Abstract
Binding of the platelet GPIb/V/IX (glycoprotein Ib/V/IX) receptor to von Willebrand factor is critical for platelet adhesion and aggregation under conditions of rapid blood flow. The adhesive function of GPIbα is regulated by its anchorage to the membrane skeleton through a specific interaction with filamin A. In the present study, we examined the amino acid residues within the cytoplasmic tail of GPIbα, which are critical for association with filamin A, using a series of 25-mer synthetic peptides that mimic the cytoplasmic tail sequences of wild-type and mutant forms of GPIbα. Peptide binding studies of purified human filamin A have demonstrated a major role for the conserved hydrophobic stretch L567FLWV571 in mediating this interaction. Progressive alanine substitutions of triple, double and single amino acid residues within the Pro561–Arg572 region suggested an important role for Trp570 and Phe568 in promoting GPIbα binding to filamin A. The importance of these two residues in promoting filamin A binding to GPIbα in vivo was confirmed from the study of Chinese-hamster ovary cells expressing GPIbα Trp570→Ala and Phe568→Ala substitutions. Phenotypic analysis of these cell lines in flow-based adhesion studies revealed a critical role for these residues in maintaining receptor anchorage to the membrane skeleton and in maintaining cell adhesion to a von Willebrand factor matrix under high-shear conditions. These studies demonstrate a novel filamin A binding motif in the cytoplasmic tail of GPIbα, which is critically dependent on both Trp570 and Phe568.
Keywords: adhesion receptor, cytoplasmic domain, filamin A, GPIb/V/IX, mutagenesis, platelet
Abbreviations: vWf, von Willebrand factor; BvWf, bovine vWf; CHO, Chinese-hamster ovary; DTT, dithiothreitol; GPIb, glycoprotein Ib; HRP, horseradish peroxidase; mAb, monoclonal antibody; RP-HPLC, reversed-phase HPLC; WT, wild-type
INTRODUCTION
Platelet adhesion and aggregation at sites of vascular injury is essential for the arrest of bleeding and is dependent on the synergistic contribution of multiple adhesion receptor–ligand interactions. Of central importance to the haemostatic response is the interaction between the platelet GPIb/V/IX (glycoprotein Ib/V/IX) receptor and vWf (von Willebrand factor), an adhesive event that is critical for initiating platelet–vessel wall interactions, particularly under high-shear conditions [1]. This receptor–ligand interaction also plays an important role in initiating platelet activation through the generation of intracellular signals that up-regulate the adhesive function of integrin αIIbβ3, a key adhesion receptor that promotes platelet aggregation and thrombus growth (reviewed in [2]).
The GPIb/V/IX receptor complex is composed of four subunits, GPIbα, GPIbβ, GPIX and GPV (reviewed in [3]), the most important of which is GPIbα from a functional perspective. This subunit consists of a large extracellular domain that incorporates the binding site for vWf (residues 1–279), a single transmembrane domain, and a 96 residue C-terminal cytoplasmic domain (residues 515–610) that binds the actin-binding protein filamin A [4], and the signalling adaptor protein, 14-3-3ζ [5]. The association between GPIbα and filamin A links the GPIb/V/IX receptor to the underlying actin membrane skeleton [6], an association that is considered important for maintaining the cytoskeletal architecture of resting platelets.
The filamins (including filamin A) are a family of actin-binding proteins that have traditionally been supposed to act principally as cytoskeletal scaffolding proteins involved in the branching and cross-linking of actin filaments [7,8]. However, it is becoming increasingly clear that their biological functions are significantly more diverse. An increasing number of transmembrane receptors and signalling molecules bind filamin A, most of them (including GPIbα) through one or more of the C-terminal repeat regions that lie beyond the first hinge region [7]. However, the specific-binding sites involved, and the regulation of these interactions have not been well characterized owing in part to a lack of homology between the different binding partners of filamin A. The function of a number of filamin A-associated receptors has recently been demonstrated to depend on filamin A association, including dopamine (D2) receptor signalling [9], trafficking of the μ opioid receptor [10] and the regulation of Kv4.2 potassium channel current density [11]. Thus an improved understanding of the molecular mechanisms underlying filamin A–protein interactions may provide insights into their mode of regulation.
Previous studies have demonstrated that the vWf–GPIb interaction can be negatively regulated by the platelet cytoskeleton, and that this regulatory mechanism is dependent on the interaction between GPIbα and filamin A [12,13]. Disruption of the GPIbα–filamin A interaction leads to an inability of GPIbα to maintain cell adhesion under high shear, due to a defect in membrane anchorage of the receptor [14]. In contrast, Schade et al. [15] recently suggested that deletions to the cytoplasmic tail of GPIbα, which prevent the association with filamin A, result in a constitutive decrease in the strength of the vWf–GPIbα bond irrespective of the prevailing shear conditions. Interestingly, a recent study by Feng et al. [16], using a cell permeable peptide corresponding to the 557–575 region of GPIbα, has suggested that the GPIbα–filamin A interaction may be dynamically regulated under conditions of high shear, promoting shear-induced platelet aggregation. However, despite the potential importance of the GPIbα–filamin A interaction for normal platelet function, the molecular basis underlying this association still remains poorly defined.
Previous studies, both from our laboratory and also by others, have attempted to delineate the specific region within the GPIbα tail required for the interaction with filamin A, and based on available evidence the primary filamin A binding domain appears to involve residues within the 551–579 region [13,14,16,17]. The majority of studies to date, examining GPIbα–filamin A interactions, have utilized transfected cell lines expressing relatively large deletions in the cytoplasmic domain of GPIbα [13,14,16–19]. Such gross deletions throughout various regions of the cytoplasmic tail may produce a variety of different conformational alterations that lead to secondary effects unrelated to changes in filamin A binding, perhaps providing one potential explanation for the apparent discrepancies among previously published studies. In the present study, we have examined critical amino acid residues within the cytoplasmic tail of GPIbα, necessary for binding filamin A. We have demonstrated a key role for the conserved hydrophobic stretch L567FLWV571 in mediating this interaction. Analysis of a series of peptides and GPIbα tail mutants containing single and double amino acid substitutions has defined an important role for Trp570 and Phe568 in promoting GPIbα binding to filamin A. We demonstrate that these residues are critical for maintaining GPIbα receptor anchorage to the membrane skeleton and for maintaining cell adhesion to a vWf matrix under high-shear conditions.
MATERIALS AND METHODS
Materials
HRP (horseradish peroxidase)-conjugated anti-mouse IgG antibody and streptavidin-coated microtitre plates were purchased from Chemicon International (Temecula, CA, U.S.A.). Anti-filamin A antibody NCL-FIL was purchased from NovoCastra Laboratories (Newcastle, U.K.). Permafluor was purchased from Immunotech (Beckman Coulter, Gladesville, NSW, Australia). Avidin, Sigma FASTTM O-phenylenediamine dihydrochloride tablets and thioanisole were purchased from Sigma (St. Louis, MO, U.S.A.). All reagents for peptide synthesis were purchased from Auspep (Melbourne, Australia). Acetonitrile and phenol were from Merck (Darmstadt, Germany). Vent® DNA polymerase and all restriction enzymes were from New England Biolabs (Beverley, MA, U.S.A.) and dNTPs were from Amersham Biosciences (Castle Hill, NSW, Australia). The expression plasmid containing full-length GPIbα (pDX-Ibα) and CHO (Chinese-hamster ovary) cells co-expressing GPIbβ and GPIX (CHO-β/IX) were kindly provided by Dr J. Lopez (Baylor College of Medicine, Houston, TX, U.S.A.). All other reagents were from sources described previously [14,19].
