Abstract
We have investigated the effect of the lipid peroxidation product, HNE (4-hydroxy-2-nonenal), on plant mitochondrial electron transport. In mitochondria isolated from Arabidopsis thaliana cell cultures, HNE inhibited succinate-dependent oxygen consumption via the Aox (alternative oxidase), but had minimal effect on respiration via Cox (cytochrome c oxidase). Maximal Cox activity, measured with reduced cytochrome c as substrate, was only slightly inhibited by high concentrations of HNE, at which Aox was completely inhibited. Incubation with HNE prevented dimerization of the Aox protein, suggesting that one site of modification was the conserved cysteine residue involved in dimerization and activation of this enzyme (CysI). However, a naturally occurring isoform of Aox lacking CysI and unable to be dimerized, LeAox1b from tomato (Lycopersicon esculentum), was equally sensitive to HNE inhibition, showing that other amino acid residues in Aox also interact with HNE. The presence of HNE in vivo in Arabidopsis cell cultures was also investigated. Induction of oxidative stress in the cell cultures by the addition of hydrogen peroxide, antimycin A or menadione, caused a significant increase in hydroxyalkenals (of which HNE is the most prominent). Western blotting of mitochondrial proteins with antibodies against HNE adducts, demonstrated significant modification of proteins during these treatments. The implications of these results for the response of plants to reactive oxygen species are discussed.
Keywords: alternative oxidase (Aox), cytochrome c oxidase (Cox), 4-hydroxy-2-nonenal (HNE), mitochondria, oxidative stress, plant mitochondria
Abbreviations: Aox, alternative oxidase; Cox, cytochrome c oxidase; DTT, dithiothreitol; HAE, hydroxyalkenal; HNE, 4-hydroxy-2-nonenal; LeAox, Aox from tomato (Lycopersicon esculentum); MDA, malondialdehyde; n-PG, n-propyl gallate; ROS, reactive oxygen species; SD, synthetic drop-out; TBARS, thiobarbituric acid-reacting substances
INTRODUCTION
A range of biotic and abiotic stresses increase levels of ROS (reactive oxygen species) in plants by perturbing chloroplast and mitochondrial metabolism. These ROS include H2O2 (hydrogen peroxide), O2− (superoxide) and the OH•− (hydroxyl radical). In plants, ROS have a dual action whereby high levels exacerbate cellular damage (to proteins, DNA and lipids), yet low concentrations can act as signalling molecules to activate defence responses [1,2]. The polyunsaturated fatty acids of membrane phospholipids are highly susceptible to peroxidation by ROS and a self-propagating chain of free radical reactions can produce various aldehydes, alkenals and HAEs (hydroxyalkenals), including MDA (malondialdehyde) and HNE (4-hydroxy-2-nonenal) [3]. These aldehydes are cytotoxic and generally more stable than ROS and can cause extensive damage to proteins and other cellular constituents. HNE has been directly measured in plant tissues [4], and indirect evidence for its increased synthesis during oxidative stress in plants has been reported [5].
The reactivity of HNE resides at its highly electrophilic C3 atom, which can form Michael adducts with the thiol group of cysteine residues, the imidazole group of histidine residues and the α-amino group of lysine residues on a myriad of proteins (reviewed in [6]). HNE also forms Schiff bases via its C1 atom and can react with the thiol groups of lipoic acid moieties on proteins. A number of lipoic acid-containing mitochondrial proteins have already been identified that are inactivated by HNE, including the acyltransferase subunits of the TCA (tricarboxylic acid) cycle, i.e., 2-oxo-glutarate and pyruvate dehydrogenase complexes [7–9], and the H protein of the mitochondrial glycine decarboxylase [5]. Investigations of the effect of HNE on animal mitochondrial respiratory electron transport chain components suggests that it inhibits Cox (cytochrome c oxidase) activity in a dose-dependent manner [10], predominantly by binding to subunit VIII of the complex [11]. However, we had previously reported little if any effect of HNE on the respiratory chain in plant mitochondria, based on assays of succinate-dependent oxygen consumption, Cox and NADH-FeCN reductase in isolated potato mitochondria [9].
