Abstract
The prodomain of TACE [TNFα (tumour necrosis factor α)-converting enzyme] is essential for the secretion of the functional enzyme. Previously, we showed that a TACE truncate was not secreted in the absence of the prodomain and that it was subjected to intracellular degradation. In the present study, we show that full-length TACE was also degraded when expressed without the prodomain. We demonstrate that the prodomain can rescue TACE's secretion in trans, suggesting an intramolecular chaperone function. We addressed the question whether a cysteine switch consensus motif is needed for the secretion of active TACE. The cysteine switch mutants [C184A (Cys184→Ala)] of TACE resembled the wild-type functionally and in their sensitivity to inhibitors. Interestingly, TACE zymogen forms expressed in the context of the C184A mutation were susceptible to intracellular degradation, suggesting that the prodomain-bound TACE zymogen may be more accessible to intracellular proteinases when compared with mature TACE. Two independent findings confirmed that the catalytic domain of TACE is in a more open state when bound to its prodomain: (i) core tryptophan residues were exposed to the solvent in the procatalytic domain complex and (ii) LysC rapidly proteolysed the procatalytic domain complex but not mature TACE. Therefore the prodomain of TACE is a specific intramolecular chaperone that aids in the secretion of this enzyme, while keeping the catalytic domain in a relatively open conformation. The cysteine switch of TACE is not essential for the secretion of the functional enzyme, but may prevent intracellular degradation of the TACE zymogen.
Keywords: a disintegrin and metalloproteinase (ADAM), cysteine switch, molecular chaperone, TNFα-converting enzyme (TACE), tumour necrosis factor (TNF), zymogen
Abbreviations: ADAM, adisintegrin and metalloproteinase; Dnp, 2,4-dinitrophenyl; HFBA, heptafluorobutyric acid; MMP, matrix metalloproteinase; MOI, multiplicity of infection; NP40, Nonidet P40; TNFα, tumour necrosis factor α; TACE, TNFα-converting enzyme
INTRODUCTION
TACE [TNFα (tumour necrosis factor α)-converting enzyme] is emerging as a centrepiece of mammalian cell signal transduction. Originally, it was described as the processing proteinase of precursor TNFα [1,2] whose release mediates inflammatory cell recruitment systemically or locally [3,4]. Later work by Black, Peschon and several groups of investigators using TACE−/− cells derived from knockout mice demonstrated its role in the shedding of a growing number of ectodomains including transforming growth factor-α [5], amyloid precursor protein [6], L-selectin [5], TNFα receptors 1 and 2 [5,7], growth hormone receptor [8], fractalkine [9,10], the epidermal growth factor receptor erbB4/HER4 [11], interleukin-6 receptor [12], Notch1 receptor [13] and the cellular prion protein [14], among many others (see [15], for a recent review). Thus the function of TACE in regulating the secretion of both receptors and ligands goes well beyond its originally described role in TNFα release and inflammation.
Despite its importance in mammalian cell biology, surprisingly, little is known about TACE's biogenesis and mechanism of activation. TACE is a multidomain, membrane-bound zinc metalloproteinase belonging to the disintegrin family of metalloproteinases known as the ADAM (a disintegrin and metalloproteinase) family (see [16] for a review). At its N-terminus, TACE contains a signal peptide followed by an approx. 200-residue prodomain that includes a consensus cysteine switch box (PKVCGY186; see Figure 3) towards its C-terminal end [1,2]. The cysteine switch was previously observed in other zinc metalloproteinases such as the MMPs (matrix metalloproteinases) [17–19]. By analogy to those proteinases, it was hypothesized that the cysteine residue at position 184 co-ordinates the zinc atom in the active site of TACE's catalytic domain, holding the enzyme in an inactive, precursor form [20]. When this co-ordination is present, the procatalytic domain complex is said to be in the ‘closed’, inhibited position. In this way, TACE is prevented from becoming proteolytically active until it has reached its resident compartment. More recently, we have shown that an intact cysteine switch is not necessary for TACE's prodomain function as an inhibitor of this enzyme [21]. However, the ‘closed’ position may still allow the prodomain to act as a chaperone during folding and/or intracellular sorting of the catalytic domain. This stems from our previous observation that this enzyme is made as an inactive zymogen containing the prodomain [20].
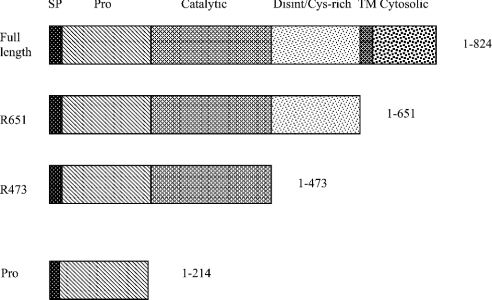
Figure 3. hTACE truncates.
Schematic representation of the domain arrangement of human hTACE truncates. SP, signal peptide; Pro, prodomain; Disint/Cys-rich, disintegrin/cysteine-rich domain; TM, transmembrane domain.
The biosynthesis of TACE requires an intact prodomain, but activation occurs only after the removal of that prodomain, since it acts as an inhibitor of this enzyme [20–22]. Proteolytic removal of the prodomain seems to be mediated by a member of the furin-like proprotein convertase family [23,24]. In vivo and in vitro, prodomain dissociation after furin cleavage requires the presence of the disintegrin/cysteine-rich region comprising residues 475–671 [20,21]. This region is followed by a single transmembrane domain and a 146-residue cytoplasmic tail that includes Src homology 2 and 3 consensus binding domains [1,2]. Induction of TACE activity in many cells requires exogenous stimulation with phorbol esters [5,6,25]. Although these findings suggest the involvement of a kinase cascade, the identity of the kinase(s) involved and the mechanism of activation are hitherto unknown. Additionally, it is not clear whether changes in the phosphorylation status of the cytoplasmic domain of TACE affect furin processing or removal of its inhibitory prodomain [26–28].
Our aim was to determine the nature of the association between TACE's pro- and catalytic domains and elucidate what determinants within the prodomain are responsible for its dual role in maintaining the zymogen state and in the secretion of functional TACE. In the present study, we report studies demonstrating that the prodomain of TACE acts as an intramolecular chaperone, aiding in the secretion of both the full-length membrane-bound enzyme and engineered soluble truncates. We show that the catalytic domain of TACE is held by its prodomain in a more open conformation relative to the mature, active enzyme. Because of this, we hypothesize that the prodomain of TACE behaves as a true intramolecular chaperone, holding the catalytic domain of TACE in a relatively open conformation that is inactive. Moreover, we show that the cysteine switch consensus sequence of the prodomain is not essential for the secretion of functional enzyme, but has a role in protecting the non-native TACE zymogen from intracellular degradation during its secretion. These results indicate that the prodomain of TACE functions in a way that is mechanistically different from the one proposed for secreted serine proteinase zymogens from several bacteria [29,30]. They are discussed in the context of existing knowledge on the prodomain's function in the biosynthesis and secretion of ADAM and other metalloproteinases.
