Abstract
Live Salmonella typhimurium phoPc bacteria were tested as mucosal vaccine vectors to deliver Helicobacter pylori antigens. The genes encoding the A and B subunits of H. pylori urease were introduced into S. typhimurium phoPc and expressed under the control of a constitutive tac promoter (tac-ureAB) or a two-phase T7 expression system (cT7-ureAB). Both recombinant Salmonella strains expressed the two urease subunits in vitro and were used to nasally immunize BALB/c mice. The plasmid carrying cT7-ureAB was stably inherited by bacteria growing or persisting in the spleen, lungs, mesenteric or cervical lymph nodes, and Peyer’s patches of immunized mice, while the plasmid carrying tac-ureAB was rapidly lost. Spleen and Peyer’s patch CD4+ lymphocytes from mice immunized with S. typhimurium phoPc cT7-ureAB proliferated in vitro in response to urease, whereas cells from mice given S. typhimurium phoPc alone did not. Splenic CD4+ cells from mice immunized with phoPc cT7-ureAB secreted gamma interferon and interleukin 10, while Peyer’s patch CD4+ cells did not secrete either cytokine. Specific H. pylori anti-urease immunoglobulin G1 (IgG1) and IgG2A antibodies were detected following immunization, confirming that both Th1- and Th2-type immune responses were generated by the live vaccine. Sixty percent of the mice (9 of 15) immunized with S. typhimurium phoPc cT7-ureAB were found to be resistant to infection by H. pylori, while all mice immunized with phoPc tac-ureAB (15 of 15) or phoPc (15 of 15) were infected. Our data demonstrate that H. pylori urease delivered nasally by using a vaccine strain of S. typhimurium can trigger Th1- and Th2-type responses and induce protective immunity against Helicobacter infection.
Helicobacter pylori causes persistent infection and inflammation in the human stomach. The infection can lead to peptic ulcer disease and is also a risk factor for gastric adenocarcinoma (32) and malignant mucosa-associated lymphoid tissue (MALT) lymphoma (42). An immunological or a vaccine approach to clear chronic H. pylori infection was initially rejected by many investigators and clinicians based on the observation that natural immunity was unable to cure or prevent Helicobacter infection and chronic atrophic gastritis. Animal studies, however, have established that immunization with Helicobacter whole-cell extracts or purified components is efficient for the prevention of infection and, more importantly, for the treatment of preexisting infections (2, 5, 7, 8, 19, 23, 25, 41).
In all successful vaccination protocols, mucosal adjuvants, i.e., cholera toxin or Escherichia coli labile toxin, had to be included to elicit protection or cure. In humans, a clinical trial has been conducted with heat-labile enterotoxin, but the dose of the toxin had to be reduced because of intestinal toxicity (26).
The purpose of the present study was to determine whether recombinant attenuated Salmonella bacteria expressing a Helicobacter antigen could be used as a vaccine delivery system. A single oral dose of Salmonella vaccines is efficient at inducing mucosal and systemic antibody and cellular responses to carried antigens (10, 21, 33, 35, 37), explained in part by the ability of Salmonella bacteria to persist in tissues for several weeks after immunization (14). The phoPc strain of Salmonella typhimurium is attenuated in macrophage survival and avirulent in mice (27), but it induces both secretory immunoglobulin A (IgA) and serum IgG responses to expressed foreign antigens, irrespective of the route of mucosal administration (14, 30, 31).
In this study, we have determined whether recombinant S. typhimurium phoPc vaccine strains expressing the urease of H. pylori would protect BALB/c mice against subsequent H. pylori infection and compared two modes of expression of the foreign protein. The two urease subunits, UreA and UreB, were either constitutively or conditionally expressed in S. typhimurium phoPc. The strain that constitutively expressed urease rapidly lost the expression plasmid and was found to be ineffective at triggering an immune response against urease, while the conditional strain retained the plasmid in vivo, elicited specific cellular and humoral responses against the foreign antigen, and conferred protection against Helicobacter.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free female BALB/c mice (Harlan, Horst, Netherlands) were housed in microisolator cages with free access to water and chow. This study was approved by the State Veterinary Office (authorization no. 836).
Bacterial strains.
The S. typhimurium phoPc strain, kindly provided by John Mekalanos (Harvard Medical School, Boston, Mass.) is derived from strain ATCC 14028 and is attenuated in both virulence and survival within macrophages in vivo (28).
The gene encoding the T7 RNA polymerase was inserted into the chromosome of the S. typhimurium phoPc strain as described elsewhere (43, 44).
H. pylori P49, kindly provided by Harry Kleanthous (OraVax Ltd., Cambridge, Mass.), is a human clinical isolate adapted to mice (17).
Construction of the expression vectors.
