Abstract
NPC (Niemann–Pick type C) disease is a progressive neurological disorder characterized by defects in intracellular cholesterol trafficking, accumulation of cholesterol in the endosomal system and impaired cholesterol homoeostasis. Although these alterations appear to occur in all NPC1-deficient cell types, the consequences are most profound in the nervous system. Since glial cells are important mediators of brain cholesterol homoeostasis, we proposed that defective generation and/or function of lipoproteins released by glia might contribute to the neurological abnormalities associated with NPC disease. We found that, as in other cell types, Npc1−/− glia accumulate cholesterol intracellularly. We hypothesized that this sequestration of cholesterol in glia might restrict the availability of cholesterol for lipoprotein production. Cerebellar astroglia were cultured from a murine model of NPC disease to compare the lipoproteins generated by these cells and wild-type glia. The experiments demonstrate that the amount of cholesterol in glia-conditioned medium is not reduced by NPC1 deficiency. Similarly, cholesterol efflux to apo (apolipoprotein) A1 or glial expression of the transporter ATP-binding-cassette transporter A1 was not decreased by NPC1 deficiency. In addition, the ratio of apo E:cholesterol and the density distribution of lipoproteins in Npc1−/− and Npc1+/+ glia-conditioned medium are indistinguishable. Importantly, in a functional assay, apo E-containing lipoproteins generated by Npc1−/− and Npc1+/+ glia each stimulate axonal elongation of neurons by approx. 35%. On the basis of these observations, we speculate that the neuropathology characteristic of NPC disease can quite probably be ascribed to impaired processes within neurons in the brain rather than defective lipoprotein production by astroglia.
Keywords: apo A1, apo E, astroglia, cholesterol secretion, glial lipoprotein, Niemann–Pick type C disease
Abbreviations: ABC transporter, ATP-binding-cassette transporter; apo, apolipoprotein; CNS, central nervous system; CSF, cerebrospinal fluid; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; GFAP, glial fibrillary acidic protein; HDL, high-density lipoproteins; LDL, low-density lipoprotein; VLDL, very low density lipoprotein; NPC, Niemann–Pick type C; RGC, retinal ganglion cells
INTRODUCTION
Lipid metabolism in the brain is distinct and separate from that in peripheral tissues. Even though the brain contains approx. 25% of total body cholesterol, it does not rely on cholesterol imported from the periphery since plasma lipoproteins do not cross the blood–brain barrier. Instead, essentially all cholesterol in the brain is derived from endogenous synthesis [1,2]. Glial cells (astrocytes, oligodendrocytes and microglia) comprise approx. 90% of all cells in the brain. It has been postulated that in adult animals neurons synthesize only small amounts of cholesterol and rely instead on uptake of cholesterol-containing lipoproteins secreted by glial cells [3]. The mechanism by which cholesterol is transported among the different cell types in the brain has not been fully elucidated. CSF (cerebrospinal fluid) contains several types of spherical lipoproteins of densities in the range of HDL (high-density lipoproteins) [4–7]. These lipoproteins contain apos (apolipoproteins) E, A1, A2, A4 and/or J in different combinations [5–7]. Apo E is synthesized in the brain and is the major CSF apo [8], whereas small amounts of apos A1 and A2 are imported from the circulation in addition to being synthesized in the endothelial lining of the brain [9,10]. Lipoproteins in the CSF are generated by glial cells; cultured astrocytes and microglia produce apo E-containing lipoproteins of density similar to that of HDL, as well as apo J-containing lipoproteins that are relatively poor in cholesterol [11–13]. Neurons synthesize little, if any, apo E [14,15]. In addition, apo D, which can bind lipids, is secreted by astroglia but its function in the brain remains unknown [16]. Whereas CSF lipoproteins contain small amounts of cholesteryl esters, practically all cholesterol secreted from cultured astrocytes is non-esterified [6].
The presence of lipoproteins in the CSF suggests that these particles play a role in cholesterol transport and homoeostasis within the brain. In the peripheral nervous system, the generation of apo E-containing lipoproteins is supposed to provide damaged nerves with cholesterol released during degeneration [17]. In the peripheral nervous system, and also in the CNS (central nervous system), the expression of apos E and D is significantly increased after injury [18–20]. Once formed, apo E-containing lipoproteins and their associated lipids can be internalized by receptors of the LDL (low-density lipoprotein) receptor superfamily, which are widely distributed in neurons [14,21–25]. Moreover, on binding apo E, several of these receptors initiate signalling cascades required for normal brain development and function [26–28]. The existence of an efficient process for cholesterol recycling within the brain is supported by the observation that cholesterol has a very long half-life (at least 5 years) in the brain (reviewed in [2]).
Results from our laboratory have recently demonstrated that glia-derived lipoproteins containing apo E and cholesterol stimulate axonal extension of cultured neurons such as RGC (retinal ganglion cells) isolated from the CNS [29]. In addition, Pfrieger and co-workers [30] showed that glia-derived cholesterol stimulates synaptogenesis of rat RGC. Apo E has also been implicated in brain function because inheritance of the apo E4 allele is a major risk factor for the development of Alzheimer's disease [31,32].
Two probable mechanisms of lipoprotein formation by glia are: (i) direct secretion of particles in which cholesterol and phospholipids are assembled with apos, and (ii) secretion of lipid-poor apos and subsequent association with cholesterol and phospholipid donated by glial cells. The formation of plasma HDL involves the ABCA1 (ATP-binding-cassette transporter A1) that promotes the efflux of cholesterol and phospholipids to an acceptor such as apo A1 (reviewed in [33]). Since glia express ABCA1 [34], extracellular apo E and/or apo A1 might contribute to lipoprotein formation in the brain by acting as acceptors for the ABCA1-mediated efflux of cholesterol and phospholipid from glia.
NPC (Niemann–Pick type C) disease is a fatal, neurodegenerative disorder caused in 95% of cases by mutation in the NPC1 protein [35]. This disease is characterized by progressive loss of cerebellar neurons, particularly Purkinje neurons [36,37]. The mechanism of action of NPC1 has been investigated primarily in fibroblasts and Chinese-hamster ovary cell mutants [38,39], with a few studies performed in primary neurons [40–42]. In all cell types examined, loss of function of NPC1 protein results in defective intracellular cholesterol trafficking, sequestration of cholesterol and other lipids in the endosomal pathway and impaired cholesterol homoeostasis [43–45]. Our laboratories have recently reported that ABCA1 expression and ABCA1-dependent efflux of cholesterol from NPC1-deficient fibroblasts is reduced [46]. The NPC1 protein is present in both neurons and glial cells [42,47]. However, it is not clear how alterations in lipid metabolism, that appear to occur in every NPC1-deficient cell type, result in such profound neurological consequences.
We have previously shown that in neurons NPC1 deficiency results in impaired cholesterol homoeostasis. For example, the amount of cholesterol is increased in cell bodies but is decreased in distal axons of neurons from NPC1-deficient newborn mice [41]. In addition, the transport of cholesterol from cell bodies to distal axons is impaired [42]. Thus a neuronal defect is already present in Npc1−/− mice at birth. Glia are supposed to be involved in cholesterol homoeostasis in the brain. We, therefore, predicted that if glia played a causative role in the development of the neuropathology of NPC disease, these cells might also display defects in cholesterol and lipoprotein metabolism at an early age, which would be evident in newborn mice. Our results show, however, that a medium conditioned by Npc1+/+ and Npc1−/− murine astroglia contains the same amount of cholesterol and apo E, and the composition of apo E-containing lipoproteins generated by the glial cells is indistinguishable. Moreover, these lipoproteins support axonal growth to the same extent.
MATERIALS AND METHODS
Materials
DMEM (Dulbecco's modified Eagle's medium) and phospholipase C (from Clostridium welchii) were purchased from Sigma (St. Louis, MO, U.S.A.). DNase I was obtained from Cedarlane (Hornby, ON, Canada). Other cell culture reagents and materials were from BD Biosciences (Bedford, MA, U.S.A.). [1-14C]acetic acid (57 mCi/mmol) was from Amersham Biosciences (Baie d’Urfé, Quebec, Canada). Silica gel G60 TLC plates were from Merck (Darmstadt, Germany). Supplies for PAGE and immunoblotting were obtained from Bio-Rad Laboratories (Mississauga, Ontario, Canada).
