Abstract
Mechanisms which inactivate NO (nitric oxide) are probably important in governing the physiological and pathological effects of this ubiquitous signalling molecule. Cells isolated from the cerebellum, a brain region rich in the NO signalling pathway, consume NO avidly. This property was preserved in brain homogenates and required both particulate and supernatant fractions. A purified fraction of the particulate component was rich in phospholipids, and NO consumption was inhibited by procedures that inhibited lipid peroxidation, namely a transition metal chelator, the vitamin E analogue Trolox and ascorbate oxidase. The requirement for the supernatant was accounted for by its content of ascorbate which catalyses metal-dependent lipid peroxidation. The NO-degrading activity of the homogenate was mimicked by a representative mixture of brain lipids together with ascorbate and, under these conditions, the lipids underwent peroxidation. In a suspension of cerebellar cells, there was a continuous low level of lipid peroxidation, and consumption of NO by the cells was decreased by approx. 50% by lipid-peroxidation inhibitors. Lipid peroxidation was also abolished when NO was supplied at a continuously low rate (∼100 nM/min), which explains why NO consumption by this process is saturable. Part of the activity remaining after the inhibition of lipid peroxidation was accounted for by contaminating red blood cells, but there was also another component whose activity was greatly enhanced when the cells were maintained under air-equilibrated conditions. A similar NO-consuming process was present in cerebellar glial cells grown in tissue culture but not in blood platelets or leucocytes, suggesting a specialized mechanism.
Keywords: ascorbate, brain cell, cerebellum, lipid peroxidation, nitric oxide, platelet
Abbreviations: AO, ascorbate oxidase; DETA, diethylenetriamine; DTPA, diethylenetriaminepenta-acetic acid; RBC, red blood cell; TBARS, thiobarbituric acid-reacting substances
INTRODUCTION
NO (nitric oxide) functions as an intercellular signalling molecule throughout the body. It is generated by NO synthase enzymes and, at low nanomolar concentrations, acts on guanylate cyclase-coupled NO receptors, leading to the accumulation of cGMP in target cells [1,2]. When NO increases to the 100 nM level or beyond, there are a number of other potential targets. One of these is mitochondrial cytochrome c oxidase, where O2 is normally reduced to water. NO binds to cytochrome c oxidase in competition with O2 and so can act as a respiratory inhibitor [3,4]. These higher NO concentrations can also participate in chemical reactions that generate noxious products, including combination with superoxide to form peroxynitrite [5,6] and reaction with O2 to generate nitrosating species such as nitrogen dioxide. With this spectrum of potential reactivity, it is important to understand how NO concentrations are regulated. This regulation may not only depend on the rate and pattern of NO synthesis but also on the rate of NO degradation. Although the mechanisms and kinetics of NO synthases are now quite well understood in biochemical terms [7], little is known regarding how NO is inactivated.
Several putative NO-consuming pathways have emerged. Well-known reactions include that of NO with haemoglobin in circulating RBCs (red blood cells), although the extent to which this reaction contributes to biological inactivation remains unclear [8]. The simple reaction of NO with O2 (autoxidation) is accelerated in the hydrophobic interior of biological membranes [9], but is too slow to account for NO inactivation at physiological concentrations. The reaction of NO with O2•− may partially account for NO breakdown [10,11], although this process may be artificially prominent in in vitro experiments [5,12]. Various enzymes have also been proposed to consume NO [13–18].
We have previously reported that suspensions of cells from a brain region (the cerebellum) rich in the NO–cGMP signalling pathway consume NO rapidly [19]. This sink functions to convert different physiological rates of NO formation into low plateau concentrations, the values of which are proportional to the NO release rate. When confronted by enduring NO release, however, the consumption mechanism fails. Further investigation [20] revealed that NO inactivation is preserved in rat brain homogenates, that it requires O2 and that it ultimately generates NO3− as the end product. Moreover, the NO-inactivating activity in homogenates was found to be lost after treatment with proteinase K or heat, indicating the involvement of a protein. Tests for an array of candidate enzymes including cytochrome c oxidase, lipoxygenases, peroxidases, prostaglandin H synthase and a flavohaemoglobin-like NO dioxygenase, however, proved negative. In the present study, we report on the identification of the prominent NO-degrading process in isolated brain cells and in brain homogenates, which, it transpires, is not dependent on protein but on lipid. Inhibition of this mechanism revealed another O2-dependent NO-degrading process.
EXPERIMENTAL
Brain homogenate preparations
Whole brain homogenate (∼20 mg of protein/ml) was prepared from 8-day-old Sprague–Dawley rats by sonication in 20 mM Tris buffer (pH 7.4). The homogenate was either stored at −20 °C until use or was further fractionated by centrifugation (at 4 °C). After an initial spin (10000 g for 30 min), the pellet was discarded and the supernatant was further spun at 100000 g for 1 h. The resultant pellet was resuspended in Tris buffer (20 mM) at 10 mg of protein/ml, whereas the supernatant was spun overnight at 2000 g through 10000 kDa cut-off filters (CENTRIPLUS®; Millipore UK, Watford, Herts., U.K.). This procedure was used to remove free haemoglobin without compromising NO consumption on recombination with the pellet (see the Results section). The 100000 g pellet and the filtered supernatant were stored at −20 °C until use.
Further purification of the pellet fraction was undertaken at 4 °C using HPLC equipment and columns from Amersham Biosciences (Little Chalfont, Bucks., U.K.). The active component in the pellet was solubilized by the addition of 0.5% CHAPS. This solution was spun at 100000 g for 1 h and the active supernatant decanted. The supernatant was then applied to a DEAE column (HiPrep™ 16/10 Sepharose DEAE) at a rate of 0.5 ml/min, and the column was washed with 5 vol. of 20 mM Tris/HCl and 0.5% CHAPS (buffer A). More than 95% of the activity bound to the DEAE column. A NaCl gradient (0–1 M) in 20 mM Tris/HCl and 0.5% CHAPS (buffer B) was then applied to the column at a rate of 0.5 ml/min. All fractions were assayed for protein, and for activity after recombination with filtered supernatant (see above). The most active fractions were pooled and transferred back into buffer A using a 25 ml desalting column (HiPrep* 26/10 Desalting Column). These pooled fractions were then concentrated into a volume of <200 μl by centrifugation at 2300 g for 2 h using a 5 kDa cut-off filter (Microcon filters; Millipore UK) and then applied to a size-exclusion column (Superdex 75) at a rate of 0.1 ml/min. The most enriched fraction was identified and examined by matrix-assisted laser-desorption ionization–time-of-flight MS [21]. Ultimately, this fraction was analysed for its lipid content (Lipid Analysis Unit, The Scottish Crop Research Institute, Dundee, Scotland, U.K.).
