Abstract
Sex and gender—biological and social constructs—significantly impact the prevalence of protective and risk factors, influencing the burden of Alzheimer's disease (AD; amyloid beta and tau) and other pathologies (e.g., cerebrovascular disease) which ultimately shape cognitive trajectories. Understanding the interplay of these factors is central to understanding resilience and resistance mechanisms explaining maintained cognitive function and reduced pathology accumulation in aging and AD. In this narrative review, the ADDRESS! Special Interest Group (Alzheimer's Association) adopted a multidisciplinary approach to provide the foundations and recommendations for future research into sex‐ and gender‐specific drivers of resilience, including a sex/gender‐oriented review of risk factors, genetics, AD and non‐AD pathologies, brain structure and function, and animal research. We urge the field to adopt a sex/gender‐aware approach to resilience to advance our understanding of the intricate interplay of biological and social determinants and consider sex/gender‐specific resilience throughout disease stages.
Highlights
Sex differences in resilience to cognitive decline vary by age and cognitive status.
Initial evidence supports sex‐specific distinctions in brain pathology.
Findings suggest sex differences in the impact of pathology on cognition.
There is a sex‐specific change in resilience in the transition to clinical stages.
Gender and sex factors warrant study: modifiable, immune, inflammatory, and vascular.
Keywords: brain maintenance, cardiovascular, cognitive decline, cognitive reserve, education, genetics, inequalities, lifestyle, TDP43
1. INTRODUCTION
There is considerable inter‐individual variability in the response to Alzheimer's disease (AD) pathology such that some individuals are able to cope better and maintain cognitive function over time. Indeed, ≈ 30% of older adults meet neuropathological criteria for AD at autopsy, yet remain cognitively unimpaired throughout life. 1 , 2 The concept of resilience aims to explain the disconnect between pathology and cognition, and refers to the brain mechanisms explaining the capacity of the brain to maintain cognitive function with aging and disease. 3 , 4 , 5 , 6 The recognition of a long preclinical phase before the appearance of the clinical symptoms in AD and the potential to intervene during this phase 7 has drawn attention to the identification of modifiable factors that may postpone the development of cognitive impairment.
Studies using in vivo AD biomarkers have also demonstrated that some individuals show absence or low burden of pathologies in the context of heightened risk, and suggested that some modifiable factors, such as physical and cognitive activities, are linked with lower burden or slower accumulation of AD pathologies. 4 The concept of resistance to pathologies refers to the notion that, in the context of heightened risk, some individuals show absence or lower‐than‐expected pathological burden. 4 , 6
Recently, there has been increasing awareness of the relevance of studying sex‐ and gender‐specific drivers of resistance and resilience in AD. Women, who make up the majority of AD patients, experience a 2‐fold higher lifetime risk and greater mortality compared to men. 8 , 9 Higher women's life expectancy in aging and AD 10 may contribute to variations in lifetime dementia risk, while incidence differences across genders remain unclear, 11 with conflicting study findings. 12 , 13 , 14 An important factor to consider is that differential risk and resilience may be driven by factors related to sex—referring to an individual's sex chromosome complement (XX vs. XY)—or gender—referring to socially constructed roles, identities, and behaviors—or an intersection of both. 15 However, there is a dearth of literature available focusing explicitly on sex/gender differences in resilience. A sex‐ and gender‐aware resilience framework in AD ought to extend beyond mere description of sex‐ and gender‐related differences and articulate sex and gender to achieve a comprehensive understanding of the sex‐ and gender‐related pathways leading to cognitive decline and dementia or the prevention of it. In line with this, it is essential to highlight that the investigation of factors associated with resilience in AD have mainly been tackled from an individual‐level perspective (e.g., the level of educational attainment). An individual‐level perspective disregards how societal‐level factors and cultural determinants, including gender, race, ethnicity, and their intersections influence individual behaviors, 16 explain differential mechanisms of risk and resilience, and interact with biological risk (e.g., social factors play a role in shaping educational attainment). The scarce available evidence from sex/gender‐stratified studies also suggests that widely investigated determinants of reserve such as education and occupation may show differential effects in women and men. 17
The overall objective of this narrative review is to present the available evidence on sex and gender differences in resilience to AD and establish the foundations for future research. We will first provide a state‐of‐the‐science review and perspective on existing studies formulating testable hypotheses. Then, we will discuss how animal research could aid in the understanding of sex differences in resilience. Finally, we will identify existing research gaps, and provide directions and recommendations to integrate sex and gender into research studies investigating cognitive resilience to aging and AD.
RESEARCH IN CONTEXT
Systematic review: Through conventional literature sources, we identified sex/gender differences in dementia risk with a gap in understanding specific risk and resilience pathways. In aging and Alzheimer's disease (AD), resilience to cognitive decline may stem from factors linked to diminished pathologies—resistance—or to their limited impact on cognition—resilience—thereby influencing dementia risk.
Interpretation: Resilience to cognitive decline should be envisaged considering age, cognitive status, and risk factors within a sex/gender‐aware framework. Initial evidence supports sex/gender differences in underlying pathologies—women show lower resistance to tau and distinct vulnerabilities to cardiovascular disease compared to men—and in the ability to cope with the effects of amyloid and atrophy—with initially enhanced resilience being lost in women as they transition to clinical stages.
Future directions: Studies on factors accounting for differential resilience including exposure to risk factors, pathologies, sex, and inflammatory and vascular pathways are warranted.
2. SEX AND GENDER IN THE RESISTANCE AND RESILIENCE FRAMEWORK: TERMINOLOGY
2.1. Sex and gender: biological versus social constructs
Sex refers to an individual's sex chromosome complement (XX vs. XY), which is reflected in the reproductive organs. 18 An individual is defined as being male or female according to genetics, anatomy, and physiology. This construct has limitations as growing evidence demonstrates the existence of non‐binary and intersex populations. 19 By contrast, gender is a social construct that refers to socially constructed roles, identities, and behaviors. 20 Gender is a multifaceted and fluid construct that is influenced by prior, as well as contemporaneous, social and cultural contexts and environments.
Conventionally, sex has been regarded as a confounding factor (and therefore controlled for) in resilience studies; here, however, we argue that this construct is central to the study of resilience and reserve. The most widely investigated determinants of reserve and resilience, for example, education, are highly gendered. Therefore, the results of studies using gendered determinants of reserve and resilience, such as years of education, need to be interpreted within a broader gender‐oriented framework in which women traditionally have had fewer opportunities for higher education. Only this level of understanding of the determinants of resilience will ensure precise approaches to building models of reserve and resilience at an individual, population, and societal level.
There are several limitations when assessing sex and gender (often self‐reported) in human research, particularly when attempting to disentangle their specific effects as they are intrinsically linked. Here we will refer to “women” and “men” when discussing human studies without explicit investigation of biological pathways, and “male” and “female” when discussing animal models or referring to potential biological pathways. We will approach gender from a binary perspective, given the very scarce existing literature on other gender identities. Health disparities have been reported in sexual and gender minorities 21 which may in turn have profound effects on individual risk and resilience for cognitive decline. Therefore, we do acknowledge the need for research in sexual and gender minorities as discussed in the Recommendations section.
2.2. Resilience and resistance to AD
The Collaboratory on Research Definitions for Reserve and Resilience in Cognitive Aging and Dementia 22 established a consensus on the use of resilience as an umbrella term 4 encompassing different mechanisms about “the capacity of the brain to maintain cognitive function with aging and disease” including brain reserve, brain maintenance, and cognitive reserve. 23 , 24 , 25 , 26 Briefly, these concepts refer to brain mechanisms including greater neurobiological capital to start with, maintenance of brain structure and function over time, or greater adaptability of cognitive strategies. 4
In the AD field, there has been an increasing consensus on the conceptual separation of the notions of “coping” with AD pathologies versus “avoiding” AD pathologies. We refer to these as resilience (coping) and resistance (avoiding). In the context of AD biomarker studies, this terminology presents with the advantage of separating factors that may help halt the development of AD pathologies (amyloid [A]/tau[T]) (“resistance”) versus delay processes downstream to AT, that is, neurodegeneration (N) and the clinical expression of the disease (“resilience”). 4 , 5
Thus, in AD, resilience refers to the ability to attenuate the detrimental effect of risk (i.e., age, apolipoprotein E [APOE] ε4) and/or pathology (i.e., amyloid beta [Aβ], tau) on cognitive performance. Therefore, a measure of resilience could be the extent to which cognitive performance is better than expected at a given level of risk or pathology. 27 Resilience is also investigated in observational studies in which risk and protective factors are considered to mitigate the associations of risk or pathology with cognitive decline. 4 The brain properties and mechanisms underlying resilience to AD are currently under intense investigation in the field. 28
In the context of AD, resistance to pathologies refers to the notion that some individuals may show absence or low pathological burden when it is expected (i.e., in the context of heightened risk). It is important to note that the absence of pathology alone does not imply resistance. 4 , 6 The rationale for this concept is that, while very old age and genetic factors may drive AD‐related pathology and cognitive decline, some individuals “avoid” (or mitigate) the effects of risk factors and show none or lower than‐expected pathologies.
When using this terminology, it is important to also consider the brain/cognition axis. Cognition theoretically operates at the highest level of abstraction, as it is the result of lifetime exposure to risk and protective factors, brain pathologies, and the ability of the brain to cope with them. Therefore, we considered two broad pathways that may result in maintained cognition in aging and disease: (1) lower presence of pathologies in the context of risk (resistance to pathologies such as amyloid or tau), and (2) more preserved brain structure/function and/or more efficient neural resources to compensate for pathology (brain resilience). 3 , 20 , 24 These mechanisms/pathways are determined by a complex interplay of genetic and environmental factors.
To ensure clarity throughout the paper, we will adopt a terminology that refers to the outcome (e.g., cognitive or brain measurements) and pathologies studied as explained in Table 1: for example, “cognitive resilience to tau,” “brain resilience to tau,” or “resistance to amyloid.” The term “cognitive resilience” will be used when talking about studies in the context of aging or risk while not directly addressing brain pathologies or brain mechanisms.
TABLE 1.
Description of the terminology resilience/resistance in the context of AD. The terminology refers to the outcomes (e.g., cognitive or brain measurements) relative to investigated pathology or risk factors. The table provides selected examples for clarity. Lower‐than‐expected pathologies or better‐than‐expected outcomes refer to superior results than expected given the level of risk.
| Primary outcome | Resistance/resilience | Pathology/risk factor | Meaning |
|---|---|---|---|
| Cognitive (cognition) | Resilience to | Tau | The study investigates factors that attenuate the impact of tau on cognition |
| Cognitive (cognition) | Resilience | – | The study investigates factors that explain better‐than‐expected cognition in the context of aging or disease |
| Cognitive (cognition) | Resilience to | APOE ε4 | The investigation evaluates factors that attenuate APOE ε4's effects on cognitive outcomes |
| Brain | Resilience to | Amyloid | The study investigates factors that minimize the effect of amyloid on brain structure and/or function |
| Brain | Resilience | The study investigates factors that explain better brain outcomes in the context of risk (aging or disease) | |
| Resistance to | Amyloid | The study investigates factors associated with absence or lower‐than‐expected amyloid levels | |
| Resistance to | Tau | The investigation focuses on factors associated with absence or lower‐than‐expected tau |
Abbreviation: AD, Alzheimer's disease; APOE, apolipoprotein.
Studies investigating factors and mechanisms associated with maintained cognitive function in aging and disease typically revolve around a framework comprising three components: (1) risk/protective factors, (2) biomarkers of aging or pathologies, and (3) cognitive measurements. Sex and gender may influence the prevalence, level and trajectory of each component, and the relations between them as illustrated in Figure 1.
FIGURE 1.
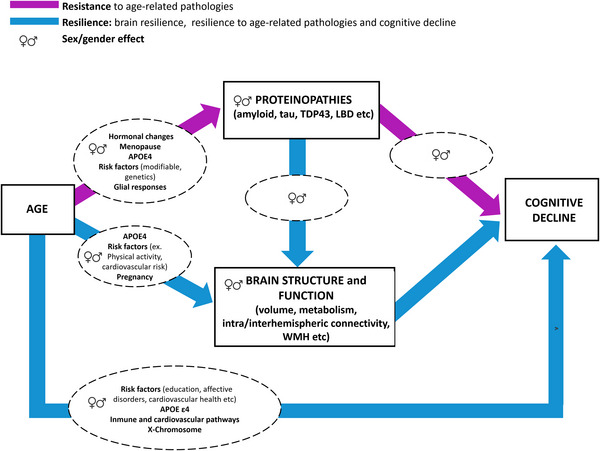
Illustration of resilience and resistance frameworks considering sex and gender effects and associated factors. Sex‐ and gender‐related protective factors may act through increasing resistance and resilience to ultimately impact cognitive resilience. We are providing examples of sex‐ and gender‐related factors shown to have an impact on pathologies, brain structure and function, or cognitive decline. APOE, apolipoprotein; TDP43, Tar DNA‐binding protein; WMH, white matter hyperintensities
3. SEX AND GENDER DIFFERENCES IN COGNITIVE RESILIENCE
In this section, we discuss sex and gender differences in baseline and cognitive trajectories. As supported by the evidence presented below, there is greater consensus on the sex/gender differences in cognitive abilities at baseline than on cognitive trajectories. While one could argue that baseline sex differences will set the stage for higher cognitive resilience and thus a delayed onset of cognitive impairment, the evidence for sex differences in cognitive trajectories is inconsistent. The findings presented in this section suggest that sex differences in cognitive resilience should be envisaged by age period, cognitive status (normal aging vs. disease), and through the consideration of health and sociocultural risk factors.
3.1. Sex/gender differences in baseline cognition and cognitive trajectories
Cross‐sectionally, women generally show better memory, notably, verbal and episodic memory, although better executive functioning in women has also been reported. 29 , 30 , 31 , 32 Men tend to show better visuospatial skills, but this is a less consistent finding. 33 , 34 The memory advantage in women seems to be stable across the lifespan and still present at very old ages. For instance, at > 75 years, women perform better in verbal episodic memory than men. 35 , 36 Overall, these findings may imply a consistent memory advantage in women as illustrated in Figure 2A. These sex differences have been reported independent of demographics including age, race/ethnicity, education, clinical measurements (i.e., blood pressure), and age‐related brain changes, 37 , 38 suggesting a robust sex‐specific advantage across cognitive domains. Recent work with a sociocultural perspective, however, has found interactions of sex/gender with race/ethnicity, 39 implying that the intersection of sociocultural variables and sex/gender requires more attention.
FIGURE 2.
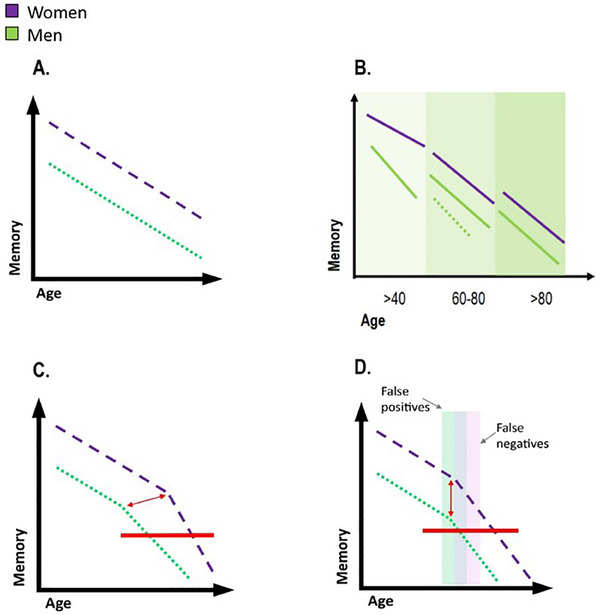
Hypothetical models explaining sex differences in memory throughout aging and disease. A, Initial memory advance provides cognitive resilience through aging (differences in level but not slope). B, Sex differences in memory may vary at different stages of the aging process. The discontinuous line in the 60s to 80s period for men illustrates the conflicting findings. C, The initial memory advantage in women diminishes over time: women cope with AD pathology for longer until they experience a faster decline (differences in level and slope). D, Diagnosis bias due to the differences in baseline memory performance. The red line in C and D illustrates the onset of AD pathology.
