Abstract
INTRODUCTION
We investigated the effect of perivascular spaces (PVS) volume on speeded executive function (sEF), as mediated by white matter hyperintensities (WMH) volume and plasma glial fibrillary acidic protein (GFAP) in neurodegenerative diseases.
METHODS
A mediation analysis was performed to assess the relationship between neuroimaging markers and plasma biomarkers on sEF in 333 participants clinically diagnosed with Alzheimer's disease/mild cognitive impairment, frontotemporal dementia, or cerebrovascular disease from the Ontario Neurodegenerative Disease Research Initiative.
RESULTS
PVS was significantly associated with sEF (c = ‐0.125 ± 0.054, 95% bootstrap confidence interval [CI] [‐0.2309, ‐0.0189], p = 0.021). This effect was mediated by both GFAP and WMH.
DISCUSSION
In this unique clinical cohort of neurodegenerative diseases, we demonstrated that the effect of PVS on sEF was mediated by the presence of elevated plasma GFAP and white matter disease. These findings highlight the potential utility of imaging and plasma biomarkers in the current landscape of therapeutics targeting dementia.
Highlights
Perivascular spaces (PVS) and white matter hyperintensities (WMH) are imaging markers of small vessel disease.
Plasma glial fibrillary protein acidic protein (GFAP) is a biomarker of astroglial injury.
PVS, WMH, and GFAP are relevant in executive dysfunction from neurodegeneration.
PVS's effect on executive function was mediated by GFAP and white matter disease.
Keywords: cerebrovascular diseases, executive function, neurodegenerative diseases, perivascular spaces, plasma glial fibrillary acidic protein, white matter hyperintensities
1. INTRODUCTION
Under normal conditions, the perivascular spaces (PVS) surrounding the brain's arterioles, capillaries, and venules, are an important component of the glymphatic system. 1 , 2 This perivascular system of tunnels serves as a fluid pathway through which interstitial fluids circulate and metabolic waste solutes can be cleared out of the brain. 3 , 4 Normal PVS are not typically seen on standard structural MRI and can only be visualized when enlarged. Thus, MRI‐visible PVS, often observed in cerebrovascular and neurodegenerative disease patients, 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 are believed to be an indicator of glymphatic dysfunction and are recently recognized as an imaging‐based feature of cerebral small vessel disease. 13
It is unclear, however, if PVS burden is associated with cognition, with mixed results demonstrated in the literature. In one recent study that applied a comprehensive neuropsychological protocol to dementia and stroke‐free older population, a higher PVS burden was related to worse cognitive performance, specifically with information processing speed and executive function (EF), even after adjusting for demographic and common risk profiles. 14 Similarly, in a longitudinal study of Alzheimer's disease (AD) patients, PVS were associated with cognitive decline, independent of amyloid burden and other markers of small vessel disease. 15 Conversely, findings from a large memory clinic population that included patients with subjective cognitive decline, mild cognitive impairment (MCI), or dementia; 16 and, a meta‐analysis of five large population‐based studies from the UNIVRSE consortium, 17 found no association of PVS burden with cognitive dysfunction. Interestingly, despite the mixed results in the literature with PVS and cognition, white matter hyperintensities (WMH)—another commonly used MRI‐based marker of cerebral small vessel disease—are often associated with decreased information processing speed and EF, with minimal controversial findings in the literature. 18 , 19 , 20
Of particular interest to glymphatic clearance and the perivascular space is the glial fibrillary acidic protein (GFAP), an intermediate filament protein found in astrocytes that is a commonly used marker of astroglial injury and glymphatic dysfunction. 21 , 22 Post‐mortem analyses of the deep white matter of patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), an inherited form of cerebral small vessel disease, found increased numbers of GFAP‐positive clasmatodendritic astrocytes, particularly in the perivascular regions of the deep white matter—suggestive of severe compromise of the gliovascular unit. 23 In AD patients, similar astrogliosis‐related increases in GFAP were observed in neuropathological analyses of WMH regions compared to normal‐appearing white matter. 24
In contrast to imaging‐based biomarkers, plasma biomarkers have garnered recent interest in clinical research as less invasive and cost‐effective diagnostic tools for various neurodegenerative diseases causing dementia. 25 Plasma GFAP has been associated with frontotemporal dementia (FTD), 26 cerebral small vessel disease, 27 AD, and vascular pathology. 28 In vivo biomarker studies demonstrated associations of plasma GFAP with cognition and EF, where higher plasma GFAP levels were associated with lower EF scores among older adults with and without cognitive impairment. 29 , 30 Along with plasma neurofilament light chain (NfL), plasma GFAP is currently being considered as a non‐specific biomarker that will be included in a Revised Criteria for the Diagnosis and Staging of Alzheimer's Disease, by the Alzheimer's Association Workgroup. 31
Thus, given the cognitive outcomes potentially related to imaging‐ and plasma‐based biomarkers in cerebral small vessel disease and neurodegeneration, the main objective of this study was to examine the direct and indirect effects of PVS on speeded executive function (sEF) as mediated by GFAP and WMH, in a clinical neurodegenerative patient population.