A rabbit anti-human ABCA1 antibody that cross-reacts with murine ABCA1 was purchased from Novus Biologicals (Littleton, CO, U.S.A.). The goat anti-human apo E antibody that recognizes murine apo E was from Biodesign (Saco, ME, U.S.A.) and mouse anti-GFAP (glial fibrillary acidic protein) antibody (clone 411A) was from BD Biosciences. Monoclonal anti-β-tubulin antibody (T 4026) and filipin complex were purchased from Sigma. Rabbit anti-human apo D antibodies were a gift from Dr S. Patel (Neurobiology Research Laboratory, Newington, CT, U.S.A.). Human apo A1 was prepared by DEAE-cellulose chromatography from delipidated HDL isolated from the plasma of healthy volunteers [48]. The anti-mouse apo A1 antibody was purchased from Calbiochem (La Jolla, CA, U.S.A.).
Primary cultures of glia
The cerebellum was dissected from 1-day-old mouse pups from a breeding colony of Balb/cNctr-npcN/+ mice established at the University of Alberta from original breeding pairs obtained from Jackson Laboratories (Bar Harbor, ME, U.S.A.). Mice were maintained under temperature-controlled conditions with a 12 h light/12 h dark cycle and supplied with food and water ad libitum. Breeders were fed a 9% fat breeder diet (Purina LabDiet, Richmond, IN, U.S.A.). Henceforth, mice homozygous or heterozygous for the Npc1 mutation will be referred to as Npc1−/− and Npc1+/− respectively, whereas wild-type mice will be termed Npc1+/+. Since Npc1−/− mice do not produce offspring, Npc1+/− mice were used for breeding. All experiments were performed by comparing littermates of the Npc1 genotypes and were approved by the Health Sciences Animal Welfare Committee of the University of Alberta. The cerebellum from each mouse pup was kept separately throughout the procedure and the genotype was determined by PCR using DNA isolated from tail clippings from each mouse pup [49]. After removal of meninges and blood vessels from the surface of the cerebellum, the cerebellum was cut into small pieces and digested with 0.125% trypsin in PBS containing 0.4 mg/ml DNase I at 37 °C for 14 min. Glial cells were then dissociated from each cerebellum by trituration and plated in a 25 cm2 flask in DMEM containing 10% (v/v) FBS (fetal bovine serum). After reaching confluency, the cells were washed three times with PBS and re-plated at one-third the original density. These glia cultures contained 90–95% astroglia as determined by immunostaining with anti-GFAP antibody and secondary detection by Texas Red-linked anti-mouse IgG (Molecular Probes, Eugene, OR, U.S.A.).
Filipin staining
Glia were grown to confluency on glass coverslips in DMEM containing 10% FBS. The cells were incubated in serum-free DMEM for 2 h or 6 days, as indicated, then washed three times with PBS and fixed for 15 min at room temperature (22 °C) in 3% (w/v) paraformaldehyde. The cells were washed thoroughly then incubated with 100 μg/ml filipin in PBS containing 1% BSA for 1 h at room temperature. The cells were washed, then mounted in Prolong Antifade mounting medium (Molecular Probes) and examined in a Leica DM IRE2 digital microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Fluotar ×63/0.70 objective and a Leica ebq100 fluorescence lamp.
Immunoblotting
Glial cells were scraped into PBS then pelleted by centrifugation at 16000 g for 2 min. Pelleted cells were resuspended in 10 mM Tris/HCl buffer (pH 7.4) containing 1 mM PMSF and a protease inhibitor cocktail (Complete Mini; Roche Diagnostics, Mannheim, Germany) and sonicated for 3 s. Brain tissue was homogenized in a Polytron homogenizer in an ice-cold homogenization buffer [20 mM Tris/HCl (pH 7.4), 1 mM EDTA and 0.25 mM sucrose] containing 1 mM PMSF and protease inhibitor cocktail. Tissue homogenates were centrifuged at 600 g for 2 min to pellet nuclei and unbroken cells and the supernatant was used for immunoblotting. For immunoblotting of ABCA1, proteins were resolved on 7% polyacrylamide gels containing 0.1% SDS under reducing conditions, then transferred on to PVDF membranes. The membranes were cut into half; the lower molecular mass portion was probed with mouse anti-tubulin antibodies as a loading control and the upper portion of the membrane was probed with rabbit anti-human ABCA1 antibodies. Immunoreactive proteins were detected by reaction with peroxidase-conjugated goat anti-rabbit or goat anti-mouse, IgG (dilution 1:10000) respectively and visualized with ECL reagent (ECL® Western Blotting System; Amersham Biosciences, Piscataway, NJ, U.S.A.). Immunoblotting for apos E, D and A1, and GFAP was performed after separation of the proteins on 12% polyacrylamide gels containing 0.1% SDS.
Isolation of glia-conditioned medium
Confluent glia were washed three times with PBS, then serum-free DMEM was added and cells were incubated for 3 days. In some experiments, as indicated, apo A1 (10 μg/ml) was added to serum-free medium. The medium was collected and centrifuged at 1000 g for 10 min to remove cell debris. The supernatant (designated as glia-conditioned medium) was used for immunoblotting, cholesterol analysis by GLC, lipoprotein isolation and FPLC over a gel filtration column (see below), as indicated.
Efflux of endogenously synthesized cholesterol
On reaching approx. 60% confluency, glia were incubated with 1 μCi/ml [14C]acetic acid in DMEM containing 5% FBS. Confluent glia were then incubated in DMEM containing 5% FBS, without radiolabel, for 24 h to minimize the presence of radio-labelled cholesterol precursors. Cells were washed three times with PBS and subsequently incubated in serum-free DMEM for 24 h. The medium was collected and centrifuged at 1000 g for 10 min to remove cell debris. Cells were washed three times with PBS and cellular lipids were extracted into hexane/propan-2-ol (3:2, v/v). Cell proteins were dissolved in 0.3 M sodium hydroxide and analysed using the BCA protein assay (Pierce, Rockford, IL, U.S.A.).
Lipid analyses
Medium from the radiolabelling experiments was extracted twice with hexane/propan-2-ol (3:2, v/v). Lipid extracts from the medium and cells were separated by TLC in the solvent system heptane/di-isopropyl ether/acetic acid (65:35:4, by vol.). The band corresponding to non-esterified cholesterol was scraped and radio-activity was measured.
Non-radioactive medium or isolated lipoproteins were extracted twice with hexane, lipids were dried under a stream of nitrogen then sialylated with N,O-bis[trimethylsialyl]trifluoro-acetamide/1% trimethylsilane in acetone and analysed by GLC using 5-α-cholestane as internal standard. Non-radioactive cellular lipids were treated with phospholipase C [50] to hydrolyse phospholipids, extracted into hexane/diethyl ether (2:1), silylated with N,O-bis[trimethylsilyl]trifluoroacetamide/1% trimethylsilane in acetone and analysed by GLC. Cell protein was determined using the BCA protein assay.
Lipoprotein isolation from glia-conditioned medium
Lipoproteins were isolated from glia-conditioned medium as described previously [51] on a discontinuous sucrose gradient consisting of the following sucrose solutions: 2 ml of density 1.30 mg/ml, 3 ml of density 1.20 mg/ml, 3 ml of density 1.10 mg/ml, 4 ml of culture medium of density 1.006 mg/ml. The gradient was centrifuged in a SW40 Ti rotor (Beckman, Palo Alto, CA, U.S.A.) at 160000 g for 48 h at 4 °C. From the top of the gradient, 12 (1 ml) fractions were taken sequentially and analysed for apo E by immunoblotting. Fractions containing apo E (typically fractions 5–7) were combined. For addition to RGC, lipoproteins were concentrated in Neurobasal medium (Invitrogen) using an Amicon Ultra Filter (100 kDa molecular mass cut-off; Millipore, Bedford, MA, U.S.A.). Cholesterol concentration was adjusted to 1 μg/ml with base medium (neurobasal medium containing additives as described in [29]) and added to distal axon-containing compartments of compartmented cultures of RGC.