Peptide synthesis and biotinylation
Peptides were synthesized using Fmoc (fluoren-9-ylmethoxycarbonyl)-solid phase peptide synthesis methods on a PS3 automated solid-phase peptide synthesizer (Ranin Instrument Company, Woburn, MA, U.S.A.). The peptide-resin was cleaved with reagent K (trifluoroacetic acid/phenol/H2O/thioanisole/1,2-ethandedithiol in the ratio 82.5:5:5:5:2.5, by vol.), and the crude peptide was purified using RP-HPLC (reversed-phase HPLC; Waters 600 Series HPLC, Milford, MA, U.S.A.). Peptides were eluted from a Phenomenex Luna C-8 column (250 mm long and 20 mm inner diameter) at 5 ml/min using a 0–60% CH3CN/H2O gradient in 0.1% trifluoroacetic acid. Fractions were analysed by liquid chromatography-MS (Micromass Technologies ZQ4000, Waters 2795 Separations Module). For retention time analysis by RP-HPLC, peptides were eluted from a Phenomenex Luna C8 analytical column (100 mm long and 4.5 mm inner diameter) at 1.0 ml/min using a 0–80% CH3CN/H2O gradient in 0.1% aqueous trifluoroacetic acid. For biotinylation of peptides, 2 mg of N-hydroxysuccinimido-linked biotin was added to 2 mg of purified peptide (10:1 molar ratio) in 1 M phosphate buffer (pH 7.4) and stirred overnight at room temperature (22 °C). Biotinylated peptide was purified using RP-HPLC as described above and analysed using liquid chromatography-MS.
Filamin A purification
Filamin A was purified from human platelets according to a modified method of Schaier [20]. Briefly, whole blood was collected from healthy volunteers and added to the anti-coagulant (100 mM NaCl, 50 mM Tris/HCl, 10 mM EDTA, 70 mM theophylline, pH 7.4 and 100 μM prostaglandin I2). Blood was centrifuged at 200 g for 30 min to obtain platelet-rich plasma followed by centrifugation at 2000 g for 20 min to obtain a platelet pellet that was resuspended in platelet wash buffer (150 mM NaCl, 2 mM EDTA, 10 mM Tris/HCl and 100 μM prostaglandin I2, pH 7.4) and washed twice more in platelet wash buffer. The final platelet pellet was lysed at 4 °C in lysis buffer [3 mM EDTA, 0.5% Triton X-100, 50 mM Tris/HCl, pH 7.5, 0.5 mM DTT (dithiothreitol), 50 μg/ml calpeptin, 1 μg/ml leupeptin and 1 tablet of Complete™ Mini EDTA-free protease inhibitor cocktail], then homogenized and sonicated at 4 °C, and the resulting slurry was centrifuged at 100000 g for 3 h at 4 °C. The pellet was resuspended in high ionic strength buffer (50 mM KH2PO4, 0.6 mM KCl, 5 mM EDTA, 10 mM ATP, 5 mM DTT, 1 μg/ml leupeptin and Complete™ EDTA-free, pH 7.0) and stirred overnight at 4 °C. Insoluble material containing mostly filamentous actin was pelleted by centrifugation at 100000 g for 90 min at 4 °C and the filamin-A-containing soluble fraction was diluted 10-fold with equilibration buffer (10 mM Tris/HCl, 5 mM EDTA and 0.5 mM DTT, pH 8.0). Filamin A was purified using anion-exchange chromatography on a BioRad Bio-Logic Duo Flow liquid chromatography system. SDS/PAGE (5% polyacrylamide) analysis identified fractions containing the correct molecular-mass protein, which was confirmed to be filamin A by immunoblotting using NCL-FIL (1:1000 dilution). Filamin-A-containing fractions were pooled and further purified using anion-exchange chromatography (Mono Q; Amersham Biosciences) to yield full-length, 280 kDa filamin A, free of any contaminating proteins as determined by Coomassie Blue staining.
Filamin A–GPIbα peptide binding assay
Biotinylated WT (wild-type; 15 pmol) or mutant GPIbα peptide was dissolved in binding buffer (10 mM Tris, 150 mM NaCl, 1 mM DTT and 0.1% BSA, pH 7.4) at 2 μg/ml and immobilized on streptavidin-coated microtitre plate wells by incubating for 30 min at room temperature. To determine maximal filamin A binding, increasing concentrations of purified filamin A (0–2.4 nmol) were incubated with immobilized WT peptide for 60 min at room temperature on a rotating platform, and based on the results from these experiments, 1.2 nmol filamin A was used in all subsequent studies (see Figure 1A). Unbound filamin A was removed by three washes in binding buffer containing 0.1% Tween 20. Bound filamin A was detected using NCL-FIL (1:1000 dilution for 1 h), followed by HRP-conjugated anti-mouse IgG mAb (monoclonal antibody; 1:10000 dilution for 30 min). HRP substrate (Sigma FASTTM O-phenylenediamine dihydrochloride) was prepared according to the manufacturer's instructions and 50 μl was added to each well and incubated for 15 min in the dark. The reaction was stopped with 50 μl of 2 M H2SO4 and the absorbance read at 490 nm using a BioRad benchmark microplate reader. For peptide competition binding studies, 1.2 nmol of purified filamin A was incubated with soluble competing peptide (0.1–10 μM for 25-mer peptides and 10–1000 μM for 5-mer peptides) for 60 min, then incubated for a further 60 min with immobilized WT peptide. Unbound filamin A and soluble peptide were removed by three washes and the amount of bound filamin A was measured as described above. The absorbance at 490 nm was expressed relative to the absorbance detected for filamin A binding to immobilized WT peptide, which was assigned as 100%. All incubations were performed in triplicate for each experiment.
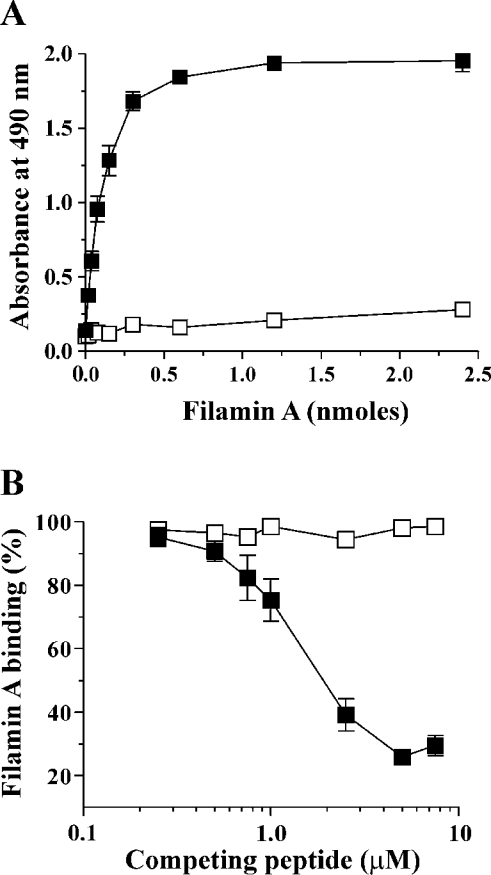
Figure 1. Binding of filamin A to WT and scrambled GPIbα peptides.
(A) To define the experimental conditions required to quantify the direct binding capacity of filamin A to WT and scrambled GPIbα peptides, filamin A (0–2.4 nmol) was incubated with 15 pmol of either immobilized biotinylated WT (■) or scrambled (Ibα-scr, □) peptide for 60 min as described in the Materials and methods section. Bound filamin A was detected using NCL-FIL mAb and HRP-conjugated secondary reagents for colorimetric detection at 490 nm as described in the Materials and methods section. Absorbances are presented as the means±S.D. for two experiments. Filamin A binding to WT peptide was saturable and reached a maximum between 0.6 and 1.2 nmol. (B) In competition binding experiments, 0.01–1 nmol of either soluble WT (■) or scrambled (Ibα-scr, □) peptide was incubated with filamin A (1.2 nmol) for 60 min, then incubated for a further 60 min with immobilized WT peptide. The level of bound filamin A was measured as described above and expressed relative to the absorbance detected for filamin A binding to immobilized WT peptide in the absence of competing soluble peptide, which was assigned as 100%. The results are presented as the means±S.E.M. for three experiments.
Generation of mutant GPIbα CHO cell lines
Alanine-substituted forms of GPIbα were generated using a two-step overlap extension PCR mutagenesis strategy by the method of Ho et al. [21], using the following mutagenic primer pairs: 5′-CAGCCTCGCCCTGTGGGTACG-3′ and 5′-CGTACCCACAGGGCGAGGCTG-3′ to generate a F568A (Phe568→Ala) GPIbα mutant; 5′-CCTCTTCCTGGCGGTACGGCC-3′ and 5′-GGCCGTACCGCCAGGAAGAGGC-3′ for W570A; 5′-GGGTAGCGCCTAATGGCC-3′ and 5′-GGCCATTAGGCGCTACCC-3′ for R572A; 5′-CCTCGCCCTGGCGGTACGGCC-3′ and 5′-GGCCGTACCGCCAGGGCGAGGC-3′ for the double FW-AA mutation. External primer sequences were 5′-CCCGTATCACTCTTAGAATCCACC-3′ and 5′-GCCTGTGTTGTGAAGACAGGGC-3′ for all mutants. The expression plasmid pDX-GPIbα containing full-length WT GPIbα was used as a template to generate single alanine-substituted plasmids (pDX-F568A, pDX-W570A and pDX-R572A), and pDX-F568A was used to generate the double mutant, pDX-FW-AA. An SmaI site upstream of the mutation site and a downstream DraIII site were utilized to clone mutagenic forms of GPIbα into SmaI–DraIII-digested pDX-GPIbα. Second round PCR product was first cloned into pUC19 and sequenced in both directions (Applied Biosystems ABI 373 DNA sequencer) to check the presence of the desired mutation and the absence of any additional PCR-generated mutations. pUC19-GPIbα was sequentially digested with SmaI and DraIII and the SmaI–DraIII-digested GPIbα fragment was ligated into SmaI–DraIII-digested pDX-GPIbα. CHO-β/IX cells were transfected using calcium phosphate precipitation as described previously [19]. The presence of GPIbα on the cell surface was confirmed by flow cytometry after labelling CHO cells with FITC-conjugated WM23 (anti-GPIbα mAb, 2 μg/ml) for 20 min. Flow cytometric analysis was performed on a FACSCalibur cytometer (Becton Dickinson, North Ryde, NSW, Australia).
Co-immunoprecipitation of GPIbα and filamin A from biotinylated CHO cells
Co-immunoprecipitation of biotinylated GPIbα and filamin A was performed as described previously [14]. Immunoprecipitated proteins were separated by SDS/PAGE (5% polyacrylamide), and transferred on to PVDF membranes. Membranes were blocked with 10% (w/v) BSA in PBS containing 0.05% Tween 20, and incubated for 1 h with a 1:25000 dilution of HRP-conjugated streptavidin. The membranes were washed three times with PBS and 0.05% Tween 20, once in PBS, and immunoprecipitated bands were detected using an enhanced chemiluminescence kit.
Flow-based CHO cell adhesion assays
Flow-based adhesion assays were performed as described previously using glass microslides (VitroCom, NJ, U.S.A.) coated with 10 μg/ml BvWf (bovine vWf) [19]. For cell-rolling velocity analysis, 25 cells were analysed from five separate fields for 20 s at each shear stress except at 6 Pa, where in some experiments there were insufficient cells remaining adherent to analyse 25 cells. In these instances, rolling velocities were calculated for all cells that still remained attached to the vWf matrix. Rolling velocities before cell detachment were analysed for WT, W570A and FW-AA cells (six cells for each cell line) by measuring the distance travelled over 2 s intervals for the 10–12 s period immediately before their detachment.
Analysis of GPIb–IX receptor extraction at high shear stress
Extraction of GPIb–IX from the cell membrane was performed as described previously [14] with minor modifications. Briefly, cells (1×106/ml) were perfused into BvWf-coated microslides (10 μg/ml) at 0.05 Pa for 5 min to maximize the number of adherent cells. Cells were then exposed to shear stresses of 6–8 Pa to detach at least 95% of cells (the shear stress required was dependent on the cell line). Microslides were then fixed with 2% (w/v) paraformaldehyde in PBS for 1 h and washed by perfusion of 500 μl of PBS. Any extracted receptor remaining on the vWf matrix was labelled with WM23 (anti-GPIbα mAb) at 1 μg/ml, diluted in PBS containing 1% BSA for 30 min, followed by Alexa488-conjugated anti-mouse IgG at 2 μg/ml under the same conditions. Microslides were washed by perfusion of 500 μl of PBS and then mounted in Permafluor. Fluorescence was visualized using confocal microscopy and images captured using Leica acquisition software (Leica TCS NT; Leica, Altona North, VIC, Australia).
Statistical analysis
Statistical analyses were performed using Student's unpaired t test and P<0.05 was considered significant. All data analysis, statistical analyses and graphic generation were performed using Prism software (Graphpad, San Diego, CA, U.S.A.).
RESULTS
Binding of filamin A to WT and triple alanine-substituted peptides
Our previous studies, using CHO cells transfected with cytoplasmic domain deletion mutants of GPIbα, suggested the involvement of a 23 amino acid region between Arg557 and Pro579 in mediating the interaction with filamin A. Within this region, lies a shorter sequence from Pro561 to Arg572, which is fully conserved between human, murine and canine GPIbα, suggesting functional importance. To define more precisely a specific region in the GPIbα cytoplasmic domain, required for the interaction with filamin A, we initially utilized synthetic peptides based on GPIbα residues Arg557–Val581 (Figure 2), and designed in vitro peptide–filamin A binding assays for this purpose. First, we established the specificity of the peptide–filamin A binding assays. In experiments designed to determine a direct interaction between GPIbα peptides and filamin A, either biotinylated WT or scrambled GPIbα (Ibα-scr) peptide was immobilized on to streptavidin-coated wells and incubated with increasing amounts of filamin A. The results in Figure 1(A) demonstrate a saturable, direct interaction between filamin A and WT peptide, which was specific, based on the inability of scrambled peptide to support filamin A binding. Filamin A binding to WT peptide reached a maximum between 0.6 and 1.2 nmol, and 1.2 nmol filamin A was used in all further experiments. An analysis of WT and scrambled peptides in a competition binding assay indicated that soluble WT peptide could efficiently compete with immobilized WT peptide for filamin A binding, achieving maximum competition at 7.5 μM of soluble competing peptide (Figure 1B). In contrast, the scrambled peptide was completely unable to compete with WT at all concentrations of competing peptide.
Figure 2. Amino acid sequences of wild-type, scrambled and alanine-substituted GPIbα peptides used in the present study.