2. METHODS
2.1. Participants
From the Ontario Neurodegenerative Disease Research Initiative (ONDRI) patient cohort, participants clinically diagnosed with Alzheimer's disease/mild cognitive impairment (AD/MCI), FTD, and cerebrovascular disease (CVD), with available clinical, demographic, MRI, neuropsychological, genetic and plasma biomarkers data were selected for analysis. ONDRI recruited participants from 14 tertiary care centers across Ontario, Canada, each of whom had passed preliminary screening. Detailed inclusion/exclusion criteria, demographics, and cohort characteristics of the ONDRI study were published previously. 32 , 33 Briefly, the prevailing consensus‐based clinical diagnostic criteria at the time of enrollment for each ONDRI‐focused disease were implemented at each tertiary clinic: AD/MCI patients met National Institute on Aging—Alzheimer's Association criteria for dementia or MCI due to probable AD, with annual tracking of MCI patients to ensure accurate characterization of the cohort; FTD patients included possible or probable behavioral variants of frontotemporal degeneration, agrammatic/non‐fluent and semantic variants of primary progressive aphasia, and possible or probable progressive supranuclear palsy. The CVD cohort comprised individuals who experienced mild to moderate acute ischemic stroke, transient ischemic attack, or subcortical infarction, at least 3 months prior to enrollment, or severe white matter disease with subcortical infarcts, in compliance with the National Institute of Neurological Disorders and Stroke and the Canadian Stroke Network vascular cognitive impairment harmonization standards. Evidence of stroke required clinical imaging confirmation; however, individuals with large vessel occlusive infarction causing severe neurological deficits were excluded. Additionally, the AD/MCI patients’ MRI scans were assessed by a neuroradiologist to exclude individuals with non‐AD‐related causes for cognitive impairment. Ethics approval was obtained from the Research Ethics Board at each participating site. All participants provided written informed consent.
RESEARCH IN CONTEXT
Systematic review: MRI‐visible perivascular spaces (PVS) suggest glymphatic waste clearance dysfunction, with conflicting findings on cognition impact. Prior work links impaired glymphatic clearance to white matter injury and astrocytic reactivity in neurodegenerative diseases. White matter hyperintensities (WMH) and plasma glial fibrillary acidic protein (GFAP) associated with executive dysfunction. No in vivo studies explored PVS's indirect effects on speeded executive function (sEF) via WMH and plasma GFAP in neurodegenerative populations.
Interpretation: We demonstrated a small but significant effect of PVS volume on sEF, mediated by plasma GFAP and WMH.
Future directions: Our findings emphasize assessing these markers in memory clinics when faced with multiple co‐pathologies. They support a cascade: glymphatic dysfunction, perivascular enlargement, astroglial GFAP activation, neuroinflammatory cycle, and WMH eventually leads to sEF deficits. Future studies should focus on longitudinal data, include larger samples across different diseases, and explore other markers of glymphatic dysfunction and neuroinflammation.
2.2. Neuroimaging
The 3 Tesla MRI included the following sequences: three‐dimensional (3D) T1‐weighted (T1), interleaved proton density and T2‐weighted (T2), T2‐fluid attenuated inversion recovery (FLAIR), and T2*‐weighted gradient echo imaging sequences. Imaging protocol details were previously published and included in a Supplemental File. 34 All MRI images were evaluated to ensure excellent imaging quality by a medical biophysicist (R.B.) and for clinical incidental findings by a licensed neuroradiologist (S.S.). Neuroimaging‐based markers for cerebral small vessel disease (WMH, PVS, lacunes, and infarcts), normal‐appearing gray matter (NAGM) volumes for gray matter (GM) atrophy, and total intracranial volume (TIV) for head‐size correction, were quantified using ONDRI's standardized image‐processing pipeline 34 in compliance with the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria. 13
In brief, volumetric data was acquired using the semi‐automated ONDRI post‐processing neuroimaging pipeline—a comprehensive, multi‐feature (T1, PD/T2, FLAIR) segmentation algorithm based on the SABRE‐LE (Semi‐automated brain extraction‐ Lesion explorer) and FLEX (Fuzzy lesion extraction) procedures 35 , 36 , 37 and optimized for the ONDRI study. 34 Each individual automated segmentation output mask was then manually checked and corrected as needed by neuroimaging analysts, who attained a DICE similarity index and intraclass correlation coefficient, both > 0.9. After the volumetric data was generated, the data was run through a novel multivariate outlier detection algorithm for data quality evaluation by an independent Neuroinformatics team 38 and fed back to the Neuroimaging team for further manual checking (and correction if required), as an iterative quality control procedure.
2.3. Neuropsychological assessment
All patients underwent the ONDRI standardized neuropsychological test protocol which provided a comprehensive assessment of cognition and behavior. 39 Screening was done for English comprehension, visual acuity, auditory acuity, and physical impairments which may confound test performance. The neuropsychological test scores were standardized (i.e., z‐transformed) using the mean and standard deviation of the normal participants from the Brain Eye Amyloid Memory Study (clinicaltrials.gov: NCT02524405). A composite measure for sEF was computed using mean z‐scores from the Digit Symbol Modalities Test, and the Trail Making Test Part B time, where increasing z‐scores were associated with increased speed, that is, better performance. The Trail Making Test Part B time was log‐transformed prior to standardization. Montreal Cognitive Assessment (MoCA) tests were also performed on all study participants.