For analysis by FPLC, glia-conditioned medium was concentrated 50-fold using an Amicon Ultra Filter (100 kDa molecular mass cut-off) and separated over a Superose 6 column (Amersham Biosciences) attached to a Beckman Systems Gold or Nouveau Gold apparatus. Cholesterol content of the eluate was monitored by a post-column, in-line detection assay (Sigma Infinity Cholesterol Reagent).
Compartmented cultures of rat RGC
Culture of RGC from rats was performed by the method of Barres et al. [52], with minor modifications [29].
Statistical analyses
Statistical significance of differences (P<0.05) was determined using the Student's t test.
RESULTS
NPC1-deficient glia accumulate cholesterol
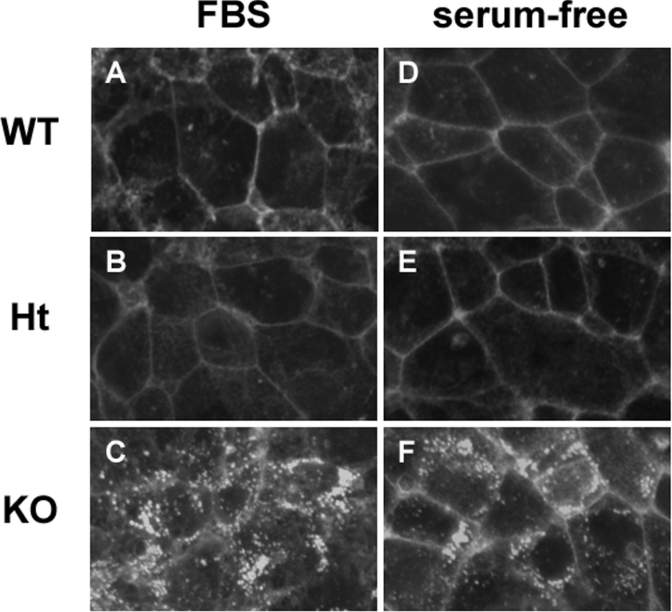
To verify whether or not cerebellar astroglia from Npc1−/− mice accumulate cholesterol, as has been described for other NPC1-deficient cells [38,53], glial cells were isolated from the cerebellum of Npc1+/+, Npc1+/− and Npc1−/− neonatal mice and stained with filipin. The filipin stain revealed an intracellular, punctate pattern of intense fluorescence in glia from Npc1−/− mice, whereas in Npc1+/+ or Npc1+/− glia the fluorescence was primarily localized to the plasma membrane (Figures 1A–1C). Moreover, the cholesterol accumulation in Npc1−/− glia diminished only slightly after 6 days of incubation in serum-free medium (Figures 1D–1F) suggesting that the sequestered cholesterol was not readily mobilized. Since filipin staining does not yield quantitative data, we also measured the cellular cholesterol content of the glial cells by GLC. Cerebellar glia from Npc1−/− mice contained approx. 20% more cholesterol/mg of protein when compared with glia from Npc1+/+ or Npc1+/− mice (42.5±1.75 μg/mg of protein in Npc1−/− versus 32.8±2.45 and 34.0±3.03 μg/mg of protein in Npc1+/+ and Npc+/− glia respectively). The amounts of triacylglycerols and cholesteryl esters in the glial cells were below the limits of detection. In accordance with the filipin staining, the cholesterol content in glial cells of all three genotypes was slightly decreased after 6 days of incubation in serum-free medium, but the difference between Npc1+/+ and Npc1−/− glia persisted (28.7±3.92 μg/mg of protein in Npc1−/− versus 22.6±1.73 and 24.3±1.84 μg/mg of protein in Npc1+/+ and Npc+/− glia respectively).
Figure 1. Intracellular sequestration of cholesterol in glia from Npc1−/− mice.
Cerebellar glia isolated from Npc1+/+ (WT, A, D), Npc1+/− (Ht, B, E) and Npc1−/− (KO, C, F) were grown to confluency on glass coverslips in DMEM containing 10% FBS, then stained with filipin after incubation for 2 h (A–C) or 6 days (D–F) in serum-free DMEM. All photographs were taken with the same exposure time. Results are representative of three independent experiments with similar results.
The amount of apo E is increased in Npc1−/− brains
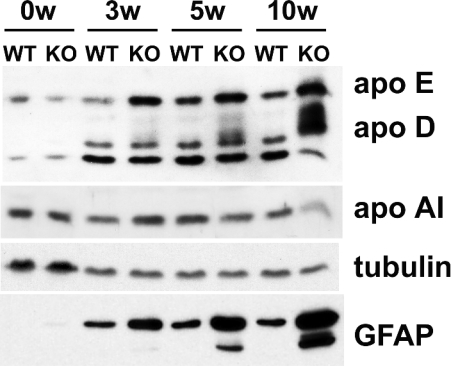
As an indication of whether or not brain lipoprotein metabolism is affected by NPC1 deficiency, we compared the amounts of apos in brains of Npc1+/+ and Npc1−/− mice of various ages by immunoblotting. Figure 2 shows that the apo E content of Npc1−/− cerebellum was higher than that of Npc1+/+ cerebellum and increased with age of the mice for both genotypes. Previous studies have shown that the apo D content of brains of Npc1−/− mice is higher than that of Npc1+/+ mice [54,55]. Our results (Figure 2) support this observation and suggest that the increase in apo D is age-dependent. The broadening of the apo D band is most probably due to different glycosylation states of the protein [54]. The amount of apo A1 was similar in brains from mice of the two NPC1 genotypes with the exception of apo A1 shown for 10-week-old Npc−/− mice (Figure 2). However, this reduction was not consistently observed. Tubulin, used as a loading control, did not show genotype-dependent differences (Figure 2). Immunoblotting of GFAP, a marker of glial filaments in astrocytes, showed a large, age-dependent increase in the amount of this protein in the brains of Npc1−/−, compared with Npc1+/+ mice (Figure 2), in agreement with the histochemical findings of German et al. [56]. This increase in GFAP content is indicative of astrogliosis and might be due to either proliferation of astrocytes in the brain or activation of astrocytes (reviewed in [57,58]). Previous studies have suggested that in NPC1-deficient murine brains, astrocytes and microglia are activated [56,59]. The cerebellum of neonatal Npc1−/− mice (0w in Figure 2) is indistinguishable from wild-type cerebellum in terms of apo content and lack of GFAP expression (Figure 2).
Figure 2. Apos E and D are increased in the cerebellum of Npc1−/− mice.
The cerebellum was dissected from neonatal (0w), 3-week- (3w), 5-week- (5w) and 10-week- (10w) old Npc1+/+ (WT) and Npc1−/− (KO) mice. Meninges and blood vessels were discarded. Tissues were homogenized and proteins were separated by electrophoresis on 12% polyacrylamide gels containing 0.1% SDS. For immunoblotting of apos E, D and A1, 40 μg of protein was applied per lane. Tubulin was used as a loading control. For GFAP and tubulin, 5.5 μg of protein was applied per lane. The experiment was repeated three times with similar results.
Production of apo by primary astrocytes
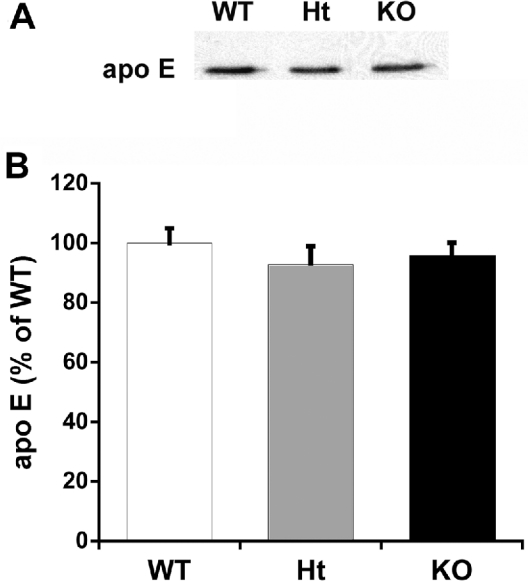
We examined lipoproteins in the culture medium conditioned by primary glia (>95% astrocytes) to determine if the differences in apo content between NPC1-deficient and wild-type brains were reflected in the lipoproteins generated by isolated primary glia. Figures 3(A) and 3(B) show that the amount of apo E in the medium of Npc1−/− and Npc1+/+ glia was the same suggesting that the release of apo E-containing lipoproteins by cultured astrocytes isolated from 1-day-old mice was not affected by NPC1 deficiency.