MATERIALS AND METHODS
Production of recombinant human TACE viruses
The construction of human TACE truncates, hR651 (Met1 to Arg651) and hR473 (Met1 to Arg473), in the baculoviral expression shuttle vector pFastBac1 has been described previously ([20] and Figure 3). Two variants of these truncates were constructed: hR473/C184A (Cys184→Ala) and hR651/C184A. Both were constructed by cassette mutagenesis. The cassettes contained a C184A substitution and were engineered with EcoRV and AccI compatible ends for insertion into hR473 and hR651. The TACE gene has naturally occurring unique EcoRV and AccI sites bracketing the cysteine switch. All recombinant plasmids were transformed into DH10Bac cells (Invitrogen). All truncates were cloned into the pFastBac1 vector and recombinant plasmids were transformed into DH10Bac cells. Recombinant bacmids were isolated, purified and used to generate baculovirus in Sf 9 (Spodoptera frugiperda) insect cells as described elsewhere [31].
Production of recombinant mouse full-length TACE viruses
The full-length mouse TACE clone (mTACE; a gift from Dr R. A. Black, Amgen, Seattle, WA, U.S.A.) was inserted by digestion with KpnI and XhoI into the pFastBac1 (pFB) vector containing an inverted multiple cloning site. In addition to the wild-type clone, the following variants were constructed: mTACEΔPro and mTACE/C184A. The variant mTACE/C184A was constructed by Stratagene's Quik Change PCR methodology using divergent primers containing a C184A substitution and pFB mTACE as the template. The variant mTACEΔPro was constructed by PCR using a forward primer containing Glu22 fused to Arg215. The PCR product was inserted into pCDNA3 mTACE using unique BspEI and EcoRI restriction sites. The variant mTACEΔPro was cloned into the pFastBac1-Inverse vector by digestion with KpnI and XhoI. Recombinant bacmids and viruses were generated as described above.
Expression of TACE truncates and mutants
Exponentially growing Trichoplusia ni cells were infected with recombinant TACE baculovirus strains at an MOI (multiplicity of infection) of 1. Cultures were harvested 24 and 48 h post-infection. The cells were separated from the media by low-speed centrifugation at 1200 g for 10 min. The cell-associated protein fraction was extracted by resuspending the cell pellet in 20 mM Tris (pH 7.5) and 1% NP40 (Nonidet P40) followed by centrifugation at 20000 g for 30 min. The supernatants were saved and pellets containing highly insoluble material were discarded. Media and cell-associated fractions were mixed with Laemmli sample buffer [32], subjected to SDS/PAGE analysis and blotted on to Hybond ECL® (enhanced chemiluminescence) nitrocellulose membranes. Blots were probed with either a mouse monoclonal antibody that recognizes the catalytic domain of TACE or a rabbit antiserum recognizing the prodomain of TACE. Blots were developed using the Pierce Supersignal West Pico ECL® kit after incubation with a sheep anti-mouse or donkey anti-rabbit horseradish peroxidase conjugate.
Purification of TACE truncates and mutants
Exponentially growing T. ni cells (3 litres) were infected with baculovirus strains encoding hR473, hR473/C184A, hR651 or hR651/C184A at an MOI of 1. Cultures were harvested 48 h post-infection. Cells were separated from media by low-speed centrifugation (2000 rev./min) for 30 min. Media supernatants were incubated with concanavalin A–Sepharose (50 ml of bed volume; Amersham Biosciences) in a batchwise manner, with overnight stirring at 4 °C. The concanavalin A beads were washed three times with 20 mM Tris/HCl (pH 8.0) containing 150 mM NaCl and twice with 20 mM Tris/HCl (pH 8.0) without salt. TACE was eluted from the concanavalin A beads with three washes (100 ml each) with the same buffer containing 0.4 M methylmannoside. Eluates were combined and applied to a 20 ml BioScale DEAE FPLC column (Bio-Rad) and washed with 400 ml of 20 mM Tris/HCl (pH 8.0). TACE was eluted with a 400 ml linear gradient of 0–0.4 M NaCl. The fractions were monitored by SDS/PAGE analysis. TACE-containing fractions were pooled and concentrated by ultrafiltration using Centriprep-30 concentrators (Amicon/Millipore, Billerica, MA, U.S.A.). The concentrates were applied on to a 16 mm×600 mm Superdex S200 size-exclusion chromatography column (Amersham Biosciences) at the flow rate of 1 ml/min, using 20 mM Tris/HCl (pH 8.0) and 150 mM NaCl as mobile phase. Fractions containing TACE were determined by SDS/PAGE analysis, pooled and concentrated using centriprep-30 concentrators (Amicon). Both for the wild-type h473 and the hC184A variant, TACE was eluted from this column as two peaks, an earlier peak corresponding to the procatalytic domain zymogen complex [Rf=0.187, where Rf stands for elution ratio from the column relative to the marker for the internal volume of the column (Phenol Red)] and a later peak corresponding to the free catalytic domain (Rf=0.267). This behaviour has been reported previously [20]. The hTACE procatalytic domain zymogen complex was stored at −20 °C and used without further modification for spectroscopic and proteolysis protection studies (see the Results section). TACE polypeptides were determined to be more than 95% pure by SDS/PAGE analysis after visualization with the Coomasie-based stain Simply Blue Safestain (Invitrogen). Protein concentrations were determined with Bradford's reagent (Bio-Rad) or based on TACE's UV light absorbance at 280 nm [33].
Activity assays
Proteolytic activities of hTACE truncates and variants were determined in an HPLC-based assay using the synthetic peptide Dnp-SPLAQAVRSSSR-NH2 (where DNp stands for 2,4-dinitrophenyl) as the substrate [1]. The sequence of this peptide corresponds to the cleavage site of TACE on pro-TNFα. The reaction cocktail contained 25 μM Dnp-serine as a standard, 20 μM peptide substrate and a 1:1000 dilution of mammalian proteinase inhibitors without EDTA (Sigma P 8340) in a reaction buffer (10 mM Hepes, pH 7.5, and 0.05% NP40). TACE was added in a tenth of the total reaction volume, at a dilution such that the turnover was between 25 and 50%. We have previously observed that the cleavage reaction is linear up to a turnover of 50%. Reaction mixtures were incubated at 37 °C for 30 min, quenched with an equal volume of 1% HFBA (heptafluorobutyric acid) and cleared by ultrafiltration through PVDF membrane filters. The product to substrate+product ratio was determined by integrating the absorbance at 350 nm of the corresponding peaks after HPLC separation of the reaction mixture using a 150 mm C18 column (Vydac) resolved with a discontinuous gradient of 0.1% HFBA in water and 0.1% HFBA in acetonitrile. Non-specific activity was determined by measuring the product formation in the presence of 10 μM GW9901, a hydroxamic acid competitive inhibitor that completely blocks TACE activity at that concentration [34].
Inhibitor profiles
The effects of various hydroxamic acid inhibitors on the activities of hR473/C184A and hR651/C184A versus hR473 and hR651 respectively were determined using the HPLC assay described above. The reactions were incubated at room temperature (23 °C) for 1 h. The inhibitors screened were GW9471 [35], GW4023 ([35a,35b]) and GW1988 [34]. Each inhibitor was screened by the addition of the following concentrations to the reaction cocktail: 10, 1 μM, 100, 10, 1 nM and 100 pM.
Fluorescence spectroscopy
Isolated hTACE catalytic domain, prodomain or procatalytic domain complexes were diluted to a final concentration of 3 μM with 20 mM Tris/HCl (pH 8.0) containing 150 mM NaCl. Intrinsic fluorescence spectra were collected between 300 and 400 nm after excitation at 390 nm using an Aviv ATF-105 spectrofluorimeter. For each sample, the detector was adjusted to 50% sensitivity at the wavelength of maximum emission (λmax). Scans were background-subtracted by placing the same buffer in the reference cell.
In vitro proteolysis of mature TACE and its zymogen
Isolated hTACE catalytic or procatalytic domain complexes at a final concentration of 40 μg/ml were incubated with highly purified LysC (Roche Molecular Biochemicals) at a final concentration of 1 μg/ml, in 20 mM Tris/HCl (pH 8.0) containing 150 mM NaCl and 10 μM GW9471. After the addition of LysC, samples were incubated at room temperature. Reactions were quenched by adding 5× Laemmli sample buffer [32] and heating to 100 °C for 3 min. Samples were resolved by SDS/PAGE followed by staining with Simply Blue Safestain.
RESULTS
Requirement of the prodomain for TACE expression
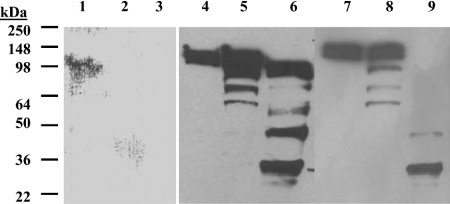
We have previously reported that a form of hTACE consisting of the signal peptide fused to the mature catalytic domain was not secreted and was extensively degraded intracellularly [20]. To confirm that the prodomain of TACE serves an essential role in the biogenesis and maturation of this enzyme, we made an equivalent prodomain deletion in full-length mouse TACE (mTACEΔPro) and compared its expression with that of the wild-type counterpart (mTACE). Owing to the human clone's extreme instability and resistance to genetic manipulation, this experiment was performed with the mouse clone. It has been shown previously by several groups that TACE's mouse orthologue is biochemically indistinguishable from its human counterpart [5,23,26,27]. Insect cells bearing the prodomain deletion mutant expressed full-length mTACE to minimal levels relative to the wild-type polypeptide (Figure 1, lanes 1–3 versus lanes 4–6). mTACEΔPro was rapidly degraded and was nearly undetectable by 48 h post-infection. This full-length enzyme result supports previous observations with the catalytic and extracellular domain truncates and confirms the essential role of the prodomain in the biogenesis and secretion of TACE [20]. This result also verifies that soluble truncated forms of TACE behave similarly to full-length TACE in terms of their biosynthesis and maturation.
Figure 1. Expression of full-length mouse TACE wild-type and variants.
Western blot of mTACEΔPro (lanes 1, 2 and 3), wild-type (lanes 4, 5 and 6) and C184A (lanes 7, 8 and 9) forms. Cell-associated fractions were analysed at 24 h (lanes 1, 4 and 7), 48 h (lanes 2 and 5) and 72 h (lanes 3, 6 and 9) post-infection. Cell associated fractions were obtained after protein extraction with 1% NP40, followed by centrifugation, SDS/PAGE separation and Western blotting using ECL® for TACE polypeptide visualization (see the Materials and methods section). The mTACEΔPro signal was weaker and the gel was overdeveloped, resulting in higher noise levels.
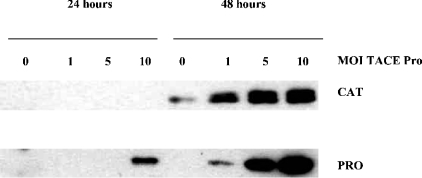
This result suggested to us that the prodomain of TACE must be acting as an intramolecular chaperone, necessary for the biosynthesis of this proteinase and its targeting to the late secretory pathway, where its activating enzyme, furin or another PC family proprotein convertase [23,24], localizes. We sought to test this hypothesis by reconstituting the expression and secretion of the prodomain-deficient catalytic domain truncate, TACE hR473ΔPro [20], by co-expression of its prodomain in trans. T. ni cells were doubly infected with a constant titre of the hR473ΔPro virus and increasing titres of a virus encoding the signal peptide followed by the prodomain (hR214, Figure 3). We observed that greater prodomain expression and secretion correlated with increased secretion of the catalytic domain into culture media (Figure 2). Thus the prodomain can rescue the secretion of TACE in trans. This suggested that the prodomain must be playing an essential role within TACE by affecting its assembly, its stability against intracellular proteinases or its trafficking through the secretory pathway.
Figure 2. Reconstitution of TACE's catalytic domain secretion by co-expression of the hR473ΔPro virus with a second virus encoding hTACE's prodomain.
T. ni cells were simultaneously infected with 5 MOI of the hR473ΔPro virus and 0, 1, 2 or 10 MOI of the prodomain virus. Culture media were harvested at 24 and 48 h post-infection and analysed by SDS/PAGE followed by Western blotting. Upper panel: anti-TACE catalytic domain mouse antibody [20]; lower panel: anti-TACE prodomain antiserum (rabbit).
Cys184 in the ‘cysteine switch’ box of TACE's prodomain is not essential for the secretion of functional TACE
TACE fails to be secreted in the absence of its prodomain (the present study and [20]). The prodomain of this enzyme contains a consensus cysteine switch box (PKVCGY186). It has been demonstrated for several zinc metalloproteinases that this motif mediates the inhibition of the zymogen through a ligation of the zinc ion in the active site by the cysteinyl thiol within the cysteine switch motif [17–19]. The role of this motif as an inhibitor of the catalytic domain within the ADAM family of metalloproteinases is less clear and our own results show that it is not important for this function [21]. Since the cysteine switch was shown to be important for the secretion of several MMPs, we constructed variants of the soluble TACE truncates hR473 and hR651 (Figure 3), in which the Cys184 was replaced with an alanine residue. Insect cells were infected with baculovirus strains harbouring wild-type hTACE or the C184A variants. The level of secretion of TACE into the culture media relative to the wild-type was monitored by Western blotting.
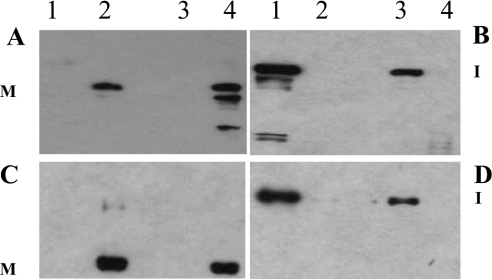
The C184A variants were secreted into media with comparable efficiency as the wild-type TACE truncates hR473 and hR651 (Figure 4). The amount of immature wild-type protein produced 24 h post-infection was somewhat higher compared with the C184A variant for both TACE hR473 (Figure 4B, lane 3 versus lane 1) and hR651 (Figure 4D, lane 3 versus lane 1); however, by 48 h, secretion of mature enzyme into culture media was similar for the mutant relative to wild-type for both hR473 (Figure 4A, lane 4 versus lane 2) and hR651 (Figure 4C, lane 4 versus lane 2). This suggested that, although TACE secretion is completely dependent on the presence of its prodomain, it does not require the Cys184 within the cysteine switch box. Other determinants within TACE's prodomain, yet to be identified, must be mediating the formation of a secretion-competent complex between the pro- and catalytic domains. Intriguingly, the only noticeable difference between the C184A variants and wild-type enzymes was the presence of proteolytic degradation products in the medium of C184A cultures 48 h post-infection. These fragments were absent from the wild-type sample. The observed differences in TACE's intracellular degradation did affect the final purification yields noticeably: 9.87 nmol for hR473 expressed in the context of a wild-type prodomain versus 1.79 nmol for the C184A cysteine switch mutant; and 16.4 nmol for wild-type hR651 versus 3.69 nmol for the C184A variant. This may indicate that a ‘closed’ cysteine-switch is essential to prevent intracellular proteolytic degradation of the TACE zymogen.
Figure 4. Expression of hTACE truncates and C184A variants.
(A) Western blot of TACE hR473 (lanes 1 and 2) and hR473/C184A (lanes 3 and 4) culture medium supernatants at 24 h (lanes 1 and 3) and 48 h (lanes 2 and 4) post-infection; M, mature TACE; I, TACE zymogen. (B) Western blot of hR473 (lanes 1 and 2) and hR473/C184A (lanes 3 and 4) cell-associated fractions obtained after protein extraction with 1% NP40. (C) Western blot of hR651 (lanes 1 and 2) and hR651/C184A (lanes 3 and 4) culture medium supernatants 24 h (lanes 1 and 3) and 48 h (lanes 2 and 4) post-infection. (D) Western blot of hR651 (lanes 1 and 2) and hR651/C184A (lanes 3 and 4) cell-associated fractions obtained after protein extraction with 1% NP40.
Both TACE hR473 and hR651 truncates lack the transmembrane and cytoplasmic domains. Therefore it could be argued that the biogenesis and maturation of these forms do not reflect the behaviour of the full-length enzyme. Under this view, mutational effects on the cysteine switch may only be readable in the context of intact full-length TACE. To address this issue, we constructed a full-length mTACE variant bearing the C184A cysteine-switch mutation and compared its expression and maturation with those of the wild-type proteinase. Expressions of the enzyme by T. ni cells infected with either wild-type or C184A full-length mTACE-encoding viruses were similar (Figure 1). The only form present 24 h post-infection was immature mTACE (Figure 1, lanes 4 and 7). By 48 h post-infection, immature mTACE, its mature form and degradation products were observed for both wild-type and C184A full-length mTACE (Figure 1, lanes 5 and 8). Interestingly, degradation products were more evident with the C184A variant. By 72 h post-infection, this variant was completely degraded, whereas the wild-type polypeptide was still detected in substantial amounts (Figure 1, lanes 6 and 9). This result obtained with the entire membrane-bound enzyme strongly indicates that the cysteine switch is not essential for TACE's expression and conversion from the zymogen into the mature form. The relative susceptibility of the defective cysteine-switch variants to intracellular proteolysis mirrored our previous observation using the complete extracellular domain of TACE (hR651). Therefore the ‘closed’ form of the cysteine switch seems to be important for protecting the enzyme from intracellular degradation during its biogenesis and maturation.
We purified to homogeneity hTACE forms expressed in the presence of a wild-type prodomain or the C184A counterpart and tested whether the variant-expressed forms were functional by assaying their catalytic activity against a synthetic peptide containing the pro-TNFα cleavage site. Both hR473/C184A and hR651/C184A cleaved this substrate only at the expected position between the alanine and valine residues and their specificity constants (kcat/Km) were similar to their respective wild-type forms (Table 1). This demonstrates that secretion of functional TACE does not depend on the presence of the ‘closed switch’ complex between the pro- and catalytic domains made by the co-ordination of the cysteinyl thiol at position 184 of the prodomain and the catalytic zinc ion within the active site of TACE.
Table 1. Peptide substrate cleavage by recombinant hTACE forms.
hTACE activity was measured using a Dnp-labelled peptide containing the cleavage site of pro-TNFα (SPLAQAVRSSR). Dnp-labelled substrate and product were separated using reversed-phase HPLC and monitored at 350 nm.
| TACE form | kcat/Km peptide (M−1·s−1) |
|---|---|
| hR473 wild-type | 1.09×106±0.54×105 |
| hR473 C184A | 1.78×105±0.20×105 |
| hR651 wild-type | 1.69×105±0.18×105 |
| hR651 C184A | 9.93×104±0.54×104 |
TACE C184A variants are equally sensitive to hydroxamic acid inhibitors relative to their wild-type counterparts
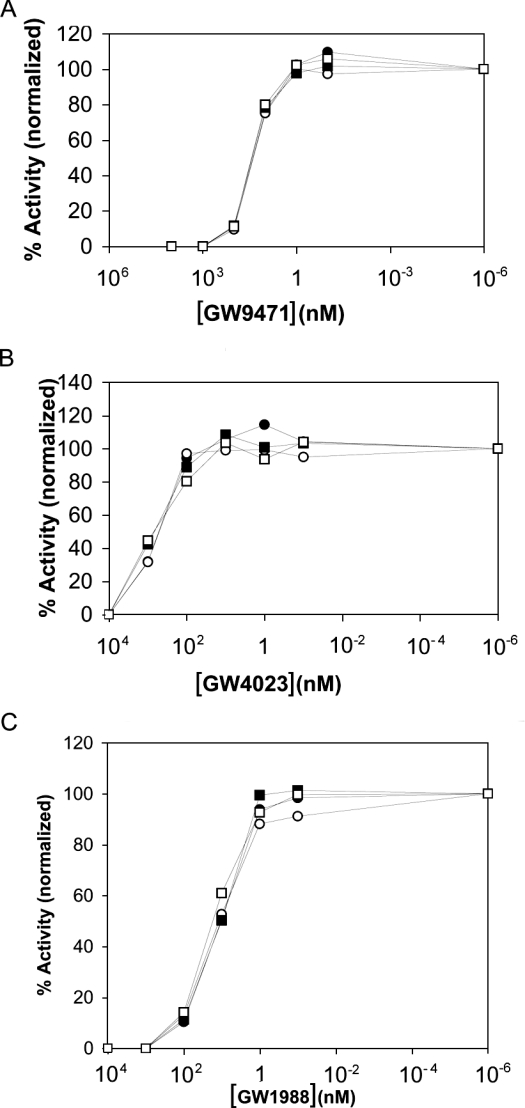
Although both hR473/C184A and hR651/C184A variants were secreted as functional enzymes, we observed modest differences in kcat and Km values (approx. 5-fold lower for hR473/C184A relative to hR473 and 2-fold lower for hR651/C184A relative to hR651). To assess further whether these variants were enzymatically equivalent to wild-type TACE hR473 and hR651, we examined their sensitivity to three hydroxamic acid inhibitors exhibiting different potencies against TACE: GW9471, GW4023 and GW1988 [34–35b]. The inhibition profiles for wild-type hTACE and the C184A variants were virtually identical for all inhibitors (Figure 5). The IC50 values calculated for wild-type hTACE and the C184A variants were also comparable (Table 2). This remarkable similarity in sensitivity to inhibitors indicates that the overall fold and substrate-binding cleft features of the C184A variants must have been preserved relative to the wild-type.
Figure 5. Effect of hydroxamic acid inhibitors on hTACE truncates and variants.
Assays were run using hR473 (●), hR473/C184A (○), hR651 (■) and hR651/C184A (□). Inhibitor curves are the averages of three or four replicate runs. The inhibitors were added at the start of the 1 h incubation: (A) GW9471, (B) GW4023 and (C) GW1988.
Table 2. IC50 values for hTACE truncates and C184A mutants.
hTACE activity was measured using a Dnp-labelled peptide containing the cleavage site of pro-TNFα in the presence and absence of three hydroxamic acid inhibitors, GW9471, GW4023 and GW1988. Each inhibitor was screened by the addition of the following concentrations to the reaction cocktail before a 1 h incubation at room temperature: 10, 1 μM, 100, 10, 1 nM and 100 pM. Dnp-labelled substrate and product were separated using reversed-phase HPLC and monitored at 350 nm.
| IC50 (nM) | |||
|---|---|---|---|
| TACE form | GW9471 | GW4023 | GW1988 |
| hR473 wild-type | 46.0±6.2 | 736±203 | 33.4±12.6 |
| hR473 C184A | 44.6±12.0 | 767±179 | 31.8±23.5 |
| hR651 wild-type | 48.2±5.6 | 851±94 | 34.4±13.0 |
| hR651 C184A | 48.7±5.3 | 836±407 | 43.2±13.5 |
The catalytic domain of TACE is in a more open conformation when bound to its prodomain
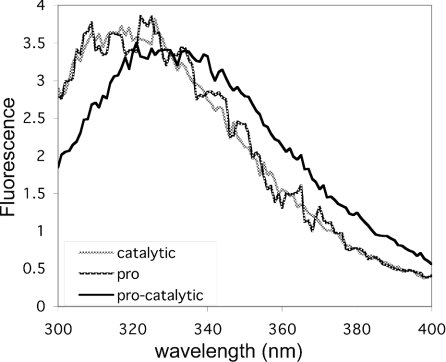
An intriguing possibility is that the pro-bound catalytic domain of TACE may be in a form different from the native, active form observed by X-ray crystallography [36]. This form may display an increased sensitivity to intracellular proteolysis, explaining the requirement for an intact, ‘closed’ cysteine switch to block access to one or more proteolysis-susceptible sites. We used two independent, complementary strategies to assess conformational differences between the catalytic domain of TACE in its zymogen (pro-bound) and mature (pro-free) forms. The pro- and catalytic domains of hTACE contain two tryptophan residues each [1]. The fluorescence emission spectra of the tryptophan residues for hTACE's isolated prodomain (Figure 6, dark grey line) and catalytic domain (light grey line) exhibited the λmax values expected for folded polypeptides with well-defined cores burying hydrophobic amino acids, including tryptophan residues (316 nm for the catalytic domain and 314 nm for the prodomain). In stark contrast, the procatalytic domain zymogen complex isolated from cell culture media exhibited a shift in the fluorescence emission λmax to 331 nm (Figure 6, black line), approaching the λmax observed for the pro- or catalytic domain fully denatured with 6 M guanidinium chloride (340 nm; results not shown). This indicates that the conformations of the catalytic domains and prodomains of TACE are more ‘open’ when complexed together in the zymogen form relative to their free forms. This results in the exposure of core tryptophan residues to the solvent environment as indicated by the change in their fluorescence properties.
Figure 6. Fluorescence emission spectra of hTACE forms.
hTACE's catalytic domain, prodomain or the procatalytic domain zymogen complex isolated from cell culture media was diluted to a final concentration of 3 μM in 20 mM Tris/HCl (pH 8.0) containing 150 mM NaCl. The excitation wavelength was 290 nm and emission was recorded between 300 and 400 nm. Dark grey line, free prodomain; light grey line, free catalytic domain; black line, procatalytic domain complex. The background-subtracted fluorescence intensity is shown in arbitrary units.
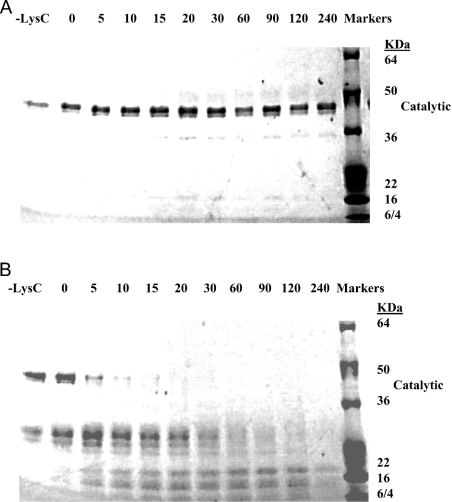
Usually, unfolded or partially folded polypeptides are better substrates for proteolytic degradation, since sites that are otherwise protected in the folded form become accessible to proteinases. A simple method to assess conformational differences consists of exposing a protein to trypsin or a trypsin-like activity and observing its degradation relative to solution conditions. We incubated the native, the isolated hTACE catalytic domain and the procatalytic domain zymogen complex with small amounts of LysC (1:40, w/w), a proteinase we have used previously for peptide digestion/Edman analysis of native hTACE [1]. The catalytic domain of native full-length hTACE is resistant to LysC (results not shown). As expected from this previous observation, the isolated catalytic domain was resistant to LysC digestion with minimal degradation visible over the time course of incubation (4 h; Figure 7A). In contrast, the catalytic domain was extremely sensitive to LysC when complexed with the prodomain; substantial to complete degradation was observed even at the shortest incubation times (5–15 min; Figure 7B). We conclude from these results that the prodomain renders the catalytic domain of TACE in a more open conformation when bound to it, probably resembling the interaction of molecular chaperones with their target polypeptides.
Figure 7. Degradation of the catalytic domain of mature hTACE and its prodomain-bound form by the endoproteinase LysC.
hTACE's catalytic domain complex or procatalytic domain complex (8 μg each) was incubated with 0.2 μg of LysC in 200 μl of Tris/HCl containing 150 mM NaCl and 10 μM GW9471 to inhibit the enzymatic activity of TACE. At the specified times, 18 μl of each incubation was processed as specified in the Materials and methods section, then resolved by SDS/PAGE (4–20% gradient gels). (A) hTACE catalytic domain degradation by LysC versus time (min). (B) Degradation of hTACE procatalytic domain complex by LysC versus time (min). The initial positions of undigested pro- and catalytic domains are shown next to the molecular-mass standards.
DISCUSSION
The role of the prodomain in the biogenesis and activity regulation of TACE and other members of the ADAM family is understood only at an incipient level. It has been shown previously that the prodomain is essential for the secretion of TACE, and in its absence, the catalytic domain is rapidly degraded intracellularly [20,27]. This observation extends to other ADAM family members (see below). We have also shown that the prodomain of TACE is a potent inhibitor of this enzyme, and its removal is required for the activation of this proteinase [20,21]. In the present study, we demonstrate that the prodomain of TACE is essential for the biogenesis and maturation of both full-length native TACE and soluble TACE truncates generated by protein engineering. We show that hTACE Pro alone can reconstitute the secretion of a TACE prodomain deletion mutant when expressed in trans. This is not due to an increase in the expression of TACE hR473Δpro, since the intracellular levels of this polypeptide remained unchanged under conditions where peak recovery of secretion was observed (results not shown). Therefore the observed reconstitution of TACE's secretion on a prodomain-dependent basis occurred after translation of both protein products. This strongly suggests that the prodomain must be serving as a chaperone, aiding in the secretion of this enzyme.
The consensus cysteine switch box within the prodomain has been demonstrated to be important for both metalloproteinase inhibition and secretion [17]. The folding or secretion of TACE may require the interaction of the thiol function of Cys184 with the catalytic zinc ion in the active site of TACE. To test this hypothesis, hTACE truncates were expressed in the context of the C184A mutation within the prodomain. They were secreted at levels comparable with those observed with the wild-type forms. It is noteworthy that the secreted hR651/C184A and full-length mTACE/C184A variants were susceptible to proteolysis, whereas their wild-type counterparts exhibited no proteolytic fragments. This degradation most probably occurred intracellularly, not in the culture media, since it was also observed with full-length, membrane-bound mTACE. This increased susceptibility to degradation was more evident at 72 h post-infection, where all of the C184A zymogen form was degraded intracellularly, whereas the wild-type form was still present at significant levels. Therefore even though a ‘closed’ complex is not essential for secretion, the Cys184–Zn2+ interaction seems to play a role by stabilizing TACE against intracellular proteolysis.
It has been suggested that the prodomain acts as a chaperone during the biosynthesis and secretion of the catalytic domain in ADAM family members [20,22,37] and the related membrane-bound MMP, MT1-MMP [38]. It is possible that the prodomain has a role in either the folding of the enzyme or its secretion (trafficking through the secretory pathway). These two models, although not mutually exclusive, are mechanistically different. In the first one, enzymes depending on the prodomain for folding reach their native state in its presence. Examples of this class of proteinases are subtilisin BPN′ and the α-lytic proteinase. For these, the prodomain effectively lowers the energy barrier between the unfolded and folded states (folding under kinetic control [39,40]). Once collapse into the native, functional state occurs, these enzymes remove the prodomain by themselves.
The second model may be more appropriate for TACE and other ADAM family members: the prodomain acts as a true intramolecular chaperone, associating with the catalytic domain in a way that actually prevents access to its native conformation. Examples of molecular chaperones affecting the conformation of their target proteins are SecB [41], Hsp90 (heat-shock protein 90) [42], rap [43,44] and Grp94 [45]. These proteins bind to partially folded intermediates of their target proteins. Although the prodomain may still be involved in the catalytic domain assembly pathway, such a process cannot be completed before its release from the catalytic domain. We have found that the tryptophan fluorescence emission of TACE hR473 was significantly different for the free, active form of this enzyme, relative to the zymogen form complexed with the prodomain. The tryptophan residues became more exposed to solvent on binding of the catalytic domain to the prodomain. Thus the prodomain of TACE may indeed hold this enzyme in a more open conformation, functionally resembling a molecular chaperone. Our discovery that the procatalytic domain complex is remarkably more sensitive to LysC digestion relative to the free enzyme strongly supports this hypothesis. Both of these results were obtained with the hTACE zymogen isolated from cell-culture media, arguing against the possibility of an artifact occurring on mixing both domains in the test tube.
Having established that an intact cysteine switch is not essential for the secretion of TACE, we sought to determine whether it was essential for the production of the active enzyme. It is important to note that the specificity constant, obtained using our methods, for the wild-type TACE hR473 truncate is higher than the previously reported value: 1.09×106 compared with 1.3×105 [20]. The prodomain of TACE inhibits the catalytic domain of this enzyme potently, with an IC50 value of 70 nM [21]. Previous purifications have used 4-aminophenylmercuric acetate in their methods to remove the prodomain from the catalytic domain. Since the time of those initial experiments, we have discovered that it is possible to remove the prodomain from the catalytic domain simply by incubating procatalytic domain complexes at room temperature. The free prodomain is completely degraded by the activated enzyme during this incubation. The previously reported constant may be an underestimate stemming from the ability of 4-aminophenylmercuric acetate to inhibit TACE itself [20].
Secreted hTACE truncates expressed in the context of the C184A mutation and purified from culture media showed activity levels similar to their wild-type counterparts. However, we did observe modest differences in specificity constants between wild-type and the C184A variants (<2-fold for hR651 and approx. 5-fold for hR473). These minor differences may be due to changes in the thermodynamic stability of the catalytic domain caused by the C184A mutation or a lower efficiency in loading the catalytic zinc ion required for enzymatic activity due to the absence of the thiol at position 184. The second hypothesis is a strong possibility, since zinc ligation by the thiol of Cys184 has been observed in the zymogen form of TACE [21]. These results do suggest that formation of a ‘closed’ TACE zymogen, although not essential for the secretion of mature enzyme, may factor into the efficiency of this process. Despite the observed differences in kcat/Km values, both hR473/C184A and hR651/C184A show identical sensitivity with a series of hydroxamic acid inhibitors with different potencies against TACE. The inhibitor screen is a more stringent evaluation of the functionality of these variants, for the fine architecture of the substrate-binding cleft within the active site of TACE must be preserved for these inhibitors to interact with similar potencies.
We have shown that the prodomain of TACE is essential for the secretion of this enzyme [20]. Several groups have reported similar observations with ADAM 9 [22], ADAM 10 [37], ADAM 12 [46,47] and ADAM 19 [48]. Of these studies, only those on ADAM 9 and ADAM 12 [22,46] specifically addressed whether an intact cysteine switch was required for secretion. Intriguingly, the equivalent C175A mutation did not affect ADAM 12 [46], but had a profound adverse effect on the secretion of ADAM 9 [22]. Given these results, the most pressing question becomes, what is the true role of the cysteine switch consensus sequence within the ADAM family? We have reported previously that a functional switch is not required for inhibition of TACE. Mutational analysis of Cys184 and vicinal residues failed to demonstrate a loss of inhibitory potency with a non-functional switch [21]. On the basis of our results, it is possible that the true physiological role of the cysteine switch, at least for TACE, is to prevent proteolytic degradation of the zymogen during its biogenesis and maturation. We have shown by two independent experiments that the TACE Pro appears to distort the conformation of the catalytic domain of TACE, making its core solvent-exposed. The open conformation resulting from prodomain binding probably exposes sites within the catalytic domain of TACE to intracellular proteinases. The presence of a functional cysteine switch in the ‘closed’ position may be necessary to prevent such an exposure in vivo, explaining the remarkable conservation of the cysteine switch motif throughout the ADAM family.
A second question is, which are the key residues determining the secretion competency of TACE and other ADAM family members? Owing to the lack of structural information on prodomains of ADAM family proteinases, we were not able to build models of TACE's prodomain or its interaction with the catalytic domain. In a search for those determinants, we are currently performing the genetic analysis of regions of TACE's prodomain that appear to be unique to this ADAM. Furthermore, efforts to solve the three-dimensional structure of TACE's zymogen forms are ongoing and showing promise. These studies may prove useful for the design of novel TACE inhibitors acting as prodomain mimetics.
Acknowledgments
We thank Dr D. Becherer from GlaxoSmithKline for discussions and his generous contribution of the TNFα peptide substrate, hydroxamic acid inhibitors and a monoclonal antibody that recognizes the catalytic domain of TACE. We also thank Dr R. A. Black (Amgen) for generously providing the mouse full-length mTACE clone. This work was supported by grant no. AR45949 from the National Institutes of Health.
References
- 1.Moss M. L., Jin S.-L. C., Milla M. E., Bickett D. M., Burkhart W., Carter H. L., Chen W.-J., Clay W. C., Didsbury J. R., Hassler D., et al. Cloning of a disintegrin metalloproteinase that processes tumour necrosis factor-α. Nature (London) 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 2.Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S., et al. A metalloproteinase disintegrin that releases tumour necrosis factor-α from cells. Nature (London) 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B., Cerami A. Tumor necrosis, cachexia, shock and inflammation: a common mediator. Annu. Rev. Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 4.Vassalli P. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 5.Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 6.Buxbaum J. D., Liu K. N., Luo Y., Slack J. L., Stocking K. L., Peshon J. J., Johnson R. S., Castner B. J., Cerretti D. P., Black R. A. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 7.Solomon K. A., Pesti N., Wu G., Newton R. C. Cutting edge: a dominant negative form of TNF-alpha converting enzyme inhibits proTNF and TNFRII secretion. J. Immunol. 1999;163:4105–4108. [PubMed] [Google Scholar]
- 8.Zhang Y., Jiang J., Black R. A., Baumann G., Frank S. J. Tumor necrosis factor-alpha converting enzyme (TACE) is a growth hormone binding protein (GHBP) sheddase: the metalloprotease TACE/ADAM-17 is critical for (PMA-induced) GH receptor proteolysis and GHBP generation. Endocrinology. 2000;141:4342–4348. doi: 10.1210/endo.141.12.7858. [DOI] [PubMed] [Google Scholar]
- 9.Tsou C. L., Haskell C. A., Charo I. F. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. J. Biol. Chem. 2001;276:44622–44626. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- 10.Garton K. J., Gough P. J., Blobel C. P., Murphy G., Greaves D. R., Dempsey P. J., Raines E. W. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J. Biol. Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 11.Rio C., Buxbaum J. D., Peschon J. J., Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J. Biol. Chem. 2000;275:10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 12.Althoff K., Reddy P., Voltz N., Rose-John S., Mullberg J. Shedding of interleukin-6 receptor and tumor necrosis factor alpha. Contribution of the stalk sequence to the cleavage pattern of transmembrane proteins. Eur. J. Biochem. 2000;267:2624–2631. doi: 10.1046/j.1432-1327.2000.01278.x. [DOI] [PubMed] [Google Scholar]
- 13.Brou C., Logeat F., Gupta N., Bessia C., LeBail O., Doedens J. R., Cumano A., Roux P., Black R. A., Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 14.Vincent B., Paitel E., Saftig P., Fobert Y., Hartmann D., De Strooper B., Grassi J., Lopez-Perez E., Checler F. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J. Biol. Chem. 2001;276:37743–37746. doi: 10.1074/jbc.M105677200. [DOI] [PubMed] [Google Scholar]
- 15.Arribas J., Borroto A. Protein ectodomain shedding. Chem. Rev. 2002;102:4627–4638. doi: 10.1021/cr010202t. [DOI] [PubMed] [Google Scholar]
- 16.Becherer J. D., Blobel C. P. Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs) Curr. Top. Dev. Biol. 2003;54:101–123. doi: 10.1016/s0070-2153(03)54006-6. [DOI] [PubMed] [Google Scholar]
- 17.van Wart H. E., Birkedal-Hansen B. The cysteine switch: a principle of regulation of metalloprotease activity with potential applicability to the entire matrix metalloprotease gene family. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5578–5581. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Springman E. B., Angleton E. L., Birkedal-Hansen H., Van Wart H. E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a ‘cysteine switch’ mechanism for activation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grams F., Huber R., Kress L. F., Moroder L., Bode W. Activation of snake venom metalloproteinases by a cysteine switch-like mechanism. FEBS Lett. 1993;335:76–80. doi: 10.1016/0014-5793(93)80443-x. [DOI] [PubMed] [Google Scholar]
- 20.Milla M. E., Leesnitzer M. A., Moss M. L., Clay W. C., Carter H. L., Miller A. B., Su J.-L., Lambert M. H., Willard D. H., Sheeley D. M., et al. Specific sequence determinants are required for the expression of functional tumor necrosis factor-α converting enzyme (TACE) J. Biol. Chem. 1999;274:30563–30570. doi: 10.1074/jbc.274.43.30563. [DOI] [PubMed] [Google Scholar]
- 21.Gonzales P., Solomon A., Leesnitzer M. A., Miller A. B., Sagi I., Milla M. E. Inhibition of TACE by its prodomain. J. Biol. Chem. 2004;279:31638–31645. doi: 10.1074/jbc.M401311200. [DOI] [PubMed] [Google Scholar]
- 22.Roghani M., Becherer J. D., Moss M. L., Atherton R. E., Erdjument-Bromage H., Arribas J., Blackburn R. K., Weskamp G., Tempst P., Blobel C. P. Metalloprotease-disintegrin MDC9: intracellular maturation and catalytic activity. J. Biol. Chem. 1999;274:3531–3540. doi: 10.1074/jbc.274.6.3531. [DOI] [PubMed] [Google Scholar]
- 23.Srour N., Lebel A., McMahon S., Fournier I., Fugere M., Day R., Dubois C. M. TACE/ADAM-17 maturation and activation of sheddase activity require proprotein convertase activity. FEBS Lett. 2003;554:275–283. doi: 10.1016/s0014-5793(03)01159-1. [DOI] [PubMed] [Google Scholar]