2.4. Plasma biomarkers and genetics
Blood samples were drawn from all participants at baseline and shipped to LifeLabs (Etobicoke, Ontario, Canada) where they were immediately processed upon receipt for plasma isolation by centrifugation at 2000 × g rpm for 15 min at 4°C, and then stored at ‐80°C. They were then shipped on dry ice to the OBI Biobank Sample Reception at the Robarts Research Institute (Western University, London, ON, Canada), and stored there until shipment to the Clinical Neurochemistry Laboratory (University of Gothenburg, Mölndal, Sweden) for measurements. The concentrations of GFAP and NfL were measured using the Human Neurology 4‐plex E ultra‐sensitive Single molecule array (Simoa) immunoassay (Item 103670, Quanterix). The measurements were performed on an HD‐X Analyzer according to instructions from the manufacturer (Quanterix, Billerica, MA, USA). The measurements were performed in one round of experiments using one batch of reagents by board‐certified laboratory technicians who were blinded to clinical data. As a general index of directionality, higher GFAP and NfL are considered more pathological. Plasma NfL was included in the analysis as a relevant biomarker that is being considered in future clinical criteria. 31
Apolipoprotein E epsilon 4 (APOE E4) carrier statuses for each participant were mapped using the calls for the APOE risk alleles rs429358(CT) and rs7412(CT) extracted from the ONDRISeq custom next‐generation sequencing‐based panel data, as previously described. 40 , 41
2.5. Statistics
To assess the effect of PVS volumes on sEF through GFAP level and WMH volume, a mediation analysis was applied using the PROCESS macro v3.5 in IBM SPSS (v28.0.1.1, SPSS Inc., Chicago, IL, USA). Bias‐corrected bootstrapping with 5000 replications and 95% confidence interval (CI) was performed to estimate the variability of direct, indirect, and total effects. We identified covariates by conducting separate Pearson correlations and linear regressions between demographic, medical history, and biomarker variables, on sEF and PVS. The following variables that were significant were selected as covariates in the main analyses: age, years of education, clinical diagnosis, hypertension, NAGM, NfL, lacunes, and presence of apolipoprotein E (APOE) E4. All variables were standardized with the mean and standard deviation of our sample. All neuroimaging variables were head‐size corrected via TIV; PVS and WMH were log‐transformed due to skewness.
3. RESULTS
The final sample size was 333: 155 patients with CVD, 126 patients with AD/MCI, and 52 patients with FTD. The mean age of our sample was 69.7 (± 7.7) years, the sample was mainly male (62.8%), highly educated (14.8 ± 2.9 years), with a mean total MoCA score of 23.7 ± 3.5 (Table 1). Although statistically significant, WMH and GFAP were only mildly correlated (Pearson r = 0.154, p = 0.005).
TABLE 1.
Demographics and neuroimaging volumetrics of the study population
| No. of participants | N = 333 |
|---|---|
| Demographics | |
| Age, years | 69.7 ± 7.7 |
| Sex, male | 209 (62.8%) |
| Level of education, years | 14.8 ± 2.9 |
| Montreal Cognitive Assessment, Total score | 23.7 ± 3.5 |
| Alcohol use (more than 7 drinks per week) | 60 (18%) |
| Clinical diagnosis | |
| Alzheimer's disease/Mild cognitive impairment | 125 (37.8%) |
| Cerebrovascular disease | 155 (46.5%) |
| Frontotemporal dementia | 52 (15.6%) |
| Vascular risk factors | |
| Hypertension | 166 (49.8%) |
| Dyslipidemia | 206 (61.9%) |
| Diabetes | 67 (20.1%) |
| Currently smoking | 22 (6.6%) |
| Waist/hip circumference | 0.93± 0.09 |
| Coronary artery disease | 34 (10.2%) |
| Atrial fibrillation | 38 (11.4%) |
| Neuroimaging | |
| Total intracranial volume, mean (SD), cm3 | 1232.01 ± 136.92 |
| Gray matter volume, mean (SD), cm3 | 533.18 ± 52.53 |
| MRI‐visible perivascular spaces volume, median (IQR), mm3 | 43.0 (56.50) |
| WMH, median (IQR), cm3 | 3.54 (6.22) |
| Plasma biomarkers | |
| GFAP, pg/mL | 118 ± 99.44 |
| Neurofilament light chain, pg/mL | 28.43 ± 24.37 |
Abbreviations: GFAP, glial fibrillary acidic protein; IQR, interquartile range; WHM, white matter hyperintensities.
PVS was significantly associated with sEF (c = ‐0.125 ± 0.054, 95% bootstrap [CI] [‐0.2309, ‐0.0189], p = 0.021, black on Figure 1). This effect was mediated by both GFAP and WMH. Specifically, the indirect effect of PVS on sEF via GFAP was significant (a1b1 = ‐0.0197 ± 0.0108, 95% CI [‐0.0442, ‐0.0021], a1: p = 0.005; b1: p = 0.029, blue on Figure 1); the indirect effect via WMH was also significant (a2b2 = ‐0.0316 ± 0.0180, 95% CI [‐0.0712, ‐0.0016], a2: p < 0.001; b2: p = 0.031, green on Figure 1). The total indirect effect of PVS on sEF via GFAP and WMH was significant (‐0.051 ± 0.022, 95% CI [‐0.097; ‐0.013], red in Figure 1).
FIGURE 1.
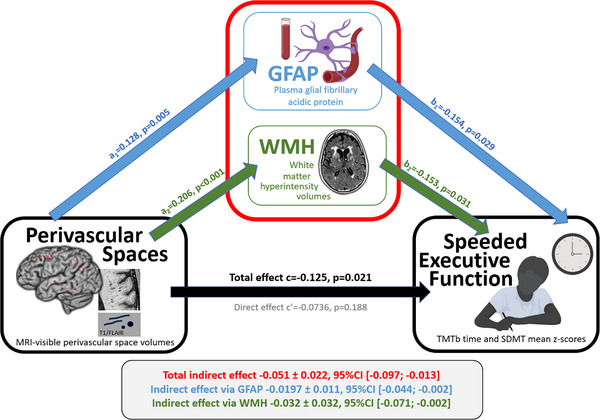
Plasma GFAP and WMH volume mediate the effect of PVS on sEF. Mediation Model showing plasma glial fibrillary acidic protein and white matter hyperintensity volumes are significantly mediating the associations of perivascular space volumes with speeded executive function. GFAP, glial fibrillary acidic protein; PVS, perivascular spaces; SDMT, Digit Symbol Modalities Test; sEF, speeded executive function; TMTb, Trail Making Test Part B; WMH, white matter hyperintensities (created with BioRender.com)
4. DISCUSSION
In this unique clinical cohort of mixed neurodegenerative and cerebrovascular patients, this study's findings shed light on the complex in vivo biomarker relationships between PVS, plasma GFAP, WMH, and cognition. Specifically, we demonstrated that PVS has a small but significant effect on sEF and that this effect was mediated via plasma GFAP and WMH in this clinical population. Our in vivo findings support the increasing neuropathological evidence that perivascular enlargement from glymphatic clearance dysfunction, commonly observed in mixed cerebral small vessels and neurodegenerative disease, may be associated with increased GFAP expression in the perivascular regions. 21 , 23 , 24 , 42 The cascade of glymphatic clearance dysfunction, perivascular enlargement, astroglial GFAP activation, the neuroinflammatory cycle, and WMH (likely due to perivascular edema from venous collagenosis 43 , 44 ), eventually leads to downstream deficits in sEF observed in our neurodegenerative and CVD patients.
Previously regarded as benign radiological anomalies, PVS has garnered recent interest due to advances in our understanding of cerebral fluid circulation and glymphatic waste clearance. PVS, previously referred to as Virchow‐Robin spaces, are anatomically defined as the fluid‐filled channels surrounding the brain's vasculature, bound by the adventitia of the vessel and the astrocytic end‐feet. 9 Facilitated by aquaporin‐4 (AQP4) water channels expressed by astrocytes, glymphatic perivascular clearance of harmful metabolic waste, such as amyloid β, has been shown in preclinical models to be active primarily during deep sleep. 4 Indeed, the clearance of amyloid β, tau, and α‐synuclein, hallmark neuropathological protein aggregates in AD and other neurodegenerative diseases, has been a therapeutic target since their discovery over 100 years ago. Additionally, the role of perivascular neuroinflammation 45 indicated by the activation of microglia and astrocytes, 46 and the GFAP expression observed in WMH regions from cerebral small vessel disease, 21 , 23 , 42 likely contributes to the overall impairment of sEF that was indicated by the PVS burden observed in our mixed neurodegenerative and CVD patients’ MRI. The mediating roles of GFAP and WMH on PVS may be the pieces to the puzzle that explain the mixed findings in the literature in regard to PVS and cognition.
Although grounded primarily from preclinical and neuropathological research findings, ample evidence regarding the clinical relevance of PVS burden is evident in the recent update to the Boston criteria. 47 Used for in vivo clinical diagnosis of cerebral amyloid angiopathy (CAA), the Boston criteria v2.0 includes the assessment of T2‐weighted MRI for severe PVS burden—defined as > 20 PVS in the centrum semiovale of one hemisphere. As this criteria would mark the first clinical diagnostic criteria that include PVS burden, it is likely that further advances that include plasma GFAP in diagnostic criteria may soon follow. 31
There are several important unanswered factors that are highly relevant to this discussion that should be given attention in future work: (1) the inclusion of a sleep quality measure, given its role in glymphatic system activation; (2) the measurement of AQP4 as driver for the movement of fluids into perivascular channels; (3) a measure of amyloid β, tau, and/or α‐synuclein burden in our patient population; and 4) the role of APOE4 and pericyte degeneration in blood‐brain barrier dysfunction. 48 , 49 In addition to these unanswered factors, it is also important to note that we did not examine longitudinal changes in cognition which prevented us from inferring directionality in our models; and, although the overall sample was decently powered, the individual patient cohorts were relatively small, which limited our statistical ability to examine individual disease cohorts. However, our heterogeneous real‐world representation of patients attending memory clinics may be considered a strength of our findings. Finally, our plasma biomarker assay did not include plasma placental growth factor, which has recently been recognized as a relevant biomarker for vascular cognitive impairment. 50
In clinical practice, our study findings emphasize the importance of T1, T2‐weighted, and T2‐FLAIR MRI for the assessment of WMH and PVS burden, and the acquisition of plasma biomarkers such as NfL and GFAP, when faced with many of the complex neurodegenerative and neurovascular diseases that cause dementia.