Figure 3. Accumulation of apo E in glia-conditioned medium is independent of Npc1 genotype.
Cerebellar glia were grown to confluency in DMEM containing 10% FBS, then washed and incubated in serum-free DMEM for 3 days. The medium was collected and probed for apo E content by immunoblotting. (A) One immunoblot representative of four independent experiments performed in duplicate. (B) Intensity of the apo E band relative to that of apo E in medium from wild-type glia. Results are calculated from densitometric scanning of the bands and are means±S.E.M. for four independent experiments performed in duplicate. White bar, Npc1+/+ (WT); grey bar, Npc1+/− (Ht); black bar, Npc1−/− (KO).
Cholesterol content of glia-conditioned medium
We next compared the release of cholesterol from isolated glial cells using both radiotracer and mass measurements. In the first set of experiments, [14C]acetate was added to 60% confluent astrocytes during their active growth phase for 3 days to radiolabel the pool of endogenously synthesized cholesterol. The confluent cells were incubated for 24 h in the absence of radio-label to minimize the presence of labelled cholesterol precursors, and incubated in serum-free medium for 24 h. The amount of radiolabelled cholesterol in cells and culture medium was determined. Table 1 shows that the percentage of total radiolabelled cholesterol released into the medium of Npc1−/− glia was approx. 40% lower than that for wild-type glia. However, importantly, the incorporation of [14C]acetate into cholesterol was significantly higher in Npc1−/− glia when compared with that in wild-type glia (Table 1). Thus the smaller percentage of cholesterol in the medium of Npc1−/− astrocytes might have been a consequence of the larger cholesterol pool in the Npc1−/− cells (Figure 1). This is also supported by the fact that [14C]cholesterol concentrations in the medium were not significantly different (Table 1). Therefore, to determine whether or not the release of cholesterol depended on Npc1 genotype, we measured the mass of cholesterol in the medium using GLC. Table 1 also shows that there was no difference between the amount of cholesterol in the medium of Npc1−/− glia and Npc1+/+ glia.
Table 1. Culture medium of cerebellar glia from Npc1−/− and Npc1+/+ mice accumulates the same amount of cholesterol.
Rows 1–3: glia (60% confluent) from Npc1+/+ (WT), Npc1+/− (Ht) and Npc1−/− (KO) mice were radiolabelled for 3 days with [14C]acetate in DMEM containing 5% FBS. Confluent cells were then incubated for 24 h in unlabelled DMEM containing 5% FBS, washed and incubated for an additional 24 h in serum-free DMEM. Lipids were extracted from cells and medium and separated by TLC. Radioactivity/mg of protein was determined in the band corresponding to non-esterified cholesterol. Data in row 1 are expressed as amount of radioactivity in the medium as a percentage of total radioactivity in cells and medium combined. In row 2 is given the radioactivity/mg of cell protein in cells and medium combined. Row 3 shows the amount of radiolabelled [14C]cholesterol (d.p.m./mg of cell protein) in the medium. Results are means±S.E.M. for three independent experiments; *P<0.005 compared with WT. Row 4: glia were grown to confluency in DMEM containing 10% FBS then incubated for 3 days in serum-free DMEM. Medium was collected and the mass of cholesterol determined by GLC. Results are means±S.E.M. for three independent experiments.
| Value | |||
|---|---|---|---|
| Parameter | WT | Ht | KO |
| [14C]Cholesterol in medium (% of total [14C]cholesterol) | 7.66±0.6 | 7.28±0.6 | 4.74±0.3* |
| Total [14C]cholesterol (d.p.m./mg of cell protein) | 58694±1984 | 54342±1154 | 78254±4006* |
| [14C]Cholesterol in medium (d.p.m./mg of cell protein) | 4496±232 | 3956±151 | 3710±112 |
| Cholesterol in medium (μg/mg of cell protein) | 6.20±0.61 | 5.63±0.37 | 5.54±0.87 |
Release of cholesterol in response to exogenously added apo A1
In addition to apo E, apo A1 has been detected in the brain ([9] and Figure 3) and CSF [6,7]. Our laboratories recently reported that, in Npc1−/− fibroblasts, the ABCA1-mediated efflux of cholesterol to the exogenously added acceptor, apo A1, is impaired [46]. We, therefore, determined whether or not apo A1 increased the amount of cholesterol released into the medium of Npc1+/+ and Npc1−/− glia. Consistent with data shown in Table 1, no differences were observed in the amounts of cholesterol in glia-conditioned medium of the three Npc1 genotypes (Table 2). Moreover, the addition of apo A1 (10 μg/ml) did not increase the amount of cholesterol released into the medium. This concentration of apo A1 has been shown to induce ABCA1-mediated efflux of cholesterol from fibroblasts [46] and other cell types [60,61].
Table 2. Apo A1 does not stimulate cholesterol release from Npc1+/+, Npc1+/− or Npc1−/− glia.
Cerebellar glia from Npc1+/+ (WT), Npc1+/− (Ht) and Npc1−/− (KO) mice were grown to confluency in DMEM containing 10% FBS, then incubated for 3 days in serum-free DMEM with or without 10 μg/ml apo A1. Medium was collected and the mass of cholesterol was determined by GLC. Results are means±S.E.M. for three independent experiments performed in duplicate.
| Concentration (μg/mg of cell protein) | |||
|---|---|---|---|
| Parameter | WT | Ht | KO |
| Cholesterol in medium without apo A1 | 5.44±0.36 | 5.73±0.32 | 5.91±0.27 |
| Cholesterol in medium with apo A1 | 5.27±0.18 | 5.50±0.13 | 6.23±0.29 |
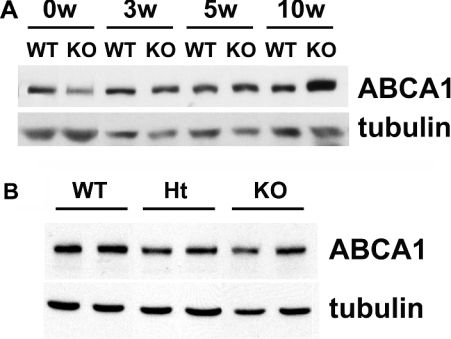
Results from our laboratories have reported that ABCA1 expression in NPC1-deficient fibroblasts is less than that in wild-type fibroblasts [46]. Thus, we next determined if the amount of ABCA1 protein was reduced in Npc1−/−, compared with Npc1+/+, glial cells. We predicted that the amount of ABCA1 protein in Npc1−/− glia might not be reduced since cholesterol release from NPC1-deficient glia was not impaired (Tables 1 and 2). Indeed, Figure 4(A) shows that the amount of ABCA1 in Npc1−/− glial cells, according to immunoblotting, was not less than that in Npc1+/+ glia. Moreover, immunoblotting revealed that the level of ABCA1 in brains of NPC1-deficient mice was similar to that in brains of wild-type mice (Figure 4B).
Figure 4. NPC1 deficiency does not decrease the amount of ABCA1 in brains or cultured glia.
(A) The cerebellum was dissected from neonatal (0w), 3-week- (3w), 5-week- (5w) and 10-week- (10w) old Npc1+/+ (WT) and Npc1−/− (KO) mice. Meninges and blood vessels were removed and then the tissues were homogenized. Proteins were separated by electrophoresis on 7% polyacrylamide gels containing 0.1% SDS. The amount of protein applied per lane was 40 μg. Immunoblotting of tubulin was used as a loading control. One blot representative of three similar independent experiments is shown. (B) Cerebellar glia were grown to confluency in DMEM containing 10% FBS. Cells were harvested and then sonicated. Proteins (20 μg per lane) were separated by electrophoresis on 7% polyacrylamide gels containing 0.1% SDS. Tubulin was used as a loading control. Results are from one experiment that is representative of four similar experiments.