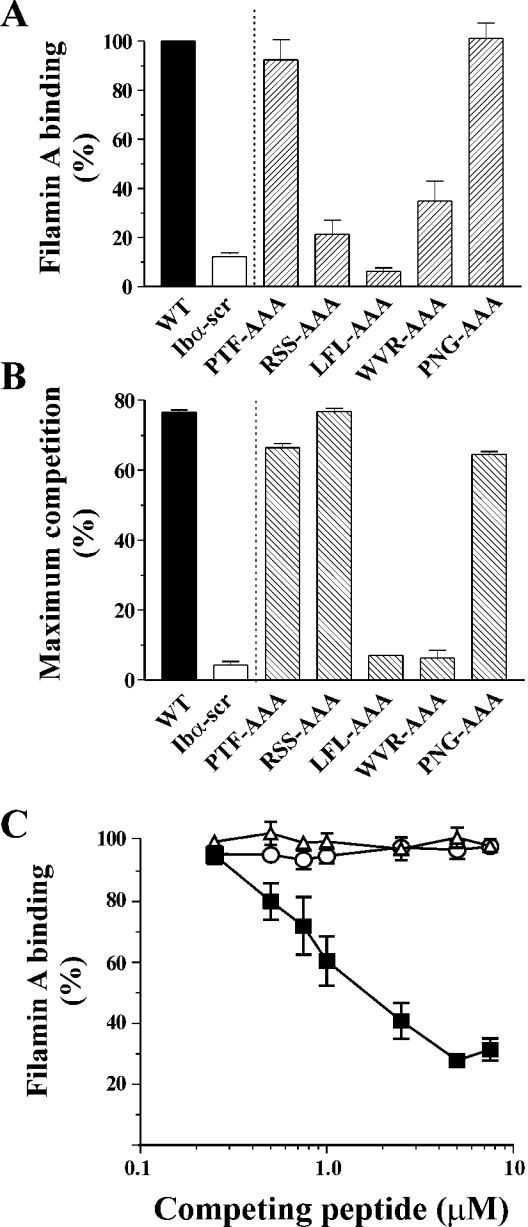
A series of five triple alanine-substituted peptides was then designed to span the conserved region of the GPIbα sequence (see Figure 2), and examined for filamin A binding. Significantly, of the triple-substituted peptides tested, only two peptides, LFL-AAA and WVR-AAA, showed a significant decrease in direct filamin A binding and a complete abrogation of competition binding (Figures 3A and 3B). The similar behaviour of the PTF-AAA and PNG-AAA peptides to WT (in both the direct and competition binding assays) suggests that a triple alanine substitution itself does not significantly affect the conformation of the peptides. The results for RSS-AAA indicated that, whereas soluble RSS-AAA peptide showed strong competition with immobilized WT for filamin A, immobilized RSS-AAA was not competent to bind filamin A, suggesting a potential difference between the ability of immobilized and soluble peptide to bind filamin A and to compete for filamin A binding. One possibility to explain this discrepancy may be that the peptide is induced to adopt a non-native binding mode on immobilization. The observation that only the LFL-AAA and WVR-AAA peptides exhibited significantly decreased filamin A binding suggested that hydrophobicity might be a key element to the binding of filamin A, and that the hydrophilic, charged residues are less important. A theoretical Hopp–Woods plot of these 25-mer peptides demonstrated a distinct hydrophobic region resulting from residues L567FLWV571, and that substitution of these residues to alanine had a marked effect on the hydrophobicity of this region. This analysis was consistent with corresponding changes in RP-HPLC retention times (results not shown). Despite the absence of any known protein-binding motifs in this sequence, the presence of a distinct hydrophobic region indicates a possible pattern contributing to protein–protein interactions. Consistent with this hypothesis, we found that peptides with single arginine to alanine substitutions across this region bound filamin A, and competed with WT for filamin A binding (see Figure 4). This result is also in agreement with the recent findings in CHO cells expressing similar arginine to alanine mutations that retained the filamin A–GPIbα interaction [16].
Figure 3. Binding of filamin A to triple alanine-substituted GPIbα peptides.
(A) Direct binding of filamin A to triple alanine-substituted peptides was performed by incubating 1.2 nmol filamin A with 15 pmol immobilized peptide as described in the Materials and methods section. Results (means±S.E.M. for four experiments) are expressed as the percentage of filamin A binding, relative to the binding measured for immobilized WT peptide which was assigned as 100%. (B) Competition binding experiments were performed as described in Figure 1 and the results (means±S.E.M. for three experiments) are expressed as the maximum percentage of competition. (C) Competition binding curves are shown for WT (■), LFL-AAA (△) and WVR-AAA (○). The level of bound filamin A is expressed relative to the binding detected for filamin A binding to immobilized WT peptide in the absence of competing soluble peptide, which was assigned as 100%. The data presented are the means±S.E.M. for six experiments.
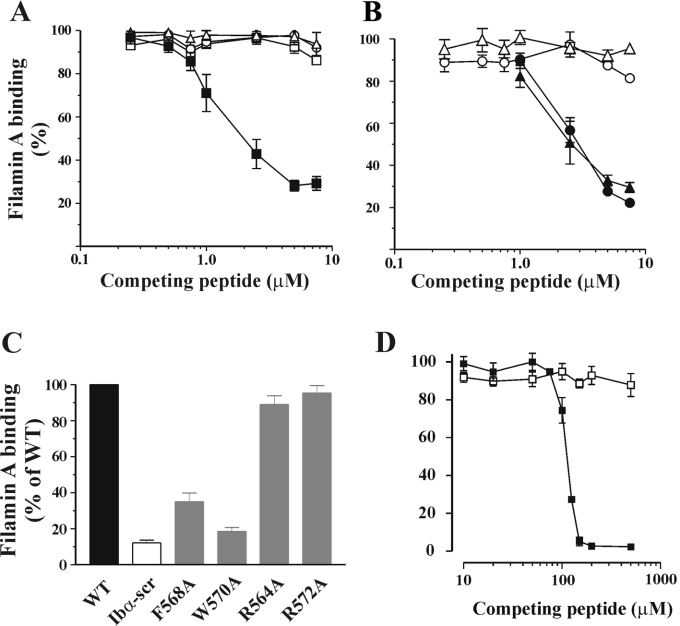
Figure 4. Binding of filamin A to double and single alanine-substituted peptides and 5-mer peptides.
(A) Competition binding experiments using double alanine-substituted peptides were performed as described for Figure 1. Competition binding curves are shown for WT (■), FW-AA (△), LF-AA (○) and FL-AA (□) and results are expressed as the percentage of filamin A binding relative to the binding measured for immobilized WT peptide in the absence of competing peptide, assigned as 100%. The results presented are the means±S.E.M. for four experiments and demonstrated that none of the double alanine mutants were able to compete with WT for filamin A binding. (B) Competition binding curves for single alanine mutants, F568A (△), W570A (○), R564A (▲) and R572A (●). The results presented are the means±S.E.M. for four experiments. Neither F568A nor W570A demonstrated any competition with WT for filamin A binding, whereas R564A and R572A exhibited similar competition as WT peptide. (C) Direct binding of filamin A to single alanine-substituted peptides was performed as described in the Materials and methods section. Filamin A binding to substituted peptides (means±S.E.M. for three or four experiments) is expressed as percentage of binding relative to WT, which was assigned as 100%. (D) Competition binding curves for 5-mer peptides LFLWV (■) and LFLAV (□). The results presented are the means±S.E.M. for five experiments.
