Abstract
INTRODUCTION
Blood‐based biomarkers are a cost‐effective and minimally invasive method for diagnosing the early and preclinical stages of amyloid positivity (AP). Our study aims to investigate our novel immunoprecipitation‐immunoassay (IP‐IA) as a test for predicting cognitive decline.
METHODS
We measured levels of amyloid beta (Aβ)X‐40 and AβX‐42 in immunoprecipitated eluates from the DELCODE cohort. Receiver‐operating characteristic (ROC) curves, regression analyses, and Cox proportional hazard regression models were constructed to predict AP by Aβ42/40 classification in cerebrospinal fluid (CSF) and conversion to mild cognitive impairment (MCI) or dementia.
RESULTS
We detected a significant correlation between AßX‐42/X‐40 in plasma and CSF (r = 0.473). Mixed‐modeling analysis revealed a substantial prediction of AßX‐42/X‐40 with an area under the curve (AUC) of 0.81 for AP (sensitivity: 0.79, specificity: 0.74, positive predictive value [PPV]: 0.71, negative predictive value [NPV]: 0.81). In addition, lower AβX‐42/X‐40 ratios were associated with negative PACC5 slopes, suggesting cognitive decline.
DISCUSSION
Our results suggest that assessing the plasma AβX‐42/X‐40 ratio via our semiautomated IP‐IA is a promising biomarker when examining patients with early or preclinical AD.
Highlights
New plasma Aβ42/Aβ40 measurement using immunoprecipitation–immunoassay
Plasma Aβ42/Aβ40 associated with longitudinal cognitive decline
Promising biomarker to detect subjective cognitive decline at‐risk for brain amyloid positivity
Keywords: Alzheimer's disease, amyloid beta, biomarker, dementia, MCI, plasma
1. BACKGROUND
Alzheimer's disease (AD) is a global public health challenge, with a growing number of people being diagnosed with AD worldwide, 1 resulting in rising health care costs and an increasing disease burden. 2 Currently, AD is diagnosed via a combination of cerebrospinal fluid (CSF) biomarkers such as phosphorylated tau protein 181 (p‐tau‐181), amyloid beta 42 (Aβ42), or rather Aβ42/Aβ40 ratio, Aβ positron emission tomography (PET), 3 as well as clinical evaluation including neuropsychological testing. 4 Most recently, the National Institute on Aging–Alzheimer's Association (NIA‐AA) presented a draft of revised clinical guidelines for AD, defining AD purely by amyloid positivity (AP) measured by PET, cerebrospinal fluid (CSF), or plasma amyloid biomarker. 5 However, CSF biomarker measurements are invasive (lumbar puncture) or expensive (PET). Measuring the Aβ42/Aβ40 ratio in plasma has recently been suggested to constitute a reliable indicator of cerebral amyloid pathology in AD. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Although several studies have indicated that plasma Aβ42/Aβ40 can differentiate cerebral amyloid pathology from normal aging, its performance in predicting cognitive decline and identifying patients at risk for AD remains thus far unclear. Currently, blood assays for supporting clinical AD diagnosis rely largely on mass spectrometry–based detection of Aβ species, with the drawbacks of high costs and limited availability. In this study, we aim to evaluate the utility of a recently described novel immunoprecipitation–immunoassay (IP‐IA) 8 as a cost‐effective, robust, and rapid assay for predicting cognitive decline in a cohort enriched for high‐risk individuals. As novel antibody‐based treatment strategies targeting amyloid in the brain have been approved, and the NIA‐AA aims to define AD based on AP, peripheral Aβ measurements are important for screening, detection, and disease monitoring.
The DELCODE (DZNE Longitudinal Cognitive Impairment and Dementia) study, 14 a longitudinal study following the clinical progression of cognitively normal individuals and patients with subjective cognitive decline (SCD) or mild cognitive impairment (MCI), provides an excellent opportunity to validate our IP‐IA method for AβX‐42/X‐40 measurement in plasma.
2. METHODS
2.1. Participants
In this study, we included 779 participants from the DELCODE study, a longitudinal study following the clinical progression of cognitively normal individuals and patients with SCD or MCI. All participants were volunteers or help‐seeking patients recruited through memory clinics within Germany. Study participants went through annual neuropsychiatric and medical evaluations, including biomarker and imaging analysis. 14 This study was registered at the German Clinical Trials Registry on May 4, 2015 (# DRKS00007966). A full neuropsychiatric assessment, including a blood draw, was performed annually during the study visits. Collected blood was processed immediately by each study center using uniform standard operating procedures (SOPs). For this study, one aliquot of ethylenediaminetetraacetic acid (EDTA) plasma was provided that was frozen within 30 minutes after blood collection and kept frozen until use.
2.2. Standard protocol approvals, registration, and patient consents
All participants provided their written informed consent to participate in this study. The research protocols for specimen sampling and data collection were approved on each study site. The study was conducted according to the Declaration of Helsinki.
2.3. Aβ measurements
A total of 500 μL EDTA plasma samples was obtained from the DELCODE cohort. All samples were collected and processed according to the highly standardized DELCODE standard operating procedures (SOPs) and stored at −80°C. The levels of AβX‐38, AβX‐40, AβX‐42, and their corresponding AβX‐42/X‐40 ratio were measured using our semi‐automated IP‐IA, as reported previously. 8 , 15 The automated Aβ immunoprecipitation (IP) from plasma was performed on a CyBio FeliX liquid‐handling instrument (Roboscreen, Leipzig, Germany), followed by the measurement of Aβ species using the Mesoscale Discovery Aβ V‐Plex immunoassay (6E10).
