH-ubiquinone segment of the respiratory chain of submitochondrial particles by endogenous free fatty acids during hyperthermia. FEBS Lett. 1978;95:181–184. doi: 10.1016/0014-5793(78)80079-9. [DOI] [PubMed] [Google Scholar]
- 32.Batayneh N., Kopacz S. J., Lee C. P. The modes of action of long chain alkyl compounds on the respiratory chain-linked energy transducing system in submitochondrial particles. Arch. Biochem. Biophys. 1986;250:476–487. doi: 10.1016/0003-9861(86)90752-6. [DOI] [PubMed] [Google Scholar]
- 33.Schewe T., Ludwig P., Rapoport S. On a slow inhibitory effect of free fatty acids on the respiratory chain of non-phosphorylating submitochondrial particles from beef heart. FEBS Lett. 1974;46:39–41. doi: 10.1016/0014-5793(74)80329-7. [DOI] [PubMed] [Google Scholar]
- 34.Schewe T., Albracht S. P. J., Ludwig P., Rapoport S. M. Two modes of irreversible inactivation of the mitochondrial electron-transfer system by tetradecanoic acid. Biochim. Biophys. Acta. 1985;807:210–215. doi: 10.1016/0005-2728(85)90124-0. [DOI] [PubMed] [Google Scholar]
- 35.Bartlett K., Eaton S. Mitochondrial β-oxidation. Eur. J. Biochem. 2004;271:462–469. doi: 10.1046/j.1432-1033.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- 36.Vinogradov A. D., Gostimskaya I. S., Loskovich M. V., Grivennikova V. G. Possible mechanisms of regulation of the mitochondrial Complex I as related to its slow active/inactive transition. Biochim. Biophys. Acta, EBEC Short Reports. 2004;13:20. [Google Scholar]
- 37.Small D. M. New York and London: Plenum Press; 1986. Hand Book of Lipid Research, Vol. 4. The Physical Chemistry of Lipids. [Google Scholar]
- 38.Vorum H., Brodersen R., Kragh-Hansen U., Pedersen A. O. Solubility of long-chain fatty acids in phosphate buffer at pH 7.4. Biochim. Biophys. Acta. 1992;1126:135–142. doi: 10.1016/0005-2760(92)90283-2. [DOI] [PubMed] [Google Scholar]
- 39.Gostimskaya I. S., Grivennikova V. G., Zharova T. V., Bakeeva L. E., Vinogradov A. D. In situ assay of the intramitochondrial enzymes: use of alamethicin for permeabilization of mitochondria. Anal. Biochem. 2003;313:46–52. doi: 10.1016/s0003-2697(02)00534-1. [DOI] [PubMed] [Google Scholar]
- 40.Wojtczak L., Schönfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim. Biophys. Acta. 1993;1183:41–165. doi: 10.1016/0005-2728(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 41.Skulachev V. P. Anion carriers in fatty acid-mediated physiological uncoupling. J. Bioenerg. Biomembr. 1999;31:431–445. doi: 10.1023/a:1005492205984. [DOI] [PubMed] [Google Scholar]
- 42.Bernardi P., Penzo D., Wojtczak L. Mitochondrial energy dissipation by fatty acids; mechanisms and implications for cell death. Vitam. Horm. 2002;65:97–126. doi: 10.1016/s0083-6729(02)65061-7. [DOI] [PubMed] [Google Scholar]
- 43.Skulachev V. P. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- 44.Skulachev V. P. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 45.Soboll S. Thyroid hormone action on mitochondrial energy transfer. Biochim. Biophys. Acta. 1993;1144:1–16. doi: 10.1016/0005-2728(93)90024-a. [DOI] [PubMed] [Google Scholar]
- 46.Boime I., Smith E. E., Hunter F. E., Jr The role of fatty acids in mitochondrial changes during liver ischemia. Arch. Biochem. Biophys. 1970;139:425–443. doi: 10.1016/0003-9861(70)90496-0. [DOI] [PubMed] [Google Scholar]
- 47.Chan S. H. P., Higgins E., Jr Uncoupling activity of endogenous free fatty acids in rat liver mitochondria. Can. J. Biochem. 1978;56:111–116. doi: 10.1139/o78-018. [DOI] [PubMed] [Google Scholar]
- 48.Veitch K., Hombroeckx A., Caucheteux D., Pouleur H., Hue L. Global ischaemia induces a biphasic response of the mitochondrial respiratory chain. Biochem. J. 1992;281:709–715. doi: 10.1042/bj2810709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McArthur M. J., Atshaves B. P., Frolov A., Foxworth W. D., Kier A. B., Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J. Lipid Res. 1999;40:1371–1383. [PubMed] [Google Scholar]
- 50.Black P. N., DiRusso C. C. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol. Mol. Biol. Rev. 2003;67:454–472. doi: 10.1128/MMBR.67.3.454-472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]