Cell preparations
Acute cerebellar cell suspensions (20×106 cells/ml; 1.25 mg of protein/ml) were prepared from 8-day-old Sprague–Dawley rats by the method described in [22] except that the pups were not pretreated with hydroxyurea. The cell incubation medium contained (mM): NaCl (130), KCl (3), CaCl2 (1.5), MgSO4 (1.2), Na2HPO4 (1.2), Tris/HCl (15) and glucose (11) adjusted to pH 7.4 at 37 °C. To determine the RBC content of the cerebellar cell preparation, the suspension was diluted 1:4 into 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and fixed for 2 h at room temperature (22 °C). Fixed cells were mounted on gelatin-coated microscope slides; RBCs were identified by their morphology and brightness under phase-contrast microscopy. The percentage of RBCs was calculated from four fields for each of 2–3 slides per preparation.
To prepare RBCs, blood from 8-day-old rats was centrifuged at 100 g for 5 min and the pellet was resuspended. The cells were then taken through the same procedure as the cerebellar tissue (except that they were not triturated and were collected by centrifugation) to be comparable with the blood cells contaminating the cerebellar cell suspension (see the Results section). Final concentrations [(0.1– 0.4)×106 RBCs/ml] were prepared by resuspension in the above cell incubation medium.
Primary cultures of cerebellar mixed glia were prepared from 8-day-old Sprague–Dawley rats by adaptation of a procedure described elsewhere [23]. In brief, cells were seeded at a density of 25×106 cells/600 cm2 dish (Nalge Europe, Hereford, U.K.) precoated with poly(D-lysine) (2 μg/cm2). The cells were maintained in 60 ml of growth media containing minimal essential medium (50%), heat-inactivated horse serum (25%, v/v), Hanks balanced salt solution (25%) and penicillin/streptomycin (100 units/ml and 100 μg/ml respectively), buffered to pH 7.3 with Tris (5 mM) and NaHCO3 (0.35 g/l) in a humidified incubator at 37 °C. Media were changed on the following day and every 2–4 days thereafter, and cells were used after 6–7 days. Before use, cultures were washed twice with 50 ml of incubation medium (as used for the acute cerebellar suspensions but lacking calcium), detached by application of 25 ml of trypsin/EDTA-containing solution (Life Technologies, Paisley, Scotland, U.K.) for 15 min at 37 °C and resuspended at (0.5–3×106/ml) in incubation medium lacking calcium.
Platelets and leucocytes were prepared by collecting whole blood from adult Sprague–Dawley rats (3–5 rats/experiment) into acid citrate dextrose solution (12.5%) and centrifuging at 300 g for 10 min at 20 °C. The platelet-rich plasma was withdrawn and re-centrifuged. Leucocytes were collected and resuspended (1 mg of protein/ml) in incubation medium containing 137 mM NaCl, 0.5 mM MgCl2, 0.5 mM NaH2PO4, 2.7 mM KCl, 25 mM Hepes and 5.6 mg mM D-glucose (pH 7.4) at 37 °C. The platelet-containing supernatant was centrifuged at 2000 g for 10 min (20 °C) and the platelet pellet was resuspended (1 mg of protein/ml) in the same buffer as that used for leucocytes.
NO and O2 measurements
NO consumption by whole brain homogenate (0.3 mg of protein/ml), the filtered supernatant (diluted 1:5 or 1:10) and/or the 100000 g pellet (0.1 mg of protein/ml) and each of the cell suspensions (concentrations as above) was studied after the addition of the NO donor diethylenetriamine/NO adduct (DETA/NO; Alexis, Nottingham, U.K.), which was prepared in 10 mM NaOH and kept on ice until use. The homogenate and pellet were sonicated briefly before use. For measurements of NO and O2 concentrations, samples (0.5 or 1 ml) were incubated in a stirred chamber (at 37 °C) equipped with an O2 electrode (Rank Brothers, Bottisham, Cambs, U.K.) and an NO electrode (ISO-NO; World Precision Instruments, Stevenage, Herts., U.K.). Except where indicated otherwise, the chamber was sealed and all experiments contained superoxide dismutase (1000 units/ml). Other stock solutions (all obtained from Sigma, Poole, Dorset, U.K.) were as follows: AO (ascorbate oxidase; 1000 units/ml) was prepared in distilled water, DTPA (diethylenetriaminepenta-acetic acid; 10 mM) in equimolar NaOH, and Trolox (100 mM) in DMSO. The final DMSO concentration did not exceed 0.1% in any experiment. Protein concentrations were measured by the bicinchoninic acid method.
Lipid peroxidation assay
Liposomes were formed at room temperature by the addition of 74 μg/ml phosphatidylcholine, 74 μg/ml phosphatidylethanolamine, 26 μg/ml phosphatidylserine and 6 μg/ml phosphatidylinositol to 20 mM Tris buffer containing 0.05% CHAPS, followed by sonication for 2–3 min. The levels of TBARS (thiobarbituric acid-reacting substances) were determined using a published assay [24]. Briefly, samples were inactivated by adding to trichloroacetic acid (10%, w/v) at 4 °C and were centrifuged to remove the precipitated protein (2000 g, 10 min). The supernatant was added to a mixture of thiobarbituric acid (0.67%, w/v) and butylated hydroxytoluene (10%, w/v) and then heated to 90 °C for 30 min. After cooling to room temperature, the absorbance of the solution was measured at 510 and 532 nm and the absorbance ratio (532–510 nm)/510 nm was calculated. The concentration was determined by reference to malondialdehyde standards.
Results are presented as means±S.E.M., each determination (n) being made on an individually prepared and treated sample. Statistical differences were analysed using one-way ANOVA with Dunnett's post hoc test; P<0.05 was regarded as significant.
RESULTS
NO inactivation by brain tissue
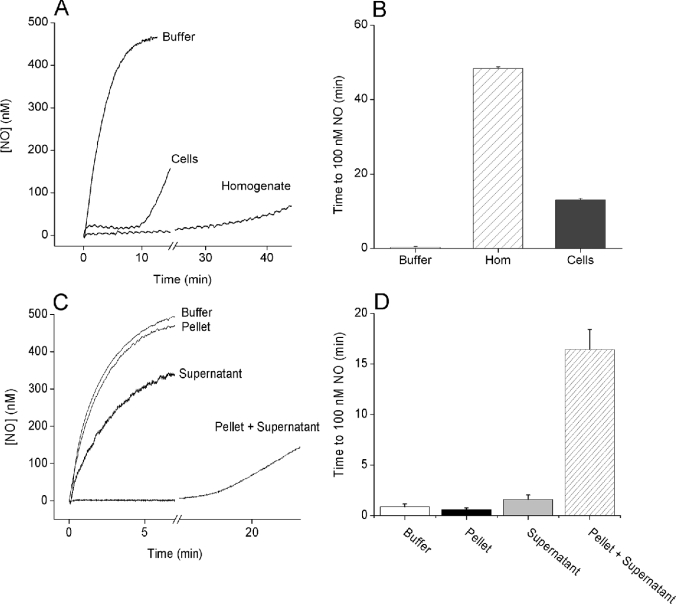
NO consumption by isolated brain cells or brain homogenates was analysed by subjecting them to the NO donor DETA/NO. The long half-life of DETA/NO (20 h at 37 °C) means that the rate of NO release will effectively be constant for several hours. When the DETA/NO (100 μM) was added to Tris buffer, the NO concentration gradually increased to a steady value of approx. 450 nM after 10 min (Figure 1A). During this steady state, the rate of NO release equals the rate of decay by interaction with O2 [25,26]. When DETA/NO was added to either cells or homogenate, a different profile was observed, in agreement with previous observations [19,20]. Within seconds, a low plateau NO concentration was formed (24±4 nM in cells, 8±1 nM in homogenate, n=3–4), but this ‘clamp’ ultimately became exhausted, as indicated by a secondary increase in the NO concentration (Figure 1A). The duration of the NO clamp was quantified as the time taken for the NO concentration to reach 100 nM, which was 0.3±0.2 min in Tris buffer, compared with 48.4±0.4 min in the homogenate and 13.1±0.4 min in cells (Figure 1B). Rate constants for NO consumption, calculated from the steady-state NO concentrations as described previously [19], were 36±2 min−1·(mg of protein)−1 in the homogenate and 3.5±0.4 min−1·(mg of protein)−1 in cells. Rate constants correlated positively with the NO clamp duration (r=0.97, P=0.001).
Figure 1. Inactivation of NO by brain tissue.
Representative traces (A) and a summary of the data (B) of NO consumption after the addition of the NO donor DETA/NO (100 μM) to buffer, cerebellar cells (20×106 cells/ml; 1.25 mg of protein/ml) or whole brain homogenate (0.3 mg of protein/ml), all in the presence of 1000 units/ml superoxide dismutase. (B) NO consumption was quantified as the time taken for the NO concentration to reach 100 nM (means±S.E.M., n=3–4). The homogenate was used at a lower concentration compared with the cells for practical reasons: since the rate of NO consumption by the brain homogenate is linearly related to the protein concentration [20], at 1.25 mg of protein/ml (equivalent to the protein concentration in cells), it would take more than 90 min for NO to reach 100 nM. (C, D) Representative traces and a summary of the data (means±S.E.M., n=3–4) respectively of NO consumption after the addition of DETA/NO to the buffer, pellet (0.1 mg of protein/ml), supernatant (10%) or recombined pellet+supernatant.
Involvement of lipid
That accelerated NO inactivation was preserved (or accentuated) in whole brain homogenate provided the opportunity to identify the underlying mechanism. Subsequent to high-speed centrifugation, there was hardly any activity in either the pellet or the supernatant fraction (filtered to remove free haemoglobin), but a combination of the two reconstituted the activity (Figures 1C and 1D). Further purification of the pellet fraction by standard ion-exchange and size-exclusion chromatography methods produced fractions that, when combined with supernatant, were enriched at least 80-fold in NO-consuming activity. Analysis of the purified fractions by matrix-assisted laser-desorption ionization–time-of-flight MS revealed a strong lipid profile (results not shown), which was subsequently found to comprise 90% phospholipid (10% phosphatidylcholine, 19% phosphatidylinositol, 27% phosphatidylserine and 34% phosphatidylethanolamine).
Inhibiting lipid peroxidation prevents NO consumption by recombined fractions
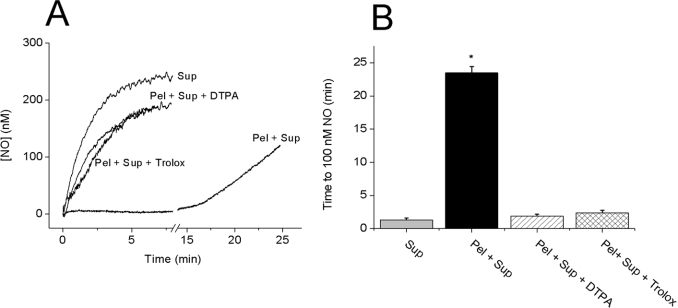
Transition-metal-catalysed reactions can result in lipid peroxidation, generating the peroxyl radical, LOO•. NO can bind avidly to LOO• and is consumed as a result [27]. To investigate whether this mechanism occurs, the effect of adding a metal chelator or an inhibitor of lipid peroxidation to the combined pellet and supernatant was tested. Preincubation with the transition metal chelator DTPA (100 μM) or the synthetic vitamin E analogue Trolox (100 μM) virtually abolished NO inactivation by the combined pellet and supernatant (Figures 2A and 2B), while having no effect on the NO profiles obtained in buffer, or in supernatant or pellet fractions alone (results not shown).
Figure 2. Role of lipid peroxidation in the consumption of NO by combined fractions.
Representative traces (A) and a summary of the data (B) of NO consumption after the addition of 100 μM DETA/NO to the supernatant (Sup; 20%) or supernatant+pellet (Pel; 0.1 mg of protein/ml). The recombined fractions were tested alone or after 10 min preincubation with DTPA (100 μM) or Trolox (100 μM). (B) Results shown are the means±S.E.M. (n=3–5; *P<0.05 versus supernatant control). The vehicle for Trolox (0.1% DMSO) had no effect (n=3; results not shown).