Unlike its animal counterpart, the plant mitochondrial respiratory electron transport chain contains two terminal oxidases, Cox and Aox (alternative oxidase). Electron transport through the two pathways is regulated by the redox state of the ubiquinone pool and the activation status of the Aox protein, with the cytochrome pathway being the ‘default’ more active pathway [12]. Aox in its reduced dimeric form, accepts electrons from the ubiquinol pool, with the concomitant reduction of molecular oxygen to water. Unlike the cytochrome pathway, the alternative pathway is non-phosphorylating and, therefore, does not contribute directly to oxidative phosphorylation. As this alternative pathway has the potential to decrease the efficiency of respiration, Aox is tightly regulated by two mechanisms. It is active as a non-covalently linked dimer, and inactive when covalently linked via disulphide bonds [13], but requires 2-oxo-acids such as pyruvate to be fully active [14]. It is generally assumed that the Aox pathway can serve to protect the plant during periods of stress [15,16]. A number of studies have shown an induction of Aox synthesis following various stress treatments of plants or cell cultures (for example, [17–19]), and studies utilizing Aox antisense tobacco cell cultures have shown higher levels of ROS present in the mitochondria while Aox over-expression resulted in lower levels of ROS [20]. However, the response of Aox protein itself to ROS and their oxidation products has not been examined.
The purpose of this study was to re-investigate the effect of HNE on the respiratory capacity of plant mitochondria, to characterize any sites of action and to further explore the presence and production of HNE in plant cells.
EXPERIMENTAL
Biological material
Arabidopsis thaliana cv. cell cultures were grown in a controlled environment incubator in the light, as described previously [19]. Saccharomyces cerevisiae strain R757, transformed with the two tomato (Lycopersicon esculentum) Aox isoforms LeAox1a and LeAox1b, was grown in 50 ml of SD (synthetic drop-out) glucose medium [6.7 g/l yeast nitrogen base, 20 g/l glucose, 10 ml/l amino acids (tryptophan, histidine, leucine, methionine, lysine and adenine)] at 30 °C for 2 days. The cells were then re-suspended in 800 ml of SD galactose medium [6.7 g/l yeast nitrogen base, 20 g/l galactose, 10 ml/l amino acids (tryptophan, histidine, leucine, methionine, lysine and adenine)] and grown at 30 °C at 200 rev./min, until they reached a D600 of between 7 and 9 (after approx. 5–6 days).
Isolation and purification of mitochondria
Mitochondria were purified from seven-day old Arabidopsis cell cultures, essentially as described previously [21]. Isolated mitochondria were incubated with 10 mM DTT (dithiothreitol) for 10 min at 4 °C to allow maximal Aox activity and finally resuspended in a small volume (approx. 0.5 ml) of wash buffer from which the BSA was omitted. S. cerevisiae mitochondria were isolated according to the method of Holtzapffel et al. [22]
Respiratory measurements
Oxygen uptake by isolated mitochondria or cell cultures was measured using a O2 electrode (Rank Brothers) in 1 ml of reaction medium [0.3 M sucrose, 5 mM KH2PO4, 10 mM Tes, 10 mM NaCl, 2 mM MgSO4, 0.1% (w/v) BSA, pH 7.2] at 25 °C. Succinate, ATP, myxothiazol, pyruvate, n-PG (n-propyl gallate), KCN, NADH and ADP were added as indicated. Protein concentrations were determined by the method of Lowry et al. [23].
Immunoanalysis
Isolated mitochondria were incubated with 200 mM diamide or 20 mM DTT for 30 min at room temperature prior to separation by SDS/PAGE (12% gel) [24]. Following separation, proteins were transferred on to nitrocellulose membranes (Amersham) and probed with 1:50 dilution of the monoclonal anti-Aox antibody [25] or 1:500 of polyclonal anti-HNE-adduct antibodies (Alpha Diagnostic International, San Antonio, TX, U.S.A.). Detection was by chemiluminescence of horseradish-peroxidase-conjugated secondary antibodies.
Oxidative stress measurements
Arabidopsis cells from 7-day-old cultures grown in the dark were treated with 88 mM H2O2, 25 μM antimycin A or 400 μM menadione. Samples for respiratory, TBARS (thiobarbituric acid-reacting substances) and HAE analysis were taken at the specified times following addition of the effector. Cells (300 μl) were suspended in reaction medium (0.3 M sucrose, 5 mM KH2PO4, 10 mM Tes, 10 mM NaCl, 2 mM MgSO4, 0.1% (w/v) BSA, pH 7.2) to give a final volume of 1 ml in the oxygen electrode chamber. Total respiration was measured following the addition of 4 mM CCCP (carbonyl cyanide m-chlorophenylhydrazone). Respiration through the alternative pathway or the cytochrome pathway was measured following the addition of 1 mM KCN or 1 mM n-PG respectively. Levels of lipid peroxidation in cells was measured using a modified TBARS assay [26]. Free HAEs in samples were analysed by a method modified from the Bioxytech HAE-586 assay kit (OXIS Research, Portland, OR, U.S.A.). Samples were ground in liquid N2, and 5 ml of dichloromethane, 5 ml of water and 50 μl of BHT were added. Tubes were vortexed rapidly for 3×30 s, and centrifuged at 3000 g for 15 min at 4 °C to separate phases. Aliquots (400 μl) of the dichloromethane (lower) phase were placed into clean glass tubes, dried at room temperature under a stream of nitrogen gas, and re-suspended in 200 μl of water. To each tube, reagents 1 and 2 were added as described in the manufacturer's instructions. Samples were mixed well and incubated at 45 °C for 60 min and absorbances measured at 586 nm (300 spectrophotometer, Varian Carey, Melbourne, Australia).