By contrast, results regarding sex differences in rates of cognitive decline are controversial. Longitudinal studies considering the adult lifespan suggest that sex discrepancies in memory performance become particularly salient at 40 or after the age of 40. 37 Slower memory decline in women at the age of 40 and greater decline in women after the age of 60 were reported in the same study. 40 In cognitively unimpaired cohorts with an average age of ≈ 60 years, however, a range of findings have been reported: no sex differences, 41 slower decline in women across cognitive domains or only in perceptuomotor speed, integration, and visuospatial ability. 34 In later life (60s–80s), results seem more consistent, with a meta‐analysis reporting no sex differences. 42 Finally, in the oldest old (> 80 years), evidence suggests no sex differences in cognitive trajectories. 36 Figure 2B illustrates sex‐specific cognitive trajectories with a lower decline after the age of 40 in women, inconsistent results around the age of 60, and no sex differences at older ages.
Another set of findings suggest that there might be a critical loss of cognitive resilience that women demonstrate as they progress from normal aging to a diagnosis of cognitive impairment and dementia as illustrated in Figure 2C. Hence, in the context of pathological aging, women with mild cognitive impairment (MCI) showed greater cognitive decline than men (as measured by the Alzheimer's Disease Assessment Scale–Cognitive subscale). 43 Further, studies looking at clinical progression have consistently reported that women progressed faster than men to a clinical diagnosis of MCI and dementia. 44 , 45 , 46 Women with AD dementia were outperformed by men in multiple domains including visuospatial, verbal processing, and semantic and episodic memory. This disadvantage was reported independent of age, education level, and dementia severity. 47 , 48
Finally, there are interesting and nuanced associations between sex and APOE ε4 on cognitive decline and clinical progression. Women APOE ε4 carriers show higher incidence rates to dementia than men carriers, 49 although this was more recently limited to the age range between 65 and 75 years. 50 Women carrying the APOE ε4 allele have been reported to show worse memory and decline faster than men. 40 , 50 In MCI patients, female APOE ε4 carriers, regardless of zygosity, show poorer delayed memory recall at the cross‐section, but only in homozygous men. 51 Finally, one study has suggested that ε 4 may only exacerbate poorer cognition in women relative to men during midlife and not later life, which may arise as a consequence of midlife menopausal changes (discussed in a later section). 52 APOE ε4 carriage and menopausal changes should therefore be considered in the context of the controversial results regarding sex differences in cognitive decline around the age of 60.
Discrepant results on cognitive trajectories as well as on the moderating effect of APOE ε4 on the association between sex and cognitive performance may arise because of different sampling frames and adjustment for different variables that may interact with sex. For example, as discussed below, the differential prevalence and incidence of risk factors across samples and age periods in men and women may differentially influence cognitive trajectories, 40 further, sociocultural factors may also interact with sex and gender. 39 Overall, studies focusing on the resilience frameworks should shift the focus from merely describing sex differences in cognition toward comprehending the underlying factors (APOE ε4, menopause, sociocultural factors) and related mechanisms that contribute to these differences.
3.2. Are sex differences in cognitive resilience the result of lower AD pathologies or preserved brain integrity?
As suggested in the previous section, there might be a critical loss of cognitive resilience in women along the transition from normal to pathological aging. Maintained cognitive performance across normal and pathological aging may be the result of lower pathologies or preserved/enhanced brain structure and function despite the presence of pathologies. Here, we address whether brain resilience to AD pathologies (i.e., differential impact of AD pathological burden on brain structure and function) or lower pathological burden (resistance to pathologies) explains the abovementioned differences in cognitive resilience in women versus men.
At the cross‐section, cognitively unimpaired women outperform men independent of baseline amyloid levels and hippocampal volume, though the hippocampal volume results are less consistent. 53 , 54 , 55 , 56 Indeed, women with amnestic MCI seem to outperform men in immediate and delayed recall tasks given equal levels of moderate hippocampal atrophy, but this advantage was only observed at moderate but not higher levels of hippocampal atrophy. 53 Longitudinally, women diagnosed with amnestic MCI or AD dementia demonstrate increased brain atrophy rates of up to 1.5% per year relative to men with the same diagnostic status. 57 Therefore, while women initially show greater cognitive resilience to hippocampal volume loss, it appears to diminish over time, particularly after a diagnosis of cognitive impairment, and probably at advanced levels of AD or neurodegenerative biomarkers. 58
Research also suggests lower cognitive resilience to AD pathologies in women compared to men. At the cross‐section, women's memory performance appears to be robust to the effects of amyloid pathology. 58 However, at comparable levels of cerebrospinal fluid (CSF) amyloid, cognitively unimpaired women decline more rapidly than men in a composite score combining episodic memory, executive function, and global cognition. 54 Similarly, greater cognitive decline in verbal memory and executive functions was observed in women with higher CSF amyloid and total tau levels and unimpaired cognition, MCI, and AD dementia, although the most pronounced sex difference was observed at the MCI stage. 58 Indeed, while there is no association between PET‐amyloid positivity and cross‐sectional memory scores in women, the effect of amyloid positivity on memory is observable at subjective cognitive decline (SCD) 55 or at MCI stages. 59 In contrast, in men with unimpaired cognition and MCI, amyloid positivity had a less significant impact compared to women, or not at all. Furthermore, women with AD dementia, including those with positive AD biomarkers, exhibit greater cognitive impairment and decline compared to men. 58 , 60
Finally, at the brain level, there might a differential impact of AD pathologies on brain structure in women versus men (sex differences in brain resilience to AD pathologies). While cross‐sectionally women may have greater brain resilience to the effects of tau, 61 women with lower CSF Aβ42 and increased CSF tau showed more rapid hippocampal atrophy, suggesting lower brain resilience to the effects of AD pathologies over time in women. 58 In AD patients, the same clinical severity of dementia was associated with greater reduction in cerebral metabolism in men than in women, which was interpreted as greater ability to cope in men. 62 Overall, these results are consistent with the notion that women show an advantage followed by a loss of compensatory mechanisms resulting in faster decline (see Figure 2B).
An alternative hypothesis is that the verbal memory advantage in women may result in delayed detection of observable cognitive decline by informants and caregivers and therefore missed diagnosis in women (see Figure 2D). In this regard, evidence suggests that sex‐adjusted norm/cut scores identify 10% of missed amnestic MCI cases among women and 10% of false positive cases among men. 53 As such, it is possible that women are diagnosed with more advanced pathology and/or more advanced cognitive decline. Because advantages in sex‐specific cognitive performances exist, models of cognitive resilience stratified by sex are warranted in future studies.
Figure 3 illustrates the changes in cognitive and brain resilience to specific brain pathologies in women versus men and aligns with the hypothesis of an initial memory advance in women.
FIGURE 3.
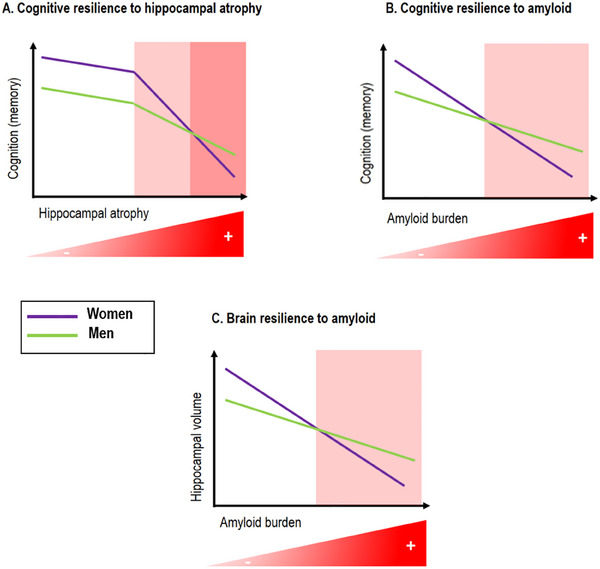
Illustration of the hypothesized sex differences in cognitive and brain resilience to amyloid burden and brain atrophy. A, Cognitive resilience to hippocampal atrophy: women show better memory function and maintained memory performance despite hippocampal atrophy, until a certain threshold is reached, beyond which women show accelerated cognitive decline compared to men. B, Cognitive resilience to amyloid: women show greater cognitive resilience to initial amyloid accumulation evidenced by superior memory performance compared to men at similar levels of amyloid deposition. However, this advantage fades away at higher amyloid levels leading to faster cognitive decline in women. C, There is greater brain resilience to amyloid burden in women evidenced by a smaller effect of amyloid on hippocampal volume compared to men at lower levels of amyloid. This trend reverses at higher levels of amyloid burden, leading to a greater effect on hippocampal volume in women. The red background in the despicted scatterplots indicates higher levels of hippocampal atrophy or amyloid burden.
4. MODIFIABLE AND BIOLOGICAL FACTORS: A SEX/GENDER AWARE FRAMEWORK
A key and shared component within theoretical frameworks to study resilience in aging and AD is the recognition that both modifiable (e.g., education or lifestyle) and biological (e.g., genetic risk) factors play roles in enhancing resilience and resistance along with the associated neural mechanisms. Modifiable risk factors, such as education, physical activity, cardiovascular health, and mood disorders, account for up to 40% of dementia cases. 63 Further, chromosomal, reproductive health, and hormonal differences are biological pathways that may also explain differences in cognitive resilience. Biological risk and modifiable factors, including social determinants of health, may independently drive differential resilience or interact with each other to increase resilience or vulnerability. In this section, we consider modifiable and genetic factors as determinants of resilience.
4.1. A sex/gender perspective on modifiable factors
Almost all the currently identified modifiable risk and protective factors for cognitive decline and dementia demonstrate sex/gender differences in rate and/or risk expression, 64 , 65 as reviewed in this section. Life course exposures and behaviors systematically differ by sex/gender due to both biological (e.g., pregnancy) and or/social (e.g., education) causes. 19 A detailed description of sex‐specific risk and protective factors for cognitive decline and dementia can be found in previous works. 64 , 65 , 66
4.1.1. Years of education
Years of education is among the most commonly used proxies of resilience and reserve in the AD field. 67 Higher education is associated with higher levels of global cognition and delayed onset of symptoms 68 , 69 and might mitigate the adverse effects of sexuality‐minority status on cognitive aging. 70 However, it is a poor reflection of the value of educational reflection among minoritized ethnic groups and its contribution to cognitive decline and resilience among racial/ethnic groups deserves further consideration. 71 , 72 Despite advances in gender equality, education and occupation levels are highly gendered as societal expectations of gender roles have historically manifested in reduced access to higher education for women. 73 While we could expect education to contribute to lower cognitive resilience in women, older women show better cognitive function than men, 35 suggesting an interplay between biological and social factors. Further, despite having overall lower levels of education, women show disproportionately larger benefits of education on both mood and cognitive outcomes compared to men. 74
Recent findings from the United Kingdom suggest that reduced disparities in education levels—due to secular increases in educational opportunities in later birth cohorts—could attenuate sex differences in dementia risk and increase cognitive resilience and reserve at the population level. 75 In Europe, a study reported that the magnitude of sex differences in three separate cognitive domains varied systematically by birth cohort and region, and was confounded by gender‐specific changes in living conditions and levels of cognitive enrichment. 76 Sex differences have also been reported in cognitive trajectories across samples from diverse ethno‐cultural groups and geographical regions with rates of cognitive decline varying across cohorts but consistent associations with education and APOE genotype. 77 As expected, older women show relatively better cognitive performances in countries characterized by more equal gender‐role attitudes, and this effect was partially mediated by education and labor‐force participation. 78 Therefore, only studies across birth cohorts and considering international differences will shed light on whether and how gender disparities may shape cognitive aging trajectories and cognitive resilience.
4.1.2. Affective disorders
Higher rates of affective disorders and social marginalization are observed in women compared to men, 74 , 79 , 80 , 81 , 82 which are in turn associated with cognitive decline and/or dementia risk. 83 , 84 , 85 , 86 Thus, mood disorders are twice as common in women than men 87 and are associated with an almost 2‐fold increased risk of incident dementia. 63 Affective disorders are increasingly being studied in the context of resilience studies, 88 , 89 while they could also be a prodromal sign of dementia. Women show stronger immune responses to pathogens, faster wound healing (e.g., including shorter recovery time from traumatic brain injury), greater antibody production, and a higher predilection for autoimmune diseases. 90 These intrinsic immunologic differences may be a common pathway directly impacting mood and cognitive resilience. Another hypothesis is that discrimination and other social standards present since birth that have systematically marginalized women contribute to women's vulnerability to mood disorders and may further impact immune system development.
FIGURE 4.
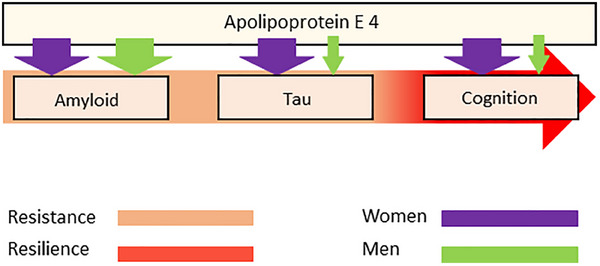
Illustration of the effects of APOE ε4 on the amyloid cascade. Sex effects are observed downstream amyloid. Illustration of the available evidence showing no sex‐specific genetic drivers of amyloidosis but different effects of APOE ε4 in tau (lowering resistance in women) and cognition (lowering cognitive resilience in women compared to men). The magnitudes of the arrows are indicative of effect sizes, with larger arrows representing larger effects. APOE: apolipoprotein E
4.1.3. Cardiovascular and health behaviors
Men have a higher prevalence of certain risk behaviors (e.g., traumatic brain injury, smoking, and substance use) 91 , 92 , 93 and also cardiovascular disease events (e.g., myocardial infarction, stroke) particularly at younger ages. 94 , 95 , 96 , 97 , 98 , 99 The larger cardiovascular advantage in women may be partially related to high estrogen exposure throughout early to midlife. 100 , 101 Precipitous decline in estrogens (> 90%) during menopause transition is associated with a shift such that women begin to show disproportionately increased vascular risk compared to men. 102 , 103 Women who are older than 60 demonstrate increased arterial stiffening, a higher prevalence and mortality from type 2 diabetes, and a stronger association between hemoglobin A1c and brain volume. 97 , 103 , 104 A recent study found that differences in stroke and hypertension between men and women contributed to differences in memory decline. 105
4.1.4. Physical activity
Sedentary behavior and physical activity, which are among the most strongly linked behaviors with dementia risk, also demonstrate gender differences. Women exercise less than men across the lifespan, which is partially related to the gender roles but also to the lack of encouragement of physical activity for women historically. 106 Nevertheless, meta‐analyses suggest that women benefit more from the effects of exercise than men 107 although the directionality is not clear across studies. 96 , 98 , 107 , 108 , 109 , 110 , 111 , 112
Overall, although there are differences in the prevalence of risk factors based on sex and gender it is important to underscore that an over‐ or under‐representation of a particular risk or protective factor in one gender does not automatically result in a more significant or lesser impact on health outcomes.
4.2. Sex‐specific genetic influences on cognitive resilience
Besides modifiable factors, an important biological determinant of resilience is genetics. While, as discussed below, robust evidence exists of genetic contributions to resilience, there has been less work on the degree to which genetic drivers of resilience differ by sex and gender. Nevertheless, initial evidence presented in this section suggests that immune, inflammatory, cardiovascular, and sex hormone pathways drive differences in resilience. This section will cover the current literature of sex‐specific genetic drivers of resilience and protection to AD pathology.
4.2.1. APOE associations with AD risk differ by sex
The studies on APOE suggest that the genetic architecture of amyloidosis is largely shared across sexes and is driven primarily by APOE with only minor sex‐specific genetic effects on amyloidosis outside of APOE. By contrast, the genetic architecture downstream of amyloidosis seems to include substantial sex differences on tau biomarkers, 113 cognitive decline, 114 and clinical AD 50 (see Figure 4), including several biological pathways of resilience that could serve as precision targets for intervention, 115 some of which will be described below.
4.2.2. Sex differences in the genetic architecture of resilience: immune, inflammatory, and cardiovascular pathways
Current evidence suggests that genetic drivers of sex hormone signaling, X‐chromosome function, immune/inflammatory signaling, and vascular hemodynamics may drive resilience to AD pathology in a sex‐specific manner.
A genome‐wide association study on CSF Aβ42 levels identified a genetic locus with a sex interaction. The top variant in the region was an expression quantitative trait locus (eQTL) for the SERPIN gene family, which is implicated in inflammatory processes. 116 A recent study revealed that female cis‐eQTLs, but not male cis‐eQTLs, colocalized with immune traits. 117 Furthermore, sex‐aware genetic correlation analyses on cognitive resilience (against amyloid) demonstrated sex‐specific associations with autoimmune traits, such as multiple sclerosis. That is, greater resilience in women was associated with lower genetic susceptibility but higher genetic susceptibility in men. 118 Autoimmune trait prevalence is higher in females, and autoimmune traits and AD have shared biology, 119 , 120 making the crosstalk between immune/inflammatory pathways and resilience pathways an exciting future direction for sex and gender research.
In the same set of sex‐aware genetic correlation analyses, resilience and heart rate variability were shown to share sex‐specific genetic architecture: more resilient males had a higher genetic susceptibility for more favorable heart rate, while no association was seen in females. 118 Previous studies have shown that heart rate variability is a good marker of heart health, lowers with aging, and has sex‐dependent effects, whereby older males decline in heart rate variability faster than older females. 121 , 122 , 123 As such, findings suggest sex‐specific genetic etiology of resilience may partially be explained by immune pathways in females and cardiovascular pathways in males.
4.2.3. Sex chromosomes and hormones contribute to sex differences in resilience
In addition, sex chromosomes and hormone pathways may contribute to the sex‐specific genetic etiology of resilience. A recent meta‐analysis on resilience identified shared genetic architecture between resilience and hormone‐related traits. 115 Sex‐biased gene expression has been linked to enrichment of hormone‐related transcription factors. 117 Importantly, loss of circulating estrogen and lifetime estrogen exposure have an established relationship with cognitive decline, 124 , 125 , 126 making the convergence of sex hormones and resilience an important future direction for genetic studies. Additionally, the X chromosome has been implicated in sex‐specific genetic pathways of vulnerability to AD. The X chromosome is enriched in brain‐related genes, and shows a reduced ploidy during typical aging in females. 127 Specifically, X‐chromosome aneuploidy is enriched in the hippocampus and cerebrum, both regions disrupted in AD. 127 , 128 To date, few genomic studies have been conducted on the X chromosome, despite its role in aging and AD, due to added complexities from dosage differences between sexes. Future X‐wide association studies on AD protective endophenotypes are needed to further elucidate the X chromosome's exact role in resilience to AD pathology in each biological sex. Taken together, resilience is a highly heritable trait with genetic contributions, and genetic factors of resilience may relate to pathways of protection to AD in a sex‐specific manner. Future sex‐aware genomic studies with large sample sizes are necessary to fully elucidate the genetic etiology of resilience to AD pathology.
5. SEX DIFFERENCES IN BRAIN RESILIENCE: BRAIN STRUCTURE AND FUNCTION
Maintained or enhanced brain structure and function 3 , 24 , 28 throughout aging and disease have been proposed as mechanisms explaining why some individuals are able to cope better with pathologies and maintain function, that is, mechanisms underlying resilience. However, measurements of brain structure and function may reflect both pathological processes as well as the protective effect of several risk and protective factors throughout the lifespan leading to brain resilience. 5
In this section, we first review sex/gender differences in brain structure and function as they may set the stage for further differences in brain resilience throughout aging and disease. Second, we review the scarce literature available suggesting sex/gender as modifier of the association between modifiable factors and brain structure/function. As presented below, the evidence suggesting sexual dimorphism in brain structure and function are consistent across studies, while the evidence for sex‐specific rates of change in structure and function is mixed.
5.1. Baseline differences in brain structure and function
There are consistent sex differences in brain structure and function. 129 , 130 , 131 , 132 Men show, on average, larger brain size 133 , 134 , 135 , 136 through larger absolute gray matter (GM) volume, and white matter (WM) volume. 129 , 137 , 138 By contrast, women show thicker cortices 129 and larger GM and WM volumes, adjusted by total skull size, than men. 138 With respect to microstructural features of the brain, men exhibit higher global fractional anisotropy and stronger intrahemispheric connectivity, while women have higher fiber dispersion and stronger interhemispheric connectivity. 129 , 139 , 140 , 141 , 142 Overall, these studies suggest sex differences in the anatomy and structural connectivity of the brain that should be considered when investigating sex‐specific brain mechanisms of resilience.
Findings regarding functional connectivity seem to suggest a women's advantage. Women demonstrated stronger default mode network (DMN) connectivity, 129 , 143 , 144 , 145 which some studies have associated with reduced cognitive decline 146 and impairment. 147 Furthermore, greater glucose metabolism in the anterior cingulate cortex, as well as in the anterior temporal pole and medial prefrontal cortex, has been associated with slower cognitive decline, particularly in older women. 148
5.2. Rates of change in brain structure and function: differential impact of aging and disease
Conflicting evidence exists regarding differences between men and women in rates of change in brain structure. Greater age‐related decline in WM integrity was reported in women 141 , 149 but also in men. 150 In addition, increased GM atrophy was observed in women 138 , 151 , 152 yet also in men. 129 , 153 , 154 , 155 Previous work suggested faster cortical thinning in both women 156 but also in men. 157 , 158 Last, accelerated brain aging (as reflected by increased brain‐predicted age difference) was found in women 159 , 160 but also in men. 161 , 162 , 163 As discussed above, a consistent sex difference across studies is the increased rate of hippocampal atrophy in women compared to men, 152 , 164 , 165 which may be exacerbated in the presence of AD pathology in women. 58 Despite the dearth of literature examining sex/gender‐specific mechanisms of resilience, studies suggest that women may have higher functional brain maintenance as they have younger metabolic brain‐predicted ages 166 and weaker and less widespread age‐related changes in glucose metabolism than males. 167 Moreover, women have significantly less pronounced age‐related decline in DMN connectivity. 168 Overall, while sex differences in the trajectories of age‐related WM and GM atrophy are unclear, women may be more vulnerable to the effects of pathologies in the structure of the medial temporal lobe but also show a metabolic and functional advantage compared to men.
5.3. Do the effects of risk and protective factors on brain structure and function differ by sex?
Initial or longitudinal sex differences in brain structure and function alone may not explain differences in cognitive decline between men and women. Adding to the complexity is the question of how lifestyle factors, or even sex/gender‐related factors (e.g., reproductive health, pregnancy), impact brain structure and function in women versus men.
Research exploring sex/gender differences in the association of lifestyle factors with brain structure and function is very scarce. High levels of objectively measured low‐intensity daily walking were associated with larger hippocampal volumes only among women. 169 Another study reported an association of maintaining physical activity over 10 years with larger hippocampal volumes in men but larger dorsolateral prefrontal cortex volumes in women, as well as sex‐specific benefits on cognitive domains. 170 Higher cardiovascular risk scores have been associated with lower GM volumes and worse memory among men only. 171
Finally, considering sex‐related factors, it is known that pregnancy is associated with reductions in cerebral GM volume, lasting for at least 2 years post partum 172 and it has been reported that hypertensive pregnancy disorders are associated with smaller brain volumes and worse performance on processing speed cognitive tests, compared to normotensive pregnancies. 173
6. RESISTANCE TO PRIMARY AD PATHOLOGIES
In this section, we review sex/gender differences in primary AD pathologies. Understanding these differences will set the stage to identify sex/gender‐specific vulnerabilities and resiliencies as well as the factors associated to them, as discussed at the end of the section.
6.1. Resistance to Aβ
The majority of studies examining levels of Aβ have not found differences between men and women, including in amyloid positron emission tomography (PET), 37 , 54 CSF Aβ42 174 , 175 or Aβ42/Aβ40 ratio, 176 , 177 , 178 and blood Aβ42/40 ratio. 179 , 180 However, a few studies found that women showed a trend toward higher levels of amyloid in post mortem neuropathological data. 181 , 182 Further, some studies suggest that higher levels of Aβ may appear during the age of menopause relative to age‐matched males, suggesting lower resistance to amyloid in women. 183 , 184
6.2. Resistance to tau
By contrast, several studies showed higher levels of tau pathology in women, as evidenced by PET imaging, 185 , 186 , 187 , 188 , 189 and post mortem data 181 , 182 , 189 implying that women may have lower resistance to tau pathology. Interestingly, PET findings suggest that sex differences in tau deposition occur across multiple cortical regions outside the temporal lobe, 185 , 186 , 187 , 188 , 190 even in clinically normal older adults. 191 Although few studies have reported a main effect of sex on levels of CSF markers of p‐tau, 177 , 192 there are consistent sex by APOE ε4 interaction effects. That is, higher levels of both total tau and p‐tau exist in women APOE ε4 carriers relative to men carriers and all non‐carriers, particularly if they have a diagnosis of MCI or dementia. 113 , 174 , 175 , 193
Most studies to date investigating blood‐based biomarkers have not specifically examined sex differences. In the few that did, conflicting findings have been reported. In one study, levels of p‐tau181 or 217 and markers of neurofibrillary tangle pathology did not differ between men and women. 178 By contrast, a study showed that higher plasma p‐tau181 was associated with greater amyloid and entorhinal cortex tau accumulation, lower brain glucose metabolism, and faster cognitive decline in women, relative to men. 194 Moreover, in a longitudinal sample of individuals with subjective cognitive concerns, 195 women exhibited faster rates of change in plasma total tau concentration than men. Some recent reports suggest that past hormone therapy use might also be associated with lower tau deposition in women. 186 As such, the area of sex differences in tau markers in plasma is an evolving area of the literature.
6.3. Do differences in resistance to AD pathologies result in poorer neurodegenerative outcomes?
While there seem to be vulnerabilities to tau deposition in women, it remains somewhat unclear at what point this may result in poorer neurodegenerative outcomes. Studies examining sex differences in CSF markers of neurodegeneration showed higher neurofilament chain light (NfL) levels in males compared to females. 196 , 197 In apparent disagreement with these findings, women exhibited greater tau‐mediated brain glucose hypometabolism than men. 198 Similarly, a recent study in autosomal dominant AD, showed that as disease progresses, women Presenilin‐1 E280A carriers have a greater plasma NfL increase than men carriers. 199
6.4. Glial cells and resistance to AD pathologies: microglia, astrocytes, and oligodendrocytes
Microglia activation has a stronger mediation effect between Aβ and tau burden in women. 200 As for oligodendrocytes, their global transcriptional activation is positively associated with increased AD pathology in males, whereas not in females. 201 Astrocyte sex differences are less explored, but in animal models, females present a higher astrocyte density in some regions, such as the cortex and hippocampus. 202 , 203 , 204 The hippocampus shows an increase in hypertrophic astrocytes near Aβ plaques in the female brain. 205 Few studies have investigated sex‐specific changes in glial cells, including microglia, astrocytes, and oligodendrocytes. 206 , 207 , 208 , 209 Some studies have shown that microglia density and phenotype vary between women and men. 210 , 211 , 212 , 213 In AD, microglia density increases in both men and women in the parietal cortex. 211 However, male AD individuals present significantly higher density and a more amoeboid microglial morphology compared to females. 211 Microglia from women are more glycolytic and ramified, indicating the presence of different microglial states in male and female brains in the AD continuum. 211 , 214 The role of glial cells in sex‐specific resistance to AD pathologies should be further explored.
6.5. Impact of sex‐ and gender‐related factors on AD pathologies
There is a lack of studies exploring sex differences in the association of lifestyle risk factors with Aβ and tau. Most existing studies have been unable to explore interactions or stratify analyses by sex due to small sample sizes (particularly men). Studies exploring the association of sleep duration 215 and body mass index (BMI) 216 with Aβ burden have not observed sex differences among cognitively normal participants. A recent cross‐sectional study, however, reported that among cognitively intact APOE ε4 carriers, higher cardiovascular risk scores are associated with higher levels of tau among women, but not among men, suggesting that women with at least one APOE ε4 allele may be particularly vulnerable to the effects of cardiovascular factors on tau deposition. 217 Due to the lack of studies, evidence is inconclusive, and future research is needed to explore possible sex differences in the association between lifestyle risk factors and AD pathology.
7. SEX DIFFERENCES IN NON‐AD PATHOLOGIES
Mixed or comorbid pathologies are common in older adults and are major contributors to cognitive decline and dementia. AD pathologies only account for ≈ 50% of the observed age‐related cognitive loss, varying greatly at the individual level. 218 , 219 Hence, additional non‐AD pathologies contribute to lower the threshold for clinical diagnosis of AD dementia 220 and therefore contribute to lower cognitive resilience. Further, non‐AD pathologies add significant variation to the mapping between AD pathology and cognition, and are therefore important factors to study in the context of cognitive resilience to AD. Consequently, in this section, we review sex/gender differences in non‐AD pathologies.
It is important to note that even when accounting for a wide array of neuropathologies, a large proportion of the variation in late‐life cognitive decline remains unexplained. 219 Figure 5 shows a framework in which AD pathologies, risk/protective factors (reviewed above), and non‐AD pathologies are associated with cognitive resilience through their association with brain structure and function (brain resilience). Cognitive and brain resilience, therefore, are the result of the interplay of pathologies and exposure to risk and protective factors throughout the lifespan.
FIGURE 5.
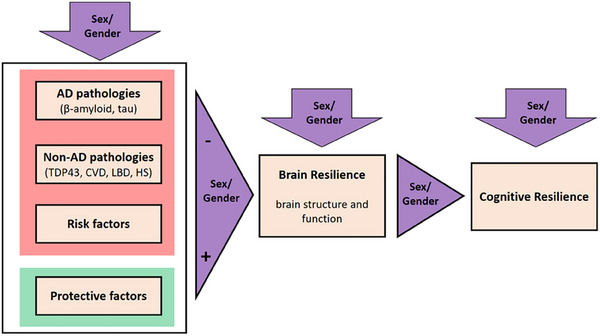
Illustration of how non‐AD pathologies may diminish brain resilience. Non‐AD pathologies are considered together with AD pathologies and risk factors (left box, red) as variables that may have a negative impact on brain resilience, while protective factors may have a positive effect (left box, green). The figure considers that sex/gender may have an effect on each box and in the relation between them. AD, Alzheimer's disease; CVD, cardiovascular disease; HS, hippocampal sclerosis; LBD, Lewy body disease; TDP43, Tar DNA‐binding protein 43
As shown in Figure 5, the most common non‐AD pathologies are cerebrovascular disease (CVD; infarcts, atherosclerosis, arteriosclerosis, cerebral amyloid angiopathy), Lewy body disease (LBD), TAR DNA‐binding protein 43 (TDP‐43), and hippocampal sclerosis (HS). 221 , 222 There are several examples in the literature supporting the need to account for multiple pathologies in resilience studies. For example, in a previous neuropathologic study in the oldest old, less CVD, less cortical aging‐related tau astrogliopathy (ARTAG), less TDP‐43, less Lewy pathology, and no HS were observed in the cognitively resilient group compared to the AD dementia group, suggesting that reduced non‐AD pathological burden explained cognitive resilience. 198 In addition, a recent study suggested that cognitive resilience to AD pathologies may be partially explained by resistance to TDP‐43. 223
Despite the same amount of AD pathology burden in women and men, the difference in these pathologies may impact their cognitive resilience, thus, sex/gender differences are reviewed below.