Briefly, ethylenediamine tetraacetic acid (EDTA) plasma samples were thawed, vortexed, and centrifuged at 10,000 × g for 10 min at room temperature. A total of 200 μL plasma was mixed with 25 μL magnetic beads (sheep anti‐mouse IgG Dynabeads [M‐280, Invitrogen/ThermoFischer Scientific Waltham, MA, USA]) coupled to monoclonal anti‐amyloid‐β antibody (1E8, nanoTools, Teningen, Germany) in 200 μL H2O and 100 μL of 5× IP‐buffer (250 mM 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid/sodium hydroxide (HEPES/NaOH), pH 7.4, 750 mM sodium cloride (NaCl), 2.5% Igepal CA630, 1.25% sodium deoxycholate, 0.25% sodium dodecyl sulfate (SDS), one tablet Complete Mini Protease Inhibitor Cocktail per 2 mL) and incubated at 17°C overnight. Beads were washed three times for 5 min with 0.1% bovine serum albumin (BSA) in phosphate‐buffered saline (PBS) and once for 3 min with 10 mM tris‐hydroxychloride (Tris‐HCL), pH 7.5. To elute Aβ peptides, the beads were incubated at 99°c for 5 min in 2 × 25 μL of 20 mM bicine, pH 7.6/0.06% 3‐[(3‐cholamidopropyl)dimethylammonio]‐1‐propanesulfonate (CHAPS). Approximately 38 μL of eluate (estimated range: 36‐40 μL) was obtained and diluted with 190 μL Diluent 35 (MSD). The diluted eluate was divided into three aliquots of 60 μL and stored at −80°C until use.
2.4. Clinical evaluation
To assess group differences in the risk of clinical progression, we analyzed follow‐up diagnostic data covering the time frame from study inception until April 2021 (follow‐up time: M = 3.27 years, SD = 1.50). Progression to dementia was assessed by the study physicians at each follow‐up visit based on published diagnostic criteria. 16 Diagnoses of incident MCI were determined in a two‐step review process adapted from the diagnostic procedures in the Wisconsin Registry for Alzheimer´s Prevention study. 17 Briefly, follow‐up neuropsychological data of SCD patients and control participants were algorithmically screened for signs of potential cognitive decline. Flagged cases were then reviewed in detail by a team of neuropsychologists, who were blinded to initial group assignment and biomarker data, and who determined the diagnostic status at follow‐up based on established diagnostic criteria. 18 Diagnostic assignments were reviewed and validated by a consensus committee, which resolved potential inconsistencies and established final diagnoses.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the most recent literature on PubMed to evaluate the current state of blood‐based amyloid beta (Aβ) biomarkers to detect brain amyloid positivity. Although several studies have been published in the past on plasma biomarkers for Alzheimer's disease (AD), the prognostic value of plasma amyloid detection in patients with subjective cognitive decline (SCD) remains inconclusive.
Interpretation: This study proposes the use of immunoprecipitation–immunoassay for early detection of amyloid positivity. Our findings suggest that plasma Aβ42/Aβ40 is associated with longitudinal cognitive decline in early AD, particularly SCD. Blood‐based amyloid biomarkers will gain further importance due to the novel National Institute on Aging–Alzheimer's Association (NIA‐AA) definition of AD based purely on brain amyloid positivity and novel antibody treatment options addressing amyloid deposition in the brain.
Future directions: The proposed technique of immunoprecipitation–immunoassay should be evaluated further in larger community‐based cohorts to determine its use as a screening biomarker in the general population.
2.5. Statistical analysis
All Mesoscale Aβ measurements were conducted in duplicate, and the variation between duplicates was calculated to ensure accurate results and reproducibility. Samples with a coefficient of variation (CV) of 10% or higher were re‐measured or excluded from further downstream analysis. Forty‐one samples with a CV >10% between the two technical replicates were re‐measured. One hundred ninety‐five samples were excluded, presumably due to inappropriate sample handling. Downstream analysis was performed with 779 remaining samples. All analyses (Deming regression, receiver‐operating characteristic (ROC) analysis, t‐tests, mixture model, Cox proportional hazards regression) were conducted using R version 3.5.1 and packages MethComp (version 1.22.2), pROC (version 1.18.0), mixtools (version 1.2.0), survival (version 3.5‐0), and survminer (version0.4.9). For the mixture model, we used an EM algorithm for mixtures of normal distributions without further constraints on mean or SD. The logistic regression (univariate or multivariate) was performed using a 10‐fold cross‐validation to avoid overfitting.
For the calculation of the Preclinical Alzheimer's Cognitive Composite (PACC5) slopes, we used as much data as available for each patient (visits 0, 1, 2, 3, and 4, if available) and calculated a linear model assuming yearly time points. To calculate the slope, PACC5 values for at least two visits had to be available. The resulting slope can be interpreted as the average yearly change of the PACC5 value.
The association between baseline AβX‐42/AβX‐40 in the IP eluates and the risk of incident dementia was analyzed with Cox proportional hazards regression models. In addition, we conducted subgroup analyses investigating the progression to dementia in the MCI group and the progression to MCI in the control and SCD groups.
2.6. Data access and availability
All data are available through the German Center of Neurodegenerative Diseases (“Deutsches Zentrum fuer Neurodegenerative Erkrankungen, DZNE,” klinische-studien@dzne.de). Anonymized data not published in this article will be made available by request from any qualified investigator.
3. RESULTS
3.1. Study cohort
The study included 779 participants for downstream analysis, consisting of 230 controls, 429 individuals with SCD, 188 with MCI, 122 with dementia due to AD, and 80 AD relatives. The proportion of women was highest in the AD dementia group (59.0%) compared to MCI (48.9%), SCD (32.6%), or controls (57.4%). The AD dementia group had a higher percentage of apolipoprotein E (APOE) ε4 carriers, as well as a higher average age, higher total tau (t‐tau) and phospho‐tau (p‐tau) CSF levels, along with lower Aβ42/Aβ 40 ratios in CSF and lower PACC5 scores compared to the MCI, SCD, or control groups (Table 1).
TABLE 1.
