Abstract
INTRODUCTION
Motor function has correlated with longevity and functionality; however, there is limited research on those with Alzheimer's disease (AD). We studied the association between motor functionality and AD pathology in primary motor and medial temporal cortices.
METHODS
A total of 206 participants with a clinical diagnosis of cognitively healthy, AD, or mild cognitive impairment (MCI) underwent imaging and motor assessment. Linear regressions and analyses of variance were applied to test the prediction from AD imaging biomarkers to motor performance and the diagnosis group differences in motor performance.
RESULTS
Increased neurodegeneration was associated with worsening dexterity and lower walking speed, and increased amyloid and tau were associated with worsening dexterity. AD and MCI participants had lower motor performance than the cognitively healthy participants.
DISCUSSION
Increased AD pathology is associated with worsening dexterity performance. The decline in dexterity in those with AD pathology may offer an opportunity for non‐pharmacological therapy intervention.
Highlights
Noted worsening dexterity performance was associated with greater Alzheimer's disease (AD) pathology (tau, amyloid beta, and neurodegeneration) in primary motor cortices.
Similarly, increased neurodegeneration and tau pathology in parahippocampal, hippocampal, and entorhinal cortices is associated with worsening dexterity performance.
Motor performance declined in those with clinical and preclinical AD among an array of motor assessments.
Keywords: Alzheimer's disease pathology, dexterity performance in clinical and preclinical Alzheimer's disease, magnetic resonance imaging, motor decline in Alzheimer's disease, motor function, physiotherapy, positron emission tomography imaging
1. INTRODUCTION
Alzheimer's disease (AD) has been known to negatively affect motor functions including speech and facial expressions, rigidity, gait, and posture, leading to bradykinesia and tremor. 1 Although these have traditionally been thought to manifest late in the disease course, improvements in motor assessment tools and earlier disease diagnosis have contributed to increasing awareness of early motor performance decline in mild to moderate AD. 2 Moreover, motor function decline in AD may impact disease morbidity and caretaker fatigue. Motor decline is a ubiquitous, though variable, process as individuals age. Studies have shown that various motor function assessments may be good predictors of elderly longevity and functionality in those with normal cognitive status. 3 , 4 Early motor performance decline may also be related to the accrual of AD pathology, such as amyloid beta (Aβ) plaques and tau tangles, which accumulate decades prior to the onset of clinical symptoms. 5 However, few studies have examined the pathological correlates of motor decline among those with impaired cognition, particularly in those with mild cognitive impairment (MCI) and AD. Existing studies on various motor dexterity, strength, and agility assessments have limited generalizability, as they have incorporated only a small number of participants, often with clinically diagnosed AD or MCI. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 As such, we sought to address this gap in knowledge and examine the association between AD pathology and clinical motor function using a large cohort that is well characterized along the AD clinicopathologic spectrum.
To test the relationship between AD pathology and motor function, we leveraged data from the National Institutes of Health (NIH)–funded Alzheimer's Disease Connectome Project (ADCP). Participants in the ADCP underwent comprehensive neuroimaging, neuropsychological testing, and motor function assessments of grip strength, walking speed, and dexterity performance with the NIH Toolbox. 14 We hypothesized that those with greater AD pathology in primary motor cortices would have poorer motor performance overall. A secondary analysis tested whether motor performance was associated with worse clinical status along the AD continuum. We hypothesized that worse motor function would be associated with worse cognitive status.
2. METHODS
2.1. Participants
Participants along the AD clinical and biologic continuum were enrolled in the ADCP, which collected data across two academic centers (University of Wisconsin and Medical College of Wisconsin), recruiting from established cohorts as well as from the surrounding community. Exclusion criteria included those under age 55, non‐English speakers, magnetic resonance (MR) imaging exclusionary bio‐hardware or body habitus, inability to complete motor assessments, those without a secondary informant, and those with an additional non‐AD neurological condition such as Parkinson's, chronic migraine, multiple sclerosis, meningitis, hydrocephalus, and so forth. A total of 206 participants were included in this study.