The expression plasmid pYZ97 (43) is referred to as construct cT7-ureAB, and the expression plasmid pDB3 which contains H. pylori urease A and B genes controlled by the tac promoter is referred to as construct tac-ureAB. The ureA and ureB genes were cloned from H. pylori by PCR. A 5′ primer (GGAATTCCGAGATGAAACTCACCCCAAAAG) and a 3′ primer (GGAATTCTGCAGCTAGAAAATGCTAAAGAGT) were used in a PCR with Taq polymerase (Pharmacia Biotech, Dübendorf, Switzerland) to amplify the 2.4-kb fragment (EMBL accession no. M60398; nucleotides 2656 to 5085) containing the sequences for ureA and ureB flanked by EcoRI and PstI restriction enzyme digestion sites. The amplified product was digested and ligated into the corresponding sites of pKK223-3 (Pharmacia). The plasmid was introduced into the phoPc strain by electroporation.
Immunization.
For each immunization, a single colony of Salmonella was grown at 37°C in L broth with or without 100 μg of ampicillin per ml to an optical density at 600 nm of 0.6 to 0.8, corresponding to ∼0.8 × 108 bacteria/ml. After a 10-min centrifugation at 5,000 × g, the bacterial pellet was resuspended in phosphate-buffered saline to yield ∼2.5 × 109 bacteria/ml. BALB/c mice were slightly anesthetized with halothane (Halocarbon Laboratories, River Edge, N.J.) and given two nasal doses of 5 × 107 CFU (20 μl/mouse) at a 2-week interval.
Infection.
H. pylori P49 was grown on GC agar plates supplemented with IsoVitaleX and horse serum or in brain heart infusion broth supplemented with 0.25% yeast extract and 10% horse serum under microaerophilic conditions as described previously (2, 11). BALB/c mice were infected 2 weeks after the last immunization with two doses of 5 × 108 bacteria by gastric intubation at a 2-day interval.
Assessment of H. pylori colonization.
The stomach of each mouse was isolated and cut longitudinally in half. One moiety was submitted to a rapid urease test (RUT; Jatrox test; Procter and Gamble, Weiterstadt, Germany); the results were quantified by spectrophotometric analysis at an optical density of 550 nm. The cutoff value of the RUT used to discriminate between infection and cure corresponded to the mean + 2 standard deviations (SD) of the absorbance values obtained for gastric biopsy specimens of naive mice (2). The other half was processed for histology; gastric fragments were fixed in neutral buffered 10% formalin, embedded in paraffin, and routinely processed. Five-micrometer-thick sections were stained with cresyl violet and hematoxylin-eosin, and the number of H. pylori organisms was assessed in the antral and fundal mucosa on coded sections (25).
Antigen-induced CD4+-T-cell proliferation.
Lymphocytes were isolated from the spleen by forcing the tissue through nylon cell strainers (Falcon; Becton Dickinson, Franklin Lakes, N.J.) and resuspended in complete medium containing 10% fetal calf serum as previously described (34). CD4+ T cells were separated by positive selection with magnetic beads as described in the manufacturer’s instructions (Mini-Macs; Myltenyi Biotec, Bergisch-Gladbach, Germany). The resulting lymphocyte population was over 95% CD4+, as determined by fluorescence-activated cell sorting (34). The CD4+-cell suspension was adjusted to 105 cells/well in 96-well flat-bottom cell culture plates (Costar, Cambridge, Mass.) together with 105 syngeneic irradiated (3,000 rads) splenic feeder cells from naive mice. Recombinant H. pylori urease (apoenzyme kindly provided by OraVax) was added at 100 μg/ml. Cells were incubated at 37°C and 5% CO2 for 5 days in triplicate and pulsed with 0.5 μCi of [3H]thymidine (NEN Life Science Products, Brussels, Belgium) for the last 16 h of incubation. Thymidine incorporation was measured by liquid scintillation. The values are expressed as Δcpm, derived by subtracting the counts obtained for unstimulated cells from the counts obtained for antigen-stimulated cells. All groups were also stimulated for 3 days with 2 μg of concanavalin A (Boehringer, Mannheim, Germany) per ml for assessment of viability.
Cytokine measurement.
The presence of gamma interferon (IFN-γ) and interleukin 10 (IL-10) was determined by sandwich enzyme-linked immunosorbent assay (ELISA), and the presence of IL-4 was determined by bioassay by using the CT.4S cell line, as described previously (1, 36). Values were calculated from a standard curve by using recombinant mouse IFN-γ expressed in L1210 cells, recombinant IL-10 (PharMingen Deutschland GmbH), and recombinant mouse IL-4 expressed in X63Ag-653 cells. The limits of detection of these assays were 8 U/ml for IFN-γ, 4 pg/ml for IL-4, and 125 pg for IL-10.
Serum antibody titers.
Serum antibody titers specific for urease were determined by ELISA as previously described (34). Microtiter plates were coated with 0.5 μg of recombinant urease per well. Specific antibodies were detected with rabbit anti-mouse IgG, IgG1, and IgG2a (Dako, Zug, Switzerland) used at a dilution of 1:500. Bound antibodies were detected with horseradish peroxidase-conjugated rabbit anti-mouse antibodies (Amersham, Zürich, Switzerland) at a dilution of 1:1,000. Immune complexes were detected with o-phenylenediamine (Sigma, Buchs, Switzerland) in the presence of 0.03% H2O2 at 15 min after incubation. The values are given as the mean optical densities from duplicates read at 492 nm for a serum dilution of 1:200. Antibody titers were estimated by end point dilutions of mouse sera (n = 3) and expressed as geometric means of reciprocal dilutions estimated as greater than four times the values of naive animals for IgG1 and greater than six times the values of naive animals for IgG2a.
Saliva was collected after intraperitoneal injection of 100 μg of pilocarpine (Sigma) by using absorbent wicks (12), which were then centrifuged at 15,000 × g for 5 min to recover the secretions. Urease-specific IgAs in saliva (1/5 dilution) were detected by ELISA as described above by using a biotinylated anti-IgA secondary antibody (KPL Inc., Gaithersburg, Md.).
Western blots.
Bacterial lysates were analyzed on polyacrylamide gels and transferred to nitrocellulose by use of a Bio-Rad (Richmond, Calif.) transblot apparatus. Transferred proteins were detected with a rabbit polyclonal antiurease antibody. A goat anti-rabbit IgG conjugated with horseradish peroxidase was then applied, and the blots were developed with enhanced chemiluminescence as described in the supplier’s instructions (Amersham).
Statistical analysis.
The absence or presence of H. pylori infection was evaluated by the two-tailed Fisher exact test; the levels of IgAs in saliva specimens of different immunized mice and the numbers of H. pylori in gastric biopsy specimens of different animals were both compared by the Wilcoxon rank sum test.
RESULTS
Expression of H. pylori urease A and B subunits by S. typhimurium phoPc in vitro.
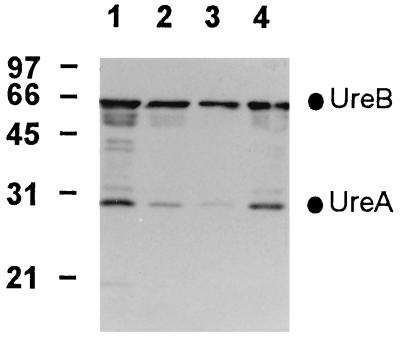
Whole-cell lysates of recombinant S. typhimurium phoPc strains expressing H. pylori urease A and B genes were analyzed by Western blotting (Fig. 1). Reaction of the extracts with polyclonal sera against urease revealed two immunoreactive bands with Mrs of 31 and 61 corresponding to the A and B subunits, respectively. The constitutive tac promoter-based expression system (tac-ureAB) leads to a larger accumulation of H. pylori urease in S. typhimurium phoPc than the two-phase T7 expression system (cT7-ureAB). The expressed antigen was found to be stable in both instances.
FIG. 1.
In vitro expression of H. pylori urease A and B subunits by S. typhimurium phoPc. Bacterial lysates of S. typhimurium phoPc expressing the two subunits of H. pylori urease were analyzed by Western blotting. The urease A and B subunits were detected with rabbit sera. Lanes: 1 and 2, 3 and 1 μg of S. typhimurium phoPc tac-ureAB, respectively; 3 and 4, 1 and 3 μg of S. typhimurium phoPc cT7-ureAB, respectively. The mobilities of the prestained molecular size standards (in kilodaltons) are indicated on the left.
Growth and stability of the bacteria.
The growth curve of S. typhimurium phoPc was not affected by transformation (data not shown). The plasmid cT7-ureAB was retained in S. typhimurium phoPc following extended cultures without selective pressure, while more than 90% of S. typhimurium phoPc transformed with tac-ureAB had lost their plasmid after 12 h in culture (data not shown).
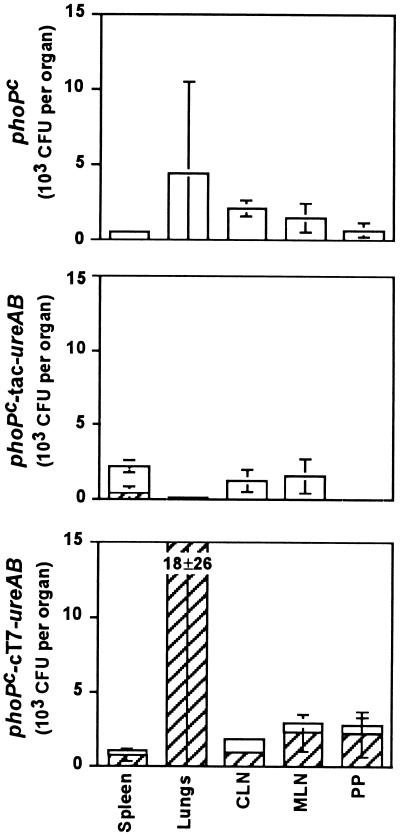
We then analyzed the stability of the transformed S. typhimurium phoPc in vivo. Groups of three mice received nasally 5 × 107 organisms. The spleens, lungs, mesenteric and cervical lymph nodes, and Peyer’s patches (PP) were removed 2 and 4 weeks after administration. The bacteria were recovered from tissue lysates, and the numbers of CFU were determined in the presence or absence of ampicillin (Fig. 2). Although variations were observed between individual animals, the overall survival levels of bacteria were comparable for the three different groups of mice, and the same numbers of bacteria were recovered from the various organs whether the mice received recombinant or control phoPc strains. The cT7-ureAB plasmid was retained for at least 4 weeks, whereas the tac-ureAB plasmid was almost completely lost after the same time period in the recovered bacteria (data not shown).