Demographics of patients.
| Controls | SCD | MCI | AD | AD relatives | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| Gender (F) | 0.57 | 230 | 0.46 | 429 | 0.45 | 188 | 0.59 | 122 | 0.61 | 80 | |||||
| APOE ε4 | 0.21 | 229 | 0.33 | 426 | 0.49 | 184 | 0.64 | 121 | 0.36 | 80 | |||||
| Age, years | 68.87 | 5.40 | 230 | 70.89 | 6.06 | 429 | 72.51 | 5.56 | 188 | 74.69 | 6.30 | 122 | 65.50 | 4.37 | 80 |
| Education, years | 14.70 | 2.72 | 230 | 14.91 | 2.97 | 429 | 14.05 | 3.13 | 188 | 12.84 | 3.04 | 122 | 14.58 | 2.73 | 80 |
| Aβ38 | 3218.18 | 879.35 | 90 | 3267.50 | 1028.01 | 207 | 3165.40 | 1064.27 | 111 | 3105.98 | 1070.00 | 65 | 3404.90 | 1119.23 | 44 |
| Aβ40 | 8749.85 | 2382.35 | 90 | 8399.13 | 2208.04 | 207 | 8168.70 | 2366.70 | 111 | 8222.74 | 2417.21 | 65 | 8539.24 | 2303.94 | 44 |
| Aβ42 | 838.27 | 300.04 | 90 | 774.51 | 336.54 | 207 | 585.68 | 307.12 | 111 | 411.52 | 198.46 | 65 | 853.08 | 338.33 | 44 |
| Aβ42/Aβ40 | 0.10 | 0.02 | 90 | 0.09 | 0.03 | 207 | 0.07 | 0.03 | 111 | 0.05 | 0.02 | 65 | 0.10 | 0.02 | 44 |
| Total tau | 366.00 | 159.29 | 90 | 369.26 | 185.25 | 207 | 544.48 | 298.64 | 111 | 805.34 | 374.44 | 65 | 342.94 | 127.01 | 44 |
| Phosphor‐tau‐181 | 49.85 | 18.02 | 90 | 53.83 | 24.16 | 207 | 70.92 | 41.88 | 111 | 96.56 | 43.63 | 65 | 49.79 | 18.32 | 44 |
| PACC5_v0 | 0.19 | 0.53 | 230 | −0.11 | 0.67 | 424 | −1.48 | 1.02 | 174 | −3.71 | 1.25 | 63 | 0.14 | 0.71 | 80 |
| PACC5_v1 | 0.32 | 0.55 | 196 | −0.08 | 0.71 | 369 | −1.48 | 1.11 | 132 | −4.43 | 1.71 | 30 | 0.31 | 0.61 | 64 |
| PACC5_v2 | 0.32 | 0.56 | 180 | −0.18 | 0.82 | 303 | −1.72 | 1.28 | 89 | −4.41 | 1.52 | 11 | 0.23 | 0.80 | 55 |
Abbreviations: APOE ε4, apolipoprotein E ε4; Aβ38, amyloid beta 38; Aβ40, amyloid beta 40; Aβ42, amyloid beta 42; Aβ42/Aβ40, amyloid beta 42/amyloid beta 40; F, female; PACC5_v0, preclinical alzheimer's cognitive composite score at baseline; PACC5_v1, preclinical alzheimer's cognitive composite score at year one follow‐up visit; PACC5_v2, preclinical alzheimer's cognitive composite score at year two follow‐up visit.
3.2. Exploratory plasma Aβ measurements and quality assessments
We conducted a total of six repetitions of measuring pooled samples to assess technical reproducibility. Interassay variability was 8.3%, 8.0%, and 6.9% for AβX‐40, AβX‐42, and the AβX‐42/AβX40 ratio, respectively. We found no effects of batch, plate, or center in the entire cohort. The data from AβX‐38 were not analyzed as part of the present investigation.
3.3. Cross‐sectional baseline plasma measurements
CSF Aβ measures were available for 375 samples. There was no correlation between IP eluates from plasma and CSF for Aβ40 (r = 0.013, p = 0.802), but correlations for Aβ42 (r = 0.169, p < 0.0001) and AβX‐42/AβX‐40 (r = 0.466, p < 0.0001) (Figure 1). As expected, CSF Aβ42/Aβ40 showed a typical bimodal distribution resulting in a calculated cutoff value of 0.08 (Gaussian mixture modeling analysis; Jessen et al., 2022 19 ) (Figure 2A). In contrast, we did not observe a bimodal distribution in AβX‐42/AβX‐40 in the IP eluates from plasma (Figure 2B). Given its unimodal distribution, we could not calculate a mixture model‐based cutoff value for plasma AβX‐42/AβX‐40. CSF Aβ42/Aβ40 was, therefore, used to classify amyloid‐positive and amyloid‐negative participants. Specifically, to calculate a cutoff value for AβX‐42/AβX‐40 in plasma IP eluates, we projected the CSF Aβ42/Aβ40 cutoff value onto the corresponding plasma AβX‐42/AβX‐40 measurements in a linear regression model (Figure 2C), resulting in a cutoff value of 0.106 for plasma AβX‐42/AβX‐40.
FIGURE 1.
