Summary
Extrahepatic portal vein obstruction (EHPVO) is a rare disease with myeloproliferative neoplasm (MPN) as the most common cause. We report that hypersplenic hematologic changes in EHPVO might be eliminated by MPN. Through experience with splenectomy for variceal control with EHPVO, we suspected that spleen might mask MPN-induced thrombocytosis, and that MPN might have a significant influence on excessive thrombocytosis after splenectomy. To clarify the influence of MPN and spleen on platelet trends, we conducted a retrospective hospital database analysis, evaluating 8 EHPVO patients with splenectomy (2 males, 6 females; from 17 years to 64 years, mean 38.3 years). Three (37.5%) of 8 were diagnosed as MPN by JAK2V617F mutation. The perioperative serum platelet counts in EHPVO without MPN were 10.5, 35.4, and 36.6 (x104/μL) preoperatively, after 1 week and 3 weeks, respectively. The platelet counts in EHPVO with MPN were 34.2, 86.4, and 137.0 (x104/μL), respectively. Splenectomy and MPN showed positive interaction on platelet increasing with statistical significance. We also examined the spleen volume index (SpVI: splenic volume (cm3) / body surface area (m2) and postoperative platelet elevations ratio (PER: 3-week postoperative platelet counts / preoperative platelet counts). However, both SpVI and PER showed no significant difference with or without MPN. Histological examination revealed splenic congestion in all 8 EHPVO cases, and splenic extramedullary hematopoiesis in 2 of 3 MPN. In EHPVO with MPN, hypersplenism causes feigned normalization of platelet count by masking MPN-induced thrombocytosis; however, splenectomy unveils postoperative thrombocytosis. Spleen in EHPVO with MPN also participates in extramedullary hematopoiesis.
Keywords: extrahepatic portal vein obstruction, myeloproliferative neoplasm, splenectomy, thrombocytosis, extramedullary hematopoiesis
1. Introduction
Extrahepatic portal vein obstruction (EHPVO) is a crucial cause of non-cirrhotic and prehepatic portal hypertension. EHPVO is an intractable and rare disease with incidence rates of EHPVO at 3.78 and 1.73 per 100,000 inhabitants in European males and females, respectively (1). Japan shows a lower estimated incidence of approximately 0.61 per 100,000 inhabitants (2).
Myeloproliferative neoplasm (MPN), including polycythemia vera, essential thrombocythemia, chronic idiopathic myelofibrosis, and unclassifiable type (MPN-U), is the most prominent cause of EHPVO with a prevalence of 15%-30% (3-9). The incidence of MPN is also rare with annual incidence rates for polycythemia vera, essential thrombocythemia, and chronic idiopathic myelofibrosis at 0.84, 1.03, and 0.47 per 100,000, respectively (10).
We recently suggested that hypersplenic hematologic changes of thrombo-leukocytopenia in EHPVO patients might veil MPN-induced proliferation of blood cells (9). Approximately 35% of polycythemia vera, one type of MPN, manifested no hematological sign of myeloproliferations. These are referred to as masked polycythemia vera (11). In portal hypertension, including EHPVO and Budd-Chiari syndrome, latent MPN with JAK2V617F mutation and normal blood counts was reportedly more common than overt MPN with elevated hemoglobin, white cell counts and/or platelets (12).
For the gastroenterologist, diagnosing the underlying MPN in EHPVO patients is extremely difficult because of the almost normalized blood conditions in hematological appearance due to the conflicting effects between hypersplenic hematopenia by EHPVO and blood cell proliferation by MPN. MPN is a potentially life-threatening blood malignancy with thrombotic complications, including myocardial, cerebral infarction and splanchnic vein thrombosis. For EHPVO patients with MPN, the original cause is splanchnic thrombosis due to thrombotic tendency; however, the main symptom of EHPVO is hematemesis or melaena by portal hypertensive bleeding, including esophagogastric or ectopic varix rupture. Further, MPN patients usually need antithrombotic agents for platelet hyperaggregability regardless of the high risk of variceal rupture. EHPVO patients are usually face a dilemma between thrombotic complications and variceal bleeding events, which causes a refractory situation for treatment by gastroenterologists.