Characterization of lipoproteins derived from glia
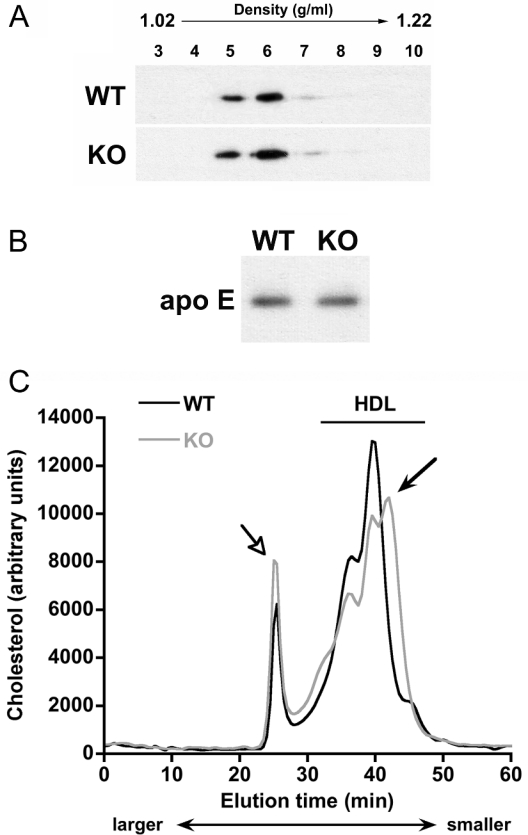
Most cholesterol released by glial cells is associated with apo E. To determine if this were the case also for Npc1−/− glia, we isolated lipoproteins from glia-conditioned medium by sucrose density centrifugation. Fractions of different densities were collected from the gradient and analysed for apo E content by immunoblotting. Most of the apo E was in fractions 5 and 6 (Figure 5A) for which the density is equivalent to that of HDL (1.07–1.10 g/ml respectively). The apo E-containing lipoproteins derived from Npc1+/+ and Npc1−/− glia had an identical density distribution (Figure 5A). Moreover, immunoblotting of apo E in medium samples that contained equal amounts of cholesterol (Figure 5B) demonstrated that the cholesterol:apo E ratio of these lipoproteins was the same.
Figure 5. Lipoproteins generated by Npc1+/+ and Npc1−/− glia are similar in density and size.
Cerebellar glia were grown to confluency in DMEM containing 10% FBS, then incubated for 3 days in serum-free DMEM. (A) Lipoproteins were separated on the basis of density by sucrose density-gradient centrifugation. Equal volumes of fractions (numbered at top of immunoblot) of densities from 1.02 g/ml (fraction 3) to 1.22 g/ml (fraction 10) were collected and the apo E content of each fraction was assessed by immunoblotting. (B) Immunoblotting of apo E in samples that contained equal amounts of cholesterol (37.5 μg). One immunoblot is shown, representative of three independent experiments with similar results. (C) Cerebellar glia-conditioned medium was concentrated 50-fold and lipoproteins were separated by size on a Superose 6 column. Cholesterol content was recorded by an in-line, post-column detection system. Results are means for three independent experiments with similar results. Dark line, Npc1+/+ (WT) glia; light line, Npc1−/− (KO) glia. Solid arrow indicates a subfraction of HDL-like particles present in the medium of Npc1−/−, but not Npc1+/+, glia. Open arrow indicates a lipoprotein fraction containing particles of size corresponding to VLDL.
Since lipoprotein isolation by density-gradient centrifugation can, in some cases, lead to partial loss of apos [62], we also examined the lipoproteins using an FPLC gel-filtration technique with post-column detection of cholesterol. The Superose 6 column used in this method separates lipoproteins by size, not density, so that larger lipoproteins are eluted at earlier times, whereas smaller lipoproteins are eluted later [63]. The elution profile of cholesterol in concentrated medium from Npc1−/− glia was similar to that from Npc1+/+ glia, with most cholesterol being in particles the size of HDL (Figure 5C). However, the medium from Npc1−/− glia contained an additional population of lipoproteins that were smaller than most lipoproteins in the medium of wild-type glia (Figure 5C, solid arrow). Although this difference is subtle, it was consistently observed in three independent experiments. In addition, a peak of cholesterol in lipoproteins corresponding to the size of VLDL (very low density lipoproteins) (Figure 5C, open arrow) was detected in media from glia of both Npc1 genotypes. In combination, the data from Tables 1 and 2 and Figures 4 and 5 indicate that despite a modest difference in the size of a small population of lipoproteins produced by Npc1+/+ and Npc1−/− glia, the overall composition of lipoproteins derived from glia of the two Npc1 genotypes is the same, and the same amounts of cholesterol and apo E are present in medium conditioned by Npc1−/− and Npc1+/+ glia.
Lipoproteins from Npc1−/− glia-conditioned medium support axonal extension of RGC
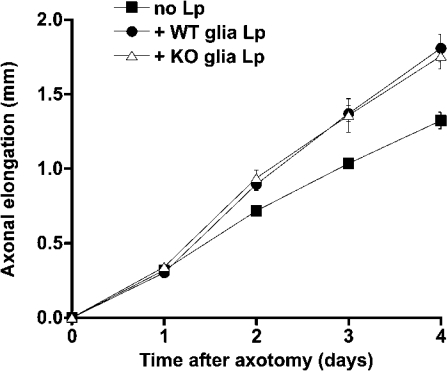
We next determined if lipoproteins produced by Npc1−/− glia were functional. Compartmented primary cultures of rat RGC were grown in the presence or absence of lipoproteins isolated from culture medium of wild-type or Npc1−/− glia. Previously, our laboratory has demonstrated that when glia-derived lipoproteins, containing cholesterol and apo E, are supplied to distal axons (but not to cell bodies) of compartmented cultures of rat RGC, the rate of axonal elongation is significantly increased [29]. We, therefore, determined if lipoproteins derived from Npc1−/− and Npc1+/+ glia stimulated axonal extension to the same degree. Lipoproteins were isolated from glia-conditioned medium and aliquots of the lipoproteins, containing equal amounts of cholesterol, were provided to the distal axon-containing compartments of RGC cultures immediately after the removal of distal axons by axotomy. Axonal elongation was measured each day for the next 4 days [29]. As a control, the same medium, without glial-derived lipoproteins, was added to some cultures. Figure 6 shows that lipoproteins from Npc1+/+ and Npc1−/− glia stimulated axonal extension to the same extent (∼35%).
Figure 6. Lipoproteins released by Npc1+/+ and Npc1−/− glia stimulate axonal extension of RGC to the same degree.
Cerebellar glia were grown to confluency in DMEM containing 10% FBS, then incubated for 3 days in serum-free DMEM. Lipoproteins from fractions 5–7 (density 1.07–1.12 g/ml) were isolated from the culture medium by sucrose density-gradient centrifugation. Compartmented cultures of RGC (10 days old) were axotomized. Medium without lipoproteins (■) or with lipoproteins derived from Npc1+/+ glia (●, WT Lp) or Npc1−/− glia (△, KO Lp) was added to distal axon-containing compartments. Axon length was measured on four consecutive days. Results are means±S.D.
From these experiments, we conclude that the cholesterol and apo composition of lipoproteins recovered from Npc1−/− glia is indistinguishable from that of lipoproteins generated by Npc1+/+ glia except that Npc1−/− lipoproteins contain a minor, subpopulation of particles that are smaller than those produced by wild-type glia. Moreover, lipoproteins generated by glial cells of both genotypes are equivalent in their ability to stimulate axonal extension of RGC.
DISCUSSION
A lack of function of the NPC1 protein leads to alterations in cholesterol homoeostasis, for example, impaired regulation of the synthesis, esterification and uptake of cholesterol, as well as impaired ABCA1-mediated cholesterol efflux [38,39,46,53]. To date, however, it is not clear how these changes in cholesterol metabolism lead to the extensive neurodegeneration characteristic of NPC disease. We previously reported that the cholesterol content of cell bodies of NPC1-deficient neurons is increased, whereas the amount of cholesterol in distal axons is decreased [41]. We also found that the anterograde transport of cholesterol into distal axons of NPC1-deficient neurons is impaired [42]. From these observations, we speculated that defects in cholesterol homoeostasis in neurons might contribute to the neurological problems of NPC disease.