2.2. Clinical diagnosis
Participants completed the majority of components of the National Alzheimer's Coordinating Center (NACC) Uniform Data Set 15 including self‐reported demographic information, and extensive cognitive function tests including the Montreal Cognitive Assessment 16 (MoCA), the Craft Story 21 Immediate and Delayed Recall, category fluency tests, Trail Making Test Parts A and B, Neuropsychiatric Inventory Questionnaire (NPI‐Q), Geriatric Depression Scale (GDS), and a functional assessment after interviewing a secondary informant on participants’ independence and ability to complete activities of daily living (ADL) and instrumental activities of daily living (IADL). Participants also completed the NIH Toolbox motor, cognitive, and emotional assessments. Participant diagnosis of MCI, AD, or cognitively healthy was determined per the National Institute on Aging–Alzheimer's Association criteria, 17 , 18 and reviewed by a team of physicians, neuropsychologists, and nurse practitioners at a consensus diagnosis conference.
2.3. AD imaging biomarker assessment
All 206 participants underwent T1‐weighted MR imaging. A subgroup of 61 and 58 participants underwent additional [C‐11] Pittsburgh compound B (PiB) positron emission tomography (PET) and [F‐18]Flortaucipir PET to assess Aβ and tau pathology, respectively. MR imaging was completed concurrently with cognitive and motor assessment visits. PET imaging was collected within a year of MR imaging and motor assessments. T1‐weighted scans were processed using FreeSurfer v6.0 to estimate regional cortical thickness as an indicator of neurodegeneration. 19 [C‐11]PiB distribution volume ratios (DVR) were estimated using Logan graphical analysis with cerebellar gray matter (GM) as the reference region. Amyloid positivity was defined as the average cortical PiB DVR ≥1.19 using a global composite from the following brain regions: bilateral anterior and posterior cingulate cortex, the precuneus, the angular gyrus, supramarginal gyrus, middle and superior temporal gyrus, and the frontal medial orbital gyrus. 20 [F‐18]Flortaucipir standardized uptake value ratios (SUVR; inferior cerebellar GM reference region) were assessed. Tau positivity was determined to be ≥ 1.29 using the threshold of two standard deviations (SDs) above the volume weight means of the anterior parahippocampal SUVRs within our amyloid negative cohort. Regions of interest (ROIs) to assess neurodegeneration included: entorhinal, parahippocampal, and precentral cortices. To further assess tau and amyloid burden we used hippocampal, parahippocampal, and precentral ROIs.
2.4. Motor function assessment
Motor function was assessed using NIH Toolbox tests including the 2‐minute walking, 9‐hole pegboard, and dynamometer grip assessments. 14 These assessments were used to analyze walking speed, dexterity, and strength, respectively. Participants did not have to complete all three motor assessments to be included in the analysis. Motor performance was analyzed using participants' uncorrected standardized scores from participant‐reported dominant hand performance, with higher scores indicating better performance.
2.5. Statistical analysis
Independent two‐sample t test was used to compare the precentral, parahippocampal, and hippocampal biomarker burden between the biomarker (Aβ or tau) positive versus negative groups. Linear regression models were used to assess the relationships between the underlying cortical burden of AD biomarker pathology in primary motor cortices and the medial temporal lobe and participants’ performance in each NIH motor assessment. Initial models included all participants regardless of diagnosis or biomarker status. Subsequent linear regression was performed only in the cognitively healthy cohort to determine whether relationships were present in individuals with no clinical impairment. The age and sex of participants were treated as covariates for these models. Due to high correlations between the ROIs, each ROI was included in a regression model separately. The Benjamini–Hochberg false discovery rate (FDR) method 21 was applied to adjust for the inflated type I error associated with multiple tests. One‐way analysis of variance (ANOVA) and post hoc Tukey tests were used to compare motor assessment performances among the consensus‐designated cognitive groups (cognitively healthy, MCI, and AD).
RESEARCH IN CONTEXT
Systematic review: The authors reviewed existing literature using online publishing databases (e.g., PubMed) to investigate the current understanding of motor function decline in those with Alzheimer's disease (AD). These relevant citations are included. We noted a pattern of declining motor functions with worsening cognition status and a dearth of research into the AD pathology presence in brain cortices and its relation to motor function.
Interpretation: Our findings led to a hypothesis that the greater presence of underlying AD pathology, particularly in primary motor cortices, was associated with declining motor performance. This hypothesis is consistent with non‐clinical and clinical findings currently in the public domain.