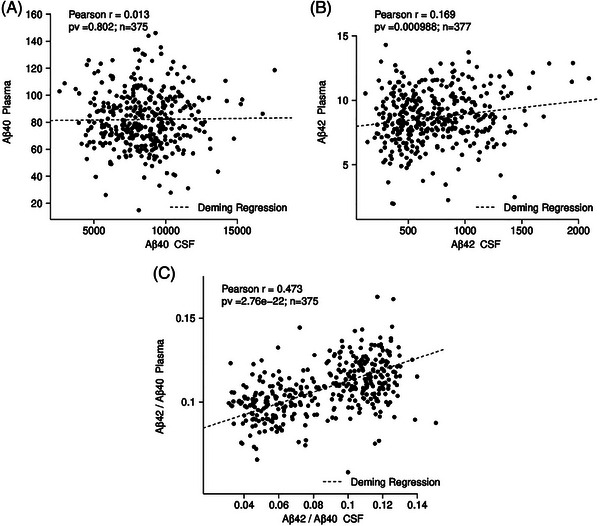
Correlation between plasma and CSF Aβ40, Aβ42, Aβ42/Aβ40. No correlation was detected between Aβ IP eluates from plasma and CSF. Note the weak correlation (r = 0.169, p = 0.000988) between IP eluates and CSF Aβ42, and a stronger correlation (r = 0.47, p = 2.76e‐22) between plasma and CSF Aβ42/Aβ40. Aβ40, amyloid beta X‐40; Aβ42, amyloid beta X‐42; Aβ42/Aβ40, amyloid beta X‐42/amyloid beta X‐40 ratio; CSF, cerebrospinal fluid; IP, immunoprecipitation. The Pearson´s correlation coefficient (r) and the corresponding p‐values are indicated in each figure part. In addition, a Deming regression is shown in each figure part.
FIGURE 2.
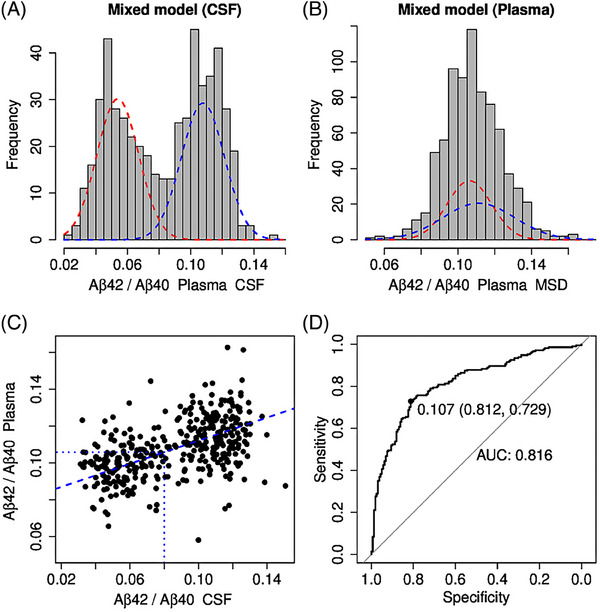
Mixture modeling analysis of the Aβ42/Aβ40 ratio in plasma and CSF. Mixture modeling analysis revealed a typical bimodal distribution for CSF (A) but not Aβ42/Aβ40 in IP eluates from plasma (B). To determine the Aβ42/Aβ40 cutoff value in plasma, Aβ42/Aβ40 values in CSF were correlated with the corresponding Aβ42/Aβ40 values in plasma via a linear regression model (C). The intersection of the dashed blue lines indicates the 0.106 cutoff value for plasma Aβ42/Aβ40. In (D), the receiver‐operating characteristic (ROC) curve was plotted with an area under the curve (AUC) of 0.81 for Aβ42/Aβ40 to predict amyloid positivity based on Aβ42/Aβ 40 classification in CSF. Aβ42/Aβ40, amyloid beta X‐42/amyloid beta X‐40; CSF, cerebrospinal fluid.
Predicting AP based on CSF Aβ42/Aβ40 classification, we calculated ROC curves for AβX‐40, AβX‐42, and AβX‐42/AβX‐40 with AUCs of 0.56 (95% confidence interval [CI]: 0.49–0.61), 0.59 (95% CI: 0.53–0.65), and 0.81 (95% CI: 0.77–0.86), respectively. Based on the Maximum Youden Index, 20 the cutoff for AβX‐42/AβX‐40 in IP eluates was 0.107, which was comparable to the cutoff of 0.106 estimated by the CSF plasma correlation (Figure 2C), resulting in a sensitivity of 0.79 and a specificity of 0.74 with a PPV of 0.71 and an NPV of 0.81. Including APOE ε4 carriage information, the AUC increased to 0.87 (95% CI: 0.83–0.90), with a corresponding specificity of 0.73 and a sensitivity of 0.87 (Figure 3, Table 2). Even more relevant is the stratification for APOE ε4 in controls and SCD: the ROC AUC can be increased from 0.76 (not stratified for APOE ε4) to 0.85 (stratified for APOE ε4, p = 0.031), with a corresponding sensitivity of 0.83, a specificity of 0.74, an NPV of 0.89, a PPV of 0.63, and a false‐positive rate of 25.6%.
FIGURE 3.
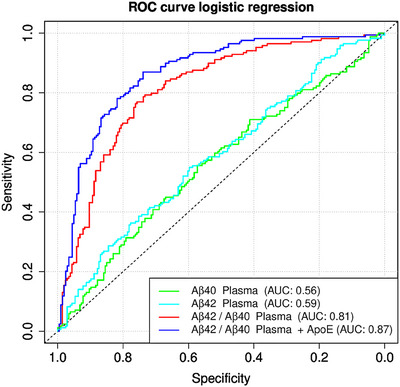
Receiver‐operating characteristic curves for the Aβ40 and Aβ42, Aβ42/Aβ40 ratio, and Aβ42/Aβ40 ratio + APOE ε4 carriage. For the plasma Aβ42/Aβ40 ratio, we calculated a receiver‐operating characteristic (ROC) curve comparing amyloid‐positive against amyloid‐negative cases with an area under the curve (AUC) of 0.81. However, when stratified by APOE ε4 positivity, the AUC rose as high as 0.87, with a 0.73 corresponding specificity and 0.87 sensitivity. Significantly lower AUCs were found for Aβ40 at 0.56 and for Aβ42 plasma at 0.59. Aβ40, amyloid beta X‐40; Aβ42, amyloid beta X‐42; Aβ42/Aβ40, amyloid beta X‐42/amyloid beta X‐40 ratio; APOE ε4, apolipoprotein E ε4.