In the brain, more than 90% of the cells are glial cells. Therefore we have now investigated whether or not the intracellular sequestration of cholesterol in NPC1-deficient glia restricted the availability of cholesterol for lipoprotein formation. In the light of recent reports that glia-derived cholesterol is crucial for synaptogenesis [30] and axonal growth [29], we predicted that a defect in lipoprotein production by NPC1-deficient glial cells might be a major factor involved in the neuropathological changes that occur in NPC1-deficient brains. Our experiments demonstrate that the quantity and composition of apo E-containing lipoproteins produced by cultured Npc1−/− glial cells are normal, and that these lipoproteins are functional, at least in their ability to stimulate axonal extension of cultured CNS neurons.
Lipoproteins produced by NPC1-deficient glia
Although the amounts of cholesterol and apo E in the medium of cultured Npc1+/+ and Npc1−/− cerebellar glia are the same, and the density distribution of the apo E-containing particles is indistinguishable, we did observe a slight but consistent difference between the gel filtration elution profile (i.e. size) of lipoproteins from Npc1+/+ and Npc1−/− glia. Unfortunately, the limited amounts of material available have so far precluded further characterization of these particles. The availability of only small amounts of glial lipoproteins also prevented our use of incubation times of <24 h. During this incubation period some lipoproteins secreted by the glia would probably have been taken up or modified. Thus the isolated lipoproteins represent particles existing at steady state rather than purely as nascent lipoproteins. As yet we have no explanation for the existence of the population of cholesterol-containing particles (produced in equal amounts by Npc1+/+ and Npc1−/− glia) whose size corresponds to that of VLDL (open arrow in Figure 5C). The presence of these particles was surprising since lipoproteins of this size in plasma contain large amounts of triacylglycerols and cholesteryl esters, which are not present in glia-conditioned medium. Nevertheless, a similar population of lipoprotein particles derived from astrocytes has been described previously [64]. It is possible that these large entities consist of stacks of discoidal particles such as those described previously [6].
In human fibroblasts, NPC1 deficiency decreases the amount of the ABCA1 transporter and reduces the efflux of cholesterol in response to apo A1 [46]. In contrast, the level of ABCA1 protein in cerebellar glia cultured from neonatal mice, and in the cerebellum of mice up to 10 weeks of age, is unaltered by NPC1 deficiency. In NPC1-deficient fibroblasts, ABCA1 expression is supposed to be decreased because the sensing of cholesterol is impaired, perhaps as a result of reduced levels of oxysterols [45]. It is possible that the regulation of cholesterol homoeostasis, in particular regulation of ABCA1 expression, is distinct in glia and fibroblasts because fibroblasts rely on an exogenous source of cholesterol to a greater extent than do glial cells or the cerebellum. We also found that the addition of apo A1 did not stimulate the efflux of cholesterol from either Npc1+/+ or Npc1−/− glia. Ito et al. [65] have similarly reported that the cholesterol efflux from astrocytes during a 24 h period is not increased by the addition of apo A1, although recently a 2.5-fold stimulation of cholesterol efflux from glia by apo A1 has been reported [66]. In the latter study, however, cortical glia were used, whereas we used cerebellar glia. Interestingly, we have also found that apo A1 modestly stimulates cholesterol efflux from cortical glia (B. Karten and J. E. Vance, unpublished work).
Another possible reason why apo A1 does not stimulate cholesterol efflux from cerebellar glia might relate to the relative concentrations of apo A1 and apo E in culture medium when compared with those in human plasma. In plasma, apo A1 is approx. 20-fold more abundant than apo E, whereas in CSF apo E and apo A1 are present at equivalent concentrations [6,7]. Apo E is the most abundant apo in glia-conditioned medium. The overall concentration of apos in the CSF is, however, only approx. 0.4% of that in plasma so that the amount of apo E in CSF is nearly 20-fold less, and apo A1 is 400-fold less, when compared with that in plasma. In glia, a factor other than the amount of acceptor apo might limit cholesterol efflux, in which case an additional acceptor (i.e. apo A1) would not be expected to stimulate cholesterol efflux. In support of this idea, we have found that, in apo E-deficient murine glia, although cholesterol efflux is negligible, the addition of apo A1 stimulates cholesterol efflux by approx. 20-fold (B. Karten and J. E. Vance, unpublished work).
The mechanism of assembly of glial lipoproteins is not clear. Two possible mechanisms are that apo E associates intracellularly with its full complement of lipids or that lipid-poor apo E is secreted and subsequently combines with cholesterol and phospholipids effluxed from the surface of glia in a process mediated by an ABC family member such as ABCA1, ABCG1, ABCG4 or ABCA7. It is important to note that although lipoproteins in CSF and glia-conditioned medium have been characterized, the types and functions of lipoproteins in intercellular spaces in the brain are not yet known.
Cholesterol and lipoprotein homoeostasis in the brain
In view of our observation that an age-dependent increase occurs in the apo E and apo D content of brains of NPC1-deficient mice, we were surprised to find no substantial difference between the apo E content of culture medium from Npc1−/− and Npc1+/+ glia. Patel and co-workers [54] reported a marked increase in the level of apo D in Npc1−/−, when compared with Npc1+/+, murine cerebellae yet a decrease in apo D in the culture medium of NPC1-deficient glia. We similarly found less apo D in culture medium from Npc1−/−, than from Npc1+/+, glia. In our experiments, we isolated cerebellar glia from 1-day-old mice. At this age, no difference was observed in the amounts of apo E or apo D in brains of Npc1+/+ and Npc1−/− mice (Figure 2). However, we cannot exclude the possibility that in the later stages of NPC disease the neuropathological symptoms might be exacerbated by changes in glial cell metabolism. It is also possible that with increasing age of the animals the number and/or differentiation state of astrocytes and microglia in NPC1-deficient brains is altered. Astrogliosis has been reported to develop in Npc1−/− mice with increasing age [56], which might account for the observed differences between levels of apos in Npc1+/+ and Npc1−/− brains. We cannot, therefore, discount the possibility that differences in amounts of cholesterol and apos in conditioned medium of Npc1+/+ and Npc1−/− glia might become apparent only in glia from more mature mice. We have attempted to culture cerebellar glia from older mice (3 weeks of age) but viability and yield of the cells were poor. Moreover, we were not able to find any report in the literature of glial cells cultured from mice as old as 3 weeks of age. Nevertheless, even in glia from neonatal Npc1−/− mice, the cholesterol trafficking defect is pronounced (Figure 1). We speculate that if defects in glial lipoproteins were causative for pathological changes in NPC disease, alterations in the lipoproteins would precede development of neuronal pathology and would be detected early in life.
It is also possible that the activation state of the astrocytes might influence lipoprotein metabolism. Glia from Npc1−/− mice become increasingly activated with increasing age of the animal, whereas Npc1+/+ glia do not, as indicated by a marked increase in inflammatory markers and GFAP expression in NPC1-deficient brains (Figure 2 and [56,59,67]). Our cultured glia do not recapitulate these changes since GFAP expression was independent of Npc1 genotype (results not shown). Moroever, cultured astrocytes are generally regarded as being more highly activated than when in their natural environment in the brain [57]. However, in our culture system most glia from both Npc1+/+ and Npc1−/− mice show a flat, polygonal morphology, rather than the fibrillary, stellate morphology characteristic of fully differentiated glia. By performing all experiments with confluent cultures from mice of similar ages, and by comparing littermates, we reduced many of these variables. Clearly, however, limitations are inherent in using cell cultures to study processes in the brain, where cross-talk between neurons and astrocytes is probably of fundamental importance [57]. Nevertheless, aberrant cholesterol trafficking is apparent in the neonatal NPC1-deficient glia that we used.
On the basis of our results, in which we detected no defects in lipoproteins produced by NPC1-deficient cerebellar glia, we speculate that the neuropathology characteristic of NPC disease can be ascribed with greater probability to dysfunctional processes within neurons, perhaps owing to a decreased cholesterol content in axons or axon terminals [41,42], compared with defects in lipoprotein production by glia.
Acknowledgments
This research was supported by grants from the Ara Parseghian Medical Research Foundation (to J. E. V.) and the Canadian Institutes for Health Research (to J. E. V.). B. K. and H. H. are recipients of postdoctoral fellowship from the Alberta Heritage Foundation for Medical Research. G. A. F. is a Scholar of the Alberta Heritage Foundation for Medical Research. We thank R. Watts and T. Chan for excellent technical assistance.