For treatment of intractable esophagogastric varices, we performed Hassab's operation including splenectomy with devascularization of the upper half of the stomach and distal esophagus. EHPVO is very rare. Furthermore, treatment with Hassab's operation including splenectomy for esophagogastric varices in EHPVO with MPN is extremely unusual. Based on experience with splenectomy in EHPVO patients, we suspected that the comorbidity of MPN might have a great influence upon the postoperative increase of platelet counts, and further, that the spleen's participation in veiling original MPN-induced blood cell proliferation might also be associated with latent MPN as a feigned normalization of hematological appearance (9,13).
This retrospective single-center study was conducted to clarify the influence of MPN on spleen and platelet count trends in EHPVO patients.
2. Patients and Methods
Between January 2000 and February 2024, 17 patients with a diagnosis of EHPVO were treated in our hospital. Eight of 17 EHPVO patients who had undergone splenectomy for treatment of esophagogastric varices were enrolled in this study. Medical records for all 8 patients were identified and reviewed retrospectively. In all patients, the diagnosis of EHPVO was confirmed by imaging modalities, including ultrasonography, contrast-enhanced computed tomography (CT), angiography, or contrast-enhanced magnetic resonance imaging. Patients with hepatocellular carcinoma, other malignancies, liver cirrhosis or operative history including pancreaticoduodenectomy and choledochotomy were excluded as a diagnosis of EHPVO.
The medical records for patient age, gender, esophagogastroduodenoscopy findings were extracted from patient charts. Regular blood tests, hepatic and renal function tests were performed in all patients. JAK2V617F mutations were tested as a screening for MPN. The diagnosis and treatment of MPN was performed by hematologists in our hospital.
In all cases of EHPVO patients with Hassab's operation including splenectomy, CT was scrutinized for preoperative simulation. Preoperative splenic volume was measured by 3-dimensional CT volumetry using Synapse Vincent® (FUJI FILM Medical, Tokyo). The volume analyzer Synapse Vincent® automatically measures splenic volume.
The splenic volume index (SpVI) was calculated as splenic volume (cm3) / body surface area (m2) (14). Platelet elevation ratio (PER) was calculated as 3-week postoperative platelet counts (/μL) / preoperative platelet counts (/μL). Continuous variables were presented as the mean ± standard deviation and compared using the Mann-Whitney U-test since the sample sizes were relatively small. Values without normal distribution were presented as medians with interquartile range (IQR). The effects of splenectomy with and without MPN, and their interaction in perioperative serum platelet counts were investigated using two-way analysis of variance (ANOVA). The data were analyzed using IBM SPSS Statistics® Ver. 28.0.1. A p-value < 0.05 was considered significant.
Histological hematoxylin and eosin-stained sections of spleen in all EHPVO cases were reviewed by pathologists in our hospital. In cases of splenic extramedullary hematopoiesis, immunohistochemical labeling of CD41, CD71 and myeloperoxidase for scrutiny of megakaryocyte, erythroid and myeloid lineage cells, respectively, were also evaluated.
All study participants provided informed consent and the study was carried out in accordance with the ethical standards set by the Declaration of Helsinki. This study was approved by the hospital ethics committee (B-2022- 615).
3. Results and Discussion
Eight EHPVO patients who underwent splenectomy were included in the analysis. The clinical presentations of these patients are summarized in Table 1. Two males (25.0%) and 6 females (75.0%); the age distribution for splenectomy ranged from 17 to 64 years, with a mean age of 38.3 years. Indication for splenectomy included 7 esophagogastric varices (87.5%) and 1 gastric varix (12.5%). All 8 EHPVO cases were referred by local gastroenterologists because of refractory esophageal and gastric varices by endoscopy and intervention radiology. Six (75.0%) of 8 cases had a history of variceal rupture, and 2 cases (25.0%) received prophylactic treatment for risky esophagogastric varices. Three patients (37.5%) of 8 were positive for JAK2V617F mutation. All 3 of the JAK2V617F mutated patients with EHPVO were diagnosed as MPN including polycythemia vera, essential thrombocythemia, and myeloproliferative neoplasm, unclassifiable (MPN-U), respectively, