Abstract
Many studies have documented racial, socioeconomic, geographic, and other disparities for United States (US) patients with multiple myeloma pertaining to diagnosis and frontline management. In contrast, very little is known about disparities in the management of relapsed/refractory multiple myeloma (RRMM) despite a plethora of novel treatment options. In this review, we discuss the manifestations of disparities in RRMM and strategies to mitigate their impact. Immunomodulatory drugs can create disparities on many axes, for example inappropriately low dosing due to Duffy-null status as well as time toxicity and financial toxicity from logistical hurdles for socioeconomically vulnerable patients. Access to myeloma expertise at high-volume centers is a critical consideration given the disconnect between how drugs like carfilzomib and dexamethasone are prescribed in trials versus optimized in real-world practice to lower toxicities. Disparities in chimeric antigen receptor T-cell therapy and bispecific antibody therapy span across racial, ethnic, and socioeconomic lines in large part due to their limited availability outside of high-volume centers. Another insidious source of disparities is supportive care in RRMM, ranging from inadequate pain control in Black patients to limited primary care provider access in rural settings. We discuss the rationales and evidence base for several solutions aimed at mitigating these disparities: for example, (1) bidirectional co-management with community-based oncologists, (2) screening for risk factors based on social determinants of health, (3) strategies to build patient trust with regard to clinical trials, and (4) longitudinal access to a primary care provider. As the treatment landscape for RRMM continues to expand, these types of efforts by the field will help ensure that this landscape is equally accessible and traversable for all US patients.
Subject terms: Health services, Myeloma
Introduction
The field of multiple myeloma (MM) has seen dramatic therapeutic advancements in the past decade, including the approvals of several novel immune effector cell (IEC) therapies such as chimeric antigen receptor T-cell (CAR-T) therapies and bispecific antibodies (bsAbs). For the average patient below age 70 diagnosed with MM today in the United States (US), a life expectancy of over 10 years is reasonable if one assumes optimal access to care. Unfortunately, the assumption of optimal care is not valid for many patients. There are many gaps in MM care created by disparities at the intersections of race, ethnicity, socioeconomic status (SES), Zonal Improvement Plan (ZIP) codes, and more. Underlying drivers of racial disparities in MM are unfortunately manifold: structural racism in healthcare delivery, bias or knowledge gaps among individual providers, mistrust in the healthcare system, and intersecting socioeconomic and geographic disparities [1–5]. The interdisciplinary expertise needed to implement cell-based therapies including autologous stem cell transplantation (ASCT) and CAR-T therapy means that such therapies are primarily offered only at academic centers in urban settings, limiting their access to patients without the means to relocate or the fortune of residing within a 30-min driving distance of a major treatment center [6].
As specific examples of disparities in MM, non-White patients are less likely to complete staging workup and must wait longer to begin modern induction regimens than White patients [7–9]. Patients who are treated for MM at higher-volume centers (typically located in urban areas) have been shown in several studies to have lower mortality than patients who are treated at lower-volume centers [10–13]. Access to and completion of frontline ASCT, a modality shown to extend progression-free survival (PFS) in newly diagnosed MM (NDMM), also varies significantly based on intersecting social determinants of health (SDOH) [14–17]. Within individual cities, patients with MM who reside in poorer neighborhoods have higher mortality than those who do not [18]. These disparities are unique to conventional healthcare systems and payment models rather than any intrinsic factors related to race, ethnicity, or ZIP code. In the Veterans Affairs (VA) healthcare system nationally where some of these inequalities are mitigated, for example, Black patients with MM actually have superior survival [19].
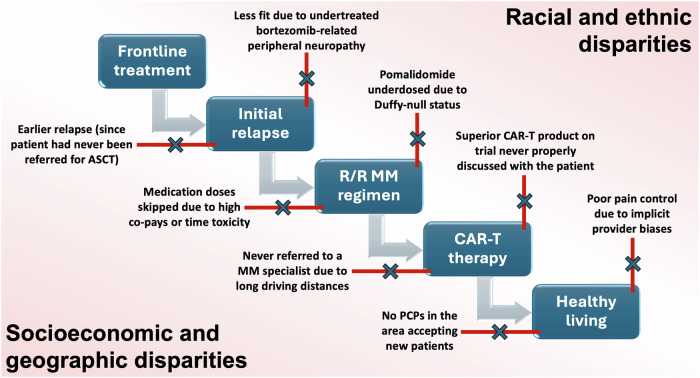
Regardless, much of this research around disparities in MM has focused on diagnosing NDMM or initiating frontline treatments (including ASCT). Relapsed/refractory MM (RRMM) has its unique predispositions to disparities that have not been examined as carefully in the literature. As illustrated in Fig. 1, these disparities can apply to dosing conventional RRMM therapies such as pomalidomide, newer IEC approaches including access to CAR-T, and global supportive care considerations including pain control. In fact, some inequalities in MM are arguably more noticeable in the relapsed/refractory setting than in the newly diagnosed setting. We convened an interprofessional task force in November 2023 (details in Supplementary Table 1) focused on mitigating disease burden and healthcare disparities in RRMM. Stakeholders included physicians, advanced practice providers (APPs), and patients with a goal of creating a position statement with expert recommendations focused on US patients living with RRMM.
Fig. 1. Representative disparities in relapsed/refractory multiple myeloma.
ASCT autologous stem cell transplantation, CAR-T chimeric antigen receptor T-cell therapy, MM multiple myeloma, PCP primary care provider.
Disparities with conventional therapies in RRMM
Conventional therapies in RRMM include monoclonal antibodies (mAbs), immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), and novel targeted agents such as selinexor. In most cases, these drugs are combined into dexamethasone-containing regimens such as Dara-Pd (daratumumab, pomalidomide, dexamethasone) or Isa-Kd (isatuximab, carfilzomib, dexamethasone). In addition to the perpetuation of the aforementioned disparities from NDMM into the relapsed/refractory setting, other unique scenarios in RRMM can predispose patients to suboptimal outcomes as discussed below.
Ensuring IMiD availability and dosing
Three IMiDs are commercially available in the US: (1) thalidomide, which is rarely used in the US today; (2) lenalidomide, which is often used in frontline therapy and maintenance; and (3) pomalidomide, which is typically used in the setting of RRMM. IMiDs are dosed orally and typically do not cause nausea or vomiting as may be seen with other oral agents in MM such as cyclophosphamide or selinexor. Unlike bortezomib or carfilzomib, IMiD-associated neuropathy is less common and IMiD-associated cardiotoxicity (e.g., arterial thromboembolic events) is quite rare. In principle, IMiDs may thus appear to be less susceptible to disparities created by long driving distances, itinerant lifestyles, or pre-existing comorbidities such as hypertension or diabetes. However, several issues may interfere with optimal IMiD access in MM: (1) inappropriate dose reductions or omissions, (2) restricted IMiD availability due to Risk Evaluation and Mitigation Strategy (REMS) restrictions, and (3) high out-of-pocket costs.
With regard to inappropriate IMiD dose reductions or omissions, two frequent causes are chronic kidney disease (CKD) and Duffy-null antigen status. Lenalidomide has been studied prospectively in CKD, and the corresponding FDA package insert offers dose reduction recommendations for its use even in patients who are on dialysis [20, 21]. However, renal function appears to be a key determinant by which physicians decide whether to use lenalidomide at any dose versus withholding it entirely in MM [22]. Although lenalidomide is primarily used only in the frontline setting in the US, withholding lenalidomide indefinitely in patients with CKD (and relying on bortezomib maintenance instead) may mean that patients have more sequelae from neuropathy and time toxicity at subsequent relapse. More pertinently to RRMM, avoiding frontline lenalidomide may have the added negative repercussion of complicating CAR-T eligibility later; this possibility stems from the updated FDA package insert for ciltacabtagene autoleucel based on the CARTITUDE-4 trial, which permits dosing in the second line and beyond but requires lenalidomide refractoriness [23].
While pomalidomide does not have any dosing difficulties in CKD [24], patients with a Duffy-null genotype may have inappropriate dose reductions with both lenalidomide and pomalidomide due to perceived neutropenia. Duffy-null status, a mutation in the ACKR1/DARC gene more commonly found in patients of African or Middle Eastern origin, is the cause of the condition previously characterized as benign ethnic neutropenia [25, 26]. Patients with Duffy-null status have lower absolute neutrophil count (ANC) values, with one study suggesting a reference range of 1210–5390 cells per microliter [27]. Patients with Duffy-null status are not at higher risk of infections or other adverse events (AEs); however, they may be undertreated for cancer due to concerns about low ANC counts [28–30]. Two-thirds of Black patients in the US have Duffy-null status, and Black patients comprise up to 20% of patients with MM [1, 31]. These facts underscore the importance of considering this phenomenon (ideally with genotyping confirmation) before reducing lenalidomide or pomalidomide doses. Ongoing clinical trials, including the SWOG S2209 trial, are attempting to mitigate this disparity with IMiDs systematically [32].