Ascorbate in the supernatant is required for NO consumption
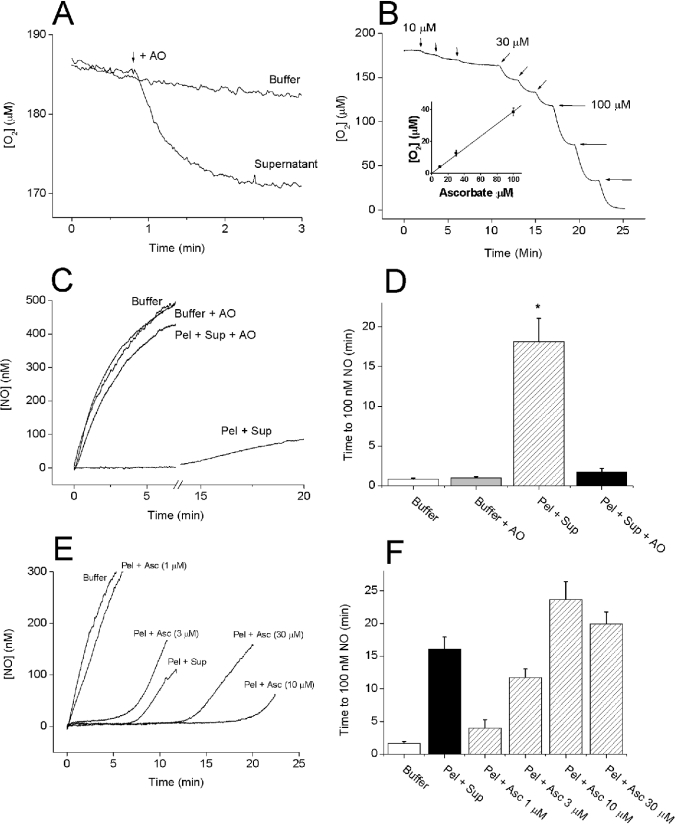
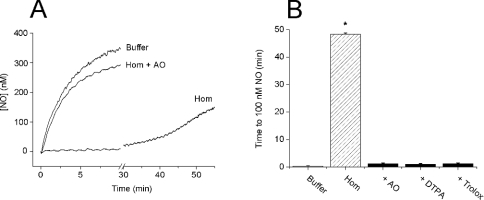
The formation of lipid peroxides by transition-metal-catalysed redox reactions is often enhanced by the addition of ascorbate. At low concentrations, ascorbate acts as a reducing agent and so may redox-cycle metals (e.g. Fe3+) that can catalyse lipid peroxidation [28]. To determine whether ascorbate was present in the supernatant fraction, we added AO, which converts ascorbate and O2 into water and dehydroascorbate, and measured changes in the O2 concentration. When added to the supernatant (10% in Tris buffer), AO (4 units/ml) caused a sharp decrease in [O2] that was complete within 2 min and totalled 11.3±0.8 μM, n=4 (Figure 3A). Further addition of AO had no effect, and no decrease was observed if AO was added to Tris buffer (results not shown). When quantified using standards (Figure 3B), the 10% supernatant contained 25–30 μM ascorbate. Moreover, after preincubation with AO for 3–5 min, the recombined pellet and supernatant failed to clamp NO (Figures 3C and 3D), and ascorbate, at the concentrations estimated to be present, was able to substitute for the supernatant. Finally, preincubation (3–5 min) of the whole homogenate (0.3 mg of protein/ml) with 100 μM DTPA, 100 μM Trolox or 4 units/ml AO almost abolished NO consumption (Figures 4A and 4B), suggesting that the same mechanism explains the behaviour of the homogenate. The ascorbate content in the homogenate at 3 mg of protein/ml was estimated to be 54±1 μM, n=4.
Figure 3. Requirement of ascorbate in the supernatant for NO consumption.
(A) Ascorbate depletion was monitored by measuring O2 consumption on the addition of AO (4 units/ml; as indicated by the arrow) to the supernatant (10%). Each trace is representative of at least three experiments. Further additions had no effect (results not shown). (B) Ascorbate was added (10–100 μM; arrows) to Tris buffer containing AO (4 units/ml) and [O2] was monitored. There was a linear relationship (inset). (C) Representative traces of NO accumulation on the addition of DETA/NO (100 μM) to Tris buffer and pellet (Pel; 0.1 mg of protein/ml)+supernatant (Sup; 10%) in the presence or absence of AO (4 units/ml). (D) A summary of the data from three or four experiments performed as in (C) (means±S.E.M.; *P<0.05 versus buffer control). (E) Representative traces of NO profiles observed on the addition of DETA/NO (100 μM) to Tris buffer, pellet (Pel; 0.1 mg of protein/ml)+supernatant (Sup; 10%) or pellet in the presence of ascorbate (Asc; 1–30 μM). (F) A summary of the data from 4–6 experiments performed as in (E) (means±S.E.M.).
Figure 4. Lipid peroxidation accounts for NO consumption in the brain homogenate.
(A) Representative traces of NO accumulation on the addition of DETA/NO (100 μM) to Tris buffer, homogenate (Hom, 0.3 mg of protein/ml) or homogenate+AO (4 units/ml). Similar traces were obtained with DTPA (100 μM) or Trolox (100 μM), and none of the test compounds affected NO accumulation in Tris buffer (results not shown). (B) A summary of the data from three experiments performed as in (A) (means±S.E.M.; *P<0.05 versus buffer control).
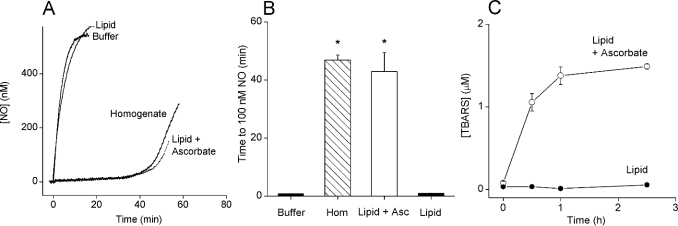
The lipid in whole rat brain homogenate is mainly phospholipid, made up of 40% phosphatidylcholine, 40% phosphatidylethanolamine, 13% phosphatidylserine and 3% phosphatidylinositol [29,30]. A preparation replicating the lipid content of a homogenate at 0.3 mg of protein/ml was tested. The final concentration was 0.2 mg of lipid/ml on the basis of the assumption that the total lipid content of rat brain is 60 mg of lipid/g wet tissue [31]. In the absence of ascorbate, the NO profile in the lipid mixture was similar to that in Tris buffer alone (Figure 5A). In contrast, after preincubation with 15 μM ascorbate (2–3 min), an NO clamp similar to that seen in whole brain homogenate was established (Figure 5A) and was maintained for approx. 35 min. There was no significant difference between the time taken to reach 100 nM NO in the lipid mix (43±7 min) and in the homogenate (47±2 min; Figure 5B). To determine whether ascorbate was required for lipid peroxidation, a time course of the accumulation of the major lipid peroxidation products (TBARS) was undertaken. In the absence of ascorbate, TBARS were not detected in the lipid mixture (Figure 5C). In the presence of ascorbate, however, TBARS accumulated to a maximum concentration of approx. 1.3 μM within 1 h. The levels of TBARS in buffer controls were below detection limits (∼0.1 μM; results not shown).
Figure 5. NO inactivation during lipid peroxidation.
(A) DETA/NO (100 μM) was added to the buffer, a mixture of phospholipids (reconstituted with 0.05% CHAPS detergent) with or without ascorbate (15 μM; 2 min preincubation) or homogenate (0.3 mg of protein/ml)+0.05% CHAPS. The inclusion of 0.05% CHAPS did not alter NO profiles either in Tris buffer or in homogenates (n=3; results not shown). (B) A summary of the data from three experiments performed as in (A) (means±S.E.M.; *P<0.05 versus buffer). (C) Time course of TBARS accumulation in the phospholipid mixture alone or in the presence of ascorbate (15 μM). Results are means±S.E.M.; n=3.
Role of lipid peroxidation in the consumption of NO by brain cell suspension
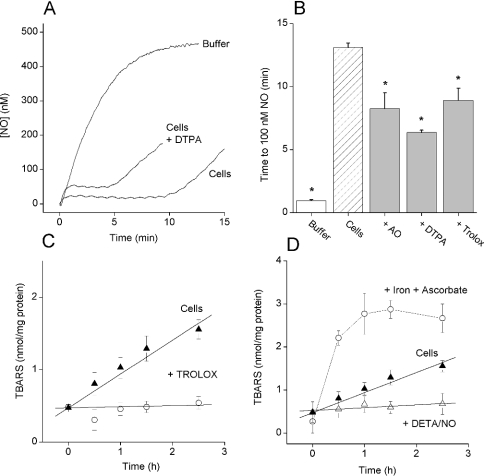
The relevance of these findings in brain homogenates to the mechanism consuming NO in suspensions of living cells freshly isolated from the cerebellum was evaluated. In the presence of 100 μM DTPA, the amplitude of the plateau attained by NO concentration after exposure to 100 μM DETA/NO was increased from 24±4 to 44±4 nM and the duration of the clamp was decreased approximately to half the value (Figure 6A). Similar decreases in clamp duration were observed when the cells were preincubated (2–3 min) with either 4 units/ml AO or 100 μM Trolox (Figure 6B), indicating that the mechanism of NO inactivation by intact cells was partially attributable to lipid peroxidation. A significant decrease (P<0.05) in clamp duration (from 14.1±0.7 to 11.3±0.5 min) was also seen subsequent to incubation with the lazaroid U-74389G (10 μM), another antioxidant [32]. To test whether there was an ongoing lipid peroxidation in the cells, the accumulation of TBARS was monitored. The quantity of TBARS in the cell preparation increased linearly at approx. 0.7 nmol·(mg of protein)−1·h−1 for 2.5 h (Figure 6C). The increase in TBARS was abolished by Trolox and also by the addition of DETA/NO (100 μM; Figure 6D). To gauge the maximum extent of lipid peroxidation in the cell preparation, excess ascorbate (1 mM) and Fe(II)SO4 (1 mM) were added. This evoked a rapid increase in TBARS [the initial rate being approx. 4 nmol·(mg of protein)−1·h−1], reaching a maximum of approx. 2.5 nmol of TBARS/mg of protein after 1 h (Figure 6D). No further increase in TBARS accumulation could be induced by the repeated application of ascorbate and Fe(II)SO4 at 1.5 h (results not shown).
Figure 6. Role of lipid peroxidation in brain cell NO consumption.
(A) Representative traces of NO accumulation on the addition of DETA/NO (100 μM) to Tris buffer or cerebellar cells (1.25 mg of protein/ml) with or without DTPA (100 μM). The effect of DTPA was mimicked by either Trolox (100 μM) or AO (4 units/ml) (results not shown). (B) A summary of the data from three to four experiments performed as in (A) (means±S.E.M.; *P<0.05 versus cells). (C) Time course of TBARS accumulation in the cell suspension under control conditions (▲) or in the presence of Trolox (100 μM; ○). Results are means±S.E.M.; n=3. (D) Time course of TBARS accumulation in the cell suspension under control conditions (▲), in the presence of Fe(II)SO4 and ascorbate (1 mM each; ○) or in the presence of DETA/NO (100 μM; Δ). Results are means±S.E.M.; n=3. Note that data for TBARS accumulation under control conditions is the same in (C and D).
Contribution of contaminant RBCs to NO consumption in cerebellar cell suspensions
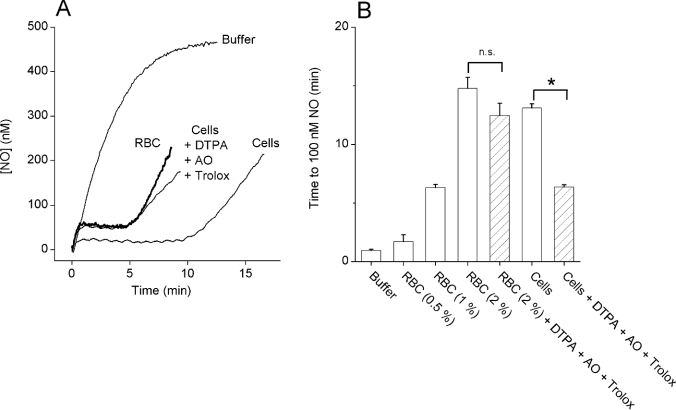
When lipid peroxidation was inhibited by a cocktail of AO, DTPA and Trolox, the cell preparation continued to consume NO, but the amplitude and duration were approximately half of the values found normally (Figures 7A and 7B). The capacity of RBCs to inactivate NO is well known [33], although we had previously discounted their contribution in cells exposed to 250 μM DETA/NO [19]. Since the current experiments used a slower NO release rate (100 μM DETA/NO), the influence of RBCs was re-investigated. The concentration of contaminant RBCs in the cerebellar cell suspension was determined to be 1.6±0.2% (n=4). When standard RBC suspensions (0.5–2%) were exposed to DETA/NO, the NO concentration became clamped in proportion to the RBC concentration (Figure 7B) and tests on a 2% RBC suspension showed that the combination of DTPA, AO and Trolox had no significant effect. The NO consumption in the cerebellar cell suspension in the presence of the lipid peroxidation inhibitors was similar to that obtained with a 1% RBC suspension, indicating that contaminant RBCs accounted for much of the residual activity (Figure 7B).
Figure 7. The contribution of contaminant RBCs to NO consumption by brain cell suspensions.
(A) Representative traces of NO accumulation on the addition of DETA/NO (100 μM) to Tris buffer, to cerebellar cells (1.25 mg of protein/ml) in the absence or presence of a combination of DTPA (100 μM), AO (4 units/ml) and Trolox (100 μM) or to a 1% RBC suspension. The addition of DTPA, AO and Trolox had no effect on the NO profile seen in the RBC suspension (results not shown). (B) A summary of the data from three independent experiments performed as in (A) (*P<0.05; n.s., not significantly different).
A third mechanism of cellular NO consumption
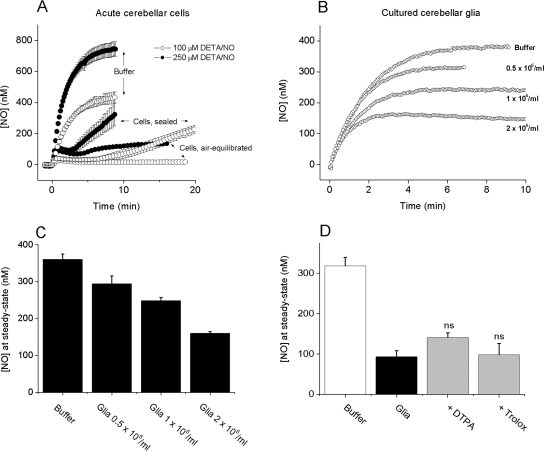
Respiring cerebellar cells, at the concentration used here (20×106/ml), consume O2 at approx. 5 μM/min [19], which inevitably causes O2 levels in a sealed chamber (as used in the above experiments) to decrease progressively. When the experiments were repeated using a chamber open to the atmosphere (thereby keeping the suspending medium equilibrated with air), a further mechanism of NO consumption was revealed. In the presence of 100 μM Trolox and at exposures exceeding the period of consumption attributable to RBCs, the NO concentration (from 100 μM DETA/NO) became persistently clamped (approx. 20 nM NO) under air-equilibrated conditions. The level of the clamp was higher (approx. 100 nM) when the NO release rate was increased (250 μM DETA/NO), but it still endured for the duration of the experiment (≥15 min; Figure 8A).
Figure 8. A third NO consumption mechanism.
(A) Traces of NO accumulation (means±S.E.M.; n=3) after the addition of 100 or 250 μM DETA/NO to buffer in a sealed chamber or to an acute cerebellar cell suspension in either a sealed or an open (air-equilibrated) chamber. Traces in buffer were unchanged in an open chamber (results not shown). (B) Representative profiles of NO accumulation after the addition of 100 μM DETA/NO to increasing concentrations (0.5–2×106/ml) of glia in air-equilibrated suspensions. (C) A summary of the data from three experiments performed as in (B). (D) Steady-state NO concentrations after the addition of 100 μM DETA/NO to buffer or glial cell suspensions (2×106/ml) in the presence or absence of DTPA or Trolox (100 μM each); ns, not significantly different from glia alone.
The additional NO consumption did not appear to be due to contaminating RBCs, because RBC suspensions (0.5–2%) incubated under the same conditions did not display a persistent component of similar magnitude (results not shown). To eliminate complications from this contaminant cell type altogether, NO consumption was measured in suspensions of glial cells from the cerebellum previously grown to confluence in tissue culture. When characterized with standard staining techniques, most of these cells (∼70%) were found to be astrocytes, with 16±3% microglia and 7±3% neurons (results not shown). These cells consumed NO at a rate that depended on cell concentration (Figures 8B and 8C). Metal chelation with DTPA or treatment with Trolox (both at 100 μM) had no significant effect (Figure 8D).
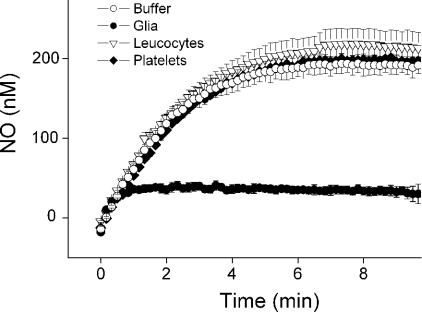
To investigate whether this additional mechanism of NO consumption was a general property of cells, similar studies were performed using platelets and a mixture of leucocytes from the blood. Neither of these preparations consumed NO significantly (Figure 9).
Figure 9. NO consumption in different cell types.
Traces of NO accumulation (means±S.E.M., n=3) after the addition of 100 μM DETA/NO to the buffer or to suspensions of leucocytes, platelets or mixed glial cells (1 mg of protein/ml each). NO concentrations were recorded in an air-equilibrated chamber.
DISCUSSION
Knowledge of the dynamics of NO signals in tissues is critical for understanding how it operates as a biological messenger, but the available information remains scanty. If present, NO inactivating pathways should participate in the shaping of NO signals and thereby influence their information content. The possible malfunctioning of inactivating pathways could help explain how NO contributes to tissue pathology. Using a suspension of brain cells as a model system, we have identified lipid peroxidation as one mechanism contributing to their avid consumption of NO and looked for a further mechanism beneath.
The preservation of NO-consuming activity in brain homogenates prompted a purification strategy that led to the identification of two components: lipid in the pellet fraction and ascorbate in the supernatant. That NO consumption in the homogenate could be replicated simply by combining brain lipids and ascorbate, both at their naturally occurring levels, indicates that these ingredients are sufficient to explain the observed activity. Evidence that the lipids in this mixture underwent peroxidation, combined with findings that NO consumption was blocked by procedures that inhibit lipid peroxidation, strongly suggest that this process is responsible.
Lipid peroxidation is initiated by radical species such as the hydroxyl radical (OH•), which can abstract a hydrogen atom from unsaturated lipid, thus generating a lipid radical (L•) and water. The lipid radical can combine with O2, forming the lipid peroxyl radical (LOO•). In addition to being capable of reacting further with unsaturated lipid, LOO• reacts with NO at a near-diffusion-limited rate {(1–3)×109 M−1·s−1; [34]}. The resulting (transient) LOONO has been suggested to have two fates, rearrangement to a more stable LONO2 or cleavage to give LO• (which may further react with NO, forming LONO) and •NO2 [27]. In the absence of any other target, •NO2 might further react with NO to generate N2O3, which ultimately will hydrolyse to NO2−. Our previous finding that NO consumption was O2-dependent [20] is consistent with lipid peroxidation being responsible and the identification of NO3− as the end-product in brain homogenates can be explained by the NO2− being oxidized by oxyhaemoproteins [35].
The formation of OH•, which initiates lipid peroxidation, may be either by the Haber–Weiss reaction (O2•−+H2O2→O2+OH−+OH•), although this is probably too slow at neutral pH [36] or, more rapidly, by the transition metal-catalysed Fenton reaction (Fe2+/Cu++H2O2→Fe3+/Cu2++OH−+OH•). Brain tissue has high iron levels and, although mainly bound to proteins such as ferritin, O2•− or ascorbate may aid in the mobilization of this iron to participate in OH• formation [37]. In vitro, trace catalytic metals are found in salt and buffer solutions, with iron often reaching 1–10 μM and copper reaching 0.1 μM, or they may be introduced into solution, e.g. from Hamilton syringes [28]. The ability of DTPA, which chelates transition metals, to inhibit NO consumption in the homogenate (or recombined pellet and supernatant fractions) indicates that the metal-catalysed reaction was the one initiating lipid peroxidation.
Fenton chemistry is substantially enhanced by redox cycling of the catalytic transition metal (reduction of Fe3+ to Fe2+ or that of Cu2+ to Cu+), which can be performed by ascorbate [28], thereby explaining the involvement of ascorbate in this component of NO consumption. Brain ascorbate is an important antioxidant, existing at an average concentration of several mM in vivo [38]. However, lower (μM) concentrations are pro-oxidant, and this ‘cross-over’ effect is dependent on the concentration of catalytic metals present [28]. The requirement for ascorbate explains the previous results that NO consumption was decreased by heat and incubation with proteinase K [20]. Ascorbate is readily oxidized and increasing the temperature accelerates its degradation [39]. After incubation with proteinase K (using the same method as before), the loss of NO consuming activity could be reversed by ascorbate (15 μM), indicating that the procedure led to ascorbate depletion (result not shown).