FIG. 2.
Recovery of S. typhimurium phoPc after nasal immunization. Groups of three mice were nasally immunized with 5 × 107 CFU of S. typhimurium phoPc (top panel), phoPc tac-ureAB (middle panel), or phoPc cT7-ureAB (bottom panel) and sacrificed after 2 weeks. The spleen, lungs, cervical and mesenteric lymph nodes (CLN and MLN, respectively) and PP were recovered, homogenized in 15% sucrose–phosphate-buffered saline, and plated on agar plates with (closed bars) or without (hatched bars) ampicillin. Data are expressed as means ± SD of CFU per tissue of three animals.
T-cell response induced by the recombinant attenuated Salmonella.
BALB/c mice received a single nasal dose of 107 S. typhimurium phoPc cT7-ureAB or untransformed phoPc bacteria. Spleen and PP were removed 4 weeks after immunization. CD4+ T cells were isolated and cultured in vitro with H. pylori urease and lethally irradiated spleen antigen-presenting cells. CD4+ T cells from mice immunized with phoPc cT7-ureAB proliferated in response to antigenic stimulation ([3H]thymidine incorporation measured at 2,034 ± 798 cpm [mean ± SD] in the spleen and 1,869 ± 1,069 cpm in the PP), while CD4+ T cells from mice immunized with control phoPc did not respond ([3H]thymidine incorporation measured at 176 ± 22 cpm in the spleen and 0 ± 1 cpm in the PP). Spleen but not PP CD4+ T cells from phoPc cT7-ureAB-immunized mice released significant amounts of IFN-γ. The Th2-type cytokine IL-10 was also detected in the supernatant of these stimulated splenic CD4+ T cells but not IL-4 (Table 1).
TABLE 1.
Cytokine secretion by CD4+ cells in spleen and PP of S. typhimurium-vaccinated mice
| Vaccine | IFN-γ concn (U/ml)a
|
IL-4 concn (pg/ml)a
|
IL-10 concn (pg/ml)a
|
|||
|---|---|---|---|---|---|---|
| Spleen | PP | Spleen | PP | Spleen | PP | |
| phoPc | <8b | ND | <4 | <4 | <125 | <125 |
| phoPc cT7-ureAB | 706 ± 10 | <8 | <4 | <4 | 1,077 ± 105 | <125 |
Secretion values for cytokines were measured in culture supernatant of CD4+ T cells after 5 days of incubation with 100 μg of recombinant H. pylori urease per ml; they are expressed as means ± SD from duplicate measurements.
Cytokine values preceded by < are under the detection threshold. In the absence of stimulation with antigen, the cytokine values were all below the threshold of detection.
Antibody response to Helicobacter urease.
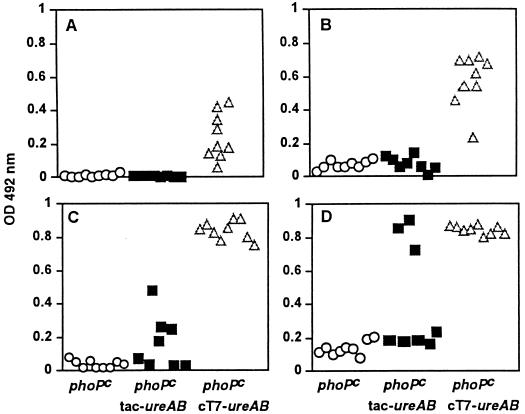
After immunization, there were no detectable specific antiurease antibodies in the sera of mice immunized with phoPc tac-ureAB or in control mice which received the attenuated live carrier only (phoPc). In contrast, sera from mice vaccinated with phoPc T7-ureAB contained significant levels of both IgG1 and IgG2A antibodies (Fig. 3A and B). Low levels of IgAs were also measured in saliva secretions; the optical density value of urease-specific IgAs for mice immunized with phoPc T7-ureAB was 0.06 ± 0.03 (n = 15; mean ± SD), compared to 0.02 ± 0.01 (n = 13) for mice immunized with phoPc (P = 0.0007). Subsequent infection of immunized mice by H. pylori did not induce detectable levels of IgG1 antibodies in phoPc-immunized mice, but a small increase in IgG2A antibodies was observed, consistent with a Th1-type response induced by infection (Fig. 3C and D).
FIG. 3.