TABLE 2.
Receiver‐operating characteristic (ROC) curves for AβX‐40, AβX‐42, and AβX‐42/AβX‐40 in plasma IP‐eluates for prediction of amyloid positivity by Aβ42/Aβ40 classification in CSF.
| tp | tn | fp | fn | ppv | npv | Sensitivity | Specificity | BACC | AUC | |
|---|---|---|---|---|---|---|---|---|---|---|
| Aβ40 | 94 | 118 | 88 | 75 | 0.516 | 0.611 | 0.556 | 0.573 | 0.565 | 0.563 |
| Aβ42 | 94 | 123 | 83 | 77 | 0.531 | 0.615 | 0.550 | 0.597 | 0.573 | 0.587 |
| Aβ42/Aβ40 | 130 | 156 | 50 | 39 | 0.722 | 0.800 | 0.769 | 0.757 | 0.763 | 0.813 |
| Aβ42/Aβ40 + ApoE ε4 | 147 | 150 | 53 | 22 | 0.735 | 0.872 | 0.870 | 0.739 | 0.804 | 0.865 |
Abbreviations: AUC, area under the curves; BACC, balanced accuracy; fn, false negative; fp, false positive; npv, negative predictive value; ppv, positive predictive value; tn, true negative; tp, true positive.
3.4. Predictive value of plasma AβX‐42/AβX‐40
We further conducted regression analysis on baseline plasma AβX‐42/AβX‐40 measurements with follow‐up cognitive measurements. For this analysis, we used PACC5 as a measure of subtle cognitive decline over time, since PACC5 is highly sensitive to preclinical cognitive decline 21 , 22 , 23 and longitudinal cognitive decline, 19 particularly in SCD. Moreover, it has substantial weight on memory, which is typically affected by brain AP. At baseline, 728 participants had plasma Aβ measured and underwent thorough neuropsychological assessment including PACC5. At the subsequent available follow‐up, one year past baseline, 594 participants underwent a PACC5 evaluation. At years 2, 3, and 5, PACC5 data were available from 447, 283, and 188 participants, respectively.
We calculated patient‐wise PACC5 slopes over time for all patients (Figure 4A) and found that low plasma AβX‐42/AβX‐40 ratios, based on the calculated cutoff value of 0.106 (Figure 2C), were associated with negative slopes, indicating cognitive decline over time (Figure 4B). This association was only present in patients with a baseline diagnosis of SCD (p = 0.0007, Figure 4D) and was not apparent in controls (p = 0.4, Figure 4C). A trend‐wise association (p = 0.09) was found in the MCI group at baseline (Figure 4E). To further verify our findings, we computed a Cox proportional hazards regression, based on clinical progression over time, using three different models: AβX‐42/AβX‐40 only without covariate adjustment (model 1), AβX‐42/AβX‐40 controlling for age, sex, and education (model 2), and AβX‐42/AβX‐40 controlling for age, sex, education, and APOE ε4 status (model 3). The results of the survival analyses are displayed in Table 3 and Figure 5. In the whole cohort, individuals with low AβX‐42/AβX‐40 had a significantly increased risk of dementia compared to those with high baseline AβX‐42/AβX‐40, even after controlling for demographic covariates and APOE ε4 (hazard ratio [HR] = 4.38, 95% CI: 1.77–10.85). Low AβX‐42/AβX‐40 was also a significant predictor of the progression from MCI to dementia as well as the progression from SCD to MCI, although these results did not remain significant after additional adjustment for APOE ε4. Within the control group, low AβX‐42/AβX‐40 was only significantly associated with an increased risk of MCI in the model that adjusted for both the demographic covariates and APOE ε4.
FIGURE 4.
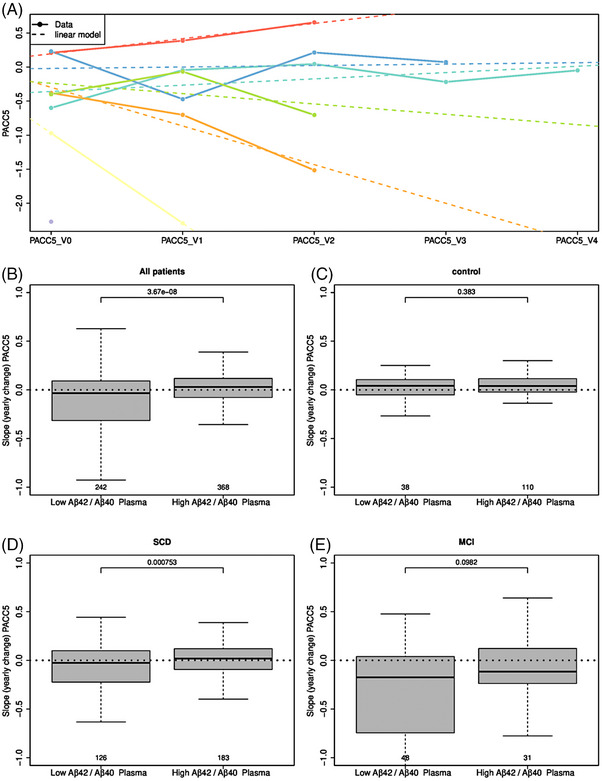
PACC5 slopes for patient groups with low and high amyloid beta peptide 42/40 ratios. (A) shows PACC5 slopes for all patients over time [visit 1 (V1)‐visit 4 (V4)]. In (B–E), box plots of low and high IP eluate Aβ42/Aβ40 ratios are shown for different patients regarding their PACC5 slope depicted as yearly change. Of interest, lower Aβ42/Aβ40 ratios in all patients in B and subjective cognitive decline (SCD) in D were associated with negative PACC5 slopes, suggesting cognitive decline. However, this association did not appear in controls in C and MCI patients in E. Aβ42/Aβ40, amyloid beta X‐42/amyloid beta X‐40; MCI, mild cognitive impairment; SCD, subjective cognitive decline.