In freshly prepared suspensions of cells from the cerebellum, NO consumption was significantly attenuated by the application of DTPA, AO or Trolox. This, together with evidence that there was an ongoing lipid peroxidation in the cell suspensions, inhibitable by Trolox, indicates that NO was undergoing partial inactivation by reaction with lipid peroxides. DTPA and AO are cell-impermeant, although Trolox will access the cell membrane. Since all three compounds were similarly effective, lipid peroxidation is probably being initiated by the metal-catalysed Fenton reaction extracellularly, promoted by ascorbate leaking from the cells [38]. NO itself, released at a rate of approx. 100 nM/min (from 100 μM DETA/NO), was a highly effective inhibitor of lipid peroxidation, in accordance with previous evidence that NO can perform this function [40–42]. The interrelationship between NO inactivation and inhibition of the process on which it depends explains why this component of NO consumption in the cell suspensions is saturable [19] and that it can be regenerated after prolonged periods without added NO [20] when, probably, significant lipid peroxidation resumes.
The results illustrate the ease with which freshly isolated brain cells become oxidatively stressed and the effect this has on their handling of NO. Apart from their cautionary value for experiments using NO in vitro, the findings may also be pertinent to the multiple situations in vivo in which there is enhanced lipid peroxidation, such as in neurodegenerative disorders, cardiovascular disease, asthma and diabetes [43–47]. Although lipid peroxidation itself is injurious to cells [48,49], the attendant consumption of NO may be sufficient to inhibit local NO signalling and contribute to tissue damage by, for example, decreasing the blood flow. In contrast, the results show that continuous low rates of NO release are capable of stopping lipid peroxidation in cells, an effect consistent with previous work using low-density lipoprotein [40]. The finding that physiological rates of NO release are effective lends support to the concept of NO serving as a protective mechanism under conditions of oxidative stress.
The elucidation of the primary mechanism of NO consumption in cerebellar cell suspensions enabled the operation of a further mechanism (in addition to that explicable by contaminant RBCs) to be revealed. This mechanism was enhanced when the concentration of O2 was increased and was preserved in cultures of cerebellar glial cells. These non-neuronal cells are unlikely to be the only ones possessing NO-degrading activity in the cerebellum, however, since acutely isolated cerebellar cells (prepared to the same protein concentration) are even more active but contain <10% glia. Nonetheless, this did not appear to be a general property of cells, because neither platelets nor leucocytes consumed NO, indicating that it may be a specialized process. Whether or not it is similar to the one inactivating NO in some tumour cell lines remains to be investigated [16], but it is notable that little NO consumption was apparent in homogenates when the lipid peroxidation-dependent mechanism was inhibited (Figure 4A), suggesting that, as in the cell lines [16], much of the activity is lost on homogenization. This lipid peroxidation-independent NO consumption appears to be the dominant mechanism in intact brain slices [50] (C. Hall, unpublished work) and its activity could explain the remarkable resistance of intact brain tissue to the toxic effects of endogenous and exogenous NO [51].
Acknowledgments
This study was funded by the Sir Jules Thorn Charitable Trust and The Wellcome Trust. We thank Dr B. Gibb, Dr R. Cramer and Dr S. Corless for contributing their scientific expertise to this work.
References
- 1.Bellamy T. C., Griffiths C., Garthwaite J. Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J. Biol. Chem. 2002;277:31801–31807. doi: 10.1074/jbc.M205936200. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths C., Wykes V., Bellamy T. C., Garthwaite J. A new and simple method for delivering clamped nitric oxide concentrations in the physiological range: application to activation of guanylyl cyclase-coupled nitric oxide receptors. Mol. Pharmacol. 2003;64:1349–1356. doi: 10.1124/mol.64.6.1349. [DOI] [PubMed] [Google Scholar]
- 3.Brown G. C., Cooper C. E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 4.Koivisto A., Matthias A., Bronnikov G., Nedergaard J. Kinetics of the inhibition of mitochondrial respiration by NO. FEBS Lett. 1997;417:75–80. doi: 10.1016/s0014-5793(97)01258-1. [DOI] [PubMed] [Google Scholar]
- 5.Beckman J. S., Koppenol W. H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 6.Huie R. E., Padmaja S. The reaction of NO with superoxide. Free Radical Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 7.Stuehr D. J., Santolini J., Wang Z. Q., Wei C. C., Adak S. Update on mechanism and catalytic regulation in the NO synthases. J. Biol. Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 8.Hobbs A. J., Gladwin M. T., Patel R. P., Williams D. L., Butler A. R. Haemoglobin: NO transporter, NO inactivator or NOne of the above? Trends Pharmacol. Sci. 2002;23:406–411. doi: 10.1016/s0165-6147(02)02067-9. [DOI] [PubMed] [Google Scholar]
- 9.Liu X., Miller M. J. S., Joshi M. S., Thomas D. D., Lancaster J. R., Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature (London) 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 11.Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature (London) 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 12.Keynes R. G., Griffiths C., Garthwaite J. Superoxide-dependent consumption of nitric oxide in biological media may confound in vitro experiments. Biochem. J. 2003;369:399–406. doi: 10.1042/BJ20020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Soud H. M., Hazen S. L. Nitric oxide is a physiological substrate for mammalian peroxidases. J. Biol. Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 14.Borutaite V., Brown G. C. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem. J. 1996;315:295–299. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffey M. J., Natarajan R., Chumley P. H., Coles B., Thimmalapura P. R., Nowell M., Kuhn H., Lewis M. J., Freeman B. A., O'Donnell V. B. Catalytic consumption of nitric oxide by 12/15-lipoxygenase: inhibition of monocyte soluble guanylate cyclase activation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8006–8011. doi: 10.1073/pnas.141136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner P. R., Martin L. A., Hall D., Gardner A. M. Dioxygen-dependent metabolism of nitric oxide in mammalian cells. Free Radical Biol. Med. 2001;31:191–204. doi: 10.1016/s0891-5849(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell V. B., Taylor K. B., Parthasarathy S., Kuhn H., Koesling D., Friebe A., Bloodsworth A., Darley-Usmar V. M., Freeman B. A. 15-Lipoxygenase catalytically consumes nitric oxide and impairs activation of guanylate cyclase. J. Biol. Chem. 1999;274:20083–20091. doi: 10.1074/jbc.274.29.20083. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell V. B., Coles B., Lewis M. J., Crews B. C., Marnett L. J., Freeman B. A. Catalytic consumption of nitric oxide by prostaglandin H synthase-1 regulates platelet function. J. Biol. Chem. 2000;275:38239–38244. doi: 10.1074/jbc.M001802200. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths C., Garthwaite J. The shaping of nitric oxide signals by a cellular sink. J. Physiol. (Cambridge, U.K.) 2001;536:855–862. doi: 10.1111/j.1469-7793.2001.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths C., Yamini B., Hall C., Garthwaite J. Nitric oxide inactivation in brain by a novel O2-dependent mechanism resulting in the formation of nitrate ions. Biochem. J. 2002;362:459–464. doi: 10.1042/0264-6021:3620459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin D., Tabb D. L., Yates J. R., III Large-scale protein identification using mass spectrometry. Biochim. Biophys. Acta. 2003;1646:1–10. doi: 10.1016/s1570-9639(02)00546-0. [DOI] [PubMed] [Google Scholar]
- 22.Garthwaite J., Garthwaite G. Cellular origins of cyclic GMP responses to excitatory amino acid receptor agonists in rat cerebellum in vitro. J. Neurochem. 1987;48:29–39. doi: 10.1111/j.1471-4159.1987.tb13123.x. [DOI] [PubMed] [Google Scholar]
- 23.Brown M. J., Bristow D. R. Molecular mechanisms of benzodiazepine-induced down-regulation of GABAA receptor alpha 1 subunit protein in rat cerebellar granule cells. Br. J. Pharmacol. 1996;118:1103–1110. doi: 10.1111/j.1476-5381.1996.tb15512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esterbauer H., Cheeseman K. H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 25.Ford P. C., Wink D. A., Stanbury D. M. Autoxidation kinetics of aqueous nitric oxide. FEBS Lett. 1993;326:1–3. doi: 10.1016/0014-5793(93)81748-o. [DOI] [PubMed] [Google Scholar]
- 26.Kharitonov V. G., Sundquist A. R., Sharma V. S. Kinetics of nitric oxide autoxidation in aqueous solution. J. Biol. Chem. 1994;269:5881–5883. [PubMed] [Google Scholar]
- 27.O'Donnell V. B., Chumley P. H., Hogg N., Bloodsworth A., Darley-Usmar V. M., Freeman B. A. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry. 1997;36:15216–15223. doi: 10.1021/bi971891z. [DOI] [PubMed] [Google Scholar]
- 28.Buettner G. R., Jurkiewicz B. A. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat. Res. 1996;145:532–541. [PubMed] [Google Scholar]
- 29.Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem. J. 1981;195:301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diagne A., Fauvel J., Record M., Chap H., Douste-Blazy L. Studies on ether phospholipids. II. Comparative composition of various tissues from human, rat and guinea pig. Biochim. Biophys. Acta. 1984;793:221–231. doi: 10.1016/0005-2760(84)90324-2. [DOI] [PubMed] [Google Scholar]
- 31.Chavko M., Nemoto E. M., Melick J. A. Regional lipid composition in the rat brain. Mol. Chem. Neuropathol. 1993;18:123–131. doi: 10.1007/BF03160026. [DOI] [PubMed] [Google Scholar]
- 32.Khalil A., Fortun A., Hebert S., Jay-Gerin J. P., El Abbouyi A., Wallach J., Fulop T., Jr Novel 21-aminosteroid U-74389G inhibits low-density lipoprotein peroxidation induced by •OH and O2•− free radicals. Life Sci. 1998;63:769–779. doi: 10.1016/s0024-3205(98)00332-4. [DOI] [PubMed] [Google Scholar]
- 33.Liu X., Miller M. J., Joshi M. S., Sadowska-Krowicka H., Clark D. A., Lancaster J. R., Jr Diffusion-limited reaction of free nitric oxide with erythrocytes. J. Biol. Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 34.Padmaja S., Huie R. E. The reaction of nitric oxide with organic peroxyl radicals. Biochem. Biophys. Res. Commun. 1993;195:539–544. doi: 10.1006/bbrc.1993.2079. [DOI] [PubMed] [Google Scholar]
- 35.Ignarro L. J., Fukuto J. M., Griscavage J. M., Rogers N. E., Byrns R. E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koppenol W. H. The Haber-Weiss cycle – 70 years later. Redox. Rep. 2001;6:229–234. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch. Biochem. Biophys. 1986;246:501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- 38.Rice M. E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 39.Meucci E., Martorana G. E., Ursitti A., Pischiutta M. G., Miggiano G. A., Castelli A. Ascorbic acid stability in aqueous solutions. Acta Vitaminol. Enzymol. 1985;7:147–153. [PubMed] [Google Scholar]
- 40.Goss S. P., Hogg N., Kalyanaraman B. The effect of nitric oxide release rates on the oxidation of human low density lipoprotein. J. Biol. Chem. 1997;272:21647–21653. doi: 10.1074/jbc.272.34.21647. [DOI] [PubMed] [Google Scholar]
- 41.Hiramoto K., Ohkawa T., Oikawa N., Kikugawa K. Is nitric oxide (NO) an antioxidant or a prooxidant for lipid peroxidation? Chem. Pharm. Bull. (Tokyo) 2003;51:1046–1050. doi: 10.1248/cpb.51.1046. [DOI] [PubMed] [Google Scholar]
- 42.Kelley E. E., Wagner B. A., Buettner G. R., Burns C. P. Nitric oxide inhibits iron-induced lipid peroxidation in HL-60 cells. Arch. Biochem. Biophys. 1999;370:97–104. doi: 10.1006/abbi.1999.1386. [DOI] [PubMed] [Google Scholar]
- 43.Behl C., Moosmann B. Antioxidant neuroprotection in Alzheimer's disease as preventive and therapeutic approach. Free Radical Biol. Med. 2002;33:182–191. doi: 10.1016/s0891-5849(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 44.Esterbauer H., Wag G., Puhl H. Lipid peroxidation and its role in atherosclerosis. Br. Med. Bull. 1993;49:566–576. doi: 10.1093/oxfordjournals.bmb.a072631. [DOI] [PubMed] [Google Scholar]
- 45.Mattson M. P. Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci. 1998;21:53–57. doi: 10.1016/s0166-2236(97)01188-0. [DOI] [PubMed] [Google Scholar]
- 46.Wood L. G., Gibson P. G., Garg M. L. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003;21:177–186. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- 47.Yorek M. A. The role of oxidative stress in diabetic vascular and neural disease. Free Radical Res. 2003;37:471–480. doi: 10.1080/1071576031000083161. [DOI] [PubMed] [Google Scholar]
- 48.Liu R., Liu W., Doctrow S. R., Baudry M. Iron toxicity in organotypic cultures of hippocampal slices: role of reactive oxygen species. J. Neurochem. 2003;85:492–502. doi: 10.1046/j.1471-4159.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 49.Robb S. J., Gaspers L. D., Wright K. J., Thomas A. P., Connor J. R. Influence of nitric oxide on cellular and mitochondrial integrity in oxidatively stressed astrocytes. J. Neurosci. Res. 1999;56:166–176. doi: 10.1002/(sici)1097-4547(19990415)56:2<166::aid-jnr6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Hall C., Griffiths C., Garthwaite J. Kinetics of nitric oxide inactivation in rat brain. J. Physiol. (Cambridge, U.K.) 2003;547P:44P. [Google Scholar]
- 51.Keynes R. G., Duport S., Garthwaite J. Hippocampal neurons in organotypic slice culture are highly resistant to damage by endogenous and exogenous nitric oxide. Eur. J. Neurosci. 2004;19:1163–1173. doi: 10.1111/j.1460-9568.2004.03217.x. [DOI] [PubMed] [Google Scholar]