Antiurease antibodies in the serum of immunized and immunized and infected mice. Sera were collected from mice after immunization with S. typhimurium phoPc (○), S. typhimurium phoPc tac-ureAB (▪), and S. typhimurium phoPc cT7-ureAB (▵) (A and B) or from mice after immunization and then infection with H. pylori 4 weeks later (C and D) (symbols the same as those for panels A and B). IgG1A (A and C) and IgG2a (B and D) were separately determined by isotype-specific ELISA for each mouse serum (dilution of 1:200). OD, optical density.
While infection of BALB/c mice does not seem to trigger specific antibody against urease, elevated titers of IgG1 and IgG2A were observed in infected mice previously immunized with phoPc cT7-ureAB (Table 2). It is of interest to note that some of the mice immunized with phoPc tac-ureAB also produced significant levels of antibodies after being infected and that this immune response did not lead to protection (see below).
TABLE 2.
Serum antibody titers in S. typhimurium-vaccinated micea
| Vaccine | IgG1 titer
|
IgG2A titer
|
||
|---|---|---|---|---|
| Preinfection | Postinfection | Preinfection | Postinfection | |
| phoPc | 0 | 0 | 0 | 0.8 ± 1 |
| phoPc tac-ureAB | 0 | 1.9 ± 1.4 | 0 | 3.4 ± 0.1 |
| phoPc cT7-ureAB | 4.4 ± 0.2 | 4.7 ± 0 | 4.2 ± 0.1 | 5 ± 0 |
Antibody titers were estimated by end point dilutions as described in Materials and Methods.
Protection against Helicobacter infection.
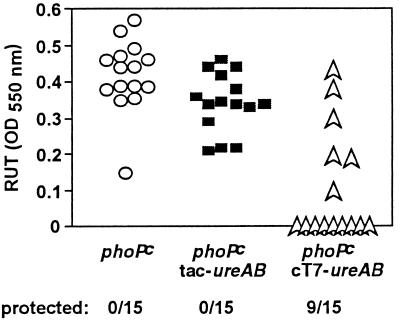
BALB/c mice were nasally immunized twice at 2-week intervals with 5 × 107 S. typhimurium phoPc bacteria expressing H. pylori urease. Control mice received untransformed phoPc. All animals were infected intragastrically with H. pylori 2 weeks after the second immunization. Sixty percent (9 of 15) of the mice vaccinated with phoPc cT7-ureAB were resistant to H. pylori infection. In contrast, all of the mice immunized with phoPc tac-ureAB (15 of 15) and all of the control mice (15 of 15) were infected, as reflected by the high urease activity detected in their stomachs (Fig. 4). Detection of H. pylori in stomach sections of the vaccinated mice confirmed the urease tests (Table 3). In one of the mice found to be negative by the urease test, a few bacteria were detected in the gastric mucosa (4 bacteria per 10 crypts); in all other cases, the two tests were 100% concordant. The degree of inflammation induced by the mouse-adapted H. pylori strain was so weak in H. pylori-infected BALB/c mice that no difference could be observed between protected and infected animals (data not shown).
FIG. 4.
Assessment of H. pylori colonization. Mice were immunized twice and infected 2 weeks later. Bacterial colonization was assessed by the RUT and quantified by spectrophotometric analysis at 550 nm. OD, optical density. P = 0.0007 (phoPccT7-ureAB versus phoPc), Fisher’s exact test.
TABLE 3.
Assessment of H. pylori presence in gastric biopsy specimens in immunized micea
| RUT results | No. of H. pylori bacteria/10 crypts
|
||
|---|---|---|---|
| phoPc | tac-ureAB | cT7-ureAB | |
| Positive | 39 ± 25 (n = 9)b | 28 ± 17 (n = 8) | 23 ± 26c (n = 2) |
| Negative | 0 (n = 0) | 0 (n = 0) | <1 ± 1 (n = 7)d |
One half of the stomach of each mouse immunized with S. typhimurium phoPc, S. typhimurium phoPc tac-ureAB, or S. typhimurium phoPc cT7-ureAB was subjected to the RUT, and the other half was processed for microscopic analysis as described in Materials and Methods.
n = number of mice.
P = 0.006 compared to value for phoPc-immunized mice; P = 0.0008 compared to value for phoPc tac-ureAB-immunized mice (by Wilcoxon rank sum test).
Six animals were found free of bacteria by microscopy, but one presented 4 H. pylori bacteria per 10 crypts.
DISCUSSION
Helicobacter infection in humans and in mice triggers a predominant Th1-type nonprotective immune response (6, 15, 29). Protective immunity can be achieved in animals when Helicobacter antigens are given together with a mucosal adjuvant such as cholera toxin or E. coli heat-labile toxin (2, 3, 5, 8, 18, 19, 23, 25).
One of the possible roles of the adjuvant is to break oral tolerance (22). Orally administered soluble antigens are known to induce a state of immune unresponsiveness known as oral tolerance (39), and proteolysis of these antigens into small peptides in the digestive tract is believed to facilitate this induction. Another essential role of adjuvants could be to direct CD4+-T-cell-subset differentiation. We have recently shown that mucosal immunization with recombinant UreB and cholera toxin progressively induces a Th2 CD4+-T-cell response which is not present during infection and correlates with protection (34). While it is becoming clear that bacterial toxins together with antigen trigger cellular effector functions that interfere with early stages of Helicobacter pathogenesis, these effectors have so far not been identified.