7.1. Sex/gender differences in CVD
Approximately 78% of all AD dementia cases have CVD and there is a growing body of evidence showing significant differences in CVD burden between men and women. 224 The most frequently reported difference is in white matter hyperintensities (WMH), which are more likely seen in women compared to men, 225 , 226 , 227 particularly post‐menopause. 228 These differences are suggested to be driven by factors such as hypertension and BMI that influence WM integrity in women 229 , 230 and increase vulnerability of WM structures in women. 231 , 232 Severe atherosclerosis is also more likely in women. 182 By contrast, higher rates of cerebral microbleeds 233 , 234 and enlarged perivascular spaces 235 are observed in men relative to women. Both these changes are associated with poor cognitive performance. 236 , 237 Additionally, the risk of stroke is higher in women compared to men and highly dependent on age. 238 Women demonstrate poorer functional outcomes post‐stroke, particularly post‐menopause. 239 , 240 These sex‐specific differences in stroke outcomes are also mediated by worse WM integrity in women. 240 , 241 Silent strokes on magnetic resonance imaging are common in the population 242 and are found to be different between men and women with differences in infarct location. 226 , 233 , 243
7.2. Sex differences in LBD and TDP‐43/hippocampal sclerosis
LBD is significantly more common in men than women; 224 , 244 however, the mixed pathology cases of AD with LBD are more common in women. 224 , 245 , 246 Severity of the clinical manifestation of LBD is also sex specific, with a more aggressive clinical disease course evident in women with dementia with LBD compared to men, 245 suggesting lower cognitive resilience to LBD in women. Neuropathology data also provide evidence of greater AD with TDP‐43/hippocampal sclerosis disease burden in women relative to men. 224 Finally, women showed greater associations between neocortical type Lewy bodies and limbic‐predominant age‐related TDP‐43 encephalopathy neuropathologic change (LATE‐NC). These co‐pathologies contribute to worse cognition in women and greater risk of AD dementia, 247 suggesting that investigating regional associations between pathologies may be important to understand sex/gender‐specific cognitive resilience.
8. INPUTS ON SEX‐SPECIFIC COGNITIVE RESILIENCE FROM ANIMAL MODELS
The study of sex‐specific resilience to AD, and its therapeutic implications, can be informed by the study of animal models. Animal models are required for the elucidation of molecular mechanisms and identification of therapeutic pathways that could improve the human condition. However, a model is only useful if the discoveries generated truly inform us about human disease. Thus, attention to aspects of AD mouse models that recapitulate clinical manifestations of human AD—such as cognition, pathology, survival, and their underlying substrates—is essential. Because there are major limitations to modeling AD, the field has historically used many models and approaches including mouse primary neurons, human amyloid precursor protein (hAPP) mice, human brains, and human cognition and included several AD‐related measures to increase the potential relevance of our findings in sex‐specific resilience against AD.
Being male or female, defined here as harboring a different sex chromosome complement (XY vs. XX), is a primary and understudied biologic variable in AD that is beginning to be explored in models. In the models studied, there are striking parallels with the human condition. Like women with AD that live to advanced ages worldwide, 10 , 248 , 249 contributing to increased female prevalence, female hAPP mice (line J20 on two different genetic backgrounds) live longer than male hAPP mice. 10 Furthermore, after hormonal depletion, female hAPP mice show less cognitive and behavioral deficits than hormone‐depleted male hAPP mice. 10 These findings in mice are congruent to human observations in aging and preclinical AD prior to the age of 85 years when women show better cognition, 54 less cognitive decline, 37 , 250 , 251 and decreased measures of neurodegeneration, 252 despite similar deposition of amyloid and tau, 37 , 253 pathological hallmarks of AD. This could underlie lower prevalence 254 and later onset of MCI in women compared to men in some populations, 255 although cognitive decline is steeper for women later in the course of AD, as pointed out above. 66
8.1. X chromosome modulates resilience against pathology in AD models
Using genetic models of sex biology combined with AD models, it has been found that the X chromosome impacts AD‐related vulnerability in hAPP mice—including survival and cognition. XX‐hAPP mice genetically modified to develop testicles or ovaries showed better survival and less deficits than XY‐hAPP mice with either gonad, indicating a sex chromosome effect. 10 After varying sex chromosome dosage, mice with two X's showed increased survival and fewer deficits than those with one. 54 Thus, adding a second X conferred resilience to survival and cognition of both XY males and XO females. The X‐mediated resilience extended to male and female primary neurons exposed to AD‐related toxicities.
A second X chromosome conferred resilience, in part, through lysine‐specific demethylase 6a (Kdm6a), a gene that escapes X‐chromosome inactivation in XX females. 10 In humans, genetic variation in KDM6A is associated with less cognitive decline over a decade in a population enriched for individuals with MCI 10 and X‐chromosome gene expression with cognitive resilience in women. 256 Collectively, the mouse, primary neuronal, and human studies support a role for the X chromosome in modulating resilience against AD.
Many factors influencing brain function are enriched on the X chromosome, 257 which represents 5% of the genome in both women and men. The X influence on human mental function is highlighted by X‐linked intellectual disability suffered primarily and more severely by males in the absence of compensatory functions from a second X chromosome. Two X chromosomes, compared to one, potentially confers an advantage in sex‐specific resilience against AD in many ways, including diversity of X gene expression from mixed parental origin of the active X—‐and increased X dose from baseline escape of the inactive X. Further study of the sex chromosomes and their contribution to sex‐specific resilience in AD may lead to novel therapeutic pathways that can benefit both sexes.
Indeed, a recent study of humans supports a role for the X chromosome in sex‐specific cognitive change and tau pathology in aging and AD. 256 Differential gene expression profiling of the X chromosome in brains from the Religious Orders Study/Memory Aging Project joint cohorts showed that X expression associated with cognitive change in women, but not men. In the majority of these changes, higher X gene expression associated with slower cognitive decline, suggesting a role for the X in female resilience. In contrast with cognition, X expression associated with neuropathological tau burden in men but not women. These findings in humans suggest that specific X factors could contribute risk or resilience to biologic pathways of AD in each sex.
9. CONCLUSIONS AND KNOWLEDGE GAPS
The general conclusions of the evidence reviewed in this work are provided below accompanied by the knowledge gaps.
Sex/gender differences in cognitive resilience might be decreasing as a function of reductions in gender inequalities, due to more opportunities for women in education, workforce participation, and improvements in their economic status and living conditions. There is a need to further understand how gender and sex disparities and inequalities relate to brain health. Notably, to mechanisms underlying cognitive resilience including brain resilience and resistance to pathologies. Education is widely acknowledged as a pivotal factor in building resilience. However, access and distribution to education have historically been gender skewed. Considering education as a gendered proxy helps prevent the missatribution of observe differences in cognitive resilience to inherent biological traits linked to sex.
The examination of sex and gender differences in cognitive trajectories is a subject of controversy, necessitating evaluation across age periods, cognitive status, and considering health and sociocultural risk factors. Factors conferring differential resilience to cognitive decline in men and women require more attention. For instance, the intersection of sociocultural variables and sex/gender variables may explain some of the inconsistent results. Similarly, mid‐ and late‐adulthood sex differences in cognitive trajectories should be investigated as a function of APOE ε4 status as well as hormonal changes.
Sex differences in coping with the effects of amyloid and brain atrophy on cognition are plausible: women may exhibit enhanced initial cognitive resilience that may fade away as they advance toward a clinical diagnosis of MCI and AD. Longitudinally, cognitively unimpaired women show lower cognitive resilience to amyloid pathology. Nevertheless, the impact of amyloid pathology on the hippocampus is mitigated in women, indicating heightened brain resilience to amyloid. Similarly, the influence of initial levels of hippocampal atrophy on cognition is minimized, suggesting higher cognitive resilience to the initial effects of atrophy. Research investigating AD pathologies, medial temporal structures, and cognition is essential to pinpoint the specific phases in the pathological cascade in which sex/gender differences may manifest. Sex differences in co‐pathologies may also explain differential cognitive trajectories.
Almost all the modifiable risk factors for cognitive decline and dementia, considered determinants of resilience, demonstrate sex/gender differences in rate and/or risk ratio. An over‐ or under‐representation of a particular risk or protective factor in one gender does not automatically result in a modified impact on brain and cognitive outcomes. Knowledge of these sex/gender‐biased risk factors should be considered when explaining cognitive trajectories.
The differential impact of modifiable factors by sex and gender on cognition, pathologies, and brain structure remains understudied. Additional focus on the differential impact of modifiable factors will inform whether a specific factor has a greater impact on cognitive or brain resilience in men or women. A recent sex‐specific analysis of the FINGER trial demonstrated that, despite differential risks by sex, intensive lifestyle modification, including physical activity, diet, and vascular risk management, showed comparable cognitive benefits in men and women. 93 Therefore, these sex‐specific vulnerabilities and resilience factors can be leveraged to inform risk stratification, as well as highlight individual‐specific pathways of disease genesis.
Sex hormone signaling, X chromosome function, immune/inflammatory signaling, and vascular hemodynamics may drive resilience to AD pathology in a sex‐specific manner. Findings suggest sex‐specific genetic etiology of resilience may partially be explained by immune pathways in females and cardiovascular pathways in men. Resilience is a trait with a significant heritable component, influenced by genetic factors that may contribute to pathways of protection against AD in a sex‐specific manner. Subsequent genomic studies, attuned to sex considerations and conducted on a substantial scale, are essential to comprehensively unravel the genetics underpinnings of resilience to AD pathology.
Initial sex differences in brain structure may present opportunities for exploration as potential moderators or mediators between pathology and cognition. There are sex/gender differences in brain structure that seem to be dependent on total intracranial volume/brain size. Because brain size is a variable studied in the context of brain resilience and reserve, it will be important to understand the role of brain size, total volumes, and regional volumes in minimizing the effects of pathology on cognition by sex/gender. Functional and structural connectivity differences could be considered candidate mechanisms explaining the differential resilience to pathologies in men and women including fractional anisotropy, intra and inter‐hemispheric connectivity, and fiber dispersion.
Growing evidence suggests a differential sex effect on resistance to AD pathologies, alongside potential biological determinants. While generally no differences in Aβ accumulation have been reported, some studies suggest great tau accumulation in women. A better understanding of the factors driving these differences whether sex or gender related (e.g., menopause, lifestyle or cardiovascular factors) is needed. Early reports suggest biological mechanisms that may be at play via microglial activation, although further investigation is needed. An intersection between sex and genetics seems clear when considering resistance; for instance, women tend to show stronger effects of APOE ε4 downstream amyloid. However, longitudinal studies are needed as it remains unclear whether women show greater‐than‐expected accumulation of AD pathology as disease progresses relative to men.
Studies with a sex/gender aware framework are lacking. While sex and gender differences in resilience and resistance to pathologies are starting to be described in the field, an articulation of a sex/gender framework will imply considering sex/gender differences in the study design, research hypothesis, and interpretation of the results to better understand the associated sex and gender differences and underlying mechanisms.
10. RECOMMENDATIONS
Conflicting results exist regarding sex and gender differences in the different outcomes reviewed in the article that require clarification in future studies. We make the following recommendations to pursue a more nuanced, accurate, and comprehensive grasp of how sex and gender influence cognitive resilience to aging and AD:
Shift the focus from merely describing to comprehending. Sex/gender‐aware studies of resilience should articulate a conceptual frame motivated by sex or gender differences in health, risk factors, pathologies, and brain structure and function. There are many demographic, genetic, social, cultural, and clinical differences that contribute to dementia risk. Each of these facets can include an important intersection with sex and gender, and which can vary markedly by geographic region. There is a need to comprehend the interconnecting contribution of sex‐ and gender‐related factors across cultures and ethnicities. 258
Publishing negative results to avoid bias. There is a risk of overestimating sex/gender differences, with a bias toward positive results. Given the conflicting results existing for some of the brain and cognitive outcomes, we recommend that all studies include results disaggregated by sex, as well as studies that are directly investigating sex/gender with neutral or negative results be published and included in systematic reviews and meta‐analyses. These include preprints, posters, and conference abstracts.
Consideration of sex/gender in a non‐binary manner and resilience research in LGTBQIA+. Emerging scientific advances suggest that sex and gender should be considered over a continuum. 259 , 260 Thus, at the individual level, both sex and gender and their interactions with other factors can have differential impact on health and disease outcomes. 261 , 262 However, sex and gender have historically been binarily characterized and labeled as indicator representing chromosome XX or XY in research. Biological sex, gender, and sexual orientation are distinct concepts and represent heterogeneous groups, therefore, they should not be interchangeably used in research. We acknowledge the limitation of the present review and call for research that combines sociocultural, demographic factors combined with intersectional disparities of sex and gender. Sex and gender minoritized populations are underrepresented in clinical research and are vulnerable to a greater disease burden of chronic conditions and diseases of aging such as AD and dementia. 19 , 72 , 263
Cross‐talk between animal models and human studies. Animal models support the role of hormones on resistance to AD neuropathology and provide evidence on likely biological mechanisms driving increased survival, brain function, and cognitive resilience associated with X chromosome. 225 Animal models, however, cannot answer some important issues on sex/gender differences that should be further addressed, for example, the complexity of the brain structure and cognitive changes related to aging in humans, the role of sociocultural variables or of menopause, as animal models of menopause can be difficult to reproduce. We recommend more model systems for human translational work and more interdisciplinary collaboration.
Gender inequalities and cognitive resilience: a contextual framework. Studies on cognitive resilience that consider education or other gendered factors must be deeply contextualized. Researchers should be equipped with historical, sociocultural, and economic frameworks to robustly interpret their findings. It is vital to incorporate other gendered proxies that might influence cognitive resilience, like occupation, social engagements, and even domestic roles. Historically influenced by gender, investigations of these variables can offer a broader understanding of their dynamic interplay to moderate levels of cognitive resilience.
Statistical power to test interaction and moderation effects. Statistical power is a critical consideration when examining sex differences. Sex differences that are apparent in large sample sizes may not be detected in smaller samples due to reduced statistical power. 264 , 265 This is particularly important when examining the interaction effect of sex and brain structure/pathology on cognition in cognitively unimpaired cohorts. For instance, a restricted dynamic range of the predictor variable (i.e., brain structure or pathology) can substantially reduce statistical power to detect an interaction effect. The effect size of a moderating effect may also be constrained by the fact that neuroimaging variables account for a relatively small amount of variance in the cognitive function of cognitively unimpaired older adults. 266 , 267 To account for these statistical issues, researchers could attempt to increase statistical power by using large harmonized cognitive datasets, 268 , 269 or a meta‐analysis approach, or they could increase the likelihood of detecting a moderation effect by oversampling individuals with very low or high values on measures of cognitive function and brain structure or pathology. 270 If these approaches are not possible and a null effect is detected in a smaller sample, researchers should, at a minimum, clarify whether they had sufficient statistical power to detect a sex‐based interaction effect. 271 , 272
Inclusion of gender measurements in resilience studies. Better measurements of gender will allow for advances in the field. Unless gender is measured, we cannot estimate the extent to which it confounds the effect of sex in the interpretation of research on cognitive resilience. However, there are no currently agreed methods for measuring gender in the field of cognitive decline and dementia. The pervasiveness of potential gender effects means that many core variables routinely included in statistical models may interact with gender (e.g., education is strongly gendered both in terms of content, type, and quantity of education). A recent framework for measuring sex and gender in cohort studies has been proposed by the “Gender Outcomes International Group: to Further Well‐being Development (GOING‐FWD).” This involves five steps to assess domains that are identified as influencing gender including identification of gender‐related variables, definitions of outcomes, building up feasible final variable lists, harmonization of data, and definition of data structure.