Binding of filamin A to double and single alanine-substituted peptides
On the basis of our observations with the triple alanine-substituted peptides, and to more precisely define the residue substitutions that would abrogate binding, a second series of synthetic peptides with more conservative double and single alanine substitutions was prepared. Consistent with the results presented in Figure 3, substitution of hydrophobic residues had a profound effect on filamin A binding. None of the doubly mutated peptides, LF-AA, FL-AA and FW-AA (Figure 4A) or the single residue mutations, F568A and W570A (Figure 4B), were able to compete with WT for filamin A binding, and in most cases demonstrated a significant decrease in direct filamin A binding (Figure 4C; results not shown). In contrast, the two peptides containing single arginine to alanine substitutions (R564A and R572A) exhibited a filamin A interaction similar to WT (Figures 4B and 4C). The observations with the W570A peptide were somewhat unexpected, since the single tryptophan substitution abolished direct filamin A binding to a greater extent when compared with the triple-substituted WVR-AAA peptide. The reason for this difference is not clear. To investigate whether the hydrophobic residues contained within the L567FLWV571 sequence were sufficient to bind filamin A, a 5-mer peptide corresponding to amino acids 567–571 was examined in both the direct and indirect filamin A binding assays. As demonstrated in Figure 4(D), LFLWV was able to inhibit filamin A binding to the immobilized 25-mer WT peptide in a dose-dependent manner, giving maximal inhibition at 150 μM, an approx. 20-fold higher concentration compared with that required for the 25-mer WT peptide (see Figure 3C). This activity was critically dependent on Trp570, since the LFLAV peptide exhibited no competition with 25-mer WT for filamin A binding even at concentrations of the peptide as high as 500 μM. Analysis of these peptides in the direct filamin A binding assay was limited by the amount of peptide that could be immobilized on the streptavidin-coated wells (maximum 15 pmol). At these concentrations of peptide, no direct binding to filamin A was observed (results not shown). Taken together, these findings support a potentially important role for the LFLWV sequence in promoting the interaction between GPIb and filamin A.
Mutation of Phe568 and Trp570 disrupts the association between GPIbα and filamin A in CHO cells
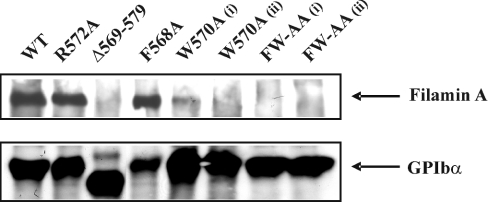
To establish more definitively the importance of hydrophobic residues in supporting the interaction between full-length GPIbα and filamin A, CHO-β/IX cells were stably transfected with GPIbα containing either one of the single mutations F568A, W570A or a combination of both mutations (FW-AA). An additional CHO cell line expressing a single alanine substitution at Arg572 (R572A) was also generated to serve as a control. Cell lines were successfully generated for all GPIbα mutations, and the surface expression of GPIbα was confirmed by flow cytometry (see Figure 6A). The ability of these substituted forms of GPIbα to interact with filamin A was determined by immunoprecipitation of the GPIb–IX complex from biotinylated CHO cell lysates. Consistent with our previous studies [14], in CHO cells expressing WT GPIbα, a 280 kDa protein band, corresponding to filamin A was co-precipitated with GPIb–IX (Figure 5). In contrast, the GPIbα–filamin A interaction was completely disrupted in cells expressing GPIbα containing the double FW-AA mutation. This result was confirmed for two different clones of this cell line (Figure 5, far right lanes). The ability of GPIbα to associate with filamin A in the cell lines expressing either of the single mutations (F568A or W570A) revealed a striking difference between the two. The specific mutation of W570A was sufficient to almost completely abolish the GPIbα–filamin A interaction, although a very weak residual association remains in two independent clones of W570A. Surprisingly, the single F568A mutation had no effect on the amount of filamin A co-immunoprecipitating with GPIb. This result is in contrast with the same mutation in the synthetic peptide binding analysis, which demonstrated that the F568A peptide exhibited no competition with WT for filamin A binding, although there was some residual direct interaction. It is possible that the profound effects induced by mutations within the hydrophobic region of the 25-mer peptide may be diluted when expressed in the full-length sequence. In control co-immunoprecipitation experiments, we confirmed that the interaction between GPIbα and 14-3-3ζ protein was preserved in all cell lines (results not shown), suggesting that neither of these residues are critical for 14-3-3ζ binding, and that these mutations have no long-range effect on the known 14-3-3ζ binding region. Overall, these results suggest that Trp570 plays a major, if not critical, role in the interaction between filamin A and GPIbα.
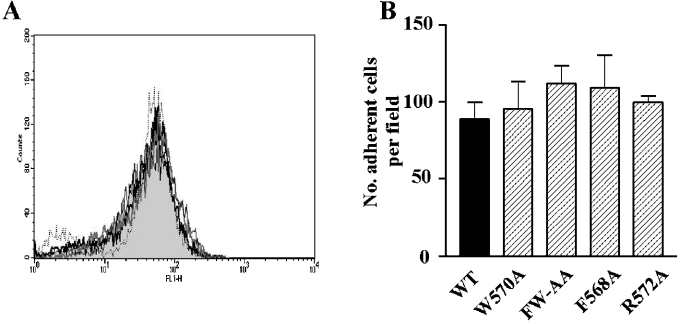
Figure 6. Analysis of GPIbα surface expression and the ability of CHO cells to adhere to vWf under shear conditions.
(A) Surface expression of GPIbα was analysed by flow cytometry as described in the Materials and methods section. GPIbα expression was similar for all cell lines; WT (grey-filled histogram), W570A (thick grey line), FW-AA, (black dotted line), F568A (thin black line) and R572A (thick black line). (B) Cells were perfused through BvWf-coated microslides (10 μg/ml) at a shear stress of 0.1 Pa. After 5 min of perfusion, the mean number of adherent cells/field over 5 fields was calculated and the results are expressed as the number of adherent cells/field (means±S.E.M.) for four to seven experiments.
Figure 5. Association of WT and mutant forms of GPIbα with filamin A.
Adherent CHO cells (8×106/ml) were detached, incubated with the membrane-permeable EZ-link-N-hydroxysuccinimidobiotin and lysed with 1% Triton X-100. GPIb–IX was immunoprecipitated using the anti-GPIbβ mAb (RAM.1), as described in the Materials and Methods section. Immunoprecipitated proteins were separated by SDS/PAGE (5% gel), transferred on to PVDF membranes, and the immunoprecipitated bands were detected by enhanced chemiluminescence. Mutation of Trp570 to alanine almost completely eliminated the co-immunoprecipitation of filamin A. The double mutation of Phe568 and Trp570 to alanine resulted in a complete disruption of the interaction between GPIb–IX complex and filamin A. These results are from one experiment, representative of five.