AUTHOR CONTRIBUTIONS
Conception of the research project: Allison A. Dilliott, Robert A. Hegele, Paula M. McLaughlin, Carmela M. Tartaglia, Ekaterina Rogaeva, Richard H. Swartz, Dar Dowlatshahi, Gustavo Saposnik, Malcolm A. Binns, Robert Bartha, Mario Masellis, and Sandra E. Black. Organization of the research project: Allison A. Dilliott, Robert A. Hegele, Paula M. McLaughlin, Courtney Berezuk, Richard H. Swartz, Gustavo Saposnik, Malcolm A. Binns, Morris Freedman, Robert Bartha, Mario Masellis, Sandra E. Black, and Joel Ramirez. Execution of the research project: Allison A. Dilliott, Robert A. Hegele, Paula M. McLaughlin, Christopher J.M. Scott, Courtney Berezuk, Miracle Ozzoude, Carmela M. Tartaglia, Ekaterina Rogaeva, David F. Tang‐Wai, Richard H. Swartz, Leanne Casaubon, Sanjeev Kumar, Dar Dowlatshahi, Jennifer Mandzia, Demetrios Sahlas, Corinne Fischer, Michael Borrie, Elizabeth Finger, Andrew Frank, Robert Bartha, Sean Symons, Henrik Zetterberg, Mario Masellis, Sandra E. Black, and Joel Ramirez. Data Curation of the research project: Allison A. Dilliott, Robert A. Hegele, Fuqiang Gao, Paula M. McLaughlin, Jennifer S. Rabin, Madeline E. Wood, Christopher J.M. Scott, Vanessa Yhap, Courtney Berezuk, Miracle Ozzoude, Walter Swardfager, Robert Bartha, Sean Symons, Henrik Zetterberg, Mario Masellis, Sandra E. Black, and Joel Ramirez. Supervision and resources of the research project: Robert A. Hegele, Carmela M. Tartaglia, Ekaterina Rogaeva, Richard H. Swartz, Leanne Casaubon, Dar Dowlatshahi, Jennifer Mandzia, Demetrios Sahlas, Gustavo Saposnik, Corinne Fischer, Michael Borrie, Ayman Hassan, Malcolm A. Binns, Morris Freedman, Elizabeth Finger, Andrew Frank, Robert Bartha, Sean Symons, Howard Chertkow, Mario Masellis, Sandra E. Black, and Joel Ramirez. Design of the statistical analysis: Daniela Andriuta, Julie Ottoy, and Joel Ramirez. Execution of the statistical analysis: Daniela Andriuta. Review and critique of the statistical analysis: Julie Ottoy, Myuri Ruthirakuhan, Malcolm A. Binns, and Joel Ramirez. Writing of the first draft of the manuscript: Daniela Andriuta. Review and critique of the manuscript: Julie Ottoy, Myuri Ruthirakuhan, Ginelle Feliciano, Allison A. Dilliott, Robert A. Hegele, Fuqiang Gao, Paula M. McLaughlin, Jennifer S. Rabin, Madeline E. Wood, Christopher J.M. Scott, Courtney Berezuk, Miracle Ozzoude, Walter Swardfager, Carmela M. Tartaglia, Ekaterina Rogaeva, David F. Tang‐Wai, Richard H. Swartz, Leanne Casaubon, Sanjeev Kumar, Dar Dowlatshahi, Jennifer Mandzia, Demetrios Sahlas, Gustavo Saposnik, Corinne Fischer, Michael Borrie, Ayman Hassan, Malcolm A. Binns, Morris Freedman, Elizabeth Finger, Andrew Frank, Robert Bartha, Sean Symons, Henrik Zetterberg, Howard Chertkow, Mario Masellis, Sandra E. Black, and Joel Ramirez. All co‐authors agree with the conditions noted on the Authorship Agreement Form.