RESULTS
Effect of HNE on respiration of isolated Arabidopsis mitochondria
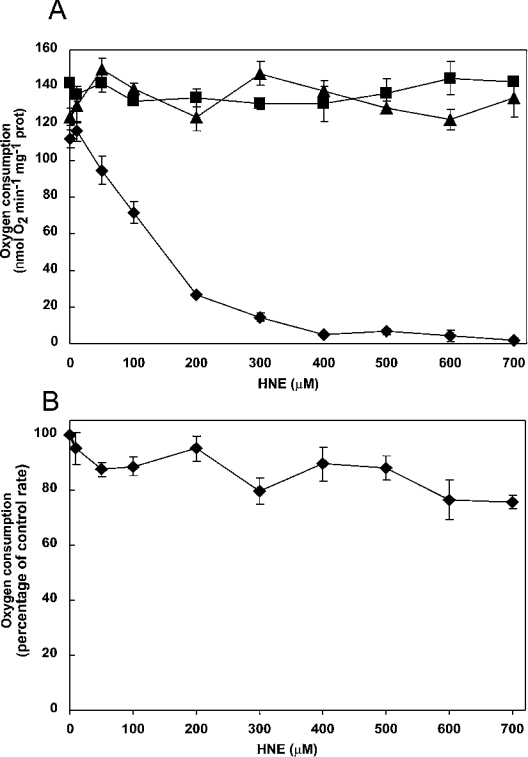
In order to determine the effects of HNE on the plant terminal oxidases, we added exogenous HNE to mitochondria purified from Arabidopsis cell cultures and measured activities of both the Aox and Cox pathways of respiration. Mitochondria were incubated for 20 min with increasing concentrations of HNE (0–700 μM), prior to measuring oxygen consumption with succinate as substrate in the presence of ADP, to maximize electron donation to the respiratory chain. The Aox inhibitor n-PG (500 μM) was added to measure whole chain respiration via Cox. Aox capacity was measured in the presence of myxothiazol (5 μM), to inhibit the cytochrome pathway, and DTT plus pyruvate to activate Aox. Uninhibited respiration and respiration via Cox was not significantly affected by HNE treatment, but electron transport through Aox was severely inhibited (Figure 1A), with 50% inhibition seen at approx. 150 μM HNE. Total inhibition of Aox-mediated respiration was observed at 400 μM HNE (Figure 1A). Since Cox activity was unlikely to be rate limiting with succinate as single substrate (adding another substrate stimulates oxygen uptake; results not shown), maximum Cox activity was measured using exogenous cytochrome c and the electron donors TMPD (N,N,N′,N′-tetramethyl-p-phenylenediamine) and ascorbate. Under these conditions, some inhibition by HNE did occur, but higher concentrations were needed than those used to inhibit Aox; 700 μM HNE caused only a 25% inhibition of electron transport through Cox (Figure 1B).
Figure 1. Effect of HNE on respiration of isolated mitochondria from Arabidopsis cell culture.
Mitochondria were isolated from 7-day-old cultures, suspended in 250 μl of reaction medium and incubated with various concentrations of HNE (0–700 μM) for 20 min. (A) Oxygen uptake with succinate as substrate: uninhibited respiration (▲); respiration through the alternative oxidase (◆); respiration through the cytochrome pathway (■). (B) Cox activity (◆), measured in the presence of myxothiazol, ascorbate and exogenously added cytochrome c. The control rate (100%) was 350 nmol·min−1·mg−1 of protein.
Aox dimerization in HNE-treated mitochondria
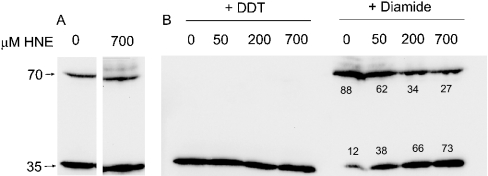
Aox is active as a reduced dimer [13] and is inactivated when a highly conserved cysteine residue (termed CysI [27]) is oxidized to covalently link the enzyme [28–30]. To determine whether modification of this cysteine residue was involved in inhibition of Aox by HNE, proteins from mitochondria incubated with HNE (0, 50, 200 or 700 μM) were separated by SDS/PAGE and Aox proteins detected via Western blotting with the anti-Aox monoclonal antibody ([25]; Figure 2), either without further treatment or after treatment with the reducing agent DTT or the oxidant diamide. Two bands were evident on the immunoblots: a 35-kDa band representing the Aox monomer in the DTT-treated mitochondria and a 70-kDa band representing the Aox dimer in the diamide-treated mitochondria. In mitochondria isolated from Arabidopsis cell cultures, Aox typically exists as a mixture of the oxidized and reduced protein (Figure 2A). Prior incubation of mitochondria with HNE had no effect on the distribution of Aox protein between the oxidized and reduced forms, nor on the ability of added DTT to fully reduce the enzyme (Figures 2A and 2B). However, HNE treatment partially blocked the ability of diamide to dimerize the Aox protein (Figure 2B), indicating that HNE does interact with CysI and raising the question of whether CysI is required for HNE inhibition of Aox.
Figure 2. Immunodetection of Aox proteins from HNE-treated mitochondria of Arabidopsis cell cultures.
Isolated mitochondria (100 μl) were suspended in 1.25 of ml of reaction medium and treated with 0–700 μM HNE as indicated, for 20 min at 25 °C. Mitochondria were pelleted and re-suspended in 100 μl of wash buffer (without BSA). Samples (50 μg) were separated by SDS/PAGE, transferred on to a nitrocellulose membrane and probed with an anti-Aox antibody. (A) No further treatment; (B) treated with either 200 mM diamide or 20 mM DTT for 30 min at room temperature immediately prior to separation, as indicated. The positions of the 70- and 35-kDa molecular-mass markers are indicated on the left. Numbers under the bands represent the percentage of Aox as a dimer or monomer at each concentration of HNE.
Effect of HNE on an Aox isoform lacking CysI
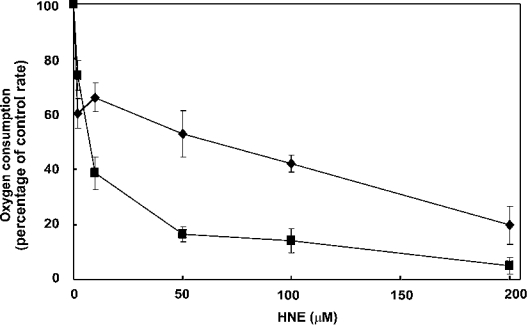
We have shown previously that cold-treated tomato fruit contain two isoforms of Aox: LeAox1a, which contains the highly conserved CysI, and LeAox1b, in which CysI is replaced by a serine residue [22]. When expressed in S. cerevisiae, LeAox1b dimers cannot be covalently linked, nor inactivated by oxidants [22]. We utilized this yeast system to test the effect of HNE on the activity of the two Aox isoforms. Isolated yeast mitochondria containing either LeAox1a or LeAox1b, were incubated for 10 min with increasing concentrations of HNE (0–200 μM) and respiration measured with succinate as substrate in the presence of myxothiazol. Oxygen uptake via both LeAox1a and LeAox1b was inhibited by HNE, with 50% inhibition of electron transport seen at 70 μM and 15 μM respectively (Figure 3). This suggests that there are other sites of modification of Aox by HNE, in addition to the regulatory CysI, that inhibit Aox function in the <100 μM concentration range.
Figure 3. Effect of HNE on the tomato Aox isoforms LeAox1a and LeAox1b.
Mitochondria were isolated from yeast expressing either LeAox1a (■) or LeAox1b (◆), suspended in reaction medium and incubated with various concentrations of HNE (0–200 μM) for 10 min. Respiration was initiated by the addition of 2 mM NADH and 1 mM ADP. Electron transport via Aox was measured in the presence of 2.5 μM myxothiazol to inhibit the cytochrome pathway, 2.5 mM DTT, and either 10 mM pyruvate (LeAox1a) or 10 mM succinate (LeAox1b), to ensure activation of Aox. Addition of 0.5 mM n-PG inhibited respiration completely. The control rate of oxygen uptake via Aox was 70 nmol·min−1·mg−1 of protein in both cases.