References
- 1.Dietschy J. M., Turley S. D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkhem I., Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 3.Pfrieger F. W. Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? Bioessays. 2003;25:486–490. doi: 10.1002/bies.10195. [DOI] [PubMed] [Google Scholar]
- 4.Pitas R. E., Boyles J. K., Lee S. H., Hui D., Weisgraber K. H. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J. Biol. Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- 5.Borghini I., Barja F., Pometta D., James R. W. Characterization of subpopulations of lipoprotein particles isolated from human cerebrospinal fluid. Biochim. Biophys. Acta. 1995;1255:192–200. doi: 10.1016/0005-2760(94)00232-n. [DOI] [PubMed] [Google Scholar]
- 6.LaDu M. J., Gilligan S. M., Lukens J. R., Cabana V. G., Reardon C. A., Van Eldik L. J., Holtzman D. M. Nascent astrocyte particles differ from lipoproteins in CSF. J. Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- 7.Koch S., Donarski N., Goetze K., Kreckel M., Stuerenburg H. J., Buhmann C., Beisiegel U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J. Lipid Res. 2001;42:1143–1151. [PubMed] [Google Scholar]
- 8.Linton M. F., Gish R., Hubl S. T., Butler E., Esquivel C., Bry W. I., Boyles J. K., Wardell M. R., Young S. G. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 1991;88:270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiler-Guttler H., Sommerfeldt M., Papandrikopoulou A., Mischek U., Bonitz D., Frey A., Grupe M., Scheerer J., Gassen H. G. Synthesis of apolipoprotein A-1 in pig brain microvascular endothelial cells. J. Neurochem. 1990;54:444–450. doi: 10.1111/j.1471-4159.1990.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 10.Panzenboeck U., Balazs Z., Sovic A., Hrzenjak A., Levak-Frank S., Wintersperger A., Malle E., Sattler W. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 2002;277:42781–42789. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- 11.Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 12.Fagan A. M., Holtzman D. M., Munson G., Mathur T., Schneider D., Chang L. K., Getz G. S., Reardon C. A., Lukens J., Shah J. A., et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (–/–), and human apoE transgenic mice. J. Biol. Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- 13.Mori K., Yokoyama A., Yang L., Maeda N., Mitsuda N., Tanaka J. L-serine-mediated release of apolipoprotein E and lipids from microglial cells. Exp. Neurol. 2004;185:220–231. doi: 10.1016/j.expneurol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Boyles J. K., Zoellner C. D., Anderson L. J., Kosik L. M., Pitas R. E., Weisgraber K. H., Hui D. Y., Mahley R. W., Gebicke-Haerter P. J., Ignatius M. J., et al. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J. Clin. Invest. 1989;83:1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brecht W. J., Harris F. M., Chang S., Tesseur I., Yu G. Q., Xu Q., Dee Fish J., Wyss-Coray T., Buttini M., Mucke L., et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J. Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S. C., Asotra K., Patel Y. C., McConathy W. J., Patel R. C., Suresh S. Astrocytes synthesize and secrete the lipophilic ligand carrier apolipoprotein D. Neuroreport. 1995;6:653–657. doi: 10.1097/00001756-199503000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 18.Holtzman D. M., Fagan A. M. Potential role of apoE in structural plasticity in the nervous system; implications for disorders of the central nervous system. Trends Cardiovasc. Med. 1998;8:250–255. doi: 10.1016/s1050-1738(98)00017-6. [DOI] [PubMed] [Google Scholar]
- 19.Franz G., Reindl M., Patel S. C., Beer R., Unterrichter I., Berger T., Schmutzhard E., Poewe W., Kampfl A. Increased expression of apolipoprotein D following experimental traumatic brain injury. J. Neurochem. 1999;73:1615–1625. doi: 10.1046/j.1471-4159.1999.0731615.x. [DOI] [PubMed] [Google Scholar]
- 20.Boyles J. K., Notterpek L. M., Anderson L. J. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J. Biol. Chem. 1990;265:17805–17815. [PubMed] [Google Scholar]
- 21.Handelman G. E., Boyles J. K., Weisgraber K. H., Mahley R. W., Pitas R. E. Effects of apolipoprotein E, β-very low density lipoproteins, and cholesterol on the extension of neurites by rabbit dorsal root ganglion neurons in vitro. J. Lipid Res. 1992;33:1677–1688. [PubMed] [Google Scholar]
- 22.Kim D.-H., Ijima H., Goto K., Sakai J., Ishi H., Kim H.-J., Suzuki H., Kondo H., Saeki S., Yamamoto T. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J. Biol. Chem. 1996;271:8373–8380. doi: 10.1074/jbc.271.14.8373. [DOI] [PubMed] [Google Scholar]
- 23.Schneider W. J., Nimpf J., Bujo H. Novel members of the low density lipoprotein receptor superfamily and their potential roles in lipid metabolism. Curr. Opin. Lipidol. 1997;8:315–319. doi: 10.1097/00041433-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Brown M. D., Banker G. A., Hussaini I. M., Gonias S. L., VandenBerg S. R. Low density lipoprotein receptor-related protein is expressed early and becomes restricted to a somatodendritic domain during neuronal differentiation in culture. Brain Res. 1997;747:313–317. doi: 10.1016/s0006-8993(96)01321-2. [DOI] [PubMed] [Google Scholar]
- 25.Posse de Chaves E. I., Vance D. E., Campenot R. B., Kiss R. S., Vance J. E. Uptake of lipoproteins for axonal growth of sympathetic neurons. J. Biol. Chem. 2000;275:19883–19890. doi: 10.1074/jbc.275.26.19883. [DOI] [PubMed] [Google Scholar]
- 26.Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R. E., Richardson J. A., Herz J. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and apoE receptor 2. Cell (Cambridge, Mass.) 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 27.Trommsdorff M., Borg J.-P., Margolis B., Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 28.Herz J., Bock H. H. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi H., Campenot R. B., Vance D. E., Vance J. E. Glial cell lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 2004;279:14009–14015. doi: 10.1074/jbc.M313828200. [DOI] [PubMed] [Google Scholar]
- 30.Mauch D. H., Nagler K., Schumacher S., Goritz C., Muller E. C., Otto A., Pfrieger F. W. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 31.Schmechel D. E., Saunders A. M., Strittmatter W. J., Crain B. J., Hulette C. M., Joo S. H., Pericak-Vance M. A., Goldgaber D., Roses A. D. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 33.Oram J. F. HDL apolipoproteins and ABCA1: partners in the removal of excess cellular cholesterol. Arterioscler. Thromb. Vasc. Biol. 2003;23:720–727. doi: 10.1161/01.ATV.0000054662.44688.9A. [DOI] [PubMed] [Google Scholar]
- 34.Koldamova R. P., Lefterov I. M., Ikonomovic M. D., Skoko J., Lefterov P. I., Isanski B. A., DeKosky S. T., Lazo J. S. 22R-hydroxycholesterol and 9-cisretinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J. Biol. Chem. 2003;278:13244–13256. doi: 10.1074/jbc.M300044200. [DOI] [PubMed] [Google Scholar]
- 35.Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B., et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol momeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 36.Pentchev P. G., Vanier M. T., Suzuki K., Patterson M. C. Niemann-Pick disease type C: a cellular cholesterol lipidosis. In: Scriver A. L. B. C. R., Sly W. S., Valle D., editors. The Metabolic and Molecular Bases of Inherited Disease. New York: Mc-Graw Hill; 1995. pp. 2625–2639. [Google Scholar]
- 37.Vanier M. T. Lipid changes in Niemann-Pick disease type C: personal experience and review of the literature. Neurochem. Res. 1999;24:481–489. doi: 10.1023/a:1022575511354. [DOI] [PubMed] [Google Scholar]