CONFLICT OF INTEREST STATEMENT
D.A. reports no disclosures relevant to the manuscript. J.O. reports no disclosures relevant to the manuscript. M.R. reports no disclosures relevant to the manuscript. G.F. reports no disclosures relevant to the manuscript. A.A.D. reports no disclosures relevant to the manuscript. R.A.H. reports no disclosures relevant to the manuscript. F.G. reports no disclosures relevant to the manuscript. P.M.M. reports no disclosures relevant to the manuscript. J.S.R. reports no disclosures relevant to the manuscript. M.W.A. reports no disclosures relevant to the manuscript. C.J.M.S. reports no disclosures relevant to the manuscript. V.Y. reports no disclosures relevant to the manuscript. C.B. reports no disclosures relevant to the manuscript. M.O. reports no disclosures relevant to the manuscript. W.S. reports no disclosures relevant to the manuscript. J.Z. reports no disclosures relevant to the manuscript. M.C.T. reports no disclosures relevant to the manuscript. E.R. reports no disclosures relevant to the manuscript. D.F.T.‐W. reports no disclosures relevant to the manuscript. L.C. reports no disclosures relevant to the manuscript. S.K. reports no disclosures relevant to the manuscript. D.D. reports no disclosures relevant to the manuscript. J.M. reports no disclosures relevant to the manuscript. D.S. reports no disclosures relevant to the manuscript. G.S. reports no disclosures relevant to the manuscript. C.E.F. reports no disclosures relevant to the manuscript. M.B. reports no disclosures relevant to the manuscript. A.H. reports no disclosures relevant to the manuscript. M.A.B. reports no disclosures relevant to the manuscript. M.F. reports no disclosures relevant to the manuscript. H.C. reports no disclosures relevant to the manuscript. E.F. reports no disclosures relevant to the manuscript. A.F. reports no disclosures relevant to the manuscript. R.B. reports no disclosures relevant to the manuscript. S.S. reports no disclosures relevant to the manuscript. H.Z. reports no disclosures relevant to the manuscript. R.H.S. reports no disclosures relevant to the manuscript. M.M. reports no disclosures relevant to the manuscript. S.E.B. reports no disclosures relevant to the manuscript. J.R. reports no disclosures relevant to the manuscript. D.A. reports funding (travel and meetings) from Biogen, Roche, Teva, Novartis, Bristol‐Myers Squibb, Genzyme, and Sanofi outside the submitted work. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). C.E.F. receives grant funding from NIA, NIH, CCNA, CIHR, ADDF, TDRA, Mito2i, the Hilary and Galen Weston Foundation, and Novo Nordisk. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We thank the ONDRI participants for their time and participation in our study. J.O. is funded by the Vascular Training (VAST) Platform (CIHR‐funded), in Canada. A.D.D. was supported by the Alzheimer Society of London and the Middlesex Doctoral Graduate Research Scholarship. J.R. acknowledges the Dr. Sandra Black Centre for Brain Resilience and Recovery, the Linda C. Campbell Foundation, the Weston UK Brain Institute, and the Foundation Leducq Transatlantic Network of Excellence for their support of PVS‐related studies. Special thanks to the BrainLab.ca team. H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2023‐00356; #2022‐01018 and #2019‐02397), the European Union's Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG‐71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809‐2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF‐21‐831376‐C, #ADSF‐21‐831381‐C, and #ADSF‐21‐831377‐C), the Bluefield Project, Cure Alzheimer's Fund, the Olav Thon Foundation, the Erling‐Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022‐0270), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme—Neurodegenerative Disease Research (JPND2021‐00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI‐1003). S.K. acknowledges research support from the Brain and Behavior Foundation, National Institute on Ageing, BrightFocus Foundation, Brain Canada, Canadian Institute of Health Research, Canadian Consortium on Neurodegeneration in Aging, Centre for Ageing and Brain Health Innovation, Centre for Addiction and Mental Health, and an Academic Scholars Award from the Department of Psychiatry, University of Toronto. Equipment support from Soterix Medical. The ONDRI study was conducted in part with the support of the Ontario Brain Institute, an independent non‐profit corporation, funded partially by the Ontario government (GRANT# ONDRI/OBI‐34739). The opinions, results, and conclusions are those of the authors, and no endorsement by the Ontario Brain Institute is intended or should be inferred. Matching funds were provided by participant hospitals and research foundations, including the Baycrest Foundation, Bruyere Research Institute, Centre for Addiction and Mental Health Foundation, London Health Sciences Foundation, McMaster University Faculty of Health Sciences, Ottawa Brain and Mind Research Institute, Queen's University Faculty of Health Sciences, the Thunder Bay Regional Health Sciences Centre, the University of Ottawa Faculty of Medicine, and the Windsor/Essex County ALS Association. The Temerty Family Foundation provided the major infrastructure matching funds.
Andriuta D, Ottoy J, Ruthirakuhan M, et al. Perivascular spaces, plasma GFAP, and speeded executive function in neurodegenerative diseases. Alzheimer's Dement. 2024;20:5800–5808. 10.1002/alz.14081
Sandra E. Black and Joel Ramirez contributed equally to this work and are considered co‐senior authors.
Clinical trials registration: NCT04104373
DATA AVAILABILITY STATEMENT
The ONDRI data are publicly available via the Ontario Brain Institute (https://braininstitute.ca/).