A second cause of IMiD-related disparities involves logistical considerations that can lead to dosing delays and “time toxicity” for vulnerable patients. Time toxicity is defined as frequent healthcare-related encounters and phone calls that interfere with patient wellbeing [33, 34]. In the US, IMiDs can only be prescribed under specialized REMS programs given their risk of teratogenicity and are largely dispensed via specialty pharmacies rather than patients’ own pharmacies. Coordinating an IMiD shipment can thus require several phone calls with specialty pharmacies, courier services, and MM clinics each month. For mail-dispensed IMiDs or premenopausal patients, the respective needs for a real-time signature during delivery or monthly pregnancy tests create additional time toxicity. The fear of delays in medication delivery if not all steps are carefully calibrated each month is a commonly cited frustration by REMS-enrolled patients [35]. Patients of low SES who are dependent on hour-to-hour employment requiring time away from home may be disproportionately affected. While no easy solutions exist, pharmacist-led “medication synchronization programs” in MM to consolidate prescriptions and workflows within a single pharmacy are feasible and should be encouraged [36].
Thirdly, even if the above issues are addressed satisfactorily, the high cost of IMiDs in RRMM can lead to substantial financial toxicity (FT) [33, 37]. For example, out-of-pocket costs for pomalidomide can exceed $21,000 per year even for patients with Medicare insurance [38]. For underinsured or underprivileged patients (particularly those from racial minorities), FT can drive dose interruptions and severely impact quality of life (QOL): for example, by requiring patients to draw down from retirement savings or to ration food and electricity [39–41]. FT screening is thus an important component of care for both NDMM and RRMM. For example, pairing patients with a financial navigator has been shown to lead to a threefold increase in the completion of financial assistance applications [42]. As a caveat, such services have not yet been shown to lower FT and require institutional commitment to be implemented sustainably. Without a doubt, a more durable solution would involve government regulations and incentives to promote competition and to lower the outsized role of pharmacy benefit managers. However, this is particularly difficult in the US with oral specialty medications and doubly so with IMiDs where patents and REMS requirements have been used to thwart competition from generic competitors [38, 43, 44].
Optimizing real-world medication dosing
One of the biggest challenges in the treatment of MM is the stubborn disconnect between how drugs are prescribed in clinical trials versus in real-world (RW) practice, both in the frontline and relapsed/refractory settings. As shown in Table 1, there are many dosing strategies that can improve a drug’s safety and even efficacy but that – because they were not employed in the trials leading to regulatory approval – are often excluded from package inserts and dosing guidance [21, 23, 45–56]. Such considerations apply to nearly every drug in RRMM and include dose reductions (e.g., with pomalidomide 2 mg instead of 4 mg) [51], dose frequency reductions (e.g., once-weekly carfilzomib instead of twice-weekly carfilzomib) [48], or omitted pre-medications to lower time toxicity (e.g., with daratumumab) [52]. Similar considerations also apply to bispecific antibodies (bsAbs) in MM as discussed in the next section, for example with de-escalating talquetamab dose frequency to lower skin-related and nail-related AEs [56].
Table 1.
Selected opportunities to optimize medication dosing in multiple myeloma.
| Drug | Trial-studied dosing | Optimized dosing | Potential benefits of optimized dosing strategy |
|---|---|---|---|
| Bortezomib | Twice-weekly dosing [45] | Once-weekly dosing | Equivalent PFS with less neuropathy [46] |
| Carfilzomib | Twice-weekly dosing [47] | Once-weekly dosing | Equivalent PFS with less time toxicity [48] |
| Lenalidomide | Avoided if CrCl <30 mL/min [49] | Dose-reduced to 5–15 mg if CrCl <30 mL/min | Benefits of IMiD exposure (e.g., PFS & CAR-T eligibility) [21, 23] |
| Pomalidomide | 4 mg for 21 out of 28 days [50] | 2 mg for 21 out of 28 days | Equivalent PFS with likely fewer cytopenias [51] |
| Daratumumab | Pre-medications with every dose [50] | Pre-medications removed after C1 | No increase in IRRs and less time toxicity per clinic visit [52] |
| Selinexor | Target dose of 100 mg weekly [53] | Starting dose of 40–60 mg weekly | Longer PFS with fewer GI toxicities [54] |
| Dexamethasone | Target dose of 40 mg weekly [50] | De-escalation once ≥PR | Fewer long-term toxicities such as cataracts and hyperglycemia [55] |
| Talquetamab | q1-2wk dosing until PD | q4wk dosing once ≥PR | Lower rates of most skin- and nail-related toxicities [56] |
Selected drugs, clinical trials, and simplified examples of dose optimization schemas are shown. Because the suggested optimized dosing schemas have generally not been studied prospectively, patients without access to a MM specialist may not necessarily be able to benefit from the advantages that such dose optimization strategies may confer.
CAR-T chimeric antigen receptor T-cell therapy, CrCl creatinine clearance, C1 cycle 1, GI gastrointestinal, IMiD immunomodulatory imide drug, IRR infusion-related reaction, mg milligrams, mL/min milliliters per minute, MM multiple myeloma, PD progressive disease, PFS progression-free survival, PR partial response, q1-2wk every 1-2 weeks (dependent on dose), q4wk every 4 weeks.
Evidently, maintaining the knowledge to optimize RRMM drug dosing requires considerable personal experience with RRMM patients and RRMM literature. Patients with MM treated at high-volume centers have better outcomes [10–13], likely in part due to the sub-specialization and allied resources in myeloma necessary to know these therapeutic nuances. Physicians at high-volume centers may also have better awareness and access to interdisciplinary tools (e.g., financial navigation as discussed above) to mitigate barriers to care in RRMM [57]. However, if access to CAR-T therapy is viewed as a surrogate for access to MM expertise, over a quarter of US patients live over 2 h away from such centers [6]. Additionally, patients with low health literacy or high time toxicity from treatment may not know to (or have time to) seek second opinions. Put plainly, this may result in two standards of care for patients with RRMM: traditional regimens based on historical trials for patients who do not have access to MM specialists, versus strategically dose-optimized regimens for patients who do.
How can we bridge this gap? Co-management of patients between community-based oncologists and MM specialists may be an effective strategy [13, 58], albeit this can be limited by driving distances or fragmented access to telehealth across state lines. The VA medical system has a long history of success here, for example by allowing patients without Internet connectivity to attend subspecialty telehealth appointments while physically located within their local community-based clinic [59]. Physician-to-physician “curbside” services may play a role, albeit incentives are needed to encourage both referring oncologists and MM specialists to take the time to use such services. Alternatively, while many excellent resources exist online for patients living with RRMM and their caregivers, direct-to-patient telephone support lines (Table 2) may be uniquely helpful to assist patients with identifying resources or securing second-opinion consultations; this may be particularly true for patients with limited digital literacy or Internet access [60]. Patient navigators from treatment centers can play a similar role in eliminating disparities in hematologic malignancies through personalized longitudinal attention [61–64], although more work is needed to standardize their scope of practice and improve payment models [62].
Table 2.
Selected resources for US patients with relapsed/refractory multiple myeloma.
| Resource | Website | Phone number |
|---|---|---|
| International Myeloma Foundation | www.myeloma.org | 1-800-452-2873 |
| Multiple Myeloma Research Foundation | www.themmrf.org | 1-888-841-6673 |
| Leukemia & Lymphoma Society | www.lls.org | 1-800-955-4572 |
| HealthTree Foundation | www.healthtree.org | 1-800-709-1113 |
| Cancer Support Community | cancersupportcommunity.org | 1-888-793-9355 |
These organizations include patient-facing hotlines (with phone numbers listed) for US patients and caregivers to call for support and navigation. This list is not meant to be exhaustive. Contact information is accurate as of publication.
US, United States.
Disparities with IEC therapies in RRMM
Collectively, CAR-T and bispecific antibody (bsAb) therapies have offered the highest single-agent response rates ever seen in the history of drug development in MM. While initially fewer than 20% of patients with MM might have lived long enough to qualify for IEC therapies based on their initial approvals [65], this proportion will rise dramatically in coming years with expanded approvals and increased adoption. However, IEC therapies come with novel toxicities such as cytokine release syndrome (CRS) for which dedicated REMS programs are required. Beyond physician familiarity with AEs, these IEC therapies also require interprofessional expertise such as apheresis-trained nurses and cell therapy personnel for CAR-T therapies. IEC therapies are thus generally only administered at large-volume centers, a reality that sets the stage for several potential inequities.
Before IEC therapy: disparities in access
All of the considerations discussed in the previous section apply to IEC therapies as well: patients can only receive IEC therapies if they are referred to an IEC-performing center. The nuances around patient selection and treatment logistics can be difficult even for MM experts to navigate given their rapid evolution. For example, patients with advanced organ dysfunction (including chronic kidney or liver disease) were historically excluded from CAR-T and bsAb trials despite emerging data suggesting the safety of IEC therapies in these patients [3, 66]. Similarly, given that the US package inserts for teclistamab and elranatamab both currently require at least 6 months of once-weekly dosing before frequency de-escalation, patients who live remotely may not traditionally be thought of as candidates for these bsAb therapies. However, over a third of teclistamab recipients in the RW setting are de-escalated to less frequent dosing sooner than the 6-month mark [67]. Efforts to improve bsAb access in smaller community-based settings by lowering the clinical risks and financial costs of bsAb initiation all warrant further investigation: for example, alternative CRS-related strategies (e.g., prophylactic tocilizumab or first-line dexamethasone) or limited provision of free elranatamab vials for the inpatient setting to help recoup costs [68–70].
The most promising advances to make IEC therapy safer and more effective are currently under investigation in clinical trials: for example, rapid-manufacturing CAR-T protocols or bsAbs paired with other medications to enhance T-cell function. Unfortunately, Black patients are vastly underrepresented in MM trials for a variety of reasons: trial eligibility criteria (including Duffy antigen status as discussed earlier), implicit bias by healthcare providers, mistrust of the consenting process, and more [1, 4, 5, 71–74]. IEC trial access in MM also depends heavily on differences in geographic location that may exacerbate these disparities: for example, of the 10 US states with the highest proportions of Black residents, fewer than half have several IEC trials open [72]. Strategies to mitigate these racial disparities have been reviewed previously and broadly include: (1) diversity plans and patient involvement during MM trial development, (2) broadened eligibility criteria, (3) more diverse study site selection and implicit bias training, and (4) better patient education as part of the consent process [1, 4, 5, 32].
After IEC therapy: disparities in outcomes
Given the disparities in trial enrollment discussed above, much of our understanding of differences in outcomes following CAR-T therapy in MM has emerged from RW data. In a recently published analysis of 207 recipients of ide-cel, 28% of patients who received CAR-T belonged to racial or ethnic minorities [75]. Non-Hispanic Black patients had higher inflammatory markers at baseline and were more likely to develop any-grade CRS compared to Hispanic and non-Hispanic White patients; however, there were no differences in the incidence of Grade 3 + CRS or use of tocilizumab or corticosteroids. Black patients had longer hospital stays than non-Hispanic White or Hispanic patients. There were no differences in survival outcomes, albeit with the important caveat of selection bias: namely, that minority patients who were never referred for CAR-T therapy could not have been included in this post-CAR-T analysis.
Less is known about disparities in outcomes following treatment with bsAbs in RRMM. Geographic distances from bsAb-capable centers certainly can create disparities given their frequent dosing, even if de-escalation for responding patients is feasible as noted above [67]. Even for subcutaneously administered bispecific antibodies, the routine use of intravenous immunoglobulin (IVIG, which typically requires several hours to infuse) can mean additional time in clinic every month. While underused, subcutaneous immunoglobulin repletion is effective in MM and may lower time toxicity for vulnerable patients [76, 77]. Talquetamab-related toxicities ranging from hyperpigmentation to onychomadesis (nail bed separation) can be managed by early recognition and dose de-escalation to maintain responses while preserving QOL [56]. Given prior evidence of racial disparities in the recognition of skin-related toxicities in MM [78], adequate provider training and dermatology co-management are important considerations.
Disparities with supportive care in RRMM
In addition to the treatment-related disparities discussed above, there is no doubt that disparities in RRMM exist with regard to supportive care. Patient needs in this domain range from pain control to psychosocial distress management to age-appropriate cancer screening and more. One of the field’s most robust tools to mitigate disparities in RRMM supportive care is to capitalize on the strengths of an interdisciplinary team: medical oncologists, APPs, registered nurses, pharmacists, patient navigators, social workers, nutritionists, schedulers, and consulting teams including palliative care providers [79–81]. Scaling these team members to smaller oncology practices is an important priority for the field in addition to the specific considerations below.
Symptom management in RRMM
Unfortunately, the presence of racial and ethnic disparities in the treatment of cancer-associated pain is well known. With regard to MM, Black patients are less likely to receive palliative radiation therapy (RT) within 1 year of diagnosis [82]. This same study also found that Black patients were also less likely to receive RT within 1 month of death, suggesting a lower usage of RT for end-of-life symptom relief in this population. In a matched analysis of Black patients versus non-Black patients with NDMM, bortezomib-induced peripheral neuropathy (which can often be painful) was more common in Black patients than their non-Black counterparts [83]. Despite these observations, Black and Hispanic patients with MM who are admitted to the hospital are less likely to receive palliative care consultations than their non-Hispanic White counterparts [84].
With these observations in mind, further research into understanding and mitigating disparities in symptom palliation for patients with MM is needed. In one study of Black patients with MM who required scheduled opioid pain medications for symptom control, half of patients met clinical criteria for depression [85]. While the interplay between pain and depressive symptoms in MM is complex and bidirectional [86, 87], workflows to recognize and treat both symptoms with racially and culturally responsive strategies may be helpful. Integrative medicine modalities may potentially help with pain management in MM, but these tools are often underused in patients from racial or ethnic minorities living with cancer [88, 89]. Furthermore, inconsistent insurance coverage for these important services may lead to additional FT for vulnerable patients. For other common RRMM symptoms like fatigue and insomnia, chronic weekly dexamethasone may be an underlying cause. As noted previously, co-management with a MM specialist may help identify settings where dexamethasone can safely be lowered or stopped.
Optimal management of medical comorbidities
Medical comorbidities are common in patients with RRMM. As an example, cardiovascular disease (CVD) is much more prevalent in patients with MM than in the general population [90]. Many factors can predispose patients with RRMM to developing CVD: carfilzomib, IMiDs (which carry a risk of arterial thromboembolism as well), anthracyclines, thoracic radiation therapy, concurrent AL amyloidosis, and more [91–93]. In ASCT recipients, pre-existing clonal hematopoiesis can predispose to CVD as well [94]. While little is known about cardiovascular disparities in MM, one analysis of over 60,000 hospitalizations found that in-hospital deaths due to arrhythmias in patients with MM were significantly more likely in Black versus non-Black patients [95]. Proposed solutions include standardized referral pathways to cardio-oncologists (or general cardiologists in the community) and implicit bias training for both oncology and cardiology care teams [96, 97].
Of course, there is no substitute for a longitudinal relationship with a primary care provider (PCP) to manage comorbidities ranging from osteoarthritis to endocrine disorders to CVD and more. Longitudinal screening for second cancers, a known side effect of many MM therapies including IMiDs and ASCT, also traditionally falls under the purview of PCPs. Many studies have analyzed the essential role that PCPs play in diagnosing MM [2, 98–101], but none to our knowledge have analyzed the role that PCPs play after diagnosis. Unfortunately, stark disparities exist in the US with regard to reliable PCP access based on racial/ethnic factors, age, SES, ZIP code, digital literacy, and more [102–105]. Studies have shown that PCPs who primarily care for patients from racial and ethnic minorities are themselves less paid, less likely to have access to subspecialty support, and less likely to feel that they are providing high-quality care to their patients [105–108]. Survivorship care plans in oncology, a key component of post-ASCT care guidelines in MM, are also less likely to be disseminated and integrated into PCP care for underserved communities [109, 110]. Despite these formidable headwinds, community-based PCPs can offer an important added layer of support in a familiar (and often geographically closer) setting for minoritized patients. As such, oncologists should encourage patients with RRMM to maintain longitudinal care with a PCP even if their disease is in remission [111].
Promotion of general health and wellbeing
Given that health is more than just the absence of illness, promoting healthy living in RRMM is a key element of survivorship care. This of course includes PCP visits as above for preventative measures and screening. The risk of dental AEs increases with time in MM [112], and patients with RRMM should be encouraged to undergo regular oral exams even if they have completed their planned courses of anti-resorptive agents such as zoledronic acid. The risk of cataracts also increases with time in MM, likely a function of longitudinal dexamethasone exposure [55]. Given that regular eye exams may lower the rate of visual decline or incidence of vision-related functional limitations among older adults [113], annual eye exams should be recommended as well. Finally, only a minority of patients living with cancer are able to fully adhere to guidelines for nutrition and physical activity [114]. In general, dietary and exercise considerations are often underdiscussed for patients with MM despite their potential importance to patient wellbeing [115–118]. Broader screening for SDOH that negatively impact wellbeing, for example financial toxicity or food insecurity, with appropriate referrals as indicated is another important step to mitigate barriers to living healthily [18, 119].
Overcoming the disparities that preclude the above recommendations from being practical requires a concerted effort by the MM field, individual clinics, healthcare payers, advocacy groups, and more. Time and provider availability are important considerations to help mitigate these inequalities, particularly for patients from racial and ethnic minorities where physician time spent listening and empathizing can help overcome medical mistrust [5]. Some support mechanisms for minoritized patients can originate outside of clinic walls. For example, MM-specific patient support groups and telephone hotlines (Table 2) can provide another layer of support for patients and their caregivers to learn from others undergoing similar experiences [120, 121]. The International Myeloma Foundation (IMF) has launched many such groups, including the virtual Las Voces de Mieloma group for Spanish-speaking patients. Additionally, the IMF M-Power program seeks to create city-specific initiatives for Black patients for MM through partnerships with churches, barbershops, and other trusted community sources [1, 5].
Discussion
While every patient’s journey with MM is unique, there is no doubt that the journeys for some patients are more obstacle-laden than for others. Disparities among a variety of overlapping axes can impede access to optimal MM therapies, outcomes following such therapies, and QOL in general for these patients. Most studies and reviews of disparities in MM care have focused on delays in diagnosis and access to optimal first-line treatments including ASCT. This is of course an appropriate emphasis given the larger population of patients in this scenario: namely, over 35,000 Americans per year who will be newly diagnosed with MM this year [122]. However, in the modern era of MM treatments, many patients will spend many years living with RRMM going through a myriad of possible sequencing strategies involving various therapeutic options. A better understanding of disparities in RRMM – and more importantly, a toolkit to mitigate such disparities – is thus an unmet need for the field.
As summarized in Table 3, potential strategies to mitigate these disparities largely center around overlapping layers of advocacy from physicians, APPs, and other healthcare team members. Steps that can be taken at the level of an individual clinic include optimal drug dosing, concrete steps to build patient trust (particularly around clinical trials and data collection), and screening for adverse SDOH such as financial toxicity or food insecurity. Other steps require broader levels of engagement between stakeholders, with the philosophy that it takes a village to treat RRMM satisfactorily. Given that strategic changes within a given regimen can make a considerable difference in patient outcomes, longitudinal co-management of patients with a primary oncologist (who knows the patient the best) and a MM specialist (who knows the evolving intricacies of myeloma the best) is an optimal solution. Ideally, such partnerships can be leveraged to bring novel therapies like bsAbs to centers closer to vulnerable patients as well. These partnerships require the support of an interdisciplinary team including APPs, pharmacists, nurses, schedulers, and more. PCPs remain as essential to the ongoing management of RRMM as they are to making the initial diagnosis.
Table 3.
Strategies to mitigate disparities in relapsed/refractory multiple myeloma.
| Individual advocacy | Interpersonal advocacy | |
|---|---|---|
| Conventional therapies | Avoid IMiD avoidance: Consider Duffy-null status, requirements for future CAR-T candidacy, and more | Encourage patient co-management: Build bidirectional ties between primary oncologists and MM specialists to help identify optimized dosing regimens |
| IEC therapies | Build the patient’s trust: Explain the importance of trials & data collection and take the time to answer questions | Bring bsAbs back home: Partner with manufacturers, hospitals, payers, and academic centers to implement bsAbs at smaller practices |
| Supportive care | Screen for SDOH risk factors: Understand each patient’s risk of financial toxicity, food insecurity, and other challenges | Keep the primary in PCP: Promote longitudinal access to a primary care provider regardless of MM status or current line of therapy |
bsAb bispecific antibody, CAR-T chimeric antigen receptor T-cell therapy, IEC immune effector cell therapies, IMiD immunomodulatory imide drug, MM multiple myeloma, PCP primary care provider, SDOH social determinants of health.
Given the heterogeneity of patient experiences with RRMM, our review necessarily has many limitations. Firstly, there are many additional layers of disparities at play beyond our emphasis on race, ethnicity, SES, and ZIP codes. The complexities of the terms Black versus African American in MM, for example, are beyond the scope of our position statement and have been reviewed elsewhere [1, 71]. While increasing SES may correlate with the presence of adequate health insurance coverage in the US, this is not always the case. For patients without written English proficiency or an able-bodied caregiver, many of the resources and strategies described in this review are only incompletely available. Indeed, given that many patients with MM are older and may have functional limitations, lack of access to a caregiver is clearly an understudied axis of disparity [3]. Most importantly, this review focuses on gaps in RRMM care created by disparities for patients living in the US. These disparities are dwarfed by far wider chasms in RRMM treatment options between high-income countries and the rest of the world, a topic that has been reviewed at length previously [123–127].
In conclusion, many of the disparities present in the care of MM are accentuated for patients in the setting of relapsed or refractory disease. Racial, ethnic, SES, and geographic barriers may interfere with access to optimal care and also prevent optimal outcomes thereafter. Evaluating and mitigating disparities for every patient – and every time a treatment decision is being made – must become a part of disease management. As the treatment landscape for RRMM expands each year, conscious efforts by the myeloma field are needed to ensure that this landscape is equally accessible and traversable for all patients.
Supplementary information
Acknowledgements
The authors wish to acknowledge Madelyne T. Fabrizio, CHCP; Keira P. Smith, BA; Eden E. D. Maack, PhD; and Elizabeth J. Heller, PhD from the i3 Health team for assistance with task force logistics.
Author contributions
RB and SA conceptualized the review concept. RB, BF, and SM wrote the first draft of the manuscript. YB, CEC, and SA provided critical feedback and revisions. All authors (RB, YB, CEC, BF, SM, and SA) have approved the final manuscript.
Competing interests
RB reports consulting: Adaptive Biotech, BMS, Caribou Biosciences, Genentech, GSK, Janssen, Karyopharm, Legend Biotech, Pfizer, Sanofi, SparkCures; research: Abbvie, BMS, Janssen, Novartis, Pack Health, Prothena, Sanofi. CEC reports consulting: AbbVie, Binding Site, Genentech, GSK, Janssen, Pfizer: research, GSK. BF reports consulting: BMS, GSK, Janssen, Karyopharm, Sanofi. SM reports consulting: Pfizer, stock ownership: AbbVie. SA reports consulting: BeiGene, BMS, Cellectar, GSK, Janssen, Pfizer, Regeneron, Sanofi, Takeda; research: AbbVie, Amgen, Ascentage, BMS, Cellectar, GSK, Janssen, Pharmacyclics, Sanofi. The remaining authors have no disclosures.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-024-01129-0.
References
- 1.Bhutani M, Blue BJ, Cole C, Badros AZ, Usmani SZ, Nooka AK, et al. Addressing the disparities: the approach to the African American patient with multiple myeloma. Blood Cancer J. 2023;13:189 10.1038/s41408-023-00961-0. 10.1038/s41408-023-00961-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikhael J, Bhutani M, Cole CE. Multiple Myeloma for the Primary Care Provider: A Practical Review to Promote Earlier Diagnosis Among Diverse Populations. Am J Med. 2023;136:33–41. 10.1016/j.amjmed.2022.08.030. 10.1016/j.amjmed.2022.08.030 [DOI] [PubMed] [Google Scholar]
- 3.Cerchione C, Grant SJ, Ailawadhi S Partnering with all patients: ensuring shared decision making and evidence-based management for underrepresented groups with multiple myeloma. Am Soc Clin Oncol Educ Book. 2023;43. 10.1200/EDBK_390202. [DOI] [PMC free article] [PubMed]
- 4.Hartley-Brown M, Cole CE, Price P, Andreini M, Mulligan G, Young AQ, et al. Creating Equitable and Inclusive Clinical Trials for Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2024;24:32–39. 10.1016/j.clml.2023.09.004. 10.1016/j.clml.2023.09.004 [DOI] [PubMed] [Google Scholar]
- 5.Blue B, Pierre A, Mikhael J. Culturally Responsive Care Delivery in Oncology: The Example of Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2023;23:651–9. 10.1016/j.clml.2023.05.005. 10.1016/j.clml.2023.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Ahmed N, Shahzad M, Shippey E, Bansal R, Mushtaq MU, Mahmoudjafari Z, et al. Socioeconomic and Racial Disparity in Chimeric Antigen Receptor T Cell Therapy Access. Transpl Cell Ther. 2022;28:358–64. 10.1016/j.jtct.2022.04.008. 10.1016/j.jtct.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 7.Notardonato LD, Langerman SS, Zhou J, Calip GS, Chiu BCH, Derman BA. Racial Disparities in the Diagnostic Evaluation of Multiple Myeloma. Blood. 2021;1381:4116–4116. 10.1182/blood-2021-146910. 10.1182/blood-2021-146910 [DOI] [Google Scholar]
- 8.Ailawadhi S, Parikh K, Abouzaid S, Zhou Z, Tang W, Clancy Z, et al. Racial disparities in treatment patterns and outcomes among patients with multiple myeloma: a SEER-Medicare analysis. Blood Adv. 2019;3:2986–94. 10.1182/bloodadvances.2019000308. 10.1182/bloodadvances.2019000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasoyan H, Fiala MA, Doering M, Vij R, Halpern M, Colditz GA. Disparities in Multiple Myeloma Treatment Patterns in the United States: A Systematic Review. Clin Lymphoma Myeloma Leuk. 2023;23:e420–e427. 10.1016/j.clml.2023.08.008. 10.1016/j.clml.2023.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go RS, Bartley AC, Crowson CS, Shah ND, Habermann EB, Holton SJ, et al. Association Between Treatment Facility Volume and Mortality of Patients With Multiple Myeloma. J Clin Oncol. 2017;35:598–604. 10.1200/JCO.2016.68.3805. 10.1200/JCO.2016.68.3805 [DOI] [PubMed] [Google Scholar]
- 11.Vardell VA, Ermann DA, Tantravahi SK, Haaland B, McClune B, Godara A, et al. Impact of academic medical center access on outcomes in multiple myeloma. Am J Hematol. 2023;98:41–48. 10.1002/ajh.26759. 10.1002/ajh.26759 [DOI] [PubMed] [Google Scholar]
- 12.Freeman AT, Kuo M, Zhou L, Trogdon JG, Baggett CD, Tuchman SA, et al. Influence of Treating Facility, Provider Volume, and Patient-Sharing on Survival of Patients With Multiple Myeloma. J Natl Compr Canc Netw. 2019;17:1100–8. 10.6004/jnccn.2019.7298. 10.6004/jnccn.2019.7298 [DOI] [PubMed] [Google Scholar]
- 13.Ailawadhi S, Advani P, Yang D, Ghosh R, Swaika A, Roy V, et al. Impact of access to NCI- and NCCN-designated cancer centers on outcomes for multiple myeloma patients: A SEER registry analysis. Cancer. 2016;122:618–25. 10.1002/cncr.29771. 10.1002/cncr.29771 [DOI] [PubMed] [Google Scholar]
- 14.Saunders A, Slaff S, Subbiah K, Gu T, Ang KK, Quan MA, et al. Clinical characteristics, treatment patterns, and outcomes among African American and White patients with multiple myeloma in the United States. Leuk Lymphoma. 2024;65:109–17. 10.1080/10428194.2023.2273746. 10.1080/10428194.2023.2273746 [DOI] [PubMed] [Google Scholar]
- 15.Schriber JR, Hari PN, Ahn KW, Fei M, Costa LJ, Kharfan-Dabaja MA, et al. Hispanics have the lowest stem cell transplant utilization rate for autologous hematopoietic cell transplantation for multiple myeloma in the United States: A CIBMTR report. Cancer. 2017;123:3141–9. 10.1002/cncr.30747. 10.1002/cncr.30747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasoyan H, Anwer F, Casacchia NJ, Kovach JD, Valent J, Wang M, et al. Role of Patient Characteristics and Insurance Type in Newly Diagnosed Multiple Myeloma Care Disparities. JCO Oncol Pract. 2024:OP2300672, 10.1200/OP.23.00672. [DOI] [PMC free article] [PubMed]
- 17.Ailawadhi S, Adu Y, Frank RD, Das S, Hodge DO, Fernandez A, et al. Factors determining utilization of stem cell transplant for initial therapy of multiple myeloma by patient race: exploring intra-racial healthcare disparities. Blood Cancer J. 2024;14:86 10.1038/s41408-024-01067-x. 10.1038/s41408-024-01067-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath GR, Renteria AS, Jagannath S, Gallagher EJ, Parekh S, Bickell NA. Where you live can impact your cancer risk: a look at multiple myeloma in New York City. Ann Epidemiol. 2020;48:e4 10.1016/j.annepidem.2020.05.005. 10.1016/j.annepidem.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 19.Fillmore NR, Yellapragada SV, Ifeorah C, Mehta A, Cirstea D, White PS, et al. With equal access, African American patients have superior survival compared to white patients with multiple myeloma: a VA study. Blood. 2019;133:2615–8. 10.1182/blood.2019000406. 10.1182/blood.2019000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Drug Administration. Package insert - REVLIMID. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021880s067lbl.pdf.