Future directions: The article proposes a framework for the generation of hypotheses and additional studies. Examples include investigating: (a) the pathophysiology time course of AD pathology deposition in motor cortices and (b) the utility of physiotherapy treatment for motor function deficits in those with AD.
3. RESULTS
3.1. Sample characteristics
This study included data from 206 participants, aged 55 to 90 years (mean = 66.9, SD = 8.1). Participants were mostly female (n = 113, 54.9%), self‐identified as White (n = 187, 90.8%), and cognitively healthy (n = 122, 59.2%). Ten participants (4.9%) self‐identified as Black and two (1.0%) self‐identified as Asian. Twenty‐three (37.7%) of the 61 participants who underwent the PiB PET were Aβ positive, and 13 (22.4%) of the 58 participants who underwent the tau PET were tau positive. The sample characteristics of the study cohort and each Aβ and tau subgroup are summarized in Table 1.
TABLE 1.
Participant demographics of each imaging cohort.
| Patient characteristics for each imaging cohort | |||
|---|---|---|---|
| MRI | Aβ | Tau | |
| Participants, n | 206 | 61 | 58 |
| Sex, n (%) | |||
| Female | 113 (54.9) | 27 (44.3) | 34 (58.6) |
| Male | 93 (45.1) | 34 (55.7) | 24 (41.4) |
| Age (Years) | 69.9 ± 8.0 | 69.4 ± 8.4 | 69.7 ± 8.3 |
| Race | |||
| White | 187 (90.8) | 57 (93.4) | 54 (93.1) |
| Black | 10 (4.9) | 2 (3.3) | 3 (5.2) |
| Asian | 2 (1.0) | 0 (0.0) | 0 (0.0) |
| Other | 7 (3.3) | 2 (3.3) | 1 (1.7) |
| Cognitive diagnosis | |||
| Healthy | 129 (62.6) | 35 (57.4) | 33(56.9) |
| MCI | 41 (19.9) | 17 (27.9) | 17 (29.3) |
| AD | 36 (17.5) | 9 (14.8) | 8 (13.8) |
| Other | |||
| Assessment performance (Avg ± SD) | |||
| Grip strength | 96.9 ± 10.9 | 98.1 ± 10.6 | 97.8 ± 10.5 |
| Walking speed | 92.1 ± 13.6 | 91.4 ± 13.3 | 89.7 ± 12.0 |
| Dexterity | 94.0 ± 13.9 | 93.3 ± 18.9 | 92.2 ± 13.0 |
| Biomarker positive n, (%) | – | 23 (37.7) | 13 (22.4) |
| Biomarker burden, SUVR, (Avg ± SD) | |||
| Precentral cortex | – | 1.1 ± 0.1 | 0.98 ± 0.1 |
| Parahippocampus | – | 1.1 ± 0.2 | 1.2 ± 0.2 |
| Hippocampus/entorhinal cortex | – | 1.1 ± 0.1 | 1.3 ± 0.2 |
| Cortical thickness in ROIs, mm, (Avg ± SD) | |||
| Precentral cortex | 2.46 ± 0.1 | 2.45 ± 0.1 | 2.44 ± 0.1 |
| Parahippocampus | 2.58 ± 0.2 | 2.60 ± 0.2 | 2.60 ± 0.2 |
| Hippocampus/entorhinal cortex | 3.22 ± 0.4 | 3.22 ± 0.4 | 3.23 ± 0.4 |
Note: All 206 study participants underwent MR imaging. Cognitive diagnosis was determined in lieu of pathology imaging within categories: cognitively healthy, MCI, and AD. Each PET imaging cohort represents a subset of participants who underwent additional PET imaging.
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; MR, magnetic resonance; PET, positron emission tomography; ROI, region of interest; SD, standard deviation; SUVR, standardized uptake value ratio.
3.2. AD biomarkers
Lower thickness in the precentral, parahippocampal, and entorhinal cortex was associated with worse dexterity (p value = 0.0029, 0.0036, 0.000, respectively; see Table 2). Neurodegeneration in the precentral gyrus was associated with slower walking speed (p value = 0.0013); however, this relationship was not observed in the parahippocampal gyrus or entorhinal cortex ROIs. Grip strength did not show statistically significant predictive outcomes with underlying cortical atrophy. There was no relationship observed between grip strength, walking speed, nor dexterity and neurodegeneration in the precentral or parahippocampal gyri or entorhinal cortex ROIs for those with healthy cognition (Table 3).