Vigilance in interpretation. It is crucial to approach gender‐based results with caution, particularly when gendered proxies like education come into play. Researchers must differentiate between actual biological characteristics and factors that might be influenced by sociocultural biases. This differentiation ensures that observed differences in resilience are not misattributed to sex when they might be more related to gender‐based societal constructs. Our recommendation is to acknowledge potential confounds in their interpretation of findings to ensure that the reader is appropriately educated on the context.
11. GENERAL CONCLUSION
We urge the field to adopt a sex/gender‐aware approach to resilience to advance our understanding of the intricate interplay of biological and social determinants and consider sex/gender‐specific resilience throughout aging and disease.
CONFLICT OF INTEREST STATEMENT
E.M.A.U. has nothing to disclose. R.B has nothing to disclose. K.C. has nothing to disclose. M.A. has nothing to disclose. E.P. has nothing to disclose. M.E. has nothing to disclose. C.V.‐C. has nothing to disclose. M.W. has nothing to disclose. L.M. has nothing to disclose. J.M.J.V. has nothing to disclose. S.K. has nothing to disclose. T.K.S.N. has nothing to disclose. J.M.E. has nothing to disclose. H.S. has nothing to disclose. P.V. has nothing to disclose. S.T. has nothing to disclose. L.S.Z. has nothing to disclose. K.A. received honorarium from Roche for a lecture and funding from the National Health and Medical Research Council and the Australian Research Council. T.J.H. sits on the scientific advisory board for Vivid Genomics and is a senior associate editor for Alzheimer's & Dementia. O.O. has received consulting fees from Mayo Clinic Rochester and IUPUI and holds a fiduciary role in the International Neuropsychological Society (Board Member). S.L. has received honorarium from Otsuka and Lundbeck. D.B.‐F. serves as a member of the Scientific Advisory Council for Linus Health. D.B.D. is an associate editor for JAMA Neurology, serves on the Board of the Glenn Foundation for Medical Research, and has consulted for Unity Biotechnology and SV Health Investors. H.Sohrabi is a non‐executive Director of SMarT Minds WA, Australia. He has been or is receiving personal reimbursements or research support from the Australian Alzheimer's Research Foundation and Pharmaceutical and Nutraceutical companies including Alector, Alnylam Pharmaceuticals, CWEK PTY LTD (WA, Australia), and Biogen pharmaceuticals. Author disclosures are available in the supporting information.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This manuscript was facilitated by the Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART), through the ADDRESS! Special Interest Group which was founded to foster international research collaborations to study cognitive reserve and resilience across sex, gender, race, and ethnic diverse populations. The present work is a joint effort of the ADDRESS! Special Interest Group made up of members from the Reserve, Resilience and Protective Factors Professional Interest Area (PIA) and the Sex and Gender Differences in Alzheimer's Disease PIAs. The views and opinions expressed by authors in this publication represent those of the authors and do not necessarily reflect those of the PIA membership, ISTAART, or the Alzheimer's Association. We would like to express our sincere gratitude to Prof. Yaakov Stern for his feedback regarding the conceptual framework of the manuscript. E.M.A.‐U. is supported by the Spanish Ministry of Science and Innovation—State Research Agency, and the European Social Fund (ESF) (RYC2018‐026053‐I), the Spanish Ministry of Science and Innovation (PID2019‐111514RA‐I00), the Alzheimer's Association (AARG2019‐AARG‐644641, AARG2019‐AARG‐644641‐RAPID), the EU Joint Programme‐Neurodegenerative Disease Research (JPND2022‐138), and the Research and innovation Program of the Barcelona City Council and Fundació La Caixa (21S0906). K.C. is supported by the National Institute on Aging (R01AG072475 and R56AG082414). E.P. is supported by the Spanish Ministry of Science and Innovation (PRE2020‐095827). C.V.C. is supported by the National Institute on Aging (K99AG073452) A.C. is supported by the National Institute of Health (T32AG078115). T.J.H. is supported by the National Institute on Aging (R01‐AG059716). J.E. is supported by The National Institute on Aging (F31‐AG077791). J.M.J.V. is supported by the National Institutes of Health (R00AG066934). R.F.B. is supported by R00AG061238, DP2AG082342, R01AG079142. O.O. is employed by University of Wisconsin School of Medicine & Public Health and has received support from the following grants, paid to his institution: R01AG062167, R01AG077507, U19AG024904, U19AG078109, U19AG073153, R01AG066203, R01AG070028, R01AG027161, RF1AG052324. P.V. is supported by the National Institutes of Health (R01 AG056366 and U01 AG006786). P.Z. received travel scholarships from the Alzheimer's Association, the NIA‐funded Hopkins Economics of Alzheimer's Disease & Services Center, and the NIA‐funded The Science of Alzheimer's Disease and Related Dementias (AD/ADRD) for Social Scientists Program at the University of Southern California. D.B.‐F. was funded by an ICREA Academia 2019 Award from the Catalan Government, and the Spanish Ministry of Science and education (PID2022‐137234OB‐I00). D.B.D. is funded by the Simons Foundation; Bakar Aging Research Institute Grants; the National Institute on Aging at the National Institutes of Health under R01 grants AG068325 and AG079176; and philanthropy.
Arenaza‐Urquijo EM, Boyle R, Casaletto K, et al. Sex and gender differences in cognitive resilience to aging and Alzheimer's disease. Alzheimer's Dement. 2024;20:5695–5719. 10.1002/alz.13844
[Correction added on August 01, 2024, after first online publication: The order of the first and last names for the author Prashanthi Vemuri was corrected].
REFERENCES
- 1. Driscoll I, Troncoso J. Asymptomatic Alzheimer's disease: a prodrome or a state of resilience? Curr Alzheimer Res. 2011;8(4):330‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seto M, Weiner RL, Dumitrescu L, Hohman TJ. Protective genes and pathways in Alzheimer's disease: moving towards precision interventions. Mol Neurodegener. 2021;16(1):29. doi: 10.1186/s13024-021-00452-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stern Y, Arenaza‐Urquijo EM, Bartrés‐Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305‐1311. doi: 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arenaza‐Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology. 2018;90(15):695‐703. doi: 10.1212/WNL.0000000000005303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arenaza‐Urquijo EM, Vemuri P. Improving the resistance and resilience framework for aging and dementia studies. Alzheimers Res Ther. 2020;12:41. doi: 10.1186/s13195-020-00609-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montine TJ, Cholerton BA, Corrada MM, et al. Concepts for brain aging: resistance, resilience, reserve, and compensation. Alzheimers Res Ther. 2019;11(1):22. doi: 10.1186/s13195-019-0479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280‐292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Austad SN. Why women live longer than men: sex differences in longevity. Gender Medicine. 2006;3(2):79‐92. doi: 10.1016/S1550-8579(06)80198-1 [DOI] [PubMed] [Google Scholar]
- 9. Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23(6):1022‐1033. doi: 10.1016/j.cmet.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis EJ, Broestl L, Abdulai‐Saiku S, et al. A second X chromosome contributes to resilience in a mouse model of Alzheimer's disease. Sci Transl Med. 2020;12(558):eaaz5677. doi: 10.1126/scitranslmed.aaz5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shaw C, Hayes‐Larson E, Glymour MM, et al. Evaluation of selective survival and sex/gender differences in dementia incidence using a simulation model. JAMA Netw Open. 2021;4(3):e211001. doi: 10.1001/jamanetworkopen.2021.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol. 2002;59(10):1589‐1593. doi: 10.1001/archneur.59.10.1589 [DOI] [PubMed] [Google Scholar]
- 13. Fiest KM, Roberts JI, Maxwell CJ, et al. The prevalence and incidence of dementia due to Alzheimer's disease: a systematic review and meta‐analysis. Can J Neurol Sci. 2016;43(suppl 1):S51‐S82. doi: 10.1017/cjn.2016.36 [DOI] [PubMed] [Google Scholar]
- 14. Huque H, Eramudugolla R, Chidiac B, et al. Could Country‐level factors explain sex differences in dementia incidence and prevalence? A systematic review and meta‐analysis. J Alzheimers Dis. 2023;91(4):1231‐1241. doi: 10.3233/JAD-220724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayeda ER. Invited commentary: examining sex/gender differences in risk of Alzheimer disease and related dementias—Challenges and future directions. Am J Epidemiol. 2019;188(7):1224‐1227. doi: 10.1093/aje/kwz047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilmore‐Bykovskyi A, Croff R, Glover CM, et al. Traversing the aging research and health equity divide: Toward intersectional frameworks of research justice and participation. Gerontologist. 2022;62(5):711‐720. doi: 10.1093/geront/gnab107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Subramaniapillai S, Almey A, Natasha Rajah M, Einstein G. Sex and gender differences in cognitive and brain reserve: Implications for Alzheimer's disease in women. Front Neuroendocrinol. 2021;60:100879. doi: 10.1016/j.yfrne.2020.100879 [DOI] [PubMed] [Google Scholar]
- 18. Clayton JA. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav. 2018;187:2‐5. doi: 10.1016/j.physbeh.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 19. Mielke MM, Aggarwal NT, Vila‐Castelar C, et al. Consideration of sex and gender in Alzheimer's disease and related disorders from a global perspective. Alzheimers Dement. 2022;18(12):2707‐2724. doi: 10.1002/alz.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartz D, Chitnis T, Kaiser UB, et al. Clinical advances in sex‐ and gender‐informed medicine to improve the health of all: a review. JAMA Intern Med. 2020;180(4):574‐583. doi: 10.1001/jamainternmed.2019.7194 [DOI] [PubMed] [Google Scholar]
- 21. Polderman TJC, Kreukels BPC, Irwig MS, et al. The biological contributions to gender identity and gender diversity: bringing data to the table. Behav Genet. 2018;48(2):95‐108. doi: 10.1007/s10519-018-9889-z [DOI] [PubMed] [Google Scholar]
- 22. Stern Y, Albert M, Barnes CA, Cabeza R, Pascual‐Leone A, Rapp PR. A framework for concepts of reserve and resilience in aging. Neurobiol Aging. 2023;124:100‐103. doi: 10.1016/j.neurobiolaging.2022.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015‐2028. doi: 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci (Regul Ed). 2012;16(5):292‐305. doi: 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Mortimer JA. Brain reserve and the clinical expression of Alzheimer's disease. Geriatrics. 1997;52(suppl 2):S50‐S53. [PubMed] [Google Scholar]
- 26. Satz P, Cole MA, Hardy DJ, Rassovsky Y. Brain and cognitive reserve: mediator(s) and construct validity, a critique. J Clin Exp Neuropsychol. 2011;33(1):121‐130. doi: 10.1080/13803395.2010.493151 [DOI] [PubMed] [Google Scholar]
- 27. Bocancea DI, van Loenhoud AC, Groot C, Barkhof F, van der Flier WM, Ossenkoppele R. Measuring resilience and resistance in aging and Alzheimer disease using residual methods: a systematic review and meta‐analysis. Neurology. 2021;97(10):474‐488. doi: 10.1212/WNL.0000000000012499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cabeza R, Albert M, Belleville S, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19(11):701‐710. doi: 10.1038/s41583-018-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landau SM, Mintun MA, Joshi AD, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72(4):578‐586. doi: 10.1002/ana.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian imaging, biomarkers, and lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275‐1283. doi: 10.1016/j.neurobiolaging.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 31. Lim YY, Pietrzak RH, Bourgeat P, et al. Relationships between performance on the cogstate brief battery, neurodegeneration, and Aβ accumulation in cognitively normal older adults and adults with MCI. Arch Clin Neuropsychol. 2015;30(1):49‐58. doi: 10.1093/arclin/acu068 [DOI] [PubMed] [Google Scholar]
- 32. Chételat G, Villemagne VL, Pike KE, et al. Relationship between memory performance and β‐amyloid deposition at different stages of Alzheimer's disease. Neurodegener Dis. 2012;10(1‐4):141‐144. doi: 10.1159/000334295 [DOI] [PubMed] [Google Scholar]
- 33. Murre JMJ, Janssen SMJ, Rouw R, Meeter M. The rise and fall of immediate and delayed memory for verbal and visuospatial information from late childhood to late adulthood. Acta Psychol (Amst). 2013;142(1):96‐107. doi: 10.1016/j.actpsy.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 34. McCarrey AC, An Y, Kitner‐Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31(2):166‐175. doi: 10.1037/pag0000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Exel E, Gussekloo J, de Craen AJM, et al. Cognitive function in the oldest old: women perform better than men. J Neurol Neurosurg Psychiatry. 2001;71(1):29‐32. doi: 10.1136/jnnp.71.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Golchert J, Roehr S, Luck T, et al. Women outperform men in verbal episodic memory even in oldest‐old age: 13‐year longitudinal results of the agecode/agequalide study. J Alzheimers Dis. 2019;69(3):857‐869. doi: 10.3233/JAD-180949 [DOI] [PubMed] [Google Scholar]
- 37. Jack CR, Wiste HJ, Weigand SD, et al. Age, sex and APOE ε4 effects on memory, brain structure and β‐amyloid across the adult lifespan. JAMA Neurol. 2015;72(5):511‐519. doi: 10.1001/jamaneurol.2014.4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid‐related decline. JAMA Neurol. 2014;71(8):961‐970. doi: 10.1001/jamaneurol.2014.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Avila JF, Vonk JMJ, Verney SP, et al. Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimers Dement. 2019;15(12):1516‐1523. doi: 10.1016/j.jalz.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anstey KJ, Ehrenfeld L, Mortby ME, et al. Gender differences in cognitive development in cohorts of young, middle, and older adulthood over 12 years. Dev Psychol. 2021;57(8):1403‐1410. doi: 10.1037/dev0001210 [DOI] [PubMed] [Google Scholar]
- 41. Soldan A, Pettigrew C, Cai Q, et al. Cognitive reserve and long‐term change in cognition in aging and preclinical Alzheimer's disease. Neurobiol Aging. 2017;60:164‐172. doi: 10.1016/j.neurobiolaging.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferreira L, Ferreira Santos‐Galduróz R, Ferri CP, Fernandes Galduróz JC. Rate of cognitive decline in relation to sex after 60 years‐of‐age: a systematic review. Geriatr Gerontol Int. 2014;14(1):23‐31. doi: 10.1111/ggi.12093 [DOI] [PubMed] [Google Scholar]
- 43. Sohn D, Shpanskaya K, Lucas JE, et al. Sex differences in cognitive decline in subjects with high likelihood of mild cognitive impairment due to Alzheimer's disease. Sci Rep. 2018;8(1):7490. doi: 10.1038/s41598-018-25377-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y). 2015;1(2):103‐110. doi: 10.1016/j.trci.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tifratene K, Robert P, Metelkina A, Pradier C, Dartigues JF. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology. 2015;85(4):331‐338. doi: 10.1212/WNL.0000000000001788 [DOI] [PubMed] [Google Scholar]
- 46. van Loenhoud AC, van der Flier WM, Wink AM, et al. Cognitive reserve and clinical progression in Alzheimer disease: a paradoxical relationship. Neurology. 2019;93(4):e334‐e346. doi: 10.1212/WNL.0000000000007821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer's disease. World J Psychiatry. 2016;6(1):54‐65. doi: 10.5498/wjp.v6.i1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer's disease: a meta‐analysis. J Clin Exp Neuropsychol. 2012;34(9):989‐998. doi: 10.1080/13803395.2012.712676 [DOI] [PubMed] [Google Scholar]
- 49. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta‐analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349‐1356. [PubMed] [Google Scholar]