Effects of Phe568 and Trp570 mutations on CHO cell adhesion under flow
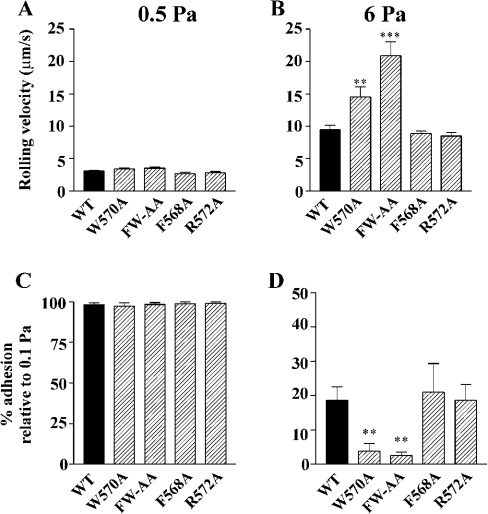
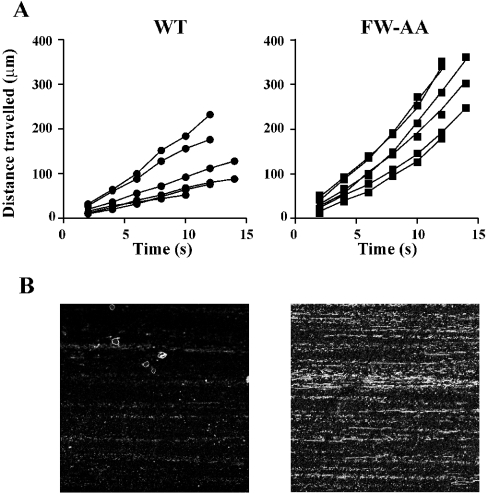
GPIb–IX-dependent adhesion of platelets and transfected cells to immobilized vWf is characterized by a reversible interaction resulting in cell translocation (rolling) on the vWf surface. We have previously demonstrated that disruption of the association between the GPIb–IX receptor complex and filamin A results in a time-dependent increase in cell-rolling velocity that is associated with increased detachment of cells from an immobilized vWf matrix at increased levels of shear stress [14]. Furthermore, we have demonstrated that this decrease in the ability of cells to remain adherent to vWf under increased shear conditions was a result of a defect in the membrane anchorage of the receptor. To determine whether the adhesion characteristics of the alanine-substituted GPIbα mutants were similarly affected, flow adhesion experiments were performed on a vWf matrix. For all flow experiments, GPIbα surface expression was measured by flow cytometry to ensure that all cell lines expressed the same level of receptor in each experiment (Figure 6A), since surface expression of GPIb–IX can have a significant effect on these results [19]. Cells were initially perfused at 0.1 Pa for 5 min followed by stepwise increases in shear stress to 0.5, 2, 4 and 6 Pa. At each shear stress, the number of adherent cells and their rolling velocity was analysed as described in the Materials and methods section. For all mutant cell lines tested, the number of adherent cells at the end of 5 min perfusion at 0.1 Pa was not significantly different from WT (Figure 6B), suggesting that none of the alanine substitutions had any effect on the association rate between GPIbα and vWf. Quantitative analysis of cell-rolling velocities at the lower shear stresses (0.5 and 2 Pa) revealed that there was no significant difference between the velocity of WT and any of the mutant cell lines (Figure 7A; results not shown), suggesting that the ligand-binding function of GPIbα is not affected by these mutations. However, at increased shear stress (6 Pa), both W570A and FW-AA cells rolled significantly faster when compared with WT (Figure 7B). In contrast, cells expressing either the F568A or R572A mutation behaved in a manner indistinguishable from WT cells (Figure 7B). An analysis of the ability of cells to remain attached to the vWf matrix at increasing levels of shear stress also revealed a significant difference between W570A, FW-AA and WT cells (Figures 7C and 7D). Both W570A and FW-AA cells exhibited a significantly impaired ability to remain adherent at high shear stress when compared with WT, whereas F568A and R572A cells were able to remain adherent at 6 Pa to a similar extent as WT cells. Taken together, these results suggest that the defect in the adhesive phenotype of W570A and FW-AA cells is restricted to a high-shear environment, since there was no significant difference between the ability of WT and any of the mutant cell lines to remain adherent at 0.5 Pa (Figure 7C). We have previously demonstrated that cell lines expressing GPIbα mutants that do not interact with filamin A exhibit accelerated rolling velocity at high shear stress, due to a critical defect in receptor anchorage [14]. A comparison of cell-rolling velocity between WT and FW-AA cells, over the period preceding the moment at which cells detached from the vWf surface, revealed that in contrast with WT cells that exhibited a constant velocity before detachment, FW-AA cells accelerated over the period before detachment (Figure 8A). These observations are consistent with our previous studies, which indicated a mechanism involving a defect in the ability of the GPIb/V/IX receptor to remain anchored in the cell membrane rather than a change in the dissociation rate of the vWf–GPIbα bond [14], as has been recently suggested by Schade et al. [15]. To confirm that this difference in adhesive behaviour was due to a defect in receptor anchorage, we performed an analysis of receptor extraction after cells had been detached at high shear as described previously [14]. This analysis revealed a clear difference between WT and FW-AA, with the presence of ‘tracks’ of GPIbα on the vWf surface only for FW-AA cells (Figure 8B). Similar results were obtained with W570A cells (results not shown). These observations indicate that the specific disruption of the GPIbα–filamin A interaction through the substitution of these two hydrophobic residues is sufficient to alter the ability of GPIb/V/IX to maintain adhesion to vWf under conditions of high shear stress.
Figure 7. Effects of alanine substitutions on GPIb–IX-dependent CHO cell-rolling velocity and detachment under shear conditions.
CHO cells were perfused through BvWf-coated microslides (10 μg/ml) for 5 min at a shear stress of 0.1 Pa, followed by stepwise increases in shear stress up to 6 Pa. (A, B) Cell-rolling velocities were analysed as described in the Materials and methods section and the results presented are the means±S.E.M. for four to seven experiments. At 0.5 Pa there were no significant differences in rolling velocities between WT and any of the mutant cell lines, whereas at 6 Pa, only W570A and FW-AA cells exhibited significantly faster velocities when compared with WT (P<0.01 and 0.001 respectively). (C, D) Cell detachment was analysed in the same experiments as described in the Materials and methods section and the results are presented as means±S.E.M. There was essentially no detachment of any of the cell lines at 0.5 Pa, but at 6 Pa both W570A and FW-AA were significantly less capable of remaining adherent (P<0.01 in both cases) when compared with WT. There was no significant difference between the adhesion of F568A and R572A cells compared with WT, for both rolling velocity and their ability to remain adherent at high shear stress.
Figure 8. Time-dependent changes to rolling velocity in detaching WT and FW-AA cells and extraction of GPIb–IX from the membrane under high-shear-stress conditions.
(A) Rolling velocities before cell detachment were analysed for WT and FW-AA cells (six cells for each cell line) by measuring the distance travelled over 2 s intervals for the 10–12 s period immediately before their detachment. WT cells exhibited a constant rate of translocation before detachment, whereas FW-AA cells exhibited a considerable time-dependent increase in translocation rate before the point of detachment. (B) Analysis of receptor extraction was performed as described in the Materials and methods section. Two representative confocal microscopic images are shown for each cell line from one experiment, representative of four independent experiments. Images were captured using identical photomultiplier tube settings on the confocal microscope and clearly demonstrate, for FW-AA cells, the presence of GPIbα receptor tracks after cells had been detached from the vWf matrix under high-shear conditions.
DISCUSSION
The results presented in this study identify a novel filamin A recognition sequence (L567FLWV571) within the cytoplasmic tail of GPIbα that is sufficient for the GPIbα–filamin A association. These residues lie within a sequence of 12 amino acids in the GPIbα cytoplasmic tail that is fully conserved amongst all mammalian species examined to date, including human, dog and mouse. Within this region we have identified an indispensable role for Trp570 and Phe568 in promoting the GPIbα–filamin A association. Moreover, through the analysis of CHO-GPIb–IX cell lines expressing GPIbα W570A and F568A substitutions, we have demonstrated an essential role for these residues in anchoring the GPIbα receptor to the membrane skeleton and for the maintenance of cell adhesion under high shear. These observations, combined with recent findings that hydrophobic interactions play an important role in mediating filamin A interaction with one other receptor [22,23], raise the possibility that a conserved binding mechanism regulates filamin A association with a subset of membrane proteins.