- 38.Cadigan K. M., Spillane D. M., Chang T.-Y. Isolation and characterization of Chinese hamster ovary cell mutants defective in intracellular low density lipoprotein cholesterol trafficking. J. Cell Biol. 1990;110:295–308. doi: 10.1083/jcb.110.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liscum L., Ruggiero R. M., Faust J. R. The intracellular transport of low density-derived cholesterol is defective in Niemann-Pick type C fibroblasts. J. Cell Biol. 1989;108:1625–1636. doi: 10.1083/jcb.108.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson L. P., Lin L., Prasad A., Paul C. A., Chang T. Y., Maue R. A. Embryonic striatal neurons from Niemann-Pick Type C mice exhibit defects in cholesterol metabolism and neurotrophin responsiveness. J. Biol. Chem. 2000;275:20179–20187. doi: 10.1074/jbc.M001793200. [DOI] [PubMed] [Google Scholar]
- 41.Karten B., Vance D. E., Campenot R. B., Vance J. E. Cholesterol accumulates in cell bodies, but is decreased in distal axons, of Niemann-Pick C1-deficient neurons. J. Neurochem. 2002;83:1154–1163. doi: 10.1046/j.1471-4159.2002.01220.x. [DOI] [PubMed] [Google Scholar]
- 42.Karten B., Vance D. E., Campenot R. B., Vance J. E. Trafficking of cholesterol from cell bodies to distal axons in Niemann Pick C1-deficient neurons. J. Biol. Chem. 2003;278:4168–4175. doi: 10.1074/jbc.M205406200. [DOI] [PubMed] [Google Scholar]
- 43.Sokol J., Blanchette-Mackie E. J., Kruth H. S., Dwyer N. K., Amende L. M., Butler J. D., Robinson E., Patel S., Brady R. O., Comly M. E., et al. Type C Niemann-Pick disease. J. Biol. Chem. 1988;263:3411–3417. [PubMed] [Google Scholar]
- 44.Liscum L., Faust J. R. Low density lipoprotein (LDL)-mediated suppression of cholesterol synthesis and LDL uptake is defective in Niemann-Pick type C fibroblasts. J. Biol. Chem. 1987;262:17002–17008. [PubMed] [Google Scholar]
- 45.Frolov A., Zielinski S. E., Crowley J. R., Dudley-Rucker N., Schaffer J. E., Ory D. S. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. J. Biol. Chem. 2003;278:25517–25525. doi: 10.1074/jbc.M302588200. [DOI] [PubMed] [Google Scholar]
- 46.Choi H. Y., Karten B., Chan T., Vance J. E., Greer W. L., Heidenreich R. A., Garver W. S., Francis G. A. Impaired ABCA1-dependent lipid efflux and hypoalphalipoproteinemia in human Niemann-Pick type C disease. J. Biol. Chem. 2003;278:32569–32577. doi: 10.1074/jbc.M304553200. [DOI] [PubMed] [Google Scholar]
- 47.Patel S. C., Suresh S., Kumar U., Hu C. Y., Cooney A., Blanchette-Mackie E. J., Neufeld E. B., Patel R. C., Brady R. O., Patel Y. C., et al. Localization of Niemann-Pick C1 protein in astrocytes: implications for neuronal degeneration in Niemann-Pick type C disease. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1657–1662. doi: 10.1073/pnas.96.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokoyama S., Tajima S., Yamamoto A. The process of dissolving apolipoprotein A-I in an aqueous buffer. J. Biochem. (Tokyo) 1982;91:1267–1272. doi: 10.1093/oxfordjournals.jbchem.a133811. [DOI] [PubMed] [Google Scholar]
- 49.Loftus S. K., Morris J. A., Carstea E. D., Gu J. Z., Cummings C., Brown A., Ellison J., Ohno K., Rosenfeld M. A., Tagle D. A., et al. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232–235. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- 50.Myher J. J., Kuksis A., Pind S. Molecular species of glycerophospholipids and sphingomyelins of human plasma: comparison to red blood cells. Lipids. 1989;24:396–407. doi: 10.1007/BF02535148. [DOI] [PubMed] [Google Scholar]
- 51.Gong J. S., Kobayashi M., Hayashi H., Zou K., Sawamura N., Fujita S. C., Yanagisawa K., Michikawa M. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J. Biol. Chem. 2002;277:29919–29926. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- 52.Barres B. A., Silverstein B. E., Corey D. P., Chun L. L. Y. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 53.Roff C. F., Goldin E., Comly M. E., Blanchette-Mackie J., Cooney A., Brady R. O., Pentchev P. G. Niemann-Pick type-C disease: deficient intracellular transport of exogenously derived cholesterol. Am. J. Med. Genet. 1992;42:593–598. doi: 10.1002/ajmg.1320420433. [DOI] [PubMed] [Google Scholar]
- 54.Suresh S., Yan Z., Patel R. C., Patel Y. C., Patel S. C. Cellular cholesterol storage in the Niemann-Pick disease type C mouse is associated with increased expression and defective processing of apolipoprotein D. J. Neurochem. 1998;70:242–251. doi: 10.1046/j.1471-4159.1998.70010242.x. [DOI] [PubMed] [Google Scholar]
- 55.Ong W. Y., Hu C. Y., Patel S. C. Apolipoprotein D in the Niemann-Pick type C disease mouse brain: an ultrastructural immunocytochemical analysis. J. Neurocytol. 2002;31:121–129. doi: 10.1023/a:1023993405851. [DOI] [PubMed] [Google Scholar]
- 56.German D. C., Liang C. L., Song T., Yazdani U., Xie C., Dietschy J. M. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience. 2002;109:437–450. doi: 10.1016/s0306-4522(01)00517-6. [DOI] [PubMed] [Google Scholar]
- 57.Eddleston M., Mucke L. Molecular profile of reactive astrocytes–implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenner M. Structure and transcriptional regulation of the GFAP gene. Brain Pathol. 1994;4:245–257. doi: 10.1111/j.1750-3639.1994.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 59.Baudry M., Yao Y., Simmons D., Liu J., Bi X. Postnatal development of inflammation in a murine model of Niemann-Pick type C disease: immunohistochemical observations of microglia and astroglia. Exp. Neurol. 2003;184:887–903. doi: 10.1016/S0014-4886(03)00345-5. [DOI] [PubMed] [Google Scholar]
- 60.Li Q., Tsujita M., Yokoyama S. Selective down-regulation by protein kinase C inhibitors of apolipoprotein-mediated cellular cholesterol efflux in macrophages. Biochemistry. 1997;36:12045–12052. doi: 10.1021/bi970079t. [DOI] [PubMed] [Google Scholar]
- 61.Selva D. M., Hirsch-Reinshagen V., Burgess B., Zhou S., Chan J., McIsaac S., Hayden M. R., Hammond G. L., Vogl A. W., Wellington C. L. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J. Lipid. Res. 2004;45:1040–1050. doi: 10.1194/jlr.M400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Castro G. R., Fielding C. J. Evidence for the distribution of apolipoprotein E between lipoprotein classes in human normocholesterolemic plasma and for the origin of unassociated apolipoprotein E (Lp-E) J. Lipid Res. 1984;25:58–67. [PubMed] [Google Scholar]
- 63.Kieft K. A., Bocan T. M., Krause B. R. Rapid on-line determination of cholesterol distribution among plasma lipoproteins after high-performance gel filtration chromatography. J. Lipid Res. 1991;32:859–866. [PubMed] [Google Scholar]
- 64.Peng D., Song C., Reardon C. A., Liao S., Getz G. S. Lipoproteins produced by ApoE–/–astrocytes infected with adenovirus expressing human ApoE. J. Neurochem. 2003;86:1391–1402. doi: 10.1046/j.1471-4159.2003.01950.x. [DOI] [PubMed] [Google Scholar]
- 65.Ito J., Zhang L. Y., Asai M., Yokoyama S. Differential generation of high-density lipoprotein by endogenous and exogenous apolipoproteins in cultured fetal rat astrocytes. J. Neurochem. 1999;72:2362–2369. doi: 10.1046/j.1471-4159.1999.0722362.x. [DOI] [PubMed] [Google Scholar]
- 66.Hirsch-Reinshagen V., Zhou S., Burgess B. L., Bernier L., McIsaac S. A., Chan J. Y., Tansley G. H., Cohn J. S., Hayden M. R., Wellington C. L. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 67.German D. C., Quintero E. M., Liang C., Xie C., Dietschy J. M. Degeneration of neurons and glia in the Niemann-Pick C mouse is unrelated to the low-density lipoprotein receptor. Neuroscience. 2001;105:999–1005. doi: 10.1016/s0306-4522(01)00230-5. [DOI] [PubMed] [Google Scholar]