- 50. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer's disease. JAMA Neurol. 2017;74(10):1178‐1189. doi: 10.1001/jamaneurol.2017.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sundermann EE, Tran M, Maki PM, Bondi MW. Sex differences in the association between apolipoprotein E ε4 allele and Alzheimer's disease markers. Alzheimers Dement (Amst). 2018;10:438‐447. doi: 10.1016/j.dadm.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Georgakis MK, Kalogirou EI, Diamantaras AA, et al. Age at menopause and duration of reproductive period in association with dementia and cognitive function: a systematic review and meta‐analysis. Psychoneuroendocrinology. 2016;73:224‐243. doi: 10.1016/j.psyneuen.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 53. Sundermann EE, Maki PM, Rubin LH, et al. Female advantage in verbal memory: evidence of sex‐specific cognitive reserve. Neurology. 2016;87(18):1916‐1924. doi: 10.1212/WNL.0000000000003288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer's disease: findings from three well‐characterized cohorts. Alzheimers Dement. 2018;14(9):1193‐1203. doi: 10.1016/j.jalz.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caldwell JZK, Cummings JL, Banks SJ, Palmqvist S, Hansson O. Cognitively normal women with Alzheimer's disease proteinopathy show relative preservation of memory but not of hippocampal volume. Alzheimers Res Ther. 2019;11(1):109. doi: 10.1186/s13195-019-0565-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vila‐Castelar C, Tariot PN, Sink KM, et al. Sex differences in cognitive resilience in preclinical autosomal‐dominant Alzheimer's disease carriers and non‐carriers: baseline findings from the API ADAD Colombia trial. Alzheimers Dement. 2022;18(11):2272‐2282. doi: 10.1002/alz.12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ardekani BA, Hadid SA, Blessing E, Bachman AH. Sexual dimorphism and hemispheric asymmetry of hippocampal volumetric integrity in normal aging and Alzheimer disease. AJNR Am J Neuroradiol. 2019;40(2):276‐282. doi: 10.3174/ajnr.A5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Koran MEI, Wagener M, Hohman TJ. Alzheimer's neuroimaging initiative. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017;11(1):205‐213. doi: 10.1007/s11682-016-9523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Caldwell JZK, Berg JL, Cummings JL, Banks SJ. Alzheimer's disease neuroimaging initiative. Moderating effects of sex on the impact of diagnosis and amyloid positivity on verbal memory and hippocampal volume. Alzheimer's Research & Therapy. 2017;9(1):72. doi: 10.1186/s13195-017-0300-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li X, Zhou S, Zhu W, et al. Sex difference in network topology and education correlated with sex difference in cognition during the disease process of Alzheimer. Front Aging Neurosci. 2021;13:639529. Accessed June 21, 2023. https://www.frontiersin.org/articles/10.3389/fnagi.2021.639529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ossenkoppele R, Lyoo CH, Jester‐Broms J, et al. Assessment of demographic, genetic, and imaging variables associated with brain resilience and cognitive resilience to pathological tau in patients with Alzheimer disease. JAMA Neurol. 2020;77(5):632‐642. doi: 10.1001/jamaneurol.2019.5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Perneczky R, Diehl‐Schmid J, Förstl H, Drzezga A, Kurz A. Male gender is associated with greater cerebral hypometabolism in frontotemporal dementia: evidence for sex‐related cognitive reserve. Int J Geriatr Psychiatry. 2007;22(11):1135‐1140. doi: 10.1002/gps.1803 [DOI] [PubMed] [Google Scholar]
- 63. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 64. Ferretti MT, Martinkova J, Biskup E, et al. Sex and gender differences in Alzheimer's disease: current challenges and implications for clinical practice. Eur J Neurol. 2020;27(6):928‐943. doi: 10.1111/ene.14174 [DOI] [PubMed] [Google Scholar]
- 65. Mielke MM. Sex and gender differences in Alzheimer's disease dementia. Psychiatr Times. 2018;35(11):14‐17. [PMC free article] [PubMed] [Google Scholar]
- 66. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37‐48. doi: 10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bartrés‐Faz D, Arenaza‐Urquijo E, Ewers M, et al. Theoretical frameworks and approaches used within the reserve, resilience and protective factors professional interest area of the Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment. Alzheimers Dement (Amst). 2020;12(1):e12115. doi: 10.1002/dad2.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, Bennett DA. Education and cognitive reserve in old age. Neurology. 2019;92(10):e1041‐e1050. doi: 10.1212/WNL.0000000000007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Singh‐Manoux A, Marmot MG, Glymour M, Sabia S, Kivimäki M, Dugravot A. Does cognitive reserve shape cognitive decline? Ann Neurol. 2011;70(2):296‐304. doi: 10.1002/ana.22391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hanes DW, Clouston SAP. Cognitive aging in same‐ and different‐sex relationships: comparing age of diagnosis and rate of cognitive decline in the health and retirement study. Gerontology. 2023;69(3):356‐369. doi: 10.1159/000526922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manly JJ, Schupf N, Tang MX, Stern Y. Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol. 2005;18(4):213‐217. doi: 10.1177/0891988705281868 [DOI] [PubMed] [Google Scholar]
- 72. Avila JF, Rentería MA, Jones RN, et al. Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimers Dement. 2021;17(1):70‐80. doi: 10.1002/alz.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer's disease: a call to action. Alzheimers Dement. 2018;14(9):1171‐1183. doi: 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ross CE, Mirowsky J. Sex differences in the effect of education on depression: resource multiplication or resource substitution? Soc Sci Med. 2006;63(5):1400‐1413. doi: 10.1016/j.socscimed.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 75. Bloomberg M, Dugravot A, Dumurgier J, et al. Sex differences and the role of education in cognitive ageing: analysis of two UK‐based prospective cohort studies. Lancet Public Health. 2021;6(2):e106‐e115. doi: 10.1016/S2468-2667(20)30258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weber D, Skirbekk V, Freund I, Herlitz A. The changing face of cognitive gender differences in Europe. Proc Natl Acad Sci USA. 2014;111(32):11673‐11678. doi: 10.1073/pnas.1319538111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lipnicki DM, Crawford JD, Dutta R, et al. Age‐related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med. 2017;14(3):e1002261. doi: 10.1371/journal.pmed.1002261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bonsang E, Skirbekk V, Staudinger UM. As you sow, so shall you reap: gender‐role attitudes and late‐life cognition. Psychol Sci. 2017;28(9):1201‐1213. doi: 10.1177/0956797617708634 [DOI] [PubMed] [Google Scholar]
- 79. Seney ML, Sibille E. Sex differences in mood disorders: perspectives from humans and rodent models. Biol Sex Differ. 2014;5(1):17. doi: 10.1186/s13293-014-0017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18(10):1413‐1420. doi: 10.1038/nn.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rainville JR, Hodes GE. Inflaming sex differences in mood disorders. Neuropsychopharmacology. 2019;44(1):184‐199. doi: 10.1038/s41386-018-0124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. SteelFisher GK, Findling MG, Bleich SN, et al. Gender discrimination in the United States: experiences of women. Health Serv Res. 2019;54(suppl 2):1442‐1453. doi: 10.1111/1475-6773.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gimson A, Schlosser M, Huntley JD, Marchant NL. Support for midlife anxiety diagnosis as an independent risk factor for dementia: a systematic review. BMJ Open. 2018;8(4):e019399. doi: 10.1136/bmjopen-2017-019399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. John A, Patel U, Rusted J, Richards M, Gaysina D. Affective problems and decline in cognitive state in older adults: a systematic review and meta‐analysis. Psychol Med. 2019;49(3):353‐365. doi: 10.1017/S0033291718001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323‐331. doi: 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marioni RE, Proust‐Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20‐year prospective cohort study. BMC Public Health. 2015;15(1):1089. doi: 10.1186/s12889-015-2426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Seney ML, Sibille E. Sex differences in mood disorders: perspectives from humans and rodent models. Biol Sex Differ. 2014;5(1):1‐10. doi: 10.1186/S13293-014-0017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marchant NL, Howard RJ. Cognitive debt and Alzheimer's disease. J Alzheimers Dis. 2015;44(3):755‐770. doi: 10.3233/JAD-141515 [DOI] [PubMed] [Google Scholar]
- 89. Strikwerda‐Brown C. Psychological well‐being: a new target for dementia prevention? Neurology. 2022;99(13):547‐548. doi: 10.1212/WNL.0000000000201110 [DOI] [PubMed] [Google Scholar]
- 90. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626‐638. doi: 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 91. Meisinger C, Thorand B, Schneider A, Stieber J, Döring A, Löwel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med. 2002;162(1):82‐89. doi: 10.1001/archinte.162.1.82 [DOI] [PubMed] [Google Scholar]
- 92. Mollayeva T, Mollayeva S, Colantonio A. Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat Rev Neurol. 2018;14(12):711‐722. doi: 10.1038/s41582-018-0091-y [DOI] [PubMed] [Google Scholar]
- 93. Sindi S, Kåreholt I, Ngandu T, et al. Sex differences in dementia and response to a lifestyle intervention: evidence from Nordic population‐based studies and a prevention trial. Alzheimer's & Dementia. 2021;17(7):1166. doi: 10.1002/ALZ.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Soriano‐Maldonado A, Aparicio VA, Félix‐Redondo FJ, Fernández‐Bergés D. Severity of obesity and cardiometabolic risk factors in adults: sex differences and role of physical activity. The HERMEX study. Int J Cardiol. 2016;223:352‐359. doi: 10.1016/j.ijcard.2016.07.253 [DOI] [PubMed] [Google Scholar]
- 95. Ruixing Y, Jinzhen W, Shangling P, Weixiong L, Dezhai Y, Yuming C. Sex differences in environmental and genetic factors for hypertension. Am J Med. 2008;121(9):811‐819. doi: 10.1016/j.amjmed.2008.04.026 [DOI] [PubMed] [Google Scholar]
- 96. Remsberg KE, Rogers NL, Demerath EW, et al. Sex differences in young adulthood metabolic syndrome and physical activity: the Fels longitudinal study. Am J Hum Biol. 2007;19(4):544‐550. doi: 10.1002/ajhb.20615 [DOI] [PubMed] [Google Scholar]
- 97. Miller TM, Gilligan S, Herlache LL, Regensteiner JG. Sex differences in cardiovascular disease risk and exercise in type 2 diabetes. J Investig Med. 2012;60(4):664‐670. doi: 10.2310/JIM.0B013E31824B2DE6 [DOI] [PubMed] [Google Scholar]
- 98. Lovejoy JC, Sainsbury A, Stock Conference 2008 Working Group . Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10(2):154‐167. doi: 10.1111/j.1467-789X.2008.00529.x [DOI] [PubMed] [Google Scholar]
- 99. Pugliese DN, Booth JN, Deng L, et al. Sex differences in masked hypertension: the Coronary Artery Risk Development in Young Adults study. J Hypertens. 2019;37(12):2380‐2388. doi: 10.1097/HJH.0000000000002175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bolego C, Rossoni G, Fadini GP, et al. Selective estrogen receptor‐α agonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilization in the absence of uterotrophic action. FASEB J. 2010;24(7):2262‐2272. doi: 10.1096/FJ.09-139220 [DOI] [PubMed] [Google Scholar]
- 101. Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29(3):289‐295. doi: 10.1161/atvbaha.108.182279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. McEniery CM. Transitioning the menopause. Arterioscler Thromb Vasc Biol. 2020;40:850‐852. doi: 10.1161/ATVBAHA.120.313980 [DOI] [PubMed] [Google Scholar]
- 103. Samargandy S, Matthews KA, Brooks MM, et al. Arterial stiffness accelerates within 1 year of the final menstrual period: the SWAN heart study. Arterioscler Thromb Vasc Biol. 2020;40(4):1001‐1008. doi: 10.1161/ATVBAHA.119.313622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fatih N, Chaturvedi N, Lane CA, et al. Sex‐related differences in whole brain volumes at age 70 in association with hyperglycemia during adult life. Neurobiol Aging. 2021;112:161‐169. doi: 10.1016/J.NEUROBIOLAGING.2021.09.008 [DOI] [PubMed] [Google Scholar]
- 105. Anstey KJ, Peters R, Mortby ME, et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population‐based cohort spanning 20‐76 years. Sci Rep. 2021;11(1):7710. doi: 10.1038/s41598-021-86397-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mielke GI. Relevance of life course epidemiology for research on physical activity and sedentary behavior. J Phys Act Health. 2022;19(4):225‐226. doi: 10.1123/jpah.2022-0128 [DOI] [PubMed] [Google Scholar]
- 107. Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu‐Ambrose T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta‐analysis of randomized controlled trials in older humans. Front Neuroendocrinol. 2017;46:71‐85. doi: 10.1016/j.yfrne.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 108. Dogra S, Ashe MC, Biddle SJH, et al. Sedentary time in older men and women: an international consensus statement and research priorities. Br J Sports Med. 2017;51(21):1526‐1532. doi: 10.1136/bjsports-2016-097209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Casaletto KB, Lindbergh CA, VandeBunte A, et al. Microglial Correlates of Late Life Physical Activity: relationship with Synaptic and Cognitive Aging in Older Adults. J Neurosci. 2022;42(2):288‐298. doi: 10.1523/JNEUROSCI.1483-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stern Y, Lee S, Predovan D, P Sloan R. Sex Moderates the Effect of Aerobic Exercise on Some Aspects of Cognition in Cognitively Intact Younger and Middle‐Age Adults. J Clin Med. 2019;8(6):886. doi: 10.3390/jcm8060886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. van Ballegooijen AJ, van der Ploeg HP, Visser M. Daily sedentary time and physical activity as assessed by accelerometry and their correlates in older adults. Eur Rev Aging Phys Act. 2019;16(1):3. doi: 10.1186/s11556-019-0210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chen T, Kishimoto H, Honda T, et al. Patterns and levels of sedentary behavior and physical activity in a general Japanese population: the Hisayama Study. J Epidemiol. 2018;28(5):260‐265. doi: 10.2188/jea.JE20170012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hohman TJ, Dumitrescu L, Barnes LL, et al. Sex‐specific association of apolipoprotein E With cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989‐998. doi: 10.1001/jamaneurol.2018.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sampedro F, Vilaplana E, de Leon MJ, et al. APOE‐by‐sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget. 2015;6(29):26663‐26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dumitrescu L, Mayeda ER, Sharman K, Moore AM, Hohman TJ. Sex differences in the genetic architecture of Alzheimer's disease. Curr Genet Med Rep. 2019;7(1):13‐21. doi: 10.1007/s40142-019-0157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Deming Y, Dumitrescu L, Barnes LL, et al. Sex‐specific genetic predictors of Alzheimer's disease biomarkers. Acta Neuropathol. 2018;136(6):857‐872. doi: 10.1007/s00401-018-1881-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Oliva M, Muñoz‐Aguirre M, Kim‐Hellmuth S, et al. The impact of sex on gene expression across human tissues. Science. 2020;369(6509):eaba3066. doi: 10.1126/science.aba3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Eissman JM, Dumitrescu L, Mahoney ER, et al. Sex differences in the genetic architecture of cognitive resilience to Alzheimer's disease. Brain. 2022;145(7):2541‐2554. doi: 10.1093/brain/awac177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Voskuhl R. Sex differences in autoimmune diseases. Biol Sex Differ. 2011;2(1):1. doi: 10.1186/2042-6410-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Voskuhl RR. The effect of sex on multiple sclerosis risk and disease progression. Mult Scler. 2020;26(5):554‐560. doi: 10.1177/1352458519892491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Grässler B, Dordevic M, Herold F, et al. Relationship between resting state heart rate variability and sleep quality in older adults with mild cognitive impairment. Int J Environ Res Public Health. 2021;18(24):13321. doi: 10.3390/ijerph182413321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Umetani K, Singer DH, McCraty R, Atkinson M. Twenty‐four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31(3):593‐601. doi: 10.1016/s0735-1097(97)00554-8 [DOI] [PubMed] [Google Scholar]
- 123. Umetani K, Duda CL CL, Singer DH. Aging Effects on Cycle Length Dependence of Heart Rate Variability: Proceedings of the 1996 Fifteenth Southern Biomedical Engineering Conference, IEEE Conference Publication, Dayton, OH, USA, 29‐31 March, 1996; 361‐364. doi: 10.1109/SBEC.1996.493226 I. [DOI] [Google Scholar]
- 124. McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. JNP. 2003;15(2):161‐167. doi: 10.1176/jnp.15.2.161 [DOI] [PubMed] [Google Scholar]