TABLE 2.
This table includes results from linear regression analysis using motor performance as the predictor and biomarker burden in select ROIs as outcomes.
| Linear regression analysis predicting each NIH motor assessment outcome by ROI biomarker burden | ||||||
|---|---|---|---|---|---|---|
| Grip | Walking speed | Dexterity | ||||
| Neurodegeneration | β | p | β | p | β | p |
| Precentral cortex | 0.003 | 0.957 | 0.227 | 0.001 | 0.213 | 0.003 |
| Parahippocampus | 0.109 | 0.054 | 0.087 | 0.232 | 0.212 | 0.004 |
| Entorhinal cortex | 0.079 | 0.166 | 0.127 | 0.082 | 0.278 | 0.0001 |
| Aβ burden | ||||||
| Precentral cortex | 0.071 | 0.464 | 0.191 | 0.157 | 0.426 | 0.001 |
| Parahippocampus | 0.080 | 0.403 | 0.185 | 0.166 | 0.263 | 0.046 |
| Hippocampus | 0.059 | 0.532 | 0.114 | 0.391 | 0.085 | 0.521 |
| Tau burden | ||||||
| Precentral cortex | 0.105 | 0.256 | 0.101 | 0.474 | 0.530 | <0.0001 |
| Parahippocampus | 0.045 | 0.623 | 0.010 | 0.945 | 0.477 | 0.0002 |
| Hippocampus | 0.124 | 0.182 | 0.012 | 0.932 | 0.341 | 0.011 |
Note: All models controlled for participant age and sex and corrected for multiple comparisons using the Benjamini–Hochberg (1995) procedure. The NIH motor assessment outcomes included the grip strength dynamometer, 2‐minute walking speed, and 9‐hole peg dexterity tests.
Abbreviations: Aβ, amyloid beta; NIH, National Institutes of Health; ROI, region of interest. Statistically significant P values are bolded.
TABLE 3.
This table includes results from linear regression analysis of our cognitively healthy subgroup using motor performance as the predictor and biomarker burden in select ROIs as outcomes.
| Linear regression analysis predicting each NIH motor assessment outcome by ROI biomarker burden for those cognitively healthy | ||||||
|---|---|---|---|---|---|---|
| Grip | Walking speed | Dexterity | ||||
| Neurodegeneration | β | p | β | p | β | p |
| Precentral cortex | 0.046 | 0.519 | 0.181 | 0.036 | 0.172 | 0.072 |
| Parahippocampus | 0.135 | 0.135 | 0.053 | 0.544 | 4.147 | 0.015 |
| Entorhinal | 0.053 | 0.462 | 0.111 | 0.212 | 0.283 | 0.003 |
| Aβ burden | ||||||
| Precentral cortex | −0.082 | 0.508 | −0.205 | 0.294 | −0.025 | 0.897 |
| Parahippocampus | −0.165 | 0.173 | −0.195 | 0.278 | −0.158 | 0.408 |
| Hippocampus | −0.059 | 0.622 | 0.091 | 0.686 | 0.021 | 0.911 |
| Tau burden | ||||||
| Precentral cortex | −0.120 | 0.321 | −0.386 | 0.046 | −0.065 | 0.754 |
| Parahippocampus | −0.251 | 0.036 | −0.195 | 0.276 | −0.220 | 0.292 |
| Hippocampus | −0.141 | 0.296 | −0.173 | 0.440 | −0.401 | 0.072 |
Note: All models were controlled for participant age and sex and corrected for multiple comparisons using the Benjamini–Hochberg (1995) procedure. The NIH motor assessment outcomes included the grip strength dynamometer, 2‐minute walking speed, and 9‐hole peg dexterity tests.
Abbreviations: Aβ, amyloid beta; NIH, National Institutes of Health; ROI, region of interest.