Lipid peroxidation products in cells exposed to oxidative stress
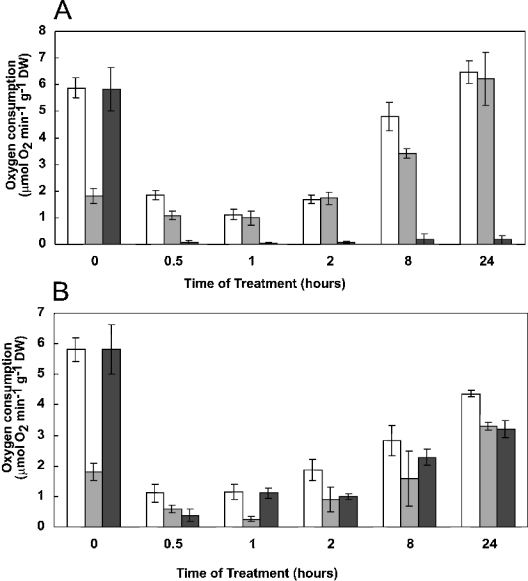
Having established that HNE is a powerful inhibitor of Aox, we investigated the production of lipid peroxidation in vivo. Arabidopsis cell cultures were incubated with 400 μM menadione, 25 μM antimycin A or 88 mM H2O2, treatments previously shown to cause detectable oxidative stress without dramatic cell death in these cell cultures [19]. These treatments inhibit cell respiration dramatically at first, but over the subsequent 24 h respiration rates slowly recover (Figure 4). In the antimycin-treated cells, the recovered respiration was entirely due to a dramatic increase in Aox activity over the time course of the experiment (Figure 4A), whereas in the H2O2-treated cells, both Aox and Cox contributed to the recovered respiration (Figure 4B). The presence of menadione prevented accurate measurements of oxygen consumption (therefore these results are not shown).
Figure 4. Respiration of Arabidopsis cells.
Cell cultures were incubated with either 25 μM antimycin (A) or 88 mM H2O2 (B), as described in the Experimental section. At the times shown, aliquots were withdrawn and oxygen consumption measured either in the absence of inhibitors (white bars), or in the presence of 1 mM KCN (to give an estimate of Aox capacity; grey bars) or 0.5 mM n-PG (to give an estimate of Cox capacity; black bars). DW, dry weight.
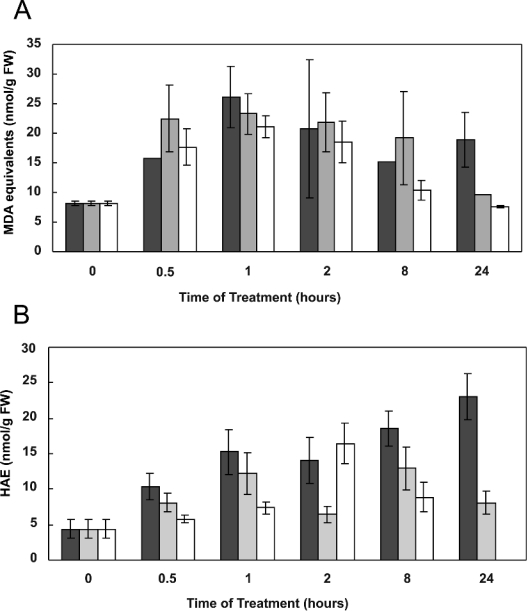
Overall lipid peroxidation was estimated by following production of MDA equivalents using the TBARS assay, at 0.5, 1, 2, 8 and 24 h following addition of each effector. Cell content of MDA equivalents increased after addition of all three effectors, reaching a maximum 1 h after addition (Figure 5A). Thereafter, MDA content began to decrease, with control levels being reached after 24 h for all treatments. HNE production in cells was estimated by measuring total HAEs, of which HNE is the most prominent. Base levels of HAEs could be detected in control (untreated) cells, and this increased substantially upon treatment with the three effectors. With antimycin and H2O2, the increase in HAE levels was transitory, peaking at 2–8 h and then declining; with menadione treatment, the increase in HAE was more sustained (Figure 5B).
Figure 5. Measurement of lipid peroxidation products as (A) MDA equivalents or (B) HAE in Arabidopsis cell cultures.
Cells were treated with 400 μM menadione (black), 25 μM antimycin A (grey) or 88 mM H2O2 (white). MDA equivalents and HAE in 5-ml samples of cells were determined using the TBARS and HAE assay at specified times after treatment. Each time point represents the mean±S.E.M. for three separate cultures. FW, fresh weight.
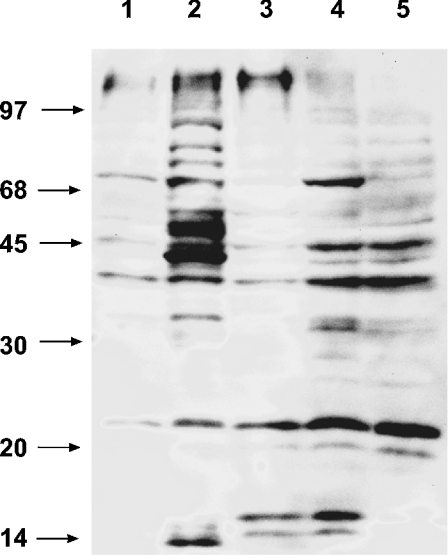
The modification of mitochondrial proteins by endogenous HNE was measured by detecting HNE-modified proteins on Western blots using polyclonal antibodies raised against the HNE adduct (Figure 6). Some reactivity was observed in the control mitochondria, but a dramatic increase in immunoreactive protein bands was seen when isolated mitochondria were treated directly with HNE (Figure 6, lane 1 and lane 2). Mitochondria isolated from cells treated with antimycin A, menadione and H2O2 also showed stronger reactivity than mitochondria from untreated cells (Figure 6, lanes 3–5 versus lane 1), although the effect of antimycin and menadione was more pronounced than that of H2O2. These results suggest that sufficient HNE accumulates in vivo, under the stresses imposed, to modify proteins, and this is consistent with the observed increases in HAE and MDA levels during periods of oxidative stress (Figure 5). Clearly, such stresses can cause direct modification of mitochondrial proteins.