- 24.Endres K., Anders A., Kojro E., Gilbert S., Fahrenholz F., Postina R. Tumor necrosis factor-alpha converting enzyme is processed by proprotein-convertases to its mature form which is degraded upon phorbol ester stimulation. Eur. J. Biochem. 2003;270:2386–2393. doi: 10.1046/j.1432-1033.2003.03606.x. [DOI] [PubMed] [Google Scholar]
- 25.Pradines-Figueres A., Raetz C. R. H. Processing and secretion of tumor necrosis factor alpha in endotoxin-treated Mono Mac 6 cells are dependent on phorbol myristate acetate. J. Biol. Chem. 1992;267:23261–23268. [PubMed] [Google Scholar]
- 26.Doedens J. R., Black R. A. Stimulation-induced down-regulation of tumor necrosis factor-alpha converting enzyme. J. Biol. Chem. 2000;275:14598–14607. doi: 10.1074/jbc.275.19.14598. [DOI] [PubMed] [Google Scholar]
- 27.Schlöndorff J., Becherer J. D., Blobel C. P. Intracellular maturation and localization of the tumour necrosis factor alpha convertase (TACE) Biochem. J. 2000;347:131–138. [PMC free article] [PubMed] [Google Scholar]
- 28.Fan H., Turck C. W., Derynck R. Characterization of growth factor-induced serine phosphorylation of tumor necrosis factor-alpha converting enzyme and of an alternatively translated polypeptide. J. Biol. Chem. 2003;278:18617–18627. doi: 10.1074/jbc.M300331200. [DOI] [PubMed] [Google Scholar]
- 29.Baker D., Shiau A. K., Agard D. A. The role of pro regions in protein folding. Curr. Opin. Cell Biol. 1993;5:966–970. doi: 10.1016/0955-0674(93)90078-5. [DOI] [PubMed] [Google Scholar]
- 30.Bryan P. N. Prodomains and protein folding catalysis. Chem. Rev. 2002;102:4805–4816. doi: 10.1021/cr010190b. [DOI] [PubMed] [Google Scholar]
- 31.Luckow V. A., Lee S. C., Barry G. F., Olins P. O. Efficient generation of recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993;67:4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Pace C. N., Vajdos F., Fee L., Grimsley G., Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss M., Becherer J. D., Milla M., Pahel G., Lambert M., Andrews R., Frye S., Haffner C., Cowan D., Maloney P., et al. TNF-α convertase. In: Bradshaw D., Nixon J. S., Bottomley K., editors. Metalloproteinases as Targets for Anti-inflammatory Drugs. Basel: Birkhauser; 1999. pp. 187–204. [Google Scholar]
- 35.McGeehan G. M., Becherer J. D., Bast R. C., Jr, Boyer C. M., Champion B., Connolly K. M., Conway J. G., Furdon P., Karp S., Kidao S., et al. Regulation of tumour necrosis factor-α processing by a metalloproteinase inhibitor. Nature (London) 1994;370:558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- 35a.Hundhausen C., Misztela D., Berkhout T. A., Broadway N., Saftig P., Reiss K., Hartmann D., Fahrenholz F., Postina R., Matthews V., et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell–cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 35b.Hussain I., Hawkins J., Shikotra A., Riddell D. R., Falter A., Dingwall C. Characterization of the ectodomain shedding of the α-site amyloid precursor protein-cleaving enzyme 1 (BACE1) J. Biol. Chem. 2003;278:36264–36268. doi: 10.1074/jbc.M304186200. [DOI] [PubMed] [Google Scholar]
- 36.Maskos K., Fernandez-Catalan C., Huber R., Bourenkov G. P., Bartunik H., Ellestad G. A., Reddy P., Wolfson M. F., Rauch C. T., Castner B. J., et al. Crystal structure of the catalytic domain of human tumor necrosis factor-α converting enzyme. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3408–3412. doi: 10.1073/pnas.95.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anders A., Gilbert S., Garten W., Postina R., Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15:1837–1839. doi: 10.1096/fj.01-0007fje. [DOI] [PubMed] [Google Scholar]
- 38.Cao J., Hymowitz M., Conner C., Bahou W. F., Zucker S. The propeptide domain of membrane type 1-matrix metalloproteinase acts as an intramolecular chaperone when expressed in trans with the mature sequence in COS-1 cells. J. Biol. Chem. 2000;275:29648–29653. doi: 10.1074/jbc.M001920200. [DOI] [PubMed] [Google Scholar]
- 39.Bryan P., Alexander P., Strausberg S., Schwarz F., Wang L., Gilliland G., Gallagher D. T. Energetics of folding of subtilysin BPN′. Biochemistry. 1992;31:4937–4945. doi: 10.1021/bi00136a003. [DOI] [PubMed] [Google Scholar]
- 40.Baker D., Sohl J. L., Agard D. A. A protein-folding reaction under kinetic control. Nature (London) 1992;356:263–265. doi: 10.1038/356263a0. [DOI] [PubMed] [Google Scholar]
- 41.Randall L. L., Hardy S. J. SecB, one small chaperone in the complex milieu of the cell. Cell. Mol. Life Sci. 2002;59:1617–1623. doi: 10.1007/PL00012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimmins S., MacRae T. H. Maturation of steroid receptors: an example of functional cooperation among molecular chaperones and their associated proteins. Cell Stress Chaperones. 2000;5:76–86. doi: 10.1379/1466-1268(2000)005<0076:mosrae>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bu G., Marzolo M. P. Role of rap in the biogenesis of lipoprotein receptors. Trends Cardiovasc. Med. 2000;10:148–155. doi: 10.1016/s1050-1738(00)00045-1. [DOI] [PubMed] [Google Scholar]
- 44.Willnow T. E. Receptor-associated protein (RAP): a specialized chaperone for endocytic receptors. Biol. Chem. 1998;379:1025–1031. [PubMed] [Google Scholar]
- 45.Argon Y., Simen B. B. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 1999;10:495–505. doi: 10.1006/scdb.1999.0320. [DOI] [PubMed] [Google Scholar]
- 46.Loechel F., Gilpin B. J., Engvall E., Albrechtsen R., Wewer U. M. Human ADAM 12 (meltrin alpha) is an active metalloprotease. J. Biol. Chem. 1998;273:16993–16997. doi: 10.1074/jbc.273.27.16993. [DOI] [PubMed] [Google Scholar]
- 47.Loechel F., Overgaard M. T., Oxvig C., Albrechtsen R., Wewer U. M. Regulation of human ADAM 12 protease by the prodomain. Evidence for a functional cysteine switch. J. Biol. Chem. 1999;274:13427–13433. doi: 10.1074/jbc.274.19.13427. [DOI] [PubMed] [Google Scholar]
- 48.Kang T., Zhao Y. G., Pei D., Sucic J. F., Sang Q. X. A. Intracellular activation of human adamalysin 19/disintegrin and metalloproteinase 19 by furin occurs via one of the two consecutive recognition sites. J. Biol. Chem. 2002;277:25583–25591. doi: 10.1074/jbc.M203532200. [DOI] [PubMed] [Google Scholar]