REFERENCES
- 1. Bakker ENTP, Bacskai BJ, Arbel‐Ornath M, et al. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol. 2016;36:181‐194. doi: 10.1007/s10571-015-0273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shulyatnikova T, Hayden MR. Why are perivascular spaces important? Medicina. 2023;59:917. doi: 10.3390/medicina59050917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The glymphatic system: a beginner's guide. Neurochem Res. 2015;40:2583‐2599. doi: 10.1007/s11064-015-1581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurology. 2018;17:1016‐1024. doi: 10.1016/S1474-4422(18)30318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charidimou A, Hong YT, Jäger HR, et al. White matter perivascular spaces on magnetic resonance imaging: marker of cerebrovascular amyloid burden? Stroke. 2015;46:1707‐1709. doi: 10.1161/STROKEAHA.115.009090 [DOI] [PubMed] [Google Scholar]
- 6. Ding J, Sigurðsson S, Jónsson PV, et al. Large Perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the age, gene/environment susceptibility–Reykjavik Study. JAMA Neurol. 2017;74:1105‐1112. doi: 10.1001/jamaneurol.2017.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845‐861. doi: 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramirez J, Berezuk C, McNeely AA, Scott CJM, Gao F, Black SE. Visible Virchow‐Robin spaces on magnetic resonance imaging of Alzheimer's disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimers Dis. 2015;43:415‐424. doi: 10.3233/JAD-132528 [DOI] [PubMed] [Google Scholar]
- 9. Ramirez J, Berezuk C, McNeely AA, Gao F, McLaurin J, Black SE. Imaging the perivascular space as a potential biomarker of neurovascular and neurodegenerative diseases. Cell Mol Neurobiol. 2016;36:289‐299. doi: 10.1007/s10571-016-0343-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smeijer D, Ikram MK, Hilal S. Enlarged perivascular spaces and dementia: a systematic review. J Alzheimers Dis. 2019;72:247‐256. doi: 10.3233/JAD-190527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarasoff‐Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457‐470. doi: 10.1038/nrneurol.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16:137‐153. doi: 10.1038/s41582-020-0312-z [DOI] [PubMed] [Google Scholar]
- 13. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822‐838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Passiak BS, Liu D, Kresge HA, et al. Perivascular spaces contribute to cognition beyond other small vessel disease markers. Neurology. 2019;92:e1309‐e1321. doi: 10.1212/WNL.0000000000007124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeong SH, Cha J, Park M, et al. Association of enlarged perivascular spaces with amyloid burden and cognitive decline in Alzheimer disease continuum. Neurology. 2022;99(16):e1791‐e1802. doi: 10.1212/WNL.0000000000200989 [DOI] [PubMed] [Google Scholar]
- 16. Choe YM, Baek H, Choi HJ, et al. Association between enlarged perivascular spaces and cognition in a memory clinic population. Neurology. 2022;99(13):e1414‐e1421. doi: 10.1212/WNL.0000000000200910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hilal S, Tan CS, Adams HHH, et al. Enlarged perivascular spaces and cognition: a meta‐analysis of 5 population‐based studies. Neurology. 2018;91:e832‐e842. doi: 10.1212/WNL.0000000000006079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small‐vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034‐2041. doi: 10.1093/brain/awh553 [DOI] [PubMed] [Google Scholar]
- 19. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ottoy J, Ozzoude M, Zukotynski K, et al. Vascular burden and cognition: mediating roles of neurodegeneration and amyloid PET. Alzheimers Dement. 2023;19:1503‐1517. doi: 10.1002/alz.12750 [DOI] [PubMed] [Google Scholar]
- 21. Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci. 2017;131:2257‐2274. doi: 10.1042/CS20160381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang Z, Wang KKW. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38:364‐374. doi: 10.1016/j.tins.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hase Y, Chen A, Bates LL, et al. Severe white matter astrocytopathy in CADASIL. Brain Pathol. 2018;28:832‐843. doi: 10.1111/bpa.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gouw AA, Seewann A, Vrenken H, et al. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post‐mortem quantitative MRI and neuropathology. Brain. 2008;131:3286‐3298. doi: 10.1093/brain/awn265 [DOI] [PubMed] [Google Scholar]
- 25. Ferreira PCL, Zhang Y, Snitz B, et al. Plasma biomarkers identify older adults at risk of Alzheimer's disease and related dementias in a real‐world population‐based cohort. Alzheimers Dement. 2023;19(10):4507‐4519. doi: 10.1002/alz.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benussi A, Ashton NJ, Karikari TK, et al. Serum Glial Fibrillary Acidic Protein (GFAP) is a marker of disease severity in frontotemporal lobar degeneration. J Alzheimers Dis. 2020;77:1129‐1141. doi: 10.3233/JAD-200608 [DOI] [PubMed] [Google Scholar]
- 27. Huss A, Abdelhak A, Mayer B, et al. Association of serum GFAP with functional and neurocognitive outcome in sporadic small vessel disease. Biomedicines. 2022;10:1869. doi: 10.3390/biomedicines10081869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shir D, Graff‐Radford J, Hofrenning EI, et al. Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer's disease and vascular pathology. Alzheimers Dement. 2022;14:e12291. doi: 10.1002/dad2.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asken BM, VandeVrede L, Rojas JC, et al. Lower white matter volume and worse executive functioning reflected in higher levels of plasma GFAP among older adults with and without cognitive impairment. J Int Neuropsychol Soc. 2022;28:588‐599. doi: 10.1017/S1355617721000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verberk IMW, Laarhuis MB, van den Bosch KA, et al. Serum markers glial fibrillary acidic protein and neurofilament light for prognosis and monitoring in cognitively normal older people: a prospective memory clinic‐based cohort study. Lancet Healthy Longev. 2021;2:e87‐e95. doi: 10.1016/S2666-7568(20)30061-1 [DOI] [PubMed] [Google Scholar]
- 31. Revised Criteria for Diagnosis and Staging of Alzheimer's. AAIC. n.d.. Accessed November 17, 2023. https://aaic.alz.org/diagnostic‐criteria.asp
- 32. Farhan SMK, Bartha R, Black SE, et al. The Ontario Neurodegenerative Disease Research Initiative (ONDRI). Can J Neurol Sci. 2017;44:196‐202. doi: 10.1017/cjn.2016.415 [DOI] [PubMed] [Google Scholar]
- 33. Sunderland KM, Beaton D, Arnott SR, et al. Characteristics of the Ontario Neurodegenerative Disease Research initiative cohort. Alzheimers Dement. 2023;19:226‐243. doi: 10.1002/alz.12632 [DOI] [PubMed] [Google Scholar]
- 34. Ramirez J, Holmes MF, Scott CJM, et al. Ontario Neurodegenerative Disease Research Initiative (ONDRI): structural MRI methods and outcome measures. Front Neurol. 2020;11:847. doi: 10.3389/fneur.2020.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kovacevic N, Lobaugh NJ, Bronskill MJ, Levine B, Feinstein A, Black SE. A robust method for extraction and automatic segmentation of brain images. Neuroimage. 2002;17:1087‐1100. doi: 10.1006/nimg.2002.1221 [DOI] [PubMed] [Google Scholar]
- 36. Ramirez J, Gibson E, Quddus A, et al. Lesion Explorer: a comprehensive segmentation and parcellation package to obtain regional volumetrics for subcortical hyperintensities and intracranial tissue. Neuroimage. 2011;54:963‐973. doi: 10.1016/j.neuroimage.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 37. Gibson E, Gao F, Black SE, Lobaugh NJ. Automatic segmentation of white matter hyperintensities in the elderly using FLAIR images at 3T. J Magn Reson Imaging. 2010;31:1311‐1322. doi: 10.1002/jmri.22004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sunderland KM, Beaton D, Fraser J, et al. The utility of multivariate outlier detection techniques for data quality evaluation in large studies: an application within the ONDRI project. BMC Med Res Methodol. 2019;19:102. doi: 10.1186/s12874-019-0737-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McLaughlin PM, Sunderland KM, Beaton D, et al. The quality assurance and quality control protocol for neuropsychological data collection and curation in the Ontario Neurodegenerative Disease Research Initiative (ONDRI) Study. Assessment. 2021;28:1267‐1286. doi: 10.1177/1073191120913933 [DOI] [PubMed] [Google Scholar]
- 40. Dilliott AA, Evans EC, Farhan SMK, et al. Genetic variation in the Ontario Neurodegenerative Disease Research Initiative. Can J Neurol Sci. 2019;46:491‐498. doi: 10.1017/cjn.2019.228 [DOI] [PubMed] [Google Scholar]
- 41. Farhan SMK, Dilliott AA, Ghani M, et al. The ONDRISeq panel: custom‐designed next‐generation sequencing of genes related to neurodegeneration. NPJ Genom Med. 2016;1:16032. doi: 10.1038/npjgenmed.2016.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lahna D, Roese N, Woltjer R, et al. Postmortem 7T MRI for guided histopathology and evaluation of cerebrovascular disease. J Neuropathol Exp Neurol. 2022;82:57‐70. doi: 10.1093/jnen/nlac103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keith J, Gao F‐Q, Noor R, et al. Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropathol Exp Neurol. 2017;76:299‐312. doi: 10.1093/jnen/nlx009 [DOI] [PubMed] [Google Scholar]
- 44. Lahna D, Schwartz DL, Woltjer R, et al. Venous collagenosis as pathogenesis of white matter hyperintensity. Ann Neurol. 2022;92:992‐1000. doi: 10.1002/ana.26487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Solé‐Guardia G, Custers E, de Lange A, et al. Association between hypertension and neurovascular inflammation in both normal‐appearing white matter and white matter hyperintensities. Acta Neuropathol Commun. 2023;11:2. doi: 10.1186/s40478-022-01497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jagust WJ, Teunissen CE, DeCarli C. The complex pathway between amyloid β and cognition: implications for therapy. Lancet Neurol. 2023;22:847‐857. doi: 10.1016/S1474-4422(23)00128-X [DOI] [PubMed] [Google Scholar]
- 47. Charidimou A, Boulouis G, Frosch MP, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI‐neuropathology diagnostic accuracy study. Lancet Neurol. 2022;21:714‐725. doi: 10.1016/S1474-4422(22)00208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hosoki S, Hansra GK, Jayasena T, et al. Molecular biomarkers for vascular cognitive impairment and dementia. Nat Rev Neurol. 2023;19:737‐753. doi: 10.1038/s41582-023-00884-1 [DOI] [PubMed] [Google Scholar]
- 49. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood‐brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71‐76. doi: 10.1038/s41586-020-2247-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hinman JD, Elahi F, Chong D, et al. Placental growth factor as a sensitive biomarker for vascular cognitive impairment. Alzheimers Dement. 2023;19:3519‐3527. doi: 10.1002/alz.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The ONDRI data are publicly available via the Ontario Brain Institute (https://braininstitute.ca/).