One major drawback with bacterial toxin adjuvants is that they are toxic in humans. In a phase I-II clinical trial, recombinant urease with heat-labile toxin was given orally to H. pylori-infected volunteers (26). The dose of toxin had to be reduced from 10 to 5 μg because of intestinal toxicity. A transient decrease in the gastric bacterial load was observed. The poor efficacy in the volunteers might have been due to low adjuvancy or to gastric degradation of the antigen. One cannot rule out, however, that the vaccination protocol and regimen used were unable to trigger the type of immune response that is effective in mice (2) and ferrets (3).
The present study was aimed at improving oral vaccine delivery systems by using a live attenuated Salmonella carrier. Live attenuated Salmonella spp. used as vectors for heterologous antigens have several advantages over conventional vaccines and are expected to circumvent most of the problems associated with adjuvants. They do not require antigen purification or formulation with adjuvants, they bypass the degradation and denaturation of antigens in the stomach, and they induce cell-mediated, humoral, and secretory IgA antibody responses (21, 24, 40). Furthermore, immune responses to foreign antigens expressed by Salmonella are independent of the route of mucosal administration (14, 30).
A major obstacle to overcome when using Salmonella vaccines, however, is the achievement of stable expression of the foreign gene at levels sufficient to trigger an immune response without impairing bacterial viability or releasing antigens at inappropriate sites. We compared the efficacy of two gene expression systems, a conventional high-copy-number expression vector (pKK223-3), mediating high-level foreign antigen expression, and the phase variation system in which only a percentage of recombinant bacteria express the antigen (43, 44). The in vitro and in vivo stability of the former vector in the absence of antibiotic was very poor, in contrast to studies using the same attenuated strain of Salmonella constitutively expressing the recombinant hepatitis B virus core antigen protein (14); the constitutive expression of urease rapidly led to the selection of plasmid-“cured” strains, and consequently, poor immune responses with no protection against H. pylori challenge were obtained.
In contrast, the phase variation system, in which only a small proportion of recombinant Salmonella at any given time expresses the foreign protein, was found to be very stable and triggered an antigen-specific cellular and humoral immune response that protected mice against H. pylori challenge. These results confirm that genetic stabilization of foreign antigen expression is a crucial step in the development of an immunogenic vaccine strain.
Nasal delivery of attenuated S. typhimurium induced urease-specific CD4+ T cells producing IFN-γ and IL-10 in the spleen, indicating that both Th1- and Th2-type responses were elicited against urease. The analysis of the subclasses of serum urease-specific IgG also supported the presence of a mixed Th1- and Th2-type response. Similar observations were reported for mice immunized with recombinant Salmonella expressing the C fragment of tetanus toxoid (40). VanCott et al. (40) indeed demonstrated the existence of an alternate Th2 pathway with CD4+ T cells producing IL-10, but not IL-4 or IL-5, in both mucosal and systemic lymphoid tissues and with macrophages releasing IL-6. We recently showed that oral administration of urease B and cholera toxin elicited a Th2 CD4+-T-cell response characterized by IL-4 secretion (34). It appears therefore that mucosal delivery of the same antigen via attenuated Salmonella results in the selective induction of CD4+ T cells secreting IL-10, confirming the observations of VanCott et al. that two different mucosal antigen delivery protocols can result in the selective induction of a distinct CD4+-T-cell-subset response (40). However, in contrast to what was observed by these authors, we have not been able to detect secretion of IFN-γ, IL-4, or IL-10 in PP CD4+ T cells despite the fact that these cells proliferated in vitro when stimulated with urease.
We have shown that recombinant S. typhimurium elicited urease-specific serum IgG1 and IgG2A antibodies as well as low IgA titers in salivary secretions. Levels of serum antibody, however, did not correlate with protection in all cases; two animals with high titers of serum antibody were not protected, while most mice with low titers were protected. Protection does not seem to always correlate with high concentrations of serum antibody but rather with the presence of antigen-specific antibody-producing plasma cells in the gastric mucosa (9, 19). In a recent study, Weltzin and coworkers (41) nasally administered H. pylori recombinant urease with or without mucosal adjuvants. In the absence of adjuvant, high levels of serum-specific IgG and secretory IgA in saliva and feces were obtained, but mice were not protected against Helicobacter felis challenge. Coadministration of cholera toxin or E. coli heat-labile toxin elicited lower levels of antibody to urease, but mice were protected against gastric infection. There were no attempts, however, to localize urease-specific antibody-producing plasma cells in the gastric mucosa, and a definitive proof of the role of antibodies in protection against Helicobacter infection awaits more experimental work.
Foreign antigens expressed in the attenuated strain Salmonella phoPc have been shown to be particularly immunogenic (14, 31). The attenuation results from a single point mutation in the phoP gene product (27), which yields constitutive expression of the two-component phoPQ system (28). Its use, however, is restricted to experimental animals, since there is an inherent risk of reversion to wild type. A number of attenuated Salmonella typhi strains, which include the phoP mutant and double mutants such as ΔaroC ΔaroD and Δcya Δcrp (4, 20, 38), have been tested in humans, and could be good candidates as vectors to deliver Helicobacter antigens. However, care must be taken when changing Salmonella strains for use in clinical trials since different attenuations and the use of S. typhi instead of S. typhimurium strains may affect the fate of the bacteria in MALT and hence the immune response leading to protection against H. pylori infection. Recently, we analyzed the fate in PP of three S. typhimurium strains expressing the same recombinant protein (Salmonella typhimurium wild type, phoPc, and Δcya Δcrp (13). All three isogenic strains were observed associated with dendritic cells present in the dome region just below the follicle-associated epithelium at early time points (16), suggesting that the fate of Salmonella vaccines in MALT is not dependent on the type of attenuation. It remains to be seen whether attenuation determines in which intracellular compartment the foreign antigen is released or whether it affects antigen presentation by dendritic cells or macrophages.
The fact that protection mediated by recombinant Salmonella does not require mucosal adjuvants makes live carriers ideal vaccine delivery systems, which should encourage the search for better H. pylori vaccines. In addition, Salmonella provides new tools to dissect the immune mechanisms mediating protection and may facilitate the identification of the immune correlates of protection.
ACKNOWLEDGMENTS
We thank E. Saraga for histology analysis, H. Kleanthous and J. Mekalanos for providing the H. pylori and S. typhimurium strains, Bertrand Ess for animal handling, C. Dieterich for experimental assistance, and P. Michetti for continuous support.
This work was supported by grants from the Swiss National Science Foundation (no. 31-043240.95 to A.L.B., no. 31-46858.96 to I.C.T., and no. 31-47110.96 to J.P.K.) and the Swiss League against Cancer (no. FOR 42 to J.P.K.). I.C.T. is a recipient of a Swiss Confederation Grant for Academic Scientists (bourse de relève de la Confédération).
REFERENCES
- 1.Abrams J S, Roncarolo M G, Yssel H, Andersson U, Gleich G J, Silver J E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 2.Corthésy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease as a treatment against Helicobacter infection. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 3.Cuenca R, Blanchard T, Lee C K, Monath T P, Redline R, Nedrud J G, Czinn S J. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets. Gastroenterology. 1995;108:A78. doi: 10.1053/gast.1996.v110.pm8964402. [DOI] [PubMed] [Google Scholar]
- 4.Curtiss R D, Kelly S M, Hassan J O. Live oral avirulent Salmonella vaccines. Vet Microbiol. 1993;37:397–405. doi: 10.1016/0378-1135(93)90038-9. [DOI] [PubMed] [Google Scholar]
- 5.Czinn S J, Nedrud J G. Oral immunization against Helicobacter pylori. Infect Immun. 1991;59:2359–2363. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Elios M M, Manghetti M, Decarli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Delprete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 7.Doidge C, Crust I, Lee A, Buck F, Hazell S, Manne U. Therapeutic immunisation against Helicobacter infection. Lancet. 1994;343:914–915. doi: 10.1016/s0140-6736(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 8.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Recombinant antigens prepared from the urease subunits of Helicobacter spp: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrero R L, Thiberge J M, Labigne A. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology. 1997;113:185–194. doi: 10.1016/s0016-5085(97)70094-5. [DOI] [PubMed] [Google Scholar]
- 10.Galen J E, Gomez-Duarte O G, Losonsky G A, Halpern J L, Lauderbaugh C S, Kaintuck S, Reymann M K, Levine M M. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 11.Haas R, Meyer T F, van Putten J P. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol Microbiol. 1993;8:753–760. doi: 10.1111/j.1365-2958.1993.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 12.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins, S., and J. P. Kraehenbuhl. Dendritic cells of the murine Peyer’s patches colocalize with Salmonella typhimurium avirulent mutants in the subepithelial dome. In Fourth International Symposium on Dendritic Cells in Fundamental and Clinical Immunology, in press. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 14.Hopkins S, Kraehenbuhl J-P, Schödel F, Potts A, Peterson D, De Grandi P, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karttunen R A, Karttunen T J, Yousfi M M, el-Zimaity H M, Graham D Y, el-Zaatari F A. Expression of mRNA for interferon-gamma, interleukin-10, and interleukin-12 (p40) in normal gastric mucosa and in mucosa infected with Helicobacter pylori. Scand J Gastroenterol. 1997;32:22–27. doi: 10.3109/00365529709025058. [DOI] [PubMed] [Google Scholar]
- 16.Kelsall B L, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome of T cell regions of the murine Peyer’s patch. J Exp Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleanthous H, Tibbits T, Bakios T J, Georgopoulos K, Myers G, Ermak T H, Fox J, Monath T. In vivo selection of a highly adapted H. pylori isolate and the development of an H. pylori mouse model for studying vaccine efficacy and attenuating lesions. Gut. 1995;37:A94. [Google Scholar]
- 18.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit–whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C K, Weltzin R, Thomas W D, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 20.Levine M M, Herrington D, Murphy J R, Morris J G, Losonsky G, Tall B, Lindberg A A, Svenson S, Baqar S, Edwards M F, Stocker B. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987;79:888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine M M, Hone D, Tacket C, Ferreccio C, Cryz S. Clinical and field trials with attenuated Salmonella typhi as live oral vaccines and as “carrier” vaccines. Res Microbiol. 1990;141:807–816. doi: 10.1016/0923-2508(90)90114-6. [DOI] [PubMed] [Google Scholar]
- 22.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 23.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 24.Mekalanos J J. Bacterial mucosal vaccines. Adv Exp Med Biol. 1992;327:43–50. doi: 10.1007/978-1-4615-3410-5_6. [DOI] [PubMed] [Google Scholar]
- 25.Michetti P, Corthésy-Theulaz I, Davin C, Haas R, Vaney A C, Heitz M, Bille J, Kraehenbuhl J P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 26.Michetti P, Kreiss C, Kotloff K, Porta N, Blanco J L, Bachmann D, Saldinger P F, Corthésy-Theulaz I, Losonsky G, Nichols R, Stolte M, Monath T, Ackerman S, Blum A L. Oral immunization of H. pylori infected adults with recombinant urease and LT adjuvant. Gastroenterology. 1997;112:A1042. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 27.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller S I, Mekalanos J J. Constitutive expression of the PhoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 30.Nardelli-Haefliger D, Kraehenbuhl J-P, Curtiss III R, Schödel F, Potts A, Kelly S, De Grandi P. Oral and rectal immunization of adult female volunteers with a recombinant attenuated Salmonella typhi vaccine strain. Infect Immun. 1996;64:5219–5224. doi: 10.1128/iai.64.12.5219-5224.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nardelli-Haefliger D, Roden R B S, Benyacoub J, Sahli R, Kraehenbuhl J-P, Schiller J T, Lachat P, Potts A, De Grandi P. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun. 1997;65:3328–3336. doi: 10.1128/iai.65.8.3328-3336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 33.Sadoff J C, Ballou W R, Baron L S, Majarian W R, Brey R N, Hockmeyer W T, Young J F, Cryz S J, Ou J, Lowell G H, Chulay J D. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988;240:336–338. doi: 10.1126/science.3281260. [DOI] [PubMed] [Google Scholar]
- 34.Saldinger, P. F., N. Porta, P. Launois, J. A. Louis, G. A. Waanders, P. Michetti, A. L. Blum, and I. Corthésy-Theulaz. Mucosal immunization of BALB/c mice with Helicobacter urease B induces a T helper type 2 response not seen during infection with Helicobacter. Submitted for publication. [DOI] [PubMed]
- 35.Schödel F. Oral vaccination using recombinant bacteria. Semin Immunol. 1990;2:341–349. [PubMed] [Google Scholar]
- 36.Swihart K, Fruth U, Messmer N, Hug K, Behin R, Huang S, Del Giudice G, Aguet M, Louis J A. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med. 1995;181:961–971. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tacket C O, Hone D M, Curtiss III R, Kelly S M, Losonsky G, Guers L, Harris A M, Edelman R, Levine M M. Comparison of the safety and immunogenicity of ΔaroC ΔaroD and Δcya Δcrp Salmonella typhi strains in adult volunteers. Infect Immun. 1992;60:536–541. doi: 10.1128/iai.60.2.536-541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacket C O, Sztein M B, Losonsky G A, Wasserman S S, Nataro J P, Edelman R, Pickard D, Dougan G, Chatfield S N, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas H C, Parrott M V. The induction of tolerance to a soluble protein antigen by oral administration. Immunology. 1974;27:631–639. [PMC free article] [PubMed] [Google Scholar]
- 40.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 41.Weltzin R, Kleanthous H, Guirakhoo F, Monath T P, Lee C K. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine. 1997;15:370–376. doi: 10.1016/s0264-410x(97)00203-x. [DOI] [PubMed] [Google Scholar]
- 42.Wotherspoon A C, Ortiz-Hidalgo C, Falzon M R, Isaacson P G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 43.Yan Z X, Meyer T F. The variation antigen expression: a natural phenomenon utilised for the construction of live recombinant Salmonella vaccines. Behring Inst Mitt. 1994;95:49–54. [PubMed] [Google Scholar]
- 44.Yan Z X, Reuss F, Meyer T F. Construction of an invertible DNA segment for improved antigen expression by a hybrid Salmonella vaccine strain. Res Microbiol. 1990;141:1003–1004. doi: 10.1016/0923-2508(90)90140-l. [DOI] [PubMed] [Google Scholar]