- 21.Mikhael J, Manola J, Dueck AC, Hayman S, Oettel K, Kanate AS, et al. Lenalidomide and dexamethasone in patients with relapsed multiple myeloma and impaired renal function: PrE1003, a PrECOG study. Blood Cancer J. 2018;8:86 10.1038/s41408-018-0110-7. 10.1038/s41408-018-0110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidana S, Kumar S, Fraser R, Estrada-Merly N, Giralt S, Agrawal V, et al. Impact of Induction Therapy with VRD versus VCD on Outcomes in Patients with Multiple Myeloma in Partial Response or Better Undergoing Upfront Autologous Stem Cell Transplantation. Transpl Cell Ther. 2022;28:e9 10.1016/j.jtct.2021.10.022. 10.1016/j.jtct.2021.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. Package Insert - CARVYKTI. https://www.fda.gov/media/156560/download.
- 24.Dimopoulos M, Weisel K, van de Donk N, Ramasamy K, Gamberi B, Streetly M, et al. Pomalidomide Plus Low-Dose Dexamethasone in Patients With Relapsed/Refractory Multiple Myeloma and Renal Impairment: Results From a Phase II Trial. J Clin Oncol. 2018;36:2035–43. 10.1200/JCO.2017.76.1742. 10.1200/JCO.2017.76.1742 [DOI] [PubMed] [Google Scholar]
- 25.Grann VR, Ziv E, Joseph CK, Neugut AI, Wei Y, Jacobson JS, et al. Duffy (Fy), DARC, and neutropenia among women from the United States, Europe and the Caribbean. Br J Haematol. 2008;143:288–93. 10.1111/j.1365-2141.2008.07335.x. 10.1111/j.1365-2141.2008.07335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charles BA, Hsieh MM, Adeyemo AA, Shriner D, Ramos E, Chin K, et al. Analyses of genome wide association data, cytokines, and gene expression in African-Americans with benign ethnic neutropenia. PLoS One. 2018;13:e0194400 10.1371/journal.pone.0194400. 10.1371/journal.pone.0194400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merz LE, Osei MA, Story CM, Freedman RY, Smeland-Wagman R, Kaufman RM, et al. Development of Duffy Null-specific absolute neutrophil count reference ranges. JAMA. 2023;329:2088–9. 10.1001/jama.2023.7467. 10.1001/jama.2023.7467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legge SE, Christensen RH, Petersen L, Pardinas AF, Bracher-Smith M, Knapper S, et al. The Duffy-null genotype and risk of infection. Hum Mol Genet. 2020;29:3341–9. 10.1093/hmg/ddaa208. 10.1093/hmg/ddaa208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naidoo KK, Ngubane A, Gaza P, Moodley A, Ndung’u T, Thobakgale CF. Neutrophil Effector Functions Are Not Impaired in Duffy Antigen Receptor for Chemokines (DARC)-Null Black South Africans. Front Immunol. 2019;10:551 10.3389/fimmu.2019.00551. 10.3389/fimmu.2019.00551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hershman D, Weinberg M, Rosner Z, Alexis K, Tiersten A, Grann VR, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;95:1545–8. 10.1093/jnci/djg073. 10.1093/jnci/djg073 [DOI] [PubMed] [Google Scholar]
- 31.Merz LE, Story CM, Osei MA, Jolley K, Ren S, Park HS, et al. Absolute neutrophil count by Duffy status among healthy Black and African American adults. Blood Adv. 2023;7:317–20. 10.1182/bloodadvances.2022007679. 10.1182/bloodadvances.2022007679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espinoza-Gutarra MR, Aiello J, Orlowski RZ, Ailawadhi S. Clinical trial design change implementation for inclusive studies. Lancet Haematol. 2023;10:e953–e954. 10.1016/S2352-3026(23)00336-8. 10.1016/S2352-3026(23)00336-8 [DOI] [PubMed] [Google Scholar]
- 33.Banerjee R, Cowan AJ, Ortega M, Missimer C, Carpenter PA, Oshima MU, et al. Financial toxicity, time toxicity, and quality of life in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2024;24:446–454.e3. 10.1016/j.clml.2024.02.013. 10.1016/j.clml.2024.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di M, Su CT, Cowan AJ, Gopal AK, Banerjee R. Mitigating time toxicity in lymphoma and multiple myeloma. Leuk Lymphoma. 2024. 10.1080/10428194.2024.2352086. [DOI] [PubMed]
- 35.Sarpatwari A, Brown BL, McGraw SA, Dejene SZ, Abdurrob A, Santiago Ortiz AJ, et al. Patient and Caregiver Experiences With and Perceptions of Risk Evaluation and Mitigation Strategy Programs With Elements to Assure Safe Use. JAMA Netw Open. 2022;5:e2144386 10.1001/jamanetworkopen.2021.44386. 10.1001/jamanetworkopen.2021.44386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costello F, Kumor L, Stubbings J. Medication synchronization service for patients with multiple myeloma within a health system-based specialty pharmacy. Am J Health Syst Pharm. 2020;77:2042–4. 10.1093/ajhp/zxaa314. 10.1093/ajhp/zxaa314 [DOI] [PubMed] [Google Scholar]
- 37.Fiala MA, Silberstein AE, Schroeder MA, Stockerl-Goldstein KE, Vij R. The Dynamics of Financial Toxicity in Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2023;23:266–72. 10.1016/j.clml.2023.01.008. 10.1016/j.clml.2023.01.008 [DOI] [PubMed] [Google Scholar]
- 38.Beck K, Sandahl T, Ailawadhi S, Khera N, Jensen C. Multiple Myeloma: Current Clinical Landscape and Compounding Costs. Curr Hematol Malig Rep. 2023;18:201–15. 10.1007/s11899-023-00705-8. 10.1007/s11899-023-00705-8 [DOI] [PubMed] [Google Scholar]
- 39.Joshi H, Lin S, Fei K, Renteria AS, Jacobs H, Mazumdar M, et al. Multiple myeloma, race, insurance and treatment. Cancer Epidemiol. 2021;73:101974 10.1016/j.canep.2021.101974. 10.1016/j.canep.2021.101974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodwin JA, Coleman EA, Sullivan E, Easley R, McNatt PK, Chowdhury N, et al. Personal financial effects of multiple myeloma and its treatment. Cancer Nurs. 2013;36:301–8. 10.1097/NCC.0b013e3182693522. 10.1097/NCC.0b013e3182693522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiala MA. Financial Toxicity and Willingness-to-Pay for Cancer Treatment Among People With Multiple Myeloma. JCO Oncol Pract. 2024:OP2400016, 10.1200/OP.24.00016. [DOI] [PubMed]
- 42.Djulbegovic M, Doherty M, Fanslau K, Chen K, Girgis S, Joslyn P, et al. A Randomized Controlled Trial of a Financial Navigation Program for Patients with Multiple Myeloma. Blood. 2023;1421:909–909. 10.1182/blood-2023-174451. 10.1182/blood-2023-174451 [DOI] [Google Scholar]
- 43.Bennett CL, Gibbons JB, Trujillo A, Carson KR, Knopf K, Nabhan C, et al. Congressional Investigation of RevAssist-Linked and General Pricing Strategies for Lenalidomide. JCO Oncol Pract. 2024:OP2300579, 10.1200/OP.23.00579. [DOI] [PubMed]
- 44.Royce TJ, Schenkel C, Kirkwood K, Levit L, Levit K, Kircher S. Impact of Pharmacy Benefit Managers on Oncology Practices and Patients. JCO Oncol Pr. 2020;16:276–84. 10.1200/JOP.19.00606. 10.1200/JOP.19.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2024;390:301–13. 10.1056/NEJMoa2312054. 10.1056/NEJMoa2312054 [DOI] [PubMed] [Google Scholar]
- 46.Hoff FW, Banerjee R, Khan AM, McCaughan G, Wang B, Wang X, et al. Once-weekly versus twice-weekly bortezomib in newly diagnosed multiple myeloma: a real-world analysis. Blood Cancer J. 2024;14:52 10.1038/s41408-024-01034-6. 10.1038/s41408-024-01034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Usmani SZ, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Final analysis of carfilzomib, dexamethasone, and daratumumab vs carfilzomib and dexamethasone in the CANDOR study. Blood Adv. 2023;7:3739–48. 10.1182/bloodadvances.2023010026. 10.1182/bloodadvances.2023010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong S, Banerjee R, Khan A, Wang M, Wang X, Afghahi A, et al. Abstract P918: Real-world carfilzomib prescribing patterns and outcomes for patients with relapsed or refractory multiple myeloma. Hemasphere. 2024;(EHA_suppl):918.