TABLE 3.
Cox proportional hazards regression based on clinical progression over time using three different models.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| Outcome: Dementia | ||||||
| Whole sample | 6.53 (2.84–15.03) | <0.001 | 6.51 (2.76–15.34) | <0.001 | 4.38 (1.77–10.85) | 0.001 |
| MCI group | 2.76 (1.12–6.82) | 0.028 | 3.28 (1.29–8.37) | 0.013 | 2.49 (0.87–7.17) | 0.09 |
| Outcome: MCI | ||||||
| Control group | 3.05 (0.76–12.20) | 0.115 | 3.75 (0.90–15.64) | 0.07 | 4.62 (1.05–20.28) | 0.043 |
| SCD group | 2.10 (1.29–3.44) | 0.003 | 1.78 (1.04–3.04) | 0.035 | 1.58 (0.90–2.77) | 0.11 |
Notes: Results of Cox proportional hazards regression models analyzing the association between low baseline Aβ42/Aβ40 IP eluates from plasma and the risk of clinical progression to dementia (whole sample, MCI group) or MCI (control group, SCD group). Model 1: no covariate adjustment; Model 2: adjusted for baseline age, sex, and years of education; Model 3: additional adjustment for APOE ε4 status.
Abbreviations: HR, hazard ratio; MCI, mild cognitive impairment; SCD, subjective cognitive decline.
FIGURE 5.
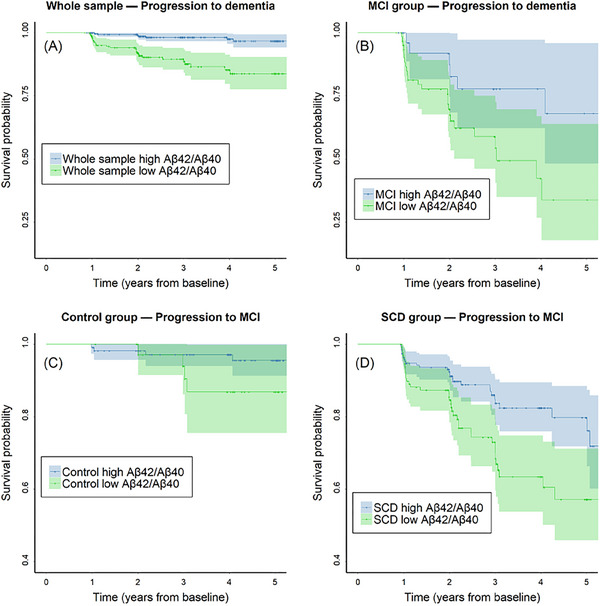
COX regression modeling conversion to MCI or dementia over time. Kaplan–Meier survival curve estimates and 95% confidence intervals displaying the risk of progression to dementia (A: whole sample, B: MCI group) or MCI (C: control group, D: SCD group). MCI, mild cognitive impairment; SCD, subjective cognitive decline.
4. DISCUSSION
The results of this study provide further evidence for the utility of the AβX‐42/AβX‐40 ratio in blood, as a biomarker for amyloid pathology in plasma, measured using our IP‐IA approach. By using the cutoff value of 0.106, the plasma AβX‐42/AβX‐40 ratio can distinguish between individuals who are positive or negative for low CSF Aβ42/Aβ40 with a sensitivity of 79% and specificity of 74%. Different assays measuring Aβ42/Aβ40 have shown sensitivity ranging from 83%–96% and specificities of 72%–87% in various , studies. 13 , 24 , 25 Depending on the intended purpose and population, cutoff values might need to be adjusted. Using our proposed cutoff values, we can detect 80% of all amyloid‐positive patients with only a 26% false‐positive rate using a minimally invasive blood assay. Adjusting the cutoff values to receive a sensitivity of 90% for screening purposes would result in a false‐positive rate of 52%. The Aβ42/40 ratio in plasma can be considered an indicator of brain amyloidosis, as it correlates well with Aβ42/40 in CSF. A meta‐analysis has recently shown that the Aβ42/40 ratio in plasma can function as an independent biomarker for brain amyloidosis detected by Aβ‐PET. 26 Compatible with this notion, the plasma Aβ42/Aβ40 ratio has been associated specifically with increased Aβ in PET, following the regional pattern typically observed in preclinical AD stages. 27 Adding the APOE ε4 genotype further increases the sensitivity and specificity of the plasma Aβ42/Aβ40 ratio, as recently reported in a study in two cohorts of individuals with MCI or without cognitive impairment. 13 Our results extend those findings by demonstrating the diagnostic and prognostic utility of the plasma Aβ42/Aβ40 ratio in the at‐risk population of individuals with SCD over a period of on average of 2 years. The plasma Aβ42/Aβ40 ratio has been shown to be related to early memory impairment in SCD individuals, 24 and we could now demonstrate its association with subsequent cognitive decline as assessed with the PACC5, a sensitive marker of early cognitive decline in SCD. The observation that a low plasma AβX‐42/AβX‐40 ratio predicts cognitive decline over 2 years in individuals with SCD, indicates that plasma AβX‐42/AβX‐40 is a promising biomarker for identifying those individuals in a population of patients with SCD who are at increased risk for progressing to MCI due to AD and ultimately dementia. In individuals with MCI, modeling and regression analysis over time support the plasma AβX‐42/AβX‐40 ratio as a relevant predictor of converting from MCI to dementia. Even when controlling for variables such as age, sex, and education in the analysis, the plasma Aβ42/Aβ40 ratio remains a relevant predictor for conversion. Including the APOE ε4 increases the predictive power of plasma Aβ42/Aβ40. ,
It should be noted, though, that, in cognitively unimpaired individuals, combining the plasma Aβ42/Aβ40 ratio measured with mass spectrometry with p‐tau231 or p‐tau217 measures obtained with immunoassays appears to be superior to relying on the plasma Aβ42/Aβ40 ratio alone to detect Aβ pathology. 7 , 28 We suggest that it should nevertheless be possible to replace mass spectrometry with the here‐described IP‐IA in a combined Aβ/tau approach, but this needs to be confirmed by future studies. Furthermore, we believe that IP‐IA can be superior to immunoassays without IP, as we have shown earlier: for example, using the Roche Elycsys analyzer, we see a 20% increase in the AUC in samples with IP compared to pure EDTA plasma. 8
Limitations of this study include the lack of controlling for covariates that might impact plasma Aβ levels, such as heart failure, medication, or weight/body mass index (BMI). It should be further noted that our measured IP‐IA Aβ corresponds to plasma Aβ levels. However, they should not be understood as true and absolute plasma Aβ concentrations.