There was a significant difference in precentral Aβ pathology between the amyloid positive and negative groups (t‐statistic: 10.7, p value: < 0.0001). Of the 23 Aβ positive participants, the average precentral Aβ burden within primary motor cortices was increased (DVR mean ± SD = 1.3 ± 0.2) compared to their AD pathology negative peers (DVR mean ± SD = 1.0 ± 0.0). Increased Aβ burden in primary motor cortices was associated with worse participant dexterity (Table 2: p value = 0.0010). Interestingly, increased parahippocampal (p value = 0.0461, α < 0.017) and hippocampal (0.5213, α < 0.019) Aβ burden was not associated with reduced dexterity. Increased Aβ burden in hippocampal, parahippocampal, or primary motor ROIs did not show significant predictive relationships to walking speed or grip strength. There was no relationship observed between grip strength, walking speed, nor dexterity and Aβ burden in precentral, parahippocampal, or hippocampal ROIs for those with healthy cognition (Table 3).
There was a significant difference in precentral tau burden between the tau positive and negative groups (t‐statistic: 5.1, p value < 0.0001). Of the 13 tau‐positive participants, the average precentral tau burden within primary motor cortices was increased (SUVR = 1.1 ± 0.2) compared to their AD pathology‐negative peers (SUVR = 0.9 ± 0.1). Increased tau burden in primary motor, parahippocampal, and hippocampal ROIs was associated with worse dexterity (Table 2: p value < 0.0001, = 0.0002, and = 0.0111, respectively). Increased tau burden in primary motor, parahippocampal, and hippocampal ROIs was not significantly associated with worse performance in either walking speed or grip strength assessments. There was no relationship observed between grip strength, walking speed, nor dexterity and tau burden in precentral, parahippocampal, or hippocampal ROIs for those with healthy cognition (Table 3).
3.3. Clinical diagnosis
Mean grip strength performance was significantly different between the cognitively healthy and MCI group (p value = 0.02, 95%). No significant difference was seen between the cognitively healthy and AD group (p value = 0.13), nor between the MCI and AD groups (p value = 0.79). Tukey honestly significant difference testing for multiple comparisons found that the mean value of walking speed was significantly different between cognitively healthy and MCI groups (p value = 0.019) and between cognitively healthy and AD groups (p value = 0.016). Similarly, dexterity motor assessments showed statistically significant differences in those with MCI and AD diagnosis relative to their cognitively healthy peers (p value = 0.0001, < 0.0001). No significant differences were found between the MCI and AD groups across all three motor performance measures (p value = 0.79, 1.0, 0.94). These findings are summarized in Figure 1.
FIGURE 1.
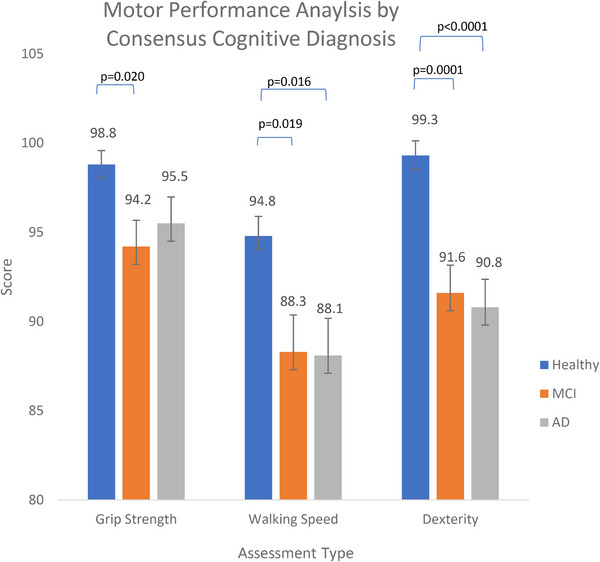
Analysis of variance least square means and Tukey pairwise comparison of motor assessment performance by each consensus cognition group: cognitively healthy, mild cognitive impairment (MCI), and Alzheimer's disease (AD) controlling for participant age and sex. National Institutes of Healthy motor assessments include grip strength dynamometer, 2‐minute walking speed, and 9‐hole dexterity. Our models demonstrated a statistically significant difference in grip strength, walking speed, and dexterity performance between the cognitive healthy and AD cohorts. Similarly, we see a statistically significant difference in walking speed and dexterity performance between the cognitively healthy and MCI cohorts.
4. DISCUSSION
The present study found that the presence of tau and Aβ biomarker burden and neurodegeneration in primary motor cortices was associated with worse dexterity. In contrast to our hypotheses, tau and Aβ burden in primary motor cortices were not shown to be associated with walking speed and grip strength performance. Cortical neurodegeneration in primary motor regions was related to performance in both dexterity and walking speed assessments, such that increased cortical atrophy was associated with worse dexterity and walking speed. Grip strength was not shown to be associated with tau, Aβ, or cortical atrophy in primary motor cortices.
Our findings also showed that increases in neurodegeneration in entorhinal and parahippocampal ROIs and increases in tau pathological burden in hippocampal and parahippocampal regions were associated with worse dexterity. Motor task performance is complex and demands integration across a variety of cortical regions. Fine motor tasks likely mandate increased supplemental cortical input and executive cognitive functions including attention, working memory, and cognitive flexibility. 22 , 23 These functions are also known to be compromised in AD, and thus it is plausible that reduced dexterity in those with increased AD pathology is due to subtle disruptions in these higher order cognitive processes. 24 To this end, these associations were not mimicked when analyzing our cognitively healthy subgroup, suggesting that these higher order cognitive functions may still be intact for these individuals. This may also explain why we did not see a similar association in grip strength, a relatively straightforward task. Similarly, walking speed may be preserved given the large input from the cerebellum, a region that is largely spared from AD pathology until far later in the disease course. 22
4.1. Research in context
Despite the presence of amyloid and tau pathology in other cortical areas, primary motor cortices are generally spared from early amyloid and tau accumulation until the later stages of AD. 25 , 26 This may suggest that motor function would deteriorate as AD pathology accumulates and cognition declines. Our study findings partially supported this hypothesis, as amyloid and tau pathology in the precentral gyrus, as well as tau burden in the hippocampus and parahippocampus, was associated with lower dexterity. Although we did not observe this relationship with regard to walking speed and grip strength, post hoc analysis showed that all indexed motor assessments differed between cognitively unimpaired individuals and those with MCI and AD. Our cognitively healthy subgroup analysis demonstrated a lack of association between motor assessment performance and underlying AD pathology and neurodegeneration, suggesting that this relative susceptibility of fine‐motor function to AD pathology presence may be silent until early clinical disease. There is mounting evidence that fine‐motor function decline is seen in tandem with clinically detectable cognitive decline for those with AD. 1 , 8 , 27 , 28 , 29 , 30 Our findings suggest a window of motor performance decline along the disease course, and a possible opportunity to implement treatment.
Non‐pharmacologic therapeutic interventions, such as physiotherapy, have shown promise in improving motor performance and physical function in those with decreased motor function related to cognitive decline. 12 , 31 , 32 , 33 These studies also demonstrated an improvement in instrumental and non‐instrumental activities of daily living (IADLs, ADLs) for those with AD. These limitations in I/ADLs are significant sources of AD treatment costs and caregiver fatigue.
Continued recognition of the decline in fine‐motor function in those with clinical and pre‐clinical AD is paramount. It could offer a novel opportunity to provide evidence‐based, non‐invasive treatment options to families that limit or slow functional decline. Further studies are needed to illuminate AD pathology progression and its role in motor function pathway disruptions.
4.2. Limitations
Participant consensus diagnosis was determined without the use of MR and PET imaging data available. As such, diagnosis relied heavily on clinical cognitive and functional data, without knowledge of participant AD pathology. Additionally, participants who were unable to complete any of the studied motor assessments were excluded from our study cohort, likely underrepresenting participants who are severely frail or have gross motor deficits. The included motor assessments examined specific, simple motor tasks. They do not encompass the entirety of measurable motor task performance nor the holistic picture of motor performance change in AD or related dementias. Alternative motor tasks should be tested to elucidate if better‐suited tasks are more valid.
Our PET imaging cohorts were smaller than our larger MR imaging cohort and thus may reduce the generalizability of these findings. Additionally, our PET imaging was collected within 1 year of motor assessments and thus there may be a slight variation in in vivo cortical biomarker burden at the time of motor assessments and imaging. Last, our data cohort comprised a predominantly White demographic distribution which is not representative of the AD population as a whole.
5. CONCLUSION
Our study evaluated the underlying brain AD pathology burden and cortical structural changes in primary motor cortices and its relation to motor performance in individuals along the AD continuum. Our findings are in concordance with previous studies demonstrating worsening motor task performance is associated with underlying AD biomarker burden or neurodegeneration. Additionally, our results support previous studies 6 , 7 , 8 , 9 , 10 , 11 which demonstrate declining motor performance in those with AD or MCI diagnosis compared to their age‐matched cognitively healthy peers.