- 49.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375:1319–31. 10.1056/NEJMoa1607751. 10.1056/NEJMoa1607751 [DOI] [PubMed] [Google Scholar]
- 50.Dimopoulos MA, Terpos E, Boccadoro M, Delimpasi S, Beksac M, Katodritou E, et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:801–12. 10.1016/S1470-2045(21)00128-5. 10.1016/S1470-2045(21)00128-5 [DOI] [PubMed] [Google Scholar]
- 51.Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970–5. 10.1182/blood-2011-04-348896. 10.1182/blood-2011-04-348896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padmaraju K, Kelly K, Jakubowiak AJ, Derman BA Evaluation of administration-related reactions with subcutaneous daratumumab with and without premedication. Oncologist. 2024. 10.1093/oncolo/oyae158. [DOI] [PMC free article] [PubMed]
- 53.Grosicki S, Simonova M, Spicka I, Pour L, Kriachok I, Gavriatopoulou M, et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet 2020;396:1563–73. 10.1016/S0140-6736(20)32292-3. 10.1016/S0140-6736(20)32292-3 [DOI] [PubMed] [Google Scholar]
- 54.Jagannath S, Delimpasi S, Grosicki S, Van Domelen DR, Bentur OS, Spicka I, et al. Association of Selinexor Dose Reductions With Clinical Outcomes in the BOSTON Study. Clin Lymphoma Myeloma Leuk. 2023;23:917–923 e3. 10.1016/j.clml.2023.08.018. 10.1016/j.clml.2023.08.018 [DOI] [PubMed] [Google Scholar]
- 55.Banerjee R, Hurtado Martinez JA, Flores Perez PA, Porras N, Hydren J, Ahlstrom JM, et al. Association between dexamethasone exposure and visually significant cataracts in multiple myeloma. Am J Hematol. 2024;99:E12–E14. 10.1002/ajh.27133. 10.1002/ajh.27133 [DOI] [PubMed] [Google Scholar]
- 56.Chari A, Oriol A, Krishnan A, Martinez Chamorro MDC, Costa L, Mateos MV, et al. Efficacy and Safety of Less Frequent/Lower Intensity Dosing of Talquetamab in Patients with Relapsed/Refractory Multiple Myeloma: Results from the Phase 1/2 MonumenTAL-1 Study. Blood. 2023;1421:1010–1010. 10.1182/blood-2023-181228. 10.1182/blood-2023-181228 [DOI] [Google Scholar]
- 57.Mikhael JR, Sullivan SL, Carter JD, Heggen CL, Gurska LM. Multisite Quality Improvement Initiative to Identify and Address Racial Disparities and Deficiencies in Delivering Equitable, Patient-Centered Care for Multiple Myeloma-Exploring the Differences between Academic and Community Oncology Centers. Curr Oncol. 2023;30:1598–613. 10.3390/curroncol30020123. 10.3390/curroncol30020123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berdeja J. Multiple myeloma: a paradigm for blending community and academic care. Hematol Am Soc Hematol Educ Program. 2023;2023:318–23. 10.1182/hematology.2023000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pimentel CB, Dryden EM, Nearing KA, Kernan LM, Kennedy MA, Hung WW, et al. The role of Department of Veterans Affairs community-based outpatient clinics in enhancing rural access to geriatrics telemedicine specialty care. J Am Geriatr Soc. 2024;72:520–8. 10.1111/jgs.18703. 10.1111/jgs.18703 [DOI] [PubMed] [Google Scholar]
- 60.Quinn SCM, Watters ED, Dempsey SL, Dolman KS, Laketic-Ljubojevic I. Providing tailored information and support through the Myeloma UK, Myeloma Infoline and Ask The Nurse services during the COVID-19 pandemic. Psychooncology 2020;29:1439–41. 10.1002/pon.5495. 10.1002/pon.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer. 2011;117:3543–52. 10.1002/cncr.26264. 10.1002/cncr.26264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez D, Pratt-Chapman ML, Rohan EA, Sheldon LK, Basen-Engquist K, Kline R, et al. Establishing effective patient navigation programs in oncology. Support Care Cancer. 2019;27:1985–96. 10.1007/s00520-019-04739-8. 10.1007/s00520-019-04739-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu B, Boselli D, Pye LM, Chen T, Bose R, Symanowski JT, et al. Equal access to care and nurse navigation leads to equitable outcomes for minorities with aggressive large B-cell lymphoma. Cancer. 2021;127:3991–7. 10.1002/cncr.33779. 10.1002/cncr.33779 [DOI] [PubMed] [Google Scholar]
- 64.Winkfield KM, Regnante JM, Miller-Sonet E, Gonzalez ET, Freund KM, Doykos PM. Development of an Actionable Framework to Address Cancer Care Disparities in Medically Underserved Populations in the United States: Expert Roundtable Recommendations. JCO Oncol Pr. 2021;17:e278–e293. 10.1200/OP.20.00630. 10.1200/OP.20.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giri S, Bal S, Godby KN, Ravi G, Clark D, Ubersax C, et al. Real-world applicability of commercial chimeric antigen receptor T-cell therapy among older adults with relapsed and/or refractory multiple myeloma. Am J Hematol. 2022;97:E153–E155. 10.1002/ajh.26472. 10.1002/ajh.26472 [DOI] [PubMed] [Google Scholar]
- 66.Sidana S, Peres LC, Hashmi H, Hosoya H, Ferreri C, Khouri J, et al. Idecabtagene vicleucel chimeric antigen receptor T-cell therapy for relapsed/refractory multiple myeloma with renal impairment. Haematologica. 2024;109:777–86. 10.3324/haematol.2023.283940. 10.3324/haematol.2023.283940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banerjee R, Cheng H, Lin D, Harper JS, Fu AZ, Kim N, et al. Teclistamab (Tec) Step-up Dosing (SUD) and Treatment Dose Schedule De-Escalation in the Real-world (RW) Setting – An Analysis of Multicenter Electronic Medical Records. Hemasphere. 2024;2024:P992. [Google Scholar]
- 68.van de Donk NW, Garfall AL, Benboubker L, Uttervall K, Groen K, Rosinol L, et al. Longer-term follow-up of patients (pts) receiving prophylactic tocilizumab (toci) for the reduction of cytokine release syndrome (CRS) in the phase 1/2 MajesTEC-1 study of teclistamab in relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2024;42:7517. 10.1200/JCO.2024.42.16_suppl.7517 [DOI] [Google Scholar]
- 69.Bansal R, Paludo J, Corraes ADMS, Spychalla M, Sandahl TB, Haugen K, et al. Outpatient Management of CAR-T and Teclistamab for Patients with Lymphoma and Multiple Myeloma. Transpl Cell Therapy. 2024;30 10.1016/j.jtct.2023.12.284.