In conclusion, determining the AβX‐42/AβX‐40 ratio in plasma by semi‐automated IP‐IA is a promising and reliable method to assess patients at early AD stages and those at risk for SCD and AP, even more, if stratifying for APOE ε4. Plasma Aβ measurements might not be able to compete against CSF or imaging biomarkers regarding diagnostic procedures. However, due to their high availability and cost‐effectiveness, they can be a very important step in early screening for AD pathology, followed by more specific testing, such as imaging, CSF biomarkers, and neuropsychiatric testing. Especially for the SCD group, we see the best correlation between the measured Aβ ratio and disease progression; it also complements other markers such as p‐tau231 or p‐tau217, which are pathological at preclinical AD stages 28 and predicts long‐term cognitive decline in preclinical AD. 29
CONFLICT OF INTEREST STATEMENT
J.V.: DFG/Clinician Scientist Kolleg (#413501650) and McLean Eric Dorris Memorial Fellowship. N.H.: DFG (530229798), Lilly AG travel support. S.T.: Advisory Board Memberships for Biogen, Eisai, Lilly; Member of the Independent Data Safety and Monitoring Board for ENVISION (Biogen). E.D.: Paid consultancy work and talks for Roche, Lilly, Eisai, Biogen, neotiv, and UCLC; Holds shares of neotiv. C.B.: Employee of MicroDiscovery GmbH. MicroDiscovery was paid by University Goettingen for supporting the statistical analysis. J.S.: Employee of MicroDiscovery GmbH. MicroDiscovery was paid by University Goettingen for supporting the statistical analysis. C.l.B.: received honoraria as a diagnostic consultant for Boehringer Ingelheim (last time: 10/2019); received honoraria for lectures from Roche (06/2021); received funding from the German Alzheimer Association (DAlzG; 2021‐2023). O.P.: Paid consultancy work and talks for Biogen, Eisai, Grifols, Lilly, Noselab, Prinnovation, Schwabe, and Roche. F.M.: Employee of ki:elements GmbH. J.W.: Paid consultancy and talks for Abbott, Actelion, Amgen, Beeijing Yibai Science and Technology Ltd., Biogen, Boehringer Ingelheim, Gloryren, Immungenetics, Janssen‐Cilag, Lilly, medUpdate GmbH, MSD Sharp & Dohme, Noselab, Pfizer, Roche, and Roboscreen; holds patents PCT/EP2011 001724 and PCT/EP 2015 052945. M.S., M.W., H.K., B.M., A.J.B., B.S., H.E., L.K., S.W., L.S.S., X.W., J.P., E.S., S.A., A.L., A.S., K.F., I.V., F.J., A.R., W.G., E.I., M.B., K.B., D.J., E.W., R.P., B.R., S.G., I.K., D.G., C.L., M.M., C.S., A.Sp., N.R.K., M.H., F.B., A.R., and M.S. did not report any disclosures. Author disclosures are available in the Supporting Information.
CONSENT STATEMENT
All participants gave their informed consent to participate in this study. The research protocols for specimen sampling and data collection were approved on each study site. The study was conducted according to the Declaration of Helsinki.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was funded by the German Federal Ministry of Education and Research (Bundesministerium fuer Bildung und Forschung, BMBF), project number 13GW0479B.
Vogelgsang J, Hansen N, Stark M, et al. Plasma amyloid beta X‐42/X‐40 ratio and cognitive decline in suspected early and preclinical Alzheimer's disease. Alzheimer's Dement. 2024;20:5132–5142. 10.1002/alz.13909
Jonathan Vogelgsang and Niels Hansen contributed equally.
Contributor Information
Jonathan Vogelgsang, Email: jvogelgsang@mclean.harvard.edu.
Jens Wiltfang, Email: jens.wiltfang@med.uni-goettingen.de.