The findings for a critical role for the 567–571 hydrophobic sequence, and specific residues therein for GPIbα–filamin A interaction, are consistent with previous results from a number of laboratories. For example, deletion mutants involving residues 551–570 [13], 557–568 and 569–579 [14] and 560–570 [16] have all been demonstrated to abrogate the association between GPIbα and filamin A. However, the interpretation of such findings has been complicated by the possibility that such large deletions may produce secondary conformational changes within the tail that indirectly influence the association between GPIbα and filamin A. The demonstration that the L567FLWV571 peptide is capable of competing with the 25-residue WT peptide for filamin A binding suggests that this motif interacts with filamin A directly. We have also demonstrated a critical role for Trp570 in this pentapeptide sequence. Whether the recognition of filamin A by L567FLWV571 is sequence-specific or primarily reflects the strong hydrophobic nature of this peptide remains to be established. In preliminary studies, we have demonstrated that a ‘scrambled’ form of the LFLWV peptide is also capable of competing with the larger 25-residue WT peptide for filamin A binding (S. L. Cranmer, unpublished work), suggesting that hydrophobicity is important for this interaction. However, more detailed mutagenic studies will be required to clarify this issue. The demonstration that the L567FLWV571 peptide had an apparent affinity 20-fold lower than the 25 residue WT peptide was not unexpected in light of previous observations with small RGD-containing peptides. For example, RGD-based peptide fragments from fibronectin or fibrinogen have a much lower affinity for integrins compared with the native protein [24], indicating the involvement of additional recognition sequences in the full-length protein. Evidence supporting the potential involvement of additional residues outside the L567FLWV571 sequence in filamin A binding includes the demonstration that deletion of sequences flanking L567FLWV571, 571–589 [16], 580–590 [14] and truncation of the GPIbα tail from the residue 576 to the C-terminus (P. Mangin, unpublished work), affects filamin A binding to GPIbα. However, with respect to the fully conserved Pro561–Arg572 region, the recent study by Feng et al. [16] failed to identify a critical role for any other residues within this region using a similar strategy involving substitutions to alanine. A more detailed mutational analysis, combined with structural information on the GPIbα tail, will be needed to delineate more clearly the role of L567FLWV571 flanking sequences in regulating the GPIbα–filamin A interaction. A preliminary NMR analysis of the peptides used in the present study has not revealed any specific recognizable structural motif for either WT or the W570A peptide in aqueous solution. Any additional insight into whether this residue is directly involved in filamin A binding or whether it affects the conformation of the filamin A binding site will require structural studies of the GPIbα tail, alone and in complex with filamin A.
The cytoplasmic tail of GPIbα has high sequence conservation and three regions in particular (containing six or more consecutive residues) are fully conserved across species, suggesting potentially important functional roles. One of these sequences represents a second hydrophobic domain, comprising residues A551WLLFL556, although our previous mutational studies have excluded a major role for this region in promoting GPIbα–filamin A interaction [14]. A second fully conserved region incorporates A582GRRPSALS590, and it remains possible that residues within this sequence are important for the GPIbα–filamin A interaction. However, it is noteworthy that the serine residues contained within this region have recently been demonstrated to play a major role in 14-3-3ζ binding [25] and it is possible that this region of GPIbα may be involved in co-ordinating receptor interaction with both filamin A and 14-3-3ζ. Finally, a stretch of seven conserved residues G592RGQDLL598 is present near the C-terminus, and although there is no evidence that it interacts with filamin A, this region may represent a binding motif for other proteins.
The ability of filamin A to form functionally important interactions with a wide range of proteins has been well established, although in virtually all cases the molecular basis for the association has not been defined. Within filamin A itself, it is primarily the C-terminal part of the protein that is involved in mediating protein–protein interactions (excluding the specialized N-terminal actin-binding domain); however, potential binding sites have only been resolved to the level of repeat regions. For GPIbα, repeat regions 17–19 of filamin A contain the binding site for GPIbα [26]; however, these regions comprise 269 amino acids and there is currently no information available on key recognition sequences and the mode of interaction between filamin A and GPIbα. Similarly, there is limited insight into the key binding sequences utilized by proteins that bind directly to filamin A. On the basis of studies using the yeast-two hybrid system, the Kv4.2 potassium channel has been proposed to bind filamin A by means of a proline-rich consensus SH3 motif, PTPP [11]. Perhaps of more direct relevance to our findings, related to GPIbα and filamin A, is the demonstration that a short hydrophobic region within the cytoplasmic tail of the type 7b glutamate receptor is essential for an association between repeats 21 and 22 of filamin A [22,23]. Although this region is distinct from the region required for the GPIbα–filamin A association, the hydrophobic nature of this region might point towards a common binding mechanism.
Four other proteins have been identified to bind within the same repeat regions of filamin A as GPIbα, including the D2 and D3 dopamine receptors [9,27], the tumour necrosis factor receptor-associated factor 2, TRAF2 [28], and the androgen receptor [29]. Of these, only the D2 and D3 dopamine receptors exhibit any sequence similarity with GPIbα [9]; however, neither receptor contains the highly conserved hydrophobic region present in GPIbα. In fact, based on findings from yeast two-hybrid analysis, a conserved serine residue may be involved in mediating dopaminergic receptor interaction with filamin A, since mutation to aspartic acid (to mimic phosphorylation), resulted in an approx. 50% decrease in filamin A binding [9]. Progress in understanding the molecular basis of association between filamin A and its binding partners and whether there are conserved binding mechanisms regulating these interactions will require more detailed analysis of the critical C-terminal binding sequences of filamin A.
Much remains to be learnt with respect to the exact nature of the filamin A binding interaction with the GPIbα cytoplasmic tail, and the contribution of residues flanking the L567FLWV571 sequence in regulating this interaction. Although our studies have clearly defined an important role for both Trp570 and Phe568 in promoting GPIbα binding to filamin A, there are clear differences in the requirement for each of these residues in this process. The identification of two key residues promoting GPIbα–filamin A binding will allow for the generation of transgenic mice that express these mutations, allowing direct analysis of the importance of the GPIbα–filamin A interaction for platelet function in vivo, while minimizing potential secondary effects related to larger deletions. In addition to the role played by the GPIbα–filamin A interaction in regulating adhesion and signalling mechanisms in platelets, such mice would also help to define the importance of the GPIbα–filamin A interaction in regulating platelet morphology and production, particularly in light of recent evidence demonstrating that the GPIbα cytoplasmic tail contributes to normal platelet morphology and thrombopoiesis [30].
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia and The Wellcome Trust. P.M. is a recipient of an Institut National de la Santé et de la Recherche Médicale/National Health and Medical Research Council Postdoctoral fellowship. M.F. is a recipient of an Australian Postgraduate Industry Award from Monash University.