CONFLICT OF INTEREST STATEMENT
Dr. Barbara Bendlin has received precursors and tracers from AVID Radiopharmaceuticals including PET precursor for AV1451 which was used in the current study. She has also received honorariums from UC‐Irvine, the University of Pittsburgh, the University of Illinois, and the Karolinska Institute for lectureships. Dr. Bendlin has received payment from New Amsterdam Inc. for consulting fees and from the Alzheimer's Association for attendance at their event. She serves as the chair of the ADRC research education national committee, the CLSA‐Healthy Brains Healthy Aging/Weston Advisory Committee, and Rush's ADRC external advisory board committee. Dr. Nathaniel A. Chin has received consulting fees from New Amsterdam Inc. He also serves as a member of the Medical & Scientific Advisory Board of the Wisconsin Alzheimer's Association and Alzheimer's Foundation of America. Dr. Ozioma C. Okonkwo is currently the treasurer of the International Neuropsychological Society. Dr. Tobey J. Betthauser has received an honorarium from Intermountain Healthcare and the NIH. He has also received travel support from the University College London, the Alzheimer's Association, and the NIH. The remaining authors have no disclosures. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This work was made possible by the staff at Wisconsin's Alzheimer's Disease Research Center (ADRC) and our devoted participants. Funds supported by the National Institutes of Health (NIH) Alzheimer's Disease Connectome Project (No: 1UF1AG051216‐01A1) and the National Institute on Aging (No: P30AG062715).
Gupta L, Ma Y, Kohli A, et al. Alzheimer's disease biomarker burden in primary motor cortices is associated with poorer dexterity performance. Alzheimer's Dement. 2024;20:5792–5799. 10.1002/alz.13899
REFERENCES
- 1. Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer's disease. Expert Rev Neurother. 2011;11(5):665‐676. doi: 10.1586/ern.11.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee G. Impaired cognitive function is associated with motor function and activities of daily living in mild to moderate Alzheimer's dementia. Curr Alzheimer Res. 2020;17(7):680‐686. doi: 10.2174/1567205017666200818193916 [DOI] [PubMed] [Google Scholar]
- 3. Dudzińska‐Griszek J, Szuster K, Szewieczek J. Grip strength as a frailty diagnostic component in geriatric inpatients. Clin Interv Aging. 2017;12:1151‐1157. doi: 10.2147/cia.s140192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quan M, Xun P, Chen C, et al. Walking pace and the risk of cognitive decline and dementia in elderly populations: a meta‐analysis of prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2017;72(2):266‐270. doi: 10.1093/gerona/glw121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jack C, Knopman D, Jagust W, et al. O3–03–01: update on hypothetical model of Alzheimer's disease biomarkers. Alzheimers Dement. 2013;9(4S_Part_13):P521‐P522. doi: 10.1016/j.jalz.2013.04.248 [DOI] [Google Scholar]
- 6. Callisaya ML, Launay CP, Srikanth VK, Verghese J, Allali G, Beauchet O. Cognitive status, fast walking speed and walking speed reserve—the Gait and Alzheimer Interactions Tracking (GAIT) study. GeroScience. 2017;39(2):231‐239. doi: 10.1007/s11357-017-9973-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan WLS, Pin TW. Reliability, validity and minimal detectable change of 2‐minute walk test, 6‐minute walk test and 10‐meter walk test in frail older adults with dementia. Exp Gerontol. 2019;115:9‐18. doi: 10.1016/j.exger.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 8. Delazer M, Zamarian L, Djamshidian A. Handwriting in Alzheimer's disease. J Alzheimers Dis. 2021;82(2):727‐735. doi: 10.3233/JAD-210279 [DOI] [PubMed] [Google Scholar]
- 9. Grande G, Triolo F, Nuara A, Welmer AK, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol. 2019;124(110625):110625. doi: 10.1016/j.exger.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 10. Knapstad MK, Steihaug OM, Aaslund MK, et al. Reduced walking speed in subjective and mild cognitive impairment: a cross‐sectional study: a cross‐sectional study. J Geriatr Phys Ther. 2019;42(3):E122‐E128. doi: 10.1519/JPT.0000000000000157 [DOI] [PubMed] [Google Scholar]
- 11. Wirths O, Bayer TA. Motor impairment in Alzheimer's disease and transgenic Alzheimer's disease mouse models. Genes Brain Behav. 2008;7(Suppl 1):1‐5. doi: 10.1111/j.1601-183X.2007.00373.x [DOI] [PubMed] [Google Scholar]