- 70.Seago K. Advancing Outcomes in Multiple Myeloma one BiTE at a Time. HOPA N. 2023;20:18. [Google Scholar]
- 71.Marinac CR, Ghobrial IM, Birmann BM, Soiffer J, Rebbeck TR. Dissecting racial disparities in multiple myeloma. Blood Cancer J. 2020;10:19 10.1038/s41408-020-0284-7. 10.1038/s41408-020-0284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alqazaqi R, Schinke C, Thanendrarajan S, Zangari M, Shaughnessy J Jr, Zhan F, et al. Geographic and Racial Disparities in Access to Chimeric Antigen Receptor-T Cells and Bispecific Antibodies Trials for Multiple Myeloma. JAMA Netw Open. 2022;5:e2228877 10.1001/jamanetworkopen.2022.28877. 10.1001/jamanetworkopen.2022.28877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al Hadidi S, Schinke C, Thanendrarajan S, Zangari M, van Rhee F. Enrollment of Black Participants in Pivotal Clinical Trials Supporting US Food and Drug Administration Approval of Chimeric Antigen Receptor-T Cell Therapy for Hematological Malignant Neoplasms. JAMA Netw Open. 2022;5:e228161 10.1001/jamanetworkopen.2022.8161. 10.1001/jamanetworkopen.2022.8161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saxton C, Cutforth A, Gonzalo MB, Nichosl H, Fearnley K. Understanding barriers and facilitators to clinical trial participation among Black patients with multiple myeloma. J Clin Oncol. 2022;40:12137. 10.1200/JCO.2022.40.16_suppl.12137 [DOI] [Google Scholar]
- 75.Peres LC, Oswald LB, Dillard CM, De Avila G, Nishihori T, Blue BJ, et al. Racial and ethnic differences in clinical outcomes among patients with multiple myeloma treated with CAR T-cell therapy. Blood Adv. 2024;8:251–9. 10.1182/bloodadvances.2023010894. 10.1182/bloodadvances.2023010894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vacca A, Melaccio A, Sportelli A, Solimando AG, Dammacco F, Ria R. Subcutaneous immunoglobulins in patients with multiple myeloma and secondary hypogammaglobulinemia: a randomized trial. Clin Immunol. 2018;191:110–5. 10.1016/j.clim.2017.11.014. 10.1016/j.clim.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 77.Wonnaparhown A, Hilal T, Squire J, Freeman C, Fonseca R. IgG replacement in multiple myeloma. Blood Cancer J. 2024;14:124 10.1038/s41408-024-01107-6. 10.1038/s41408-024-01107-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milrod CJ, Mann M, Blevins F, Hughes D, Patel P, Li KY, et al. Underrepresentation of Black participants and adverse events in clinical trials of lenalidomide for myeloma. Crit Rev Oncol Hematol. 2022;172:103644 10.1016/j.critrevonc.2022.103644. 10.1016/j.critrevonc.2022.103644 [DOI] [PubMed] [Google Scholar]
- 79.Raje N, Faiman B, Harvey RD, Kurtin SE, Lonial S, Kumar SK, et al. Identifying professional education gaps and barriers in multiple myeloma patient care: findings of the Managing Myeloma Continuing Educational Initiative Advisory Committee. Clin Lymphoma Myeloma Leuk. 2014;14:356–69. 10.1016/j.clml.2014.04.011. 10.1016/j.clml.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 80.Sweiss K, Calip GS, Wirth S, Rondelli D, Patel P. Polypharmacy and potentially inappropriate medication use is highly prevalent in multiple myeloma patients and is improved by a collaborative physician-pharmacist clinic. J Oncol Pharm Pr. 2020;26:536–42. 10.1177/1078155219851550. 10.1177/1078155219851550 [DOI] [PubMed] [Google Scholar]
- 81.Ebied M, Chan V. Multidisciplinary Professional Roles Addressing Needs in Multiple Myeloma: An Innovative ‘Virtual’ Pharmacist Surveillance Clinic. Semin Oncol Nurs. 2021;37:151173 10.1016/j.soncn.2021.151173. 10.1016/j.soncn.2021.151173 [DOI] [PubMed] [Google Scholar]
- 82.Fossum CC, Navarro S, Farias AJ, Ballas LK. Racial disparities in the use of palliative radiotherapy for black patients with multiple myeloma in the United States. Leuk Lymphoma. 2021;62:3235–43. 10.1080/10428194.2021.1953012. 10.1080/10428194.2021.1953012 [DOI] [PubMed] [Google Scholar]
- 83.Sun LF, Maples KT, Hall KH, Liu Y, Cao Y, Joseph NS, et al. Impact of Black Race on Peripheral Neuropathy in Patients With Newly Diagnosed Multiple Myeloma Receiving Bortezomib Induction. JCO Oncol Pr. 2023;19:793–8. 10.1200/OP.22.00781. 10.1200/OP.22.00781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al Hadidi S, Dongarwar D, Salihu HM, Kamble RT, Lulla P, Hill LC, et al. Health disparities experienced by Black and Hispanic Americans with multiple myeloma in the United States: a population-based study. Leuk Lymphoma. 2021;62:3256–63. 10.1080/10428194.2021.1953013. 10.1080/10428194.2021.1953013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belcher SM, Watkins Bruner D, Hofmeister CC, Kweon J, Meghani SH, Yeager KA. Characterizing Pain Experiences: African American Patients With Multiple Myeloma Taking Around-the-Clock Opioids. Clin J Oncol Nurs. 2020;24:538–46. 10.1188/20.CJON.538-546. 10.1188/20.CJON.538-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi H, Ren H, Tian Y, Chen Z, Xu C, Lu L, et al. Pain as a risk factor of depression and anxiety symptoms with multiple myeloma during maintenance therapy. Front Psychol. 2022;13:1015497 10.3389/fpsyg.2022.1015497. 10.3389/fpsyg.2022.1015497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jespersen E, Nielsen LK, Larsen RF, Moller S, Jarlbaek L. Everyday living with pain - reported by patients with multiple myeloma. Scand J Pain. 2021;21:127–34. 10.1515/sjpain-2020-0087. 10.1515/sjpain-2020-0087 [DOI] [PubMed] [Google Scholar]
- 88.Liou KT, Ashare R, Worster B, Jones KF, Yeager KA, Acevedo AM, et al. SIO-ASCO guideline on integrative medicine for cancer pain management: implications for racial and ethnic pain disparities. JNCI Cancer Spectr. 2023;7 10.1093/jncics/pkad042. [DOI] [PMC free article] [PubMed]
- 89.Ludwick A, Corey K, Meghani S. Racial and Socioeconomic Factors Associated with the Use of Complementary and Alternative Modalities for Pain in Cancer Outpatients: An Integrative Review. Pain Manag Nurs. 2020;21:142–50. 10.1016/j.pmn.2019.08.005. 10.1016/j.pmn.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 90.Kistler KD, Kalman J, Sahni G, Murphy B, Werther W, Rajangam K, et al. Incidence and Risk of Cardiac Events in Patients With Previously Treated Multiple Myeloma Versus Matched Patients Without Multiple Myeloma: An Observational, Retrospective, Cohort Study. Clin Lymphoma Myeloma Leuk. 2017;17:89–96 e3. 10.1016/j.clml.2016.11.009. 10.1016/j.clml.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 91.Fontes Oliveira M, Naaktgeboren WR, Hua A, Ghosh AK, Oakervee H, Hallam S, et al. Optimising cardiovascular care of patients with multiple myeloma. Heart. 2021;107:1774–82. 10.1136/heartjnl-2020-318748. 10.1136/heartjnl-2020-318748 [DOI] [PubMed] [Google Scholar]
- 92.Plummer C, Driessen C, Szabo Z, Mateos MV. Management of cardiovascular risk in patients with multiple myeloma. Blood Cancer J. 2019;9:26 10.1038/s41408-019-0183-y. 10.1038/s41408-019-0183-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee DH, Fradley MG. Cardiovascular Complications of Multiple Myeloma Treatment: Evaluation, Management, and Prevention. Curr Treat Options Cardiovasc Med. 2018;20:19 10.1007/s11936-018-0618-y. 10.1007/s11936-018-0618-y [DOI] [PubMed] [Google Scholar]
- 94.Rhee JW, Pillai R, He T, Bosworth A, Chen S, Atencio L, et al. Clonal Hematopoiesis and Cardiovascular Disease in Patients With Multiple Myeloma Undergoing Hematopoietic Cell Transplant. JAMA Cardiol. 2024;9:16–24. 10.1001/jamacardio.2023.4105. 10.1001/jamacardio.2023.4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balogun O, Jackson I, Oyenuga M, Oyenuga A, Etuk A, Jackson N. Burden of arrhythmias and predictors of mortality among multiple myeloma patients with arrhythmias. J Investig Med. 2022;70:1381–6. 10.1136/jim-2021-002321. 10.1136/jim-2021-002321 [DOI] [PubMed] [Google Scholar]
- 96.Ohman RE, Yang EH, Abel ML. Inequity in Cardio-Oncology: Identifying Disparities in Cardiotoxicity and Links to Cardiac and Cancer Outcomes. J Am Heart Assoc. 2021;10:e023852 10.1161/JAHA.121.023852. 10.1161/JAHA.121.023852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Camilli M, La Vecchia G, Lillo R, Iannaccone G, Lamendola P, Montone RA, et al. Cardiovascular involvement in patients affected by multiple myeloma: a comprehensive review of recent advances. Expert Rev Hematol. 2021;14:1115–28. 10.1080/17474086.2021.2003704. 10.1080/17474086.2021.2003704 [DOI] [PubMed] [Google Scholar]