REFERENCES
- 1. Collaborators G 2019 DF , Nichols E, Steinmetz JD, et al, Collaborators G 2019 DF . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Heal. 2022;7:e105‐e125. doi: 10.1016/s2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ding C, Wu Y, Chen X, et al. Global, regional, and national burden and attributable risk factors of neurological disorders: the Global Burden of Disease study 1990–2019. Frontiers Public Heal. 2022;10:952161. doi: 10.3389/fpubh.2022.952161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dementia. 2018;14:535‐562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer's disease: recommendations of the International Working Group. Lancet Neurol. 2021;20:484‐496. doi: 10.1016/s1474-4422(21)00066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carrillo MC, Snyder H, Andrews JS, et al. NIA‐AA Revised Clinical Guidelines for Alzheimer's n.d. (accessed July 17, 2023). https://aaic.alz.org/nia‐aa.asp
- 6. Brand AL, Lawler PE, Bollinger JG, et al. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer's disease: a literature review. Alzheimer's Res Ther. 2022;14:195. doi: 10.1186/s13195-022-01117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janelidze S, Palmqvist S, Leuzy A, et al. Detecting amyloid positivity in early Alzheimer's disease using combinations of plasma Aβ42/Aβ40 and p‐tau. Alzheimer's Dementia. 2022;18:283‐293. doi: 10.1002/alz.12395 [DOI] [PubMed] [Google Scholar]
- 8. Klafki H‐W, Vogelgsang J, Manuilova E, et al. Diagnostic performance of automated plasma amyloid‐β assays combined with pre‐analytical immunoprecipitation. Alzheimer's Res Ther. 2022;14:127. doi: 10.1186/s13195-022-01071-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klafki H‐W, Morgado B, Wirths O, et al. Is plasma amyloid‐β 1–42/1–40 a better biomarker for Alzheimer's disease than AβX–42/X–40? Fluids Barriers Cns. 2022;19:96. doi: 10.1186/s12987-022-00390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Schindler SE, Bollinger JG, et al. Validation of plasma amyloid‐β 42/40 for detecting Alzheimer disease amyloid plaques. Neurology. 2022;98:e688‐e699. doi: 10.1212/wnl.0000000000013211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wisch JK, Gordon BA, Boerwinkle AH, et al. Predicting continuous amyloid PET values with CSF and plasma Aβ42/Aβ40. Alzheimer's Dementia Diagnosis Assess Dis Monit. 2023;15:e12405. doi: 10.1002/dad2.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. Embo Mol Med. 2019;11:e11170. doi: 10.15252/emmm.201911170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmqvist S, Stomrud E, Cullen N, et al. An accurate fully automated panel of plasma biomarkers for Alzheimer's disease. Alzheimer's Dementia. 2023;19:1204‐1215. doi: 10.1002/alz.12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jessen F, Spottke A, Boecker H, et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer's disease (DELCODE). Alzheimer's Res Ther. 2018;10:15. doi: 10.1186/s13195-017-0314-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shahpasand‐Kroner H, Klafki H, Bauer C, et al. A two‐step immunoassay for the simultaneous assessment of Aβ38, Aβ40 and Aβ42 in human blood plasma supports the Aβ42/Aβ40 ratio as a promising biomarker candidate of Alzheimer's disease. Alzheimer's Res Ther. 2018;10:121. doi: 10.1186/s13195-018-0448-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7:263‐269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koscik RL, Hermann BP, Allison S, et al. Validity evidence for the research category, “Cognitively Unimpaired—Declining,” as a risk marker for mild cognitive impairment and Alzheimer's disease. Front Aging Neurosci. 2021;13:688478. doi: 10.3389/fnagi.2021.688478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7:270‐279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jessen F, Wolfsgruber S, Kleineindam L, et al. Subjective cognitive decline and stage 2 of Alzheimer disease in patients from memory centers. Alzheimer's Dementia. 2023;19:487‐497. doi: 10.1002/alz.12674 [DOI] [PubMed] [Google Scholar]
- 20. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32‐35. doi: [DOI] [PubMed] [Google Scholar]
- 21. Bransby L, Lim YY, Ames D, et al. Sensitivity of a preclinical Alzheimer's Cognitive Composite (PACC) to amyloid β load in preclinical Alzheimer's disease. J Clin Exp Neuropsyc. 2019;41:591‐600. doi: 10.1080/13803395.2019.1593949 [DOI] [PubMed] [Google Scholar]
- 22. Papp KV, Rofael H, Veroff AE, et al. Sensitivity of the preclinical Alzheimer's Cognitive Composite (PACC), PACC5, and Repeatable Battery for Neuropsychological Status (RBANS) to Amyloid Status in Preclinical Alzheimer's Disease ‐Atabecestat Phase 2b/3 EARLY Clinical Trial. J Prev Alzheimer's Dis. 2022;9:255‐261. doi: 10.14283/jpad.2022.17 [DOI] [PubMed] [Google Scholar]
- 23. Wolfsgruber S, Kleineidam L, Guski J, et al. Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology. 2020;95:e1134‐e1143. doi: 10.1212/wnl.0000000000010142 [DOI] [PubMed] [Google Scholar]
- 24. Pascual‐Lucas M, Allué JA, Sarasa L, et al. Clinical performance of an antibody‐free assay for plasma Aβ42/Aβ40 to detect early alterations of Alzheimer's disease in individuals with subjective cognitive decline. Alzheimer's Res Ther. 2023;15:2. doi: 10.1186/s13195-022-01143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamashita K, Miura M, Watanabe S, et al. Fully automated and highly specific plasma β‐amyloid immunoassays predict β‐amyloid status defined by amyloid positron emission tomography with high accuracy. Alzheimer's Res Ther. 2022;14:86. doi: 10.1186/s13195-022-01029-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng L, Li W, Chen Y, et al. Plasma Aβ as a biomarker for predicting Aβ‐PET status in Alzheimer's disease:a systematic review with meta‐analysis. J Neurol Neurosurg Psychiatry. 2022;93:513‐520. doi: 10.1136/jnnp-2021-327864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lemercier P, Vergallo A, Lista S, et al. Association of plasma Aβ40/Aβ42 ratio and brain Aβ accumulation: testing a whole‐brain PLS‐VIP approach in individuals at risk of Alzheimer's disease. Neurobiol Aging. 2021;107:57‐69. doi: 10.1016/j.neurobiolaging.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 28. Ashton NJ, Janelidze S, Mattsson‐Carlgren N, et al. Differential roles of Aβ42/40, p‐tau231 and p‐tau217 for Alzheimer's trial selection and disease monitoring. Nat Med. 2022;28:2555‐2562. doi: 10.1038/s41591-022-02074-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mattsson‐Carlgren N, Salvadó G, Ashton NJ, et al. Prediction of longitudinal cognitive decline in preclinical Alzheimer disease using plasma biomarkers. Jama Neurol. 2023;80(4):360‐369. doi: 10.1001/jamaneurol.2022.5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