Figure 6. Immunodetection of mitochondrial proteins modified by HNE in oxidative stress-treated cells.
Proteins from mitochondria isolated from cell cultures, treated for 16 h with either 88 mM H2O2 (lane 3), 25 μM antimycin A (lane 4) or 400 μM menadione (lane 5), were separated by SDS/PAGE, transferred on to nylon membranes and probed with polyclonal anti-HNE-adduct antibodies. Control mitochondria from untreated cells (lane 1) and mitochondria treated directly with 700 μM HNE (lane 2) are also shown. The positions of molecular-mass markers are indicated on the left in kDa.
DISCUSSION
We have provided direct evidence that Arabidopsis cells produce HAEs upon exposure to oxidative stress in the form of H2O2 and the respiratory poison antimycin A, and that antibodies raised to HNE adducts show increased reaction with mitochondrial proteins following these treatments. This supports previous studies showing likely HNE modification of lipoic acid moieties in Arabidopsis mitochondria following these treatments [19], and HNE interaction with the H-subunit of glycine decarboxylase during exposure of pea leaves to herbicide, cold and drought [5]. We also show that the respiratory terminal oxidase Aox is severely inhibited by HNE. Aox is largely absent from potato tuber mitochondria, which explains why this effect was not identified in previous studies [9]. Together with the inhibition of glycine decarboxylase and other lipoic acid-containing enzymes in the mitochondrial matrix [5,9], this Aox sensitivity could substantially impair respiratory metabolism under environmental stress in plants. It should be noted that Aox activity is a major point of control of ROS formation in isolated plant mitochondria [31] and its inhibition will, therefore, lead to further oxidative damage.
Respiration via the cytochrome pathway is clearly much less sensitive to HNE inhibition (Figure 1 and [9]). This appears to be in contrast with the reported inhibitory effect HNE has on mammalian Cox activity [10,11]. This discrepancy may lie with the difference in the experiments performed and/or the observed site of inhibition in mammalian Cox. Lengthy incubations of purified Cox were required for HNE inhibition of Cox at lower HNE concentrations in mammalian studies. Chen et al. [10] incubated purified Cox with HNE overnight to observe half-maximal inhibition at approx. 100 μM, whereas Musatov et al. [11] observed 30–50% inhibition of the purified enzyme after 2 h with 300–500 μM HNE. The shorter inhibition times here, with whole plant mitochondrial samples containing many other HNE targets, may thus miss this comparatively low level inhibition. Furthermore, MS analysis has suggested that HNE modification of His36 of subunit VIII in the Cox complex from bovine heart is probably the cause of Cox inhibition by this effector molecule [11]. The likely plant orthologue of subunit VIII is known as Cox subunit Vc [32]. The Arabidopsis Cox Vc subunit has a divergent C-terminal region compared with the mammalian Cox VIII, but does contain a histidine residue at approximately the same location (results not shown). Sequence divergence around this inhibition site, and/or access of this histidine for modification in the plant Cox structure may also be a factor in the resistance of plant Cox to HNE-induced inhibition.
Aox is generally thought to play a protective role against oxidative stress in plants by minimizing ROS formation from the electron transport chain, and direct evidence for this has been obtained in transgenic tobacco cells with varying levels of Aox expression [20]. The susceptibility of Aox to inhibition by HNE confuses this role, since ROS formation in the electron transport chain is likely to lead to HNE production (shown here in the antimycin-treated cells). Interestingly, in environmentally stressed pea leaves, a dramatic increase in Aox protein was observed, concomitant with an increase in MDA levels [5], yet this did not prevent inhibition of HNE-sensitive enzymes, such as glycine decarboxylase. Mitochondria isolated from these leaves showed increased Aox activity, but less than was expected from the increase in Aox protein [5], perhaps because of interaction between Aox and HNE. Increases seen in Aox protein and transcript levels in plants in response to a variety of treatments that induce oxidative stress [12] may be, in part, an attempt to maintain an active Aox pool in the mitochondria and not necessarily reflective of an increase in respiratory capacity. That is, the plants keep making more Aox, because the oxidase becomes inactivated during the stress. This certainly seems to be the case in the antimycin-treated cells in the present study, where Aox activity increased dramatically over 24 h to rescue respiratory rates (Figure 4). Although respiration via Aox was inhibited severely immediately after antimycin addition (Figure 4), sufficient Aox activity remained to allow cell survival and subsequent synthesis of further Aox. Similar results have been observed with tobacco cell cultures [33]. It is also interesting in this context that uncoupling protein, another protein of the inner mitochondrial membrane that is thought to play an antioxidant role by preventing over-reduction of the electron transport chain, is activated by HNE [34]. Thus, depending on whether or not HNE is produced at high levels, Aox and uncoupling protein may play complementary roles during severe oxidative stress in plants.