- 12. Koppelmans V, Silvester B, Duff K. Neural mechanisms of motor dysfunction in mild cognitive impairment and Alzheimer's disease: a systematic review. J Alzheimers Dis Rep. 2022;6(1):307‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaefer SY, Duff K, Hooyman A, Hoffman JM. Improving prediction of amyloid deposition in mild cognitive impairment with a timed motor task. Am J Alzheimers Dis Other Demen. 2022;37:15333175211048262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S65‐75. doi: 10.1212/WNL.0b013e3182872e01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32(4):351‐358. doi: 10.1097/WAD.0000000000000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 17. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sprecher KE, Bendlin BB, Racine AM, et al. Amyloid burden is associated with self‐reported sleep in nondemented late middle‐aged adults. Neurobiol Aging. 2015;36(9):2568‐2576. doi: 10.1016/j.neurobiolaging.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwarz CG, Gunter JL, Wiste HJ, et al. A large‐scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin. 2016;11:802‐812. doi: 10.1016/j.nicl.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Racine AM, Clark LR, Berman, et al. Associations between performance on an Abbreviated CogState Battery, other measures of cognitive function, and biomarkers in people at risk for Alzheimer's disease. J Alzheimers Dis. 2016;54(4):1395‐1408. doi: 10.3233/JAD-160528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289‐300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 22. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age‐related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721‐733. doi: 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quandt F, Bönstrup M, Schulz R, et al. Spectral variability in the aged brain during fine motor control. Front Aging Neurosci. 2016;8:305. doi: 10.3389/fnagi.2016.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swanberg MM, Tractenberg RE, Mohs R, Thal LJ, Cummings JL. Executive dysfunction in Alzheimer disease. Arch Neurol. 2004;61(4):556‐560. doi: 10.1001/archneur.61.4.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grothe MJ, Barthel H, Sepulcre J, et al. In vivo staging of regional amyloid deposition. Neurology. 2017;89(20):2031‐2038. doi: 10.1212/WNL.0000000000004643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braak H, Braak E. Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol. 1991;82(4):239‐259. doi: 10.1007/bf00308809 [DOI] [PubMed] [Google Scholar]
- 27. Pettersson AF, Olsson E, Wahlund LO. Motor function in subjects with mild cognitive impairment and early Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19(5‐6):299‐304. doi: 10.1159/000084555 [DOI] [PubMed] [Google Scholar]
- 28. Yan JH, Rountree S, Massman P, Doody RS, Li H. Alzheimer's disease and mild cognitive impairment deteriorate fine movement control. J Psychiatr Res. 2008;42(14):1203‐1212. doi: 10.1016/j.jpsychires.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 29. Koppelmans V, Ruitenberg MFL, Schaefer SY, et al. Delayed and more variable unimanual and bimanual finger tapping in Alzheimer's disease: associations with biomarkers and applications for classification. J Alzheimers Dis. 2023;95(3):1233‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malek‐Ahmadi M, Duff K, Chen K, et al. Volumetric regional MRI and neuropsychological predictors of motor task variability in cognitively unimpaired, mild cognitive impairment, and probable Alzheimer's disease older adults. Exp Gerontol. 2023;173:112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olczak A, Truszczyńska‐Baszak A, Stępień A, Górecki K. Functional therapeutic strategies used in different stages of Alzheimer's disease‐a systematic review. Int J Environ Res Public Health. 2022;19(18):11769. doi: 10.3390/ijerph191811769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu XC, Yu Y, Wang HF, et al. Physiotherapy intervention in Alzheimer's disease: systematic review and meta‐analysis. J Alzheimers Dis. 2015;44(1):163‐174. doi: 10.3233/JAD-141377 [DOI] [PubMed] [Google Scholar]
- 33. Venturelli M, Scarsini R, Schena F. Six‐month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26(5):381‐388. doi: 10.1177/1533317511418956 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