References
- 1.Savage B., Saldivar E., Ruggeri Z. M. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell (Cambridge, Mass.) 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 2.Berndt M. C., Shen Y., Dopheide S. M., Gardiner E. E., Andrews R. K. The vascular biology of the glycoprotein Ib-IX-V complex. Thromb. Haemostasis. 2001;86:178–188. [PubMed] [Google Scholar]
- 3.Lopez J. A., Andrews R. K., Afshar-Kharghan V., Berndt M. C. Bernard-Soulier syndrome. Blood. 1998;91:4397–4418. [PubMed] [Google Scholar]
- 4.Okita J. R., Pidard D., Newman P. J., Montgomery R. R., Kunicki T. J. On the association of glycoprotein Ib and actin-binding protein in human platelets. J. Cell Biol. 1985;100:317–321. doi: 10.1083/jcb.100.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du X., Fox J. E., Pei S. Identification of a binding sequence for the 14-3-3 protein within the cytoplasmic domain of the adhesion receptor, platelet glycoprotein Ib alpha. J. Biol. Chem. 1996;271:7362–7367. doi: 10.1074/jbc.271.13.7362. [DOI] [PubMed] [Google Scholar]
- 6.Fox J. E. Linkage of a membrane skeleton to integral membrane glycoproteins in human platelets. Identification of one of the glycoproteins as glycoprotein Ib. J. Clin. Invest. 1985;76:1673–1683. doi: 10.1172/JCI112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Flier A., Sonnenberg A. Structural and functional aspects of filamins. Biochim. Biophys. Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- 8.Stossel T. P., Condeelis J., Cooley L., Hartwig J. H., Noegel A., Schleicher M., Shapiro S. S. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell. Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 9.Li M., Bermak J. C., Wang Z. W., Zhou Q. Y. Modulation of dopamine D(2) receptor signaling by actin-binding protein (ABP-280) Mol. Pharmacol. 2000;57:446–452. doi: 10.1124/mol.57.3.446. [DOI] [PubMed] [Google Scholar]
- 10.Onoprishvili I., Andria M. L., Kramer H. K., Ancevska-Taneva N., Hiller J. M., Simon E. J. Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol. Pharmacol. 2003;64:1092–1100. doi: 10.1124/mol.64.5.1092. [DOI] [PubMed] [Google Scholar]
- 11.Petrecca K., Miller D. M., Shrier A. Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin. J. Neurosci. 2000;20:8736–8744. doi: 10.1523/JNEUROSCI.20-23-08736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistry N., Cranmer S. L., Yuan Y., Mangin P., Dopheide S. M., Harper I., Giuliano S., Dunstan D. E., Lanza F., Salem H. H., et al. Cytoskeletal regulation of the platelet glycoprotein Ib/V/IX-von Willebrand factor interaction. Blood. 2000;96:3480–3489. [PubMed] [Google Scholar]
- 13.Englund G. D., Bodnar R. J., Li Z., Ruggeri Z. M., Du X. Regulation of von Willebrand factor binding to the platelet glycoprotein Ib-IX by a membrane skeleton-dependent inside-out signal. J. Biol. Chem. 2001;276:16952–16959. doi: 10.1074/jbc.M008048200. [DOI] [PubMed] [Google Scholar]
- 14.Williamson D., Pikovski I., Cranmer S. L., Mangin P., Mistry N., Domagala T., Chehab S., Lanza F., Salem H. H., Jackson S. P. Interaction between platelet glycoprotein Ibalpha and filamin-1 is essential for glycoprotein Ib/IX receptor anchorage at high shear. J. Biol. Chem. 2002;277:2151–2159. doi: 10.1074/jbc.M109384200. [DOI] [PubMed] [Google Scholar]
- 15.Schade A. J., Arya M., Gao S., Diz-Kucukkaya R., Anvari B., McIntire L. V., Lopez J. A., Dong J. F. Cytoplasmic truncation of glycoprotein Ib alpha weakens its interaction with von Willebrand factor and impairs cell adhesion. Biochemistry. 2003;42:2245–2251. doi: 10.1021/bi026549n. [DOI] [PubMed] [Google Scholar]
- 16.Feng S., Resendiz J. C., Lu X., Kroll M. H. Filamin A binding to the cytoplasmic tail of glycoprotein Ibalpha regulates von Willebrand factor-induced platelet activation. Blood. 2003;102:2122–2129. doi: 10.1182/blood-2002-12-3805. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham J. G., Meyer S. C., Fox J. E. The cytoplasmic domain of the alpha-subunit of glycoprotein (GP) Ib mediates attachment of the entire GP Ib-IX complex to the cytoskeleton and regulates von Willebrand factor-induced changes in cell morphology. J. Biol. Chem. 1996;271:11581–11587. doi: 10.1074/jbc.271.19.11581. [DOI] [PubMed] [Google Scholar]
- 18.Dong J. F., Li C. Q., Sae-Tung G., Hyun W., Afshar-Kharghan V., Lopez J. A. The cytoplasmic domain of glycoprotein (GP) Ibalpha constrains the lateral diffusion of the GP Ib-IX complex and modulates von Willebrand factor binding. Biochemistry. 1997;36:12421–12427. doi: 10.1021/bi970636b. [DOI] [PubMed] [Google Scholar]
- 19.Cranmer S. L., Ulsemer P., Cooke B. M., Salem H. H., de la Salle C., Lanza F., Jackson S. P. Glycoprotein (GP) Ib-IX-transfected cells roll on a von Willebrand factor matrix under flow. Importance of the GPib/actin-binding protein (ABP-280) interaction in maintaining adhesion under high shear. J. Biol. Chem. 1999;274:6097–6106. doi: 10.1074/jbc.274.10.6097. [DOI] [PubMed] [Google Scholar]
- 20.Schaier S. R. Purification and characterization of platelet actin, actin-binding protein, and alpha-actinin. Methods Enzymol. 1992;215:58–77. doi: 10.1016/0076-6879(92)15053-f. [DOI] [PubMed] [Google Scholar]
- 21.Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 22.Enz R. The actin-binding protein filamin-A interacts with the metabotropic glutamate receptor type 7. FEBS Lett. 2002;514:184–188. doi: 10.1016/s0014-5793(02)02361-x. [DOI] [PubMed] [Google Scholar]
- 23.Enz R., Croci C. Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. Biochem. J. 2003;372:183–191. doi: 10.1042/BJ20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc. Natl. Acad. Sci. U.S.A. 1985;82:8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangin P., David T., Lavaud V., Cranmer S. L., Pikovski I., Jackson S. P., Berndt M. C., Cazenave J.-P., Gachet C., Lanza F. Identification of a novel 14-3-3{zeta} binding site within the cytoplasmic tail of platelet glycoprotein Ib{alpha} Blood. 2004;104:420–427. doi: 10.1182/blood-2003-08-2881. [DOI] [PubMed] [Google Scholar]
- 26.Meyer S. C., Zuerbig S., Cunningham C. C., Hartwig J. H., Bissell T., Gardner K., Fox J. E. Identification of the region in actin-binding protein that binds to the cytoplasmic domain of glycoprotein IBalpha. J. Biol. Chem. 1997;272:2914–2919. doi: 10.1074/jbc.272.5.2914. [DOI] [PubMed] [Google Scholar]
- 27.Lin R., Karpa K., Kabbani N., Goldman-Rakic P., Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5258–5263. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonardi A., Ellinger-Ziegelbauer H., Franzoso G., Brown K., Siebenlist U. Physical and functional interaction of filamin (actin-binding protein-280) and tumor necrosis factor receptor-associated factor 2. J. Biol. Chem. 2000;275:271–278. doi: 10.1074/jbc.275.1.271. [DOI] [PubMed] [Google Scholar]
- 29.Ozanne D. M., Brady M. E., Cook S., Gaughan L., Neal D. E., Robson C. N. Androgen receptor nuclear translocation is facilitated by the f-actin cross-linking protein filamin. Mol. Endocrinol. 2000;14:1618–1626. doi: 10.1210/mend.14.10.0541. [DOI] [PubMed] [Google Scholar]
- 30.Kanaji T., Russell S., Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]