Under periods of oxidative stress, plant cells have a variety of mechanisms in place to scavenge free radicals, generally converting them into non-toxic products [35]. Our data show an initial burst of lipid peroxidation, seen as increases in MDA equivalents and HAE concentration, following induction of oxidative stress in Arabidopsis cells. This burst occurred over the initial 2–8 h, after which MDA and HAE levels declined, reaching steady-state levels after 24 h in the case of antimycin and H2O2 treatments. Cell respiration rates showed corresponding changes over this period, being severely inhibited initially and then recovering over 24 h. This decline in lipid peroxidation over time is presumably due to activation of defence and detoxification mechanisms, which will remove ROS, thereby limiting further lipid peroxidation, and breakdown lipid aldehydes. Nonetheless, the initial burst of lipid peroxidation does cause protein modification and potentially inactivation of mitochondrial respiratory proteins, especially Aox. Although the amounts of HAE measured in these cells were rather low compared with those added in vitro (Figure 5), it should be remembered that HAEs may be concentrated at their site of generation within the cell and exposure times in vivo were greater than those imposed in vitro. Partial inhibition of electron transport has been observed in mitochondria isolated from Arabidopsis cells after 16 h treatment with H2O2 [19].
The results with the anti-HNE-adduct antibody show that many proteins in the mitochondria react with HNE, whether it is applied directly to isolated organelles or produced endogenously during stress, and that some protein modification occurs even in untreated cells (Figure 6). Long-term exposure to more moderate oxidative stress than that imposed in short-term experiments used in the present study, is also likely to lead to HNE-induced damage to proteins in mitochondria. These results raise the question of how these damaged proteins are repaired or removed in mitochondria.
References
- 1.Dat J., Vandenabeele S., Vranova E., Van Montagu M., Inze D., Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 3.Schneider C., Tallman K. A., Porter N. A., Brash A. R. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 4.Takamura H., Gardner H. W. Oxygenation of (3Z)-alkenal to (2E)-4-hydroxy-2-alkenal in soybean seed (Glycine max L.) Biochim. Biophys. Acta. 1996;1303:83–91. doi: 10.1016/0005-2760(96)00076-8. [DOI] [PubMed] [Google Scholar]
- 5.Taylor N. L., Day D. A., Millar A. H. Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J. Biol. Chem. 2002;277:42663–42668. doi: 10.1074/jbc.M204761200. [DOI] [PubMed] [Google Scholar]
- 6.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 7.Humphries K. M., Szweda L. I. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 8.Humphries K. M., Yoo Y., Szweda L. I. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry. 1998;37:552–557. doi: 10.1021/bi971958i. [DOI] [PubMed] [Google Scholar]
- 9.Millar A. H., Leaver C. J. The cytotoxic lipid peroxidation product, 4-hydroxy-2-nonenal, specifically inhibits decarboxylating dehydrogenases in the matrix of plant mitochondria. FEBS Lett. 2000;481:117–121. doi: 10.1016/s0014-5793(00)01976-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Henderson G. I., Freeman G. L. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J. Mol. Cell. Cardiol. 2001;33:1919–1927. doi: 10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]
- 11.Musatov A., Carroll C. A., Liu Y. C., Henderson G. I., Weintraub S. T., Robinson N. C. Identification of bovine heart cytochrome c oxidase subunits modified by the lipid peroxidation product 4-hydroxy-2-nonenal. Biochemistry. 2002;41:8212–8220. doi: 10.1021/bi025896u. [DOI] [PubMed] [Google Scholar]
- 12.Finnegan P. M., Soole K. L., Umbach A. L. In: Plant Mitochondria: from Genome to Function, vol. 17. Day D. A., Millar A. H., Whelan J., editors. Doordecht: Kluwer Academic Publishers; 2004. pp. 163–230. [Google Scholar]
- 13.Umbach A. L., Siedow J. N. Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol. 1993;103:845–854. doi: 10.1104/pp.103.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millar A. H., Wiskich J. T., Whelan J., Day D. A. Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett. 1993;329:259–262. doi: 10.1016/0014-5793(93)80233-k. [DOI] [PubMed] [Google Scholar]
- 15.Wagner A. M. A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in Petunia hybrida cells. FEBS Lett. 1995;368:339–342. doi: 10.1016/0014-5793(95)00688-6. [DOI] [PubMed] [Google Scholar]
- 16.Robson C. A., Vanlerberghe G. C. Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathways of programmed cell death. Plant Physiol. 2002;129:1908–1920. doi: 10.1104/pp.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanlerberghe G. C., McLntosh L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol. 1996;111:589–595. doi: 10.1104/pp.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amora Y., Chevionb M., Levinea A. Anoxia pretreatment protects soybean cells against H2O2-induced cell death: possible involvement of peroxidases and of alternative oxidase. FEBS Lett. 2000;477:175–180. doi: 10.1016/s0014-5793(00)01797-x. [DOI] [PubMed] [Google Scholar]
- 19.Sweetlove L. J., Heazlewood J. L., Herald V., Holtzapffel R., Day D. A., Leaver C. J., Millar A. H. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2002;32:891–904. doi: 10.1046/j.1365-313x.2002.01474.x. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell D. P., Wang Y., McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl Acad. Sci. U.S.A. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millar A. H., Sweetlove L. J., Giege P., Leaver C. J. Analysis of the Arabidopsis mitochondrial proteome. Plant Physiol. 2001;127:1711–1727. [PMC free article] [PubMed] [Google Scholar]
- 22.Holtzapffel R. C., Castelli J., Finnegan P. M., Millar A. H., Whelan J., Day D. A. A tomato alternative oxidase protein with altered regulatory properties. Biochim. Biophys. Acta. 2003;1606:153–162. doi: 10.1016/s0005-2728(03)00112-9. [DOI] [PubMed] [Google Scholar]
- 23.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Elthon T. E., McIntosh L. Characterization and solubilization of the alternative oxidase of Sauromatum guttatum mitochondria. Plant Physiol. 1986;82:1–6. doi: 10.1104/pp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodges D. M., DeLong J. M., Forney C. F., Prange R. K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- 27.Berthold D. A., Andersson M. E., Nordlund P. New insight into the structure and function of the alternative oxidase. Biochim. Biophys. Acta. 2000;1460:241–254. doi: 10.1016/s0005-2728(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 28.Rhoads D. M., Umbach A. L., Sweet C. R., Lennon A. M., Rauch G. S., Siedow J. N. Regulation of the cyanide-resistant alternative oxidase of plant mitochondria. Identification of the cysteine residue involved in alpha-keto acid stimulation and intersubunit disulfide bond formation. J. Biol. Chem. 1998;273:30750–30756. doi: 10.1074/jbc.273.46.30750. [DOI] [PubMed] [Google Scholar]
- 29.Vanlerberghe G. C., McIntosh L., Yip J. Y. Molecular localization of a redox-modulated process regulating plant mitochondrial electron transport. Plant Cell. 1998;10:1551–1560. doi: 10.1105/tpc.10.9.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djajanegara I., Holtzapffel R., Finnegan P. M., Hoefnagel M. H., Berthold D. A., Wiskich J. T., Day D. A. A single amino acid change in the plant alternative oxidase alters the specificity of organic acid activation. FEBS Lett. 1999;454:220–224. doi: 10.1016/s0014-5793(99)00808-x. [DOI] [PubMed] [Google Scholar]
- 31.Adriana C., Rafael M.-S., Irma B.-L. Control of superoxide production in mitochondria from maize mesocotyls. FEBS Lett. 2004;570:52–56. doi: 10.1016/j.febslet.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Millar A. H., Eubel H., Jänsch L., Kruft V., Heazlewood J. L., Braun H. P. Mitochondrial cytochrome c oxidase and succinate dehydrogenase complexes contain plant-specific subunits. Plant Mol. Biol. 2004;56:77–89. doi: 10.1007/s11103-004-2316-2. [DOI] [PubMed] [Google Scholar]
- 33.Vanlerberghe G. C., McIntosh L. Mitochondrial electron transport regulation of nuclear gene expression. Studies with the alternative oxidase gene of tobacco. Plant Physiol. 1994;105:867–874. doi: 10.1104/pp.105.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith A. M., Ratcliffe R. G., Sweetlove L. J. Activation and function of mitochondrial uncoupling protein in plants. J. Biol. Chem. 2004;279:51944–51952. doi: 10.1074/jbc.M408920200. [DOI] [PubMed] [Google Scholar]
- 35.Møller I. M. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]