- 98.Koshiaris C, Van den Bruel A, Nicholson BD, Lay-Flurrie S, Hobbs FR, Oke JL. Clinical prediction tools to identify patients at highest risk of myeloma in primary care: a retrospective open cohort study. Br J Gen Pr. 2021;71:e347–e355. 10.3399/BJGP.2020.0697. 10.3399/BJGP.2020.0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koshiaris C, Oke J, Abel L, Nicholson BD, Ramasamy K, Van den Bruel A. Quantifying intervals to diagnosis in myeloma: a systematic review and meta-analysis. BMJ Open. 2018;8:e019758 10.1136/bmjopen-2017-019758. 10.1136/bmjopen-2017-019758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seesaghur A, Petruski-Ivleva N, Banks VL, Wang JR, Abbasi A, Neasham D, et al. Clinical features and diagnosis of multiple myeloma: a population-based cohort study in primary care. BMJ Open. 2021;11:e052759 10.1136/bmjopen-2021-052759. 10.1136/bmjopen-2021-052759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howell DA, Hart RI, Smith AG, Macleod U, Patmore R, Cook G, et al. Myeloma: Patient accounts of their pathways to diagnosis. PLoS One. 2018;13:e0194788 10.1371/journal.pone.0194788. 10.1371/journal.pone.0194788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryskina KL, Shultz K, Zhou Y, Lautenbach G, Brown RT. Older adults’ access to primary care: Gender, racial, and ethnic disparities in telemedicine. J Am Geriatr Soc. 2021;69:2732–40. 10.1111/jgs.17354. 10.1111/jgs.17354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lueckmann SL, Hoebel J, Roick J, Markert J, Spallek J, von dem Knesebeck O, et al. Socioeconomic inequalities in primary-care and specialist physician visits: a systematic review. Int J Equity Health. 2021;20:58 10.1186/s12939-020-01375-1. 10.1186/s12939-020-01375-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown EJ, Polsky D, Barbu CM, Seymour JW, Grande D. Racial Disparities In Geographic Access To Primary Care In Philadelphia. Health Aff. 2016;35:1374–81. 10.1377/hlthaff.2015.1612. 10.1377/hlthaff.2015.1612 [DOI] [PubMed] [Google Scholar]
- 105.Landon BE, Onnela JP, Meneades L, O’Malley AJ, Keating NL. Assessment of Racial Disparities in Primary Care Physician Specialty Referrals. JAMA Netw Open. 2021;4:e2029238 10.1001/jamanetworkopen.2020.29238. 10.1001/jamanetworkopen.2020.29238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary Care Physicians Who Treat Blacks and Whites. N Engl J Med. 2004;351:575–84. 10.1056/NEJMsa040609 [DOI] [PubMed] [Google Scholar]
- 107.Chien AT, Chin MH, Alexander GC, Tang H, Peek ME. Physician financial incentives and care for the underserved in the United States. Am J Manag Care. 2014;20:121–9. [PMC free article] [PubMed] [Google Scholar]
- 108.Chien AT, Wroblewski K, Damberg C, Williams TR, Yanagihara D, Yakunina Y, et al. Do physician organizations located in lower socioeconomic status areas score lower on pay-for-performance measures. J Gen Intern Med. 2012;27:548–54. 10.1007/s11606-011-1946-8. 10.1007/s11606-011-1946-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tawfik B, Jaffe SA, Mohler L, Oomen-Hajagos J, Gil IS, Chamberlain R, et al. Developing a survivorship care plan (SCP) delivery process for patients and primary care providers serving poor, rural, and minority patients with cancer. Support Care Cancer. 2021;29:5021–8. 10.1007/s00520-021-06043-w. 10.1007/s00520-021-06043-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamlish T, Liu L, Zhang Z, Sohmer D, Moton Z, Johnson D, et al. Care Coordination for Breast Cancer Survivors in Urban Underserved Communities: Will Treatment Summaries and Survivorship Care Plans Be Enough? J Racial Ethn Health Disparities. 2020;7:577–83. 10.1007/s40615-019-00687-5. 10.1007/s40615-019-00687-5 [DOI] [PubMed] [Google Scholar]
- 111.Faiman B, Faiman M. Living with hematologic cancer: Recommendations, solutions. Cleve Clin J Med. 2017;84:528–34. 10.3949/ccjm.84a.15159. 10.3949/ccjm.84a.15159 [DOI] [PubMed] [Google Scholar]
- 112.Van Poznak CH, Unger JM, Darke AK, Moinpour C, Bagramian RA, Schubert MM, et al. Association of Osteonecrosis of the Jaw With Zoledronic Acid Treatment for Bone Metastases in Patients With Cancer. JAMA Oncol. 2021;7:246–54. 10.1001/jamaoncol.2020.6353. 10.1001/jamaoncol.2020.6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sloan FA, Picone G, Brown DS, Lee PP. Longitudinal analysis of the relationship between regular eye examinations and changes in visual and functional status. J Am Geriatr Soc. 2005;53:1867–74. 10.1111/j.1532-5415.2005.53560.x. 10.1111/j.1532-5415.2005.53560.x [DOI] [PubMed] [Google Scholar]
- 114.Baughman C, Norman K, Mukamal K. Adherence to American Cancer Society Nutrition and Physical Activity Guidelines Among Cancer Survivors. JAMA Oncol. 2024;10.1001/jamaoncol.2024.0470. [DOI] [PMC free article] [PubMed]
- 115.Malik MA, Sweeney NW, Jafri M, Derkach A, Chmielewski C, Adintori PA, et al. Nutrition perceptions, needs and practices among patients with plasma cell disorders. Blood Cancer J. 2022;12:70 10.1038/s41408-022-00666-w. 10.1038/s41408-022-00666-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nicol JL, Hill MM, Burton NW, Skinner TL. Promoting exercise for patients with multiple myeloma: attitudes and practices of clinical haematologists. J Cancer Surviv. 2022;16:688–95. 10.1007/s11764-021-01062-2. 10.1007/s11764-021-01062-2 [DOI] [PubMed] [Google Scholar]
- 117.Shah UA, Parikh R, Castro F, Bellone M, Lesokhin AM. Dietary and microbiome evidence in multiple myeloma and other plasma cell disorders. Leukemia. 2023;37:964–80. 10.1038/s41375-023-01874-4. 10.1038/s41375-023-01874-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeevanantham D, Rajendran V, McGillis Z, Tremblay L, Lariviere C, Knight A. Mobilization and Exercise Intervention for Patients With Multiple Myeloma: Clinical Practice Guidelines Endorsed by the Canadian Physiotherapy Association. Phys Ther. 2021;101:10.1093/ptj/pzaa180. [DOI] [PMC free article] [PubMed]
- 119.Neparidze N, Godara A, Lin D, Le HH, Fixler K, Shea L, et al. A Mixed Methods Study to Describe the Relationship between Burden of Illness, Social Needs, and Identity Experiences Among Patients with Multiple Myeloma. Blood. 2023;1421:7353–7353. 10.1182/blood-2023-178893. 10.1182/blood-2023-178893 [DOI] [Google Scholar]
- 120.Dowling E, Sowton M, Correia N, Simpson D. NS-63: Adapting and improving a Myeloma Support group during the pandemic. Clin Lymphoma Myeloma Leuk. 2021;21:S166–S167. 10.1016/s2152-2650(21)02357-0. 10.1016/s2152-2650(21)02357-0 [DOI] [Google Scholar]
- 121.de Wet R, Lane H, Tandon A, Augustson B, Joske D. ‘It is a journey of discovery’: living with myeloma. Support Care Cancer. 2019;27:2435–42. 10.1007/s00520-018-4502-9. 10.1007/s00520-018-4502-9 [DOI] [PubMed] [Google Scholar]
- 122.National Cancer Institute. Cancer Stat Facts: Myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed 2024 May 3.
- 123.Mateos MV, Ailawadhi S, Costa LJ, Grant SJ, Kumar L, Mohty M, et al. Global disparities in patients with multiple myeloma: a rapid evidence assessment. Blood Cancer J. 2023;13:109 10.1038/s41408-023-00877-9. 10.1038/s41408-023-00877-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ludwig H, Novis Durie S, Meckl A, Hinke A, Durie B. Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist 2020;25:e1406–e1413. 10.1634/theoncologist.2020-0141. 10.1634/theoncologist.2020-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Geng J, Zhao J, Fan R, Zhu Z, Zhang Y, Zhu Y, et al. Global, regional, and national burden and quality of care of multiple myeloma, 1990-2019. J Glob Health. 2024;14:04033 10.7189/jogh.14.04033. 10.7189/jogh.14.04033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cowan AJ, Baldomero H, Atsuta Y, Mikhael J, Aljurf M, Seber A, et al. The Global State of Hematopoietic Cell Transplantation for Multiple Myeloma: An Analysis of the Worldwide Network of Blood and Marrow Transplantation Database and the Global Burden of Disease Study. Biol Blood Marrow Transpl. 2020;26:2372–7. 10.1016/j.bbmt.2020.08.018. 10.1016/j.bbmt.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ganguly S, Mailankody S, Ailawadhi S. Many Shades of Disparities in Myeloma Care. Am Soc Clin Oncol Educ Book. 2019;39:519–29. 10.1200/EDBK_238551. 10.1200/EDBK_238551 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.