- 125. Ryan J, Carrière I, Scali J, Ritchie K, Ancelin ML. Life‐time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34(2):287‐298. doi: 10.1016/j.psyneuen.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 126. Ryan J, Scali J, Carrière I, et al. Impact of a premature menopause on cognitive function in later life. BJOG. 2014;121(13):1729‐1739. doi: 10.1111/1471-0528.12828 [DOI] [PubMed] [Google Scholar]
- 127. Yurov YB, Vorsanova SG, Liehr T, Kolotii AD, Iourov IY. X chromosome aneuploidy in the Alzheimer's disease brain. Molecular Cytogenetics. 2014;7(1):20. doi: 10.1186/1755-8166-7-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bajic VP, Essack M, Zivkovic L, et al. The X files: “The Mystery of X Chromosome Instability in Alzheimer's Disease.” Front Genet. 2020;10. Accessed June 22, 2023. https://www.frontiersin.org/articles/10.3389/fgene.2019.01368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ritchie SJ, Cox SR, Shen X, et al. Sex differences in the adult human brain: evidence from 5216 UK Biobank participants. Cereb Cortex. 2018;28(8):2959‐2975. doi: 10.1093/cercor/bhy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Weis S, Patil KR, Hoffstaedter F, Nostro A, Yeo BTT, Eickhoff SB. Sex classification by resting state brain connectivity. Cereb Cortex. 2020;30(2):824‐835. doi: 10.1093/cercor/bhz129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang N, Fan FM, Huang SY, Rodriguez MA. Mindfulness training for loneliness among Chinese college students: a pilot randomized controlled trial. Int J Psychol. 2018;53(5):373‐378. doi: 10.1002/ijop.12394 [DOI] [PubMed] [Google Scholar]
- 132. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847‐855. doi: 10.1016/j.biopsych.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Davison Ankney C. Sex differences in relative brain size: the mismeasure of woman, too? Intelligence. 1992;16(3):329‐336. doi: 10.1016/0160-2896(92)90013-H [DOI] [Google Scholar]
- 134. Rushton JP, Ankney CD. Whole brain size and general mental ability: a review. Int J Neurosci. 2009;119(5):692‐732. doi: 10.1080/00207450802325843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006;129(2):386‐398. doi: 10.1093/brain/awh696 [DOI] [PubMed] [Google Scholar]
- 136. Williams CM, Peyre H, Toro R, Ramus F. Neuroanatomical norms in the UK Biobank: the impact of allometric scaling, sex, and age. Hum Brain Mapp. 2021;42(14):4623‐4642. doi: 10.1002/hbm.25572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. van der Linden D, Dunkel CS, Madison G. Sex differences in brain size and general intelligence (g). Intelligence. 2017;63:78‐88. doi: 10.1016/J.INTELL.2017.04.007 [DOI] [Google Scholar]
- 138. Sang F, Chen Y, Chen K, Dang M, Gao S, Zhang Z. Sex differences in cortical morphometry and white matter microstructure during brain aging and their relationships to cognition. Cereb Cortex. 2021;31(11):5253‐5262. doi: 10.1093/cercor/bhab155 [DOI] [PubMed] [Google Scholar]
- 139. Ingalhalikar M, Smith A, Parker D, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA. 2014;111(2):823‐828. doi: 10.1073/pnas.1316909110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tunç B, Solmaz B, Parker D, et al. Establishing a link between sex‐related differences in the structural connectome and behaviour. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150111. doi: 10.1098/rstb.2015.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lawrence KE, Nabulsi L, Santhalingam V, et al. Age and sex effects on advanced white matter microstructure measures in 15,628 older adults: a UK biobank study. Brain Imaging Behav. 2021;15(6):2813‐2823. doi: 10.1007/s11682-021-00548-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Perry A, Wen W, Lord A, et al. The organisation of the elderly connectome. Neuroimage. 2015;114:414‐426. doi: 10.1016/j.neuroimage.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 143. Bluhm RL, Osuch EA, Lanius RA, et al. Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 2008;19(8):887‐891. doi: 10.1097/WNR.0b013e328300ebbf [DOI] [PubMed] [Google Scholar]
- 144. Jamadar SD, Sforazzini F, Raniga P, et al. Sexual dimorphism of resting‐state network connectivity in healthy ageing. J Gerontol B Psychol Sci Soc Sci. 2019;74(7):1121‐1131. doi: 10.1093/geronb/gby004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Allen EA, Erhardt EB, Damaraju E, et al. A baseline for the multivariate comparison of resting‐state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Buckley RF, Schultz AP, Hedden T, et al. Functional network integrity presages cognitive decline in preclinical Alzheimer disease. Neurology. 2017;89(1):29‐37. doi: 10.1212/WNL.0000000000004059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chhatwal JP, Schultz AP, Johnson K, et al. Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology. 2013;81(8):736‐744. doi: 10.1212/WNL.0b013e3182a1aafe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Arenaza‐Urquijo EM, Przybelski SA, Lesnick TL, et al. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain. 2019;142(4):1134‐1147. doi: 10.1093/brain/awz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Isaac Tseng WY, Hsu YC, Chen CL, et al. Microstructural differences in white matter tracts across middle to late adulthood: a diffusion MRI study on 7167 UK Biobank participants. Neurobiol Aging. 2021;98:160‐172. doi: 10.1016/j.neurobiolaging.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 150. Abel AN, Lloyd LK, Williams JS. The effects of regular yoga practice on pulmonary function in healthy individuals: a literature review. J Altern Complement Med. 2013;19(3):185‐190. doi: 10.1089/acm.2011.0516 [DOI] [PubMed] [Google Scholar]
- 151. Crivello F, Tzourio‐Mazoyer N, Tzourio C, Mazoyer B. Longitudinal assessment of global and regional rate of grey matter atrophy in 1,172 healthy older adults: modulation by sex and age. PLoS ONE. 2014;9(12):e114478. doi: 10.1371/journal.pone.0114478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hua X, Hibar DP, Lee S, et al. Sex and age differences in atrophic rates: an ADNI study with n = 1368 MRI scans. Neurobiol Aging. 2010;31(8):1463‐1480. doi: 10.1016/j.neurobiolaging.2010.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1):21‐36. doi: 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- 154. Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53(1):189‐196. [DOI] [PubMed] [Google Scholar]
- 155. Skup M, Zhu H, Wang Y, et al. Sex differences in grey matter atrophy patterns among AD and aMCI patients: results from ADNI. Neuroimage. 2011;56(3):890‐906. doi: 10.1016/j.neuroimage.2011.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Jiang J, Sachdev P, Lipnicki DM, et al. A longitudinal study of brain atrophy over two years in community‐dwelling older individuals. Neuroimage. 2014;86:203‐211. doi: 10.1016/j.neuroimage.2013.08.022 [DOI] [PubMed] [Google Scholar]
- 157. van Velsen EFS, Vernooij MW, Vrooman HA, et al. Brain cortical thickness in the general elderly population: the Rotterdam Scan Study. Neurosci Lett. 2013;550:189‐194. doi: 10.1016/j.neulet.2013.06.063 [DOI] [PubMed] [Google Scholar]
- 158. Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 2010;52(4):1215‐1223. doi: 10.1016/j.neuroimage.2010.04.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Boyle R, Jollans L, Rueda‐Delgado LM, et al. Brain‐predicted age difference score is related to specific cognitive functions: a multi‐site replication analysis. Brain Imaging Behav. 2021;15(1):327‐345. doi: 10.1007/s11682-020-00260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Smith SM, Vidaurre D, Alfaro‐Almagro F, Nichols TE, Miller KL. Estimation of brain age delta from brain imaging. Neuroimage. 2019;200:528‐539. doi: 10.1016/j.neuroimage.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cole JH, Ritchie SJ, Bastin ME, et al. Brain age predicts mortality. Mol Psychiatry. 2018;23(5):1385‐1392. doi: 10.1038/mp.2017.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Franke K, Gaser C, Manor B, Novak V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci. 2013;5. https://www.frontiersin.org/articles/10.3389/fnagi.2013.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Luders E, Cherbuin N, Gaser C. Estimating brain age using high‐resolution pattern recognition: younger brains in long‐term meditation practitioners. Neuroimage. 2016;134:508‐513. doi: 10.1016/j.neuroimage.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 164. Nobis L, Manohar SG, Smith SM, et al. Hippocampal volume across age: nomograms derived from over 19,700 people in UK Biobank. NeuroImage: Clinical. 2019;23:101904. doi: 10.1016/j.nicl.2019.101904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J Alzheimers Dis. 2016;50(3):847‐857. doi: 10.3233/JAD-150780 [DOI] [PubMed] [Google Scholar]
- 166. Goyal MS, Blazey TM, Su Y, et al. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci. 2019;116(8):3251‐3255. doi: 10.1073/pnas.1815917116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Malpetti M, Ballarini T, Presotto L, et al. Gender differences in healthy aging and Alzheimer's dementia: a 18 F‐FDG‐PET study of brain and cognitive reserve. Hum Brain Mapp. 2017;38(8):4212‐4227. doi: 10.1002/hbm.23659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Scheinost D, Finn ES, Tokoglu F, et al. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp. 2015;36(4):1524‐1535. doi: 10.1002/hbm.22720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Varma VR, Tang X, Carlson MC. Hippocampal sub‐regional shape and physical activity in older adults. Hippocampus. 2016;26(8):1051‐1060. doi: 10.1002/hipo.22586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Barha CK, Best JR, Rosano C, Yaffe K, Catov JM, Liu‐Ambrose T. Sex‐Specific relationship between long‐term maintenance of physical activity and cognition in the Health ABC study: potential role of hippocampal and dorsolateral prefrontal cortex volume. J Gerontol A Biol Sci Med Sci. 2020;75(4):764‐770. doi: 10.1093/gerona/glz093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Heger IS, Deckers K, Schram MT, et al. Associations of the lifestyle for brain health index with structural brain changes and cognition: results from the maastricht study. Neurology. 2021;97(13):e1300‐e1312. doi: 10.1212/WNL.0000000000012572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hoekzema E, Barba‐Müller E, Pozzobon C, et al. Pregnancy leads to long‐lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287‐296. doi: 10.1038/nn.4458 [DOI] [PubMed] [Google Scholar]
- 173. Mielke MM, Milic NM, Weissgerber TL, et al. Impaired cognition and brain atrophy decades after hypertensive pregnancy disorders. Circ Cardiovasc Qual Outcomes. 2016;9(2)(suppl 1):S70‐S76. doi: 10.1161/CIRCOUTCOMES.115.002461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Altmann A, Tian L, Henderson VW, Greicius MD. Alzheimer's disease neuroimaging initiative investigators. Sex modifies the APOE‐related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563‐573. doi: 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid‐beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122‐131. doi: 10.1002/ana.21843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's disease. Alzheimer's Research & Therapy. 2019;11(1):34. doi: 10.1186/s13195-019-0485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Schoonenboom NSM, Pijnenburg YaL, Mulder C, et al. Amyloid beta(1‐42) and phosphorylated tau in CSF as markers for early‐onset Alzheimer disease. Neurology. 2004;62(9):1580‐1584. doi: 10.1212/01.wnl.0000123249.58898.e0 [DOI] [PubMed] [Google Scholar]
- 178. Brickman AM, Manly JJ, Honig LS, et al. Plasma p‐tau181, p‐tau217, and other blood‐based Alzheimer's disease biomarkers in a multi‐ethnic, community study. Alzheimers Dement. 2021;17(8):1353‐1364. doi: 10.1002/alz.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Vergallo A, Mégret L, Lista S, et al. Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer's disease. Alzheimers Dement. 2019;15(6):764‐775. doi: 10.1016/j.jalz.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 180. Keshavan A, Pannee J, Karikari TK, et al. Population‐based blood screening for preclinical Alzheimer's disease in a British birth cohort at age 70. Brain. 2021;144(2):434‐449. doi: 10.1093/brain/awaa403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Liesinger AM, Graff‐Radford NR, Duara R, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer's disease. Acta Neuropathol. 2018;136(6):873‐885. doi: 10.1007/s00401-018-1908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer's disease and common neuropathologies of aging. Acta Neuropathol. 2018;136(6):887‐900. doi: 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk: brain imaging of endocrine vs chronologic aging. Neurology. 2017;89(13):1382‐1390. doi: 10.1212/WNL.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Rahman A, Schelbaum E, Hoffman K, et al. Sex‐driven modifiers of Alzheimer risk: a multimodality brain imaging study. Neurology. 2020;95(2):e166‐e178. doi: 10.1212/WNL.0000000000009781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76(5):542‐551. doi: 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wisch JK, Meeker KL, Gordon BA, et al. Sex‐related differences in tau positron emission tomography (PET) and the effects of hormone therapy (HT). Alzheimer Dis Assoc Disord. 2021;35(2):164‐168. doi: 10.1097/WAD.0000000000000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Pereira JB, Harrison TM, La Joie R, Baker SL, Jagust WJ. Spatial patterns of tau deposition are associated with amyloid, ApoE, sex, and cognitive decline in older adults. Eur J Nucl Med Mol Imaging. 2020;47(9):2155‐2164. doi: 10.1007/s00259-019-04669-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Palta P, Page G, Piferi RL, et al. Evaluation of a mindfulness‐based intervention program to decrease blood pressure in low‐income African‐American older adults. J Urban Health. 2012;89(2):308‐316. doi: 10.1007/s11524-011-9654-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685‐691. doi: 10.1001/archpsyc.62.6.685 [DOI] [PubMed] [Google Scholar]
- 190. Edwards L, La Joie R, Iaccarino L, et al. Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer's continuum: greater tau‐PET retention in females. Neurobiol Aging. 2021;105:86‐98. doi: 10.1016/j.neurobiolaging.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Buckley RF, Scott MR, Jacobs HIL, et al. Sex mediates relationships between regional tau pathology and cognitive decline. Ann Neurol. 2020;88(5):921‐932. doi: 10.1002/ana.25878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Li G, Shofer JB, Petrie EC, et al. Cerebrospinal fluid biomarkers for Alzheimer's and vascular disease vary by age, gender, and APOE genotype in cognitively normal adults. Alzheimers Res Ther. 2017;9(1):48. doi: 10.1186/s13195-017-0271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Damoiseaux JS, Seeley WW, Zhou J, et al. Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32(24):8254‐8262. doi: 10.1523/JNEUROSCI.0305-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tsiknia AA, Edland SD, Sundermann EE, et al. Sex differences in plasma p‐tau181 associations with Alzheimer's disease biomarkers, cognitive decline, and clinical progression. Mol Psychiatry. 2022;27(10):4314‐4322. doi: 10.1038/s41380-022-01675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Baldacci F, Lista S, Manca ML, et al. Age and sex impact plasma NFL and t‐Tau trajectories in individuals with subjective memory complaints: a 3‐year follow‐up study. Alzheimers Res Ther. 2020;12:147. doi: 10.1186/s13195-020-00704-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Mielke MM, Syrjanen JA, Blennow K, et al. Comparison of variables associated with CSF neurofilament, total‐tau, and neurogranin. Alzheimers Dement. 2019;15(11):1437‐1447. doi: 10.1016/j.jalz.2019.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta‐analysis. JAMA Neurol. 2019;76(9):1035‐1048. doi: 10.1001/jamaneurol.2019.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ramanan VK, Castillo AM, Knopman DS, et al. Association of apolipoprotein e ɛ4, educational level, and sex with tau deposition and tau‐mediated metabolic dysfunction in older adults. JAMA Netw Open. 2019;2(10):e1913909. doi: 10.1001/jamanetworkopen.2019.13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Vila‐Castelar C, Chen Y, Langella S, et al. Sex differences in blood biomarkers and cognitive performance in individuals with autosomal dominant Alzheimer's disease. Alzheimer's & Dementia. 2023;19(9):4127‐4138. doi: 10.1002/alz.13314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Casaletto KB, Nichols E, Aslanyan V, et al. Sex‐specific effects of microglial activation on Alzheimer's disease proteinopathy in older adults. Brain. 2022;145(10):3536‐3545. doi: 10.1093/brain/awac257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Mathys H, Davila‐Velderrain J, Peng Z, et al. Single‐cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570(7761):332‐337. doi: 10.1038/s41586-019-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Jaber SM, Bordt EA, Bhatt NM, et al. Sex differences in the mitochondrial bioenergetics of astrocytes but not microglia at a physiologically relevant brain oxygen tension. Neurochem Int. 2018;117:82‐90. doi: 10.1016/j.neuint.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol. 2008;511(5):599‐609. doi: 10.1002/cne.21859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mouton PR, Long JM, Lei DL, et al. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956(1):30‐35. doi: 10.1016/s0006-8993(02)03475-3 [DOI] [PubMed] [Google Scholar]
- 205. Richetin K, Petsophonsakul P, Roybon L, Guiard BP, Rampon C. Differential alteration of hippocampal function and plasticity in females and males of the APPxPS1 mouse model of Alzheimer's disease. Neurobiol Aging. 2017;57:220‐231. doi: 10.1016/j.neurobiolaging.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 206. Oliveira‐Pinto AV, Santos RM, Coutinho RA, et al. Sexual dimorphism in the human olfactory bulb: females have more neurons and glial cells than males. PLoS ONE. 2014;9(11):e111733. doi: 10.1371/journal.pone.0111733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Mangold CA, Wronowski B, Du M, et al. Sexually divergent induction of microglial‐associated neuroinflammation with hippocampal aging. J Neuroinflammation. 2017;14(1):141. doi: 10.1186/s12974-017-0920-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Lynch MA. Exploring sex‐related differences in microglia may be a game‐changer in precision medicine. Front Aging Neurosci. 2022;14:868448. doi: 10.3389/fnagi.2022.868448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Brawek B, Skok M, Garaschuk O. Changing functional signatures of microglia along the axis of brain aging. Int J Mol Sci. 2021;22(3):1091. doi: 10.3390/ijms22031091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Guneykaya D, Ivanov A, Hernandez DP, et al. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 2018;24(10):2773‐2783.e6. doi: 10.1016/j.celrep.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 211. Guillot‐Sestier MV, Araiz AR, Mela V, et al. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer's disease. Commun Biol. 2021;4(1):711. doi: 10.1038/s42003-021-02259-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Sala Frigerio C, Wolfs L, Fattorelli N, et al. The major risk factors for Alzheimer's disease: age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep. 2019;27(4):1293‐1306.e6. doi: 10.1016/j.celrep.2019.03.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Bonham LW, Sirkis DW, Yokoyama JS. The transcriptional landscape of microglial genes in aging and neurodegenerative disease. Front Immunol. 2019;10:1170. doi: 10.3389/fimmu.2019.01170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Vidal‐Itriago A, Radford RAW, Aramideh JA, et al. Microglia morphophysiological diversity and its implications for the CNS. Front Immunol. 2022;13:997786. doi: 10.3389/fimmu.2022.997786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Insel PS, Mohlenhoff BS, Neylan TC, Krystal AD, Mackin RS. Association of sleep and β‐amyloid pathology among older cognitively unimpaired adults. JAMA Netw Open. 2021;4(7):e2117573. doi: 10.1001/jamanetworkopen.2021.17573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Thirunavu V, McCullough A, Su Y, et al. Higher body mass index is associated with lower cortical amyloid‐β burden in cognitively normal individuals in late‐life. J Alzheimers Dis. 2019;69(3):817‐827. doi: 10.3233/JAD-190154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Tsiknia AA, Reas E, Bangen KJ, et al. Sex and APOE ɛ4 modify the effect of cardiovascular risk on tau in cognitively normal older adults. Brain Commun. 2022;4(1):fcac035. doi: 10.1093/braincomms/fcac035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person‐specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74‐83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Boyle PA, Wang T, Yu L, et al. To what degree is late life cognitive decline driven by age‐related neuropathologies? Brain. 2021;144(7):2166‐2175. doi: 10.1093/brain/awab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer's disease, and dementia in older persons. Biochim Biophys Acta. 2016;1862(5):878‐886. doi: 10.1016/j.bbadis.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Robinson JL, Corrada MM, Kovacs GG, et al. Non‐Alzheimer's contributions to dementia and cognitive resilience in the 90+ Study. Acta Neuropathol. 2018;136(3):377‐388. doi: 10.1007/s00401-018-1872-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Buciuc M, Whitwell JL, Tosakulwong N, et al. Association between transactive response DNA‐binding protein of 43 kDa type and cognitive resilience to Alzheimer's disease: a case‐control study. Neurobiol Aging. 2020;92:92‐97. doi: 10.1016/j.neurobiolaging.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Latimer CS, Burke BT, Liachko NF, et al. Resistance and resilience to Alzheimer's disease pathology are associated with reduced cortical pTau and absence of limbic‐predominant age‐related TDP‐43 encephalopathy in a community‐based cohort. Acta Neuropathol Commun. 2019;7(1):91. doi: 10.1186/s40478-019-0743-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Barnes LL, Lamar M, Schneider JA. Sex differences in mixed neuropathologies in community‐dwelling older adults. Brain Res. 2019;1719:11‐16. doi: 10.1016/j.brainres.2019.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatr. 2001;70(1):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Fatemi F, Kantarci K, Graff‐Radford J, et al. Sex differences in cerebrovascular pathologies on FLAIR in cognitively unimpaired elderly. Neurology. 2018;90(6):e466‐e473. doi: 10.1212/WNL.0000000000004913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30(6):946‐956. doi: 10.1016/j.neurobiolaging.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 228. Lohner V, Pehlivan G, Sanroma G, et al. Relation between sex, menopause, and white matter hyperintensities: the Rhineland Study. Neurology. 2022;99(9):e935‐e943. doi: 10.1212/WNL.0000000000200782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Scharf EL, Graff‐Radford J, Przybelski SA, et al. Cardiometabolic health and longitudinal progression of white matter hyperintensity: the Mayo Clinic Study of Aging. Stroke. 2019;50(11):3037‐3044. doi: 10.1161/STROKEAHA.119.025822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Alqarni A, Jiang J, Crawford JD, et al. Sex differences in risk factors for white matter hyperintensities in non‐demented older individuals. Neurobiol Aging. 2021;98:197‐204. doi: 10.1016/j.neurobiolaging.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 231. Vemuri P, Lesnick TG, Knopman DS, et al. Amyloid, vascular, and resilience pathways associated with cognitive aging. Ann Neurol. 2019;86(6):866‐877. doi: 10.1002/ana.25600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26(5):1439‐1447. doi: 10.1523/JNEUROSCI.2219-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Han F, Zhai FF, Wang Q, et al. Prevalence and risk factors of cerebral small vessel disease in a Chinese population‐based sample. J Stroke. 2018;20(2):239‐246. doi: 10.5853/jos.2017.02110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population‐based AGES‐Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79(9):1002‐1006. doi: 10.1136/jnnp.2007.121913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Ramirez J, Berezuk C, McNeely AA, Scott CJM, Gao F, Black SE. Visible Virchow‐Robin spaces on magnetic resonance imaging of Alzheimer's disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimers Dis. 2015;43(2):415‐424. doi: 10.3233/JAD-132528 [DOI] [PubMed] [Google Scholar]
- 236. Maclullich AMJ, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75(11):1519‐1523. doi: 10.1136/jnnp.2003.030858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ding J, Sigurðsson S, Jónsson PV, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the age, gene/environment susceptibility‐Reykjavik Study. JAMA Neurol. 2017;74(9):1105‐1112. doi: 10.1001/jamaneurol.2017.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res. 2022;130(4):512‐528. doi: 10.1161/CIRCRESAHA.121.319915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Di Carlo A, Lamassa M, Consoli D, et al. Sex differences in presentation, severity, and management of stroke in a population‐based study. Neurology. 2010;75(7):670‐671. doi: 10.1212/WNL.0b013e3181ec68b5. author reply 671. [DOI] [PubMed] [Google Scholar]
- 240. Etherton MR, Wu O, Cougo P, et al. Sex‐specific differences in white matter microvascular integrity after ischaemic stroke. Stroke Vasc Neurol. 2019;4(4):198‐205. doi: 10.1136/svn-2019-000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Etherton MR, Wu O, Cougo P, et al. Structural integrity of normal appearing white matter and sex‐specific outcomes after acute ischemic stroke. Stroke. 2017;48(12):3387‐3389. doi: 10.1161/STROKEAHA.117.019258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Raghavan S, Graff‐Radford J, Scharf E, et al. Study of symptomatic vs. silent brain infarctions on MRI in elderly subjects. Front Neurol. 2021;12:615024. doi: 10.3389/fneur.2021.615024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Price TR, Manolio TA, Kronmal RA, et al, CHS Collaborative Research Group . Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community‐dwelling older adults. The cardiovascular health study. Stroke. 1997;28(6):1158‐1164. doi: 10.1161/01.str.28.6.1158 [DOI] [PubMed] [Google Scholar]
- 244. Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18(4):437‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. van de Beek M, Babapour Mofrad R, van Steenoven I, et al. Sex‐specific associations with cerebrospinal fluid biomarkers in dementia with Lewy bodies. Alzheimers Res Ther. 2020;12(1):44. doi: 10.1186/s13195-020-00610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Nelson PT, Schmitt FA, Jicha GA, et al. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol. 2010;257(11):1875‐1881. doi: 10.1007/s00415-010-5630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Agrawal S, Yu L, Nag S, et al. The association of Lewy bodies with limbic‐predominant age‐related TDP‐43 encephalopathy neuropathologic changes and their role in cognition and Alzheimer's dementia in older persons. Acta Neuropathol Commun. 2021;9(1):156. doi: 10.1186/s40478-021-01260-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Hebert R, Lehmann D. Theta bursts: an EEG pattern in normal subjects practising the transcendental meditation technique. Electroencephalogr Clin Neurophysiol. 1977;42(3):397‐405. [DOI] [PubMed] [Google Scholar]
- 249. Dubal DB. Sex difference in Alzheimer's disease: an updated, balanced and emerging perspective on differing vulnerabilities. Handb Clin Neurol. 2020;175:261‐273. doi: 10.1016/B978-0-444-64123-6.00018-7 [DOI] [PubMed] [Google Scholar]
- 250. Casaletto KB, Elahi FM, Staffaroni AM, et al. Cognitive aging is not created equally: differentiating unique cognitive phenotypes in “normal” adults. Neurobiol Aging. 2019;77:13‐19. doi: 10.1016/j.neurobiolaging.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Cerhan JR, Folsom AR, Mortimer JA, et al, Atherosclerosis Risk in Communities (ARIC) Study Investigators . Correlates of cognitive function in middle‐aged adults. Gerontology. 1998;44(2):95‐105. doi: 10.1159/000021991 [DOI] [PubMed] [Google Scholar]
- 252. Jack CR, Therneau TM, Wiste HJ, et al. Transition rates between amyloid and neurodegeneration biomarker states and to dementia: a population‐based, longitudinal cohort study. Lancet Neurol. 2016;15(1):56‐64. doi: 10.1016/S1474-4422(15)00323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Jack CR, Wiste HJ, Weigand SD, et al. Age‐specific and sex‐specific prevalence of cerebral β‐amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individals aged 50‐95 years: a cross‐sectional study. Lancet Neurol. 2017;16(6):435‐444. doi: 10.1016/S1474-4422(17)30077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Jack CR, Therneau TM, Weigand SD, et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the national institute on aging‐Alzheimer's association research framework. JAMA Neurol. 2019;76(10):1174‐1183. doi: 10.1001/jamaneurol.2019.1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889‐897. doi: 10.1212/WNL.0b013e3181f11d85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Davis EJ, Solsberg CW, White CC, et al. Sex‐specific association of the X chromosome with cognitive change and tau pathology in aging and Alzheimer disease. JAMA Neurol. 2021;78(10):1‐6. doi: 10.1001/jamaneurol.2021.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Skuse DH. X‐linked genes and mental functioning. Hum Mol Genet. 2005;14(1):R27‐R32. doi: 10.1093/hmg/ddi112 [DOI] [PubMed] [Google Scholar]
- 258. Vila‐Castelar C, Udeh‐Momoh C, Aggarwal NT, Mielke MM. Sex and gender considerations in dementia: a call for global research. Nat Aging. 2023;3(5):463‐465. doi: 10.1038/s43587-023-00374-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Alex L, Fjellman Wiklund A, Lundman B, Christianson M, Hammarström A. Beyond a dichotomous view of the concepts of “sex” and “gender” focus group discussions among gender researchers at a medical faculty. PLoS ONE. 2012;7(11):e50275. doi: 10.1371/journal.pone.0050275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Morgenroth T, Ryan MK. The effects of gender trouble: an integrative theoretical framework of the perpetuation and disruption of the gender/sex binary. Perspect Psychol Sci. 2021;16(6):1113‐1142. doi: 10.1177/1745691620902442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Tan JY, Baig AA, Chin MH. High stakes for the health of sexual and gender minority patients of color. J Gen Intern Med. 2017;32(12):1390‐1395. doi: 10.1007/s11606-017-4138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Ayhan CHB, Bilgin H, Uluman OT, Sukut O, Yilmaz S, Buzlu S. A systematic review of the discrimination against sexual and gender minority in health care settings. Int J Health Serv. 2020;50(1):44‐61. doi: 10.1177/0020731419885093 [DOI] [PubMed] [Google Scholar]
- 263. Flatt JD, Cicero EC, Kittle KR, Brennan‐Ing M. Recommendations for advancing research with sexual and gender minority older adults. J Gerontol B Psychol Sci Soc Sci. 2022;77(1):1‐9. doi: 10.1093/geronb/gbab127 [DOI] [PubMed] [Google Scholar]
- 264. Porac C. Are age trends in adult hand preference best explained by developmental shifts or generational differences? Can J Exp Psychol/Revue canadienne de psychologie expérimentale. 1993;47(4):697‐713. doi: 10.1037/h0078873 [DOI] [PubMed] [Google Scholar]
- 265. Coren S. Age and handedness: patterns of change in the population and sex differences become visible with increased statistical power. Can J Exp Psychol/Revue canadienne de psychologie expérimentale. 1995;49(3):376‐386. doi: 10.1037/1196-1961.49.3.376 [DOI] [PubMed] [Google Scholar]
- 266. Whisman MA, McClelland GH. Designing, testing, and interpreting interactions and moderator effects in family research. J Fam Psychol. 2005;19(1):111‐120. doi: 10.1037/0893-3200.19.1.111 [DOI] [PubMed] [Google Scholar]
- 267. Hedden T, Schultz AP, Rieckmann A, et al. Multiple brain markers are linked to age‐related variation in cognition. Cereb Cortex. 2016;26(4):1388‐1400. doi: 10.1093/cercor/bhu238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Mukherjee S, Choi SE, Lee ML, et al. Cognitive domain harmonization and cocalibration in studies of older adults. Neuropsychology. 2023;37(4):409‐423. doi: 10.1037/neu0000835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Hampton OL, Mukherjee S, Properzi MJ, et al. Harmonizing the preclinical Alzheimer cognitive composite for multicohort studies. Neuropsychology. 2023;37(4):436‐449. doi: 10.1037/neu0000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychol Bull. 1993;114(2):376‐390. doi: 10.1037/0033-2909.114.2.376 [DOI] [PubMed] [Google Scholar]
- 271. Salvatore JE, Cho SB, Dick DM. Genes, environments, and sex differences in alcohol research. J Stud Alcohol Drugs. 2017;78(4):494‐501. doi: 10.15288/jsad.2017.78.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Beltz AM, Beery AK, Becker JB. Analysis of sex differences in pre‐clinical and clinical data sets. Neuropsychopharmacology. 2019;44(13):2155‐2158. doi: 10.1038/s41386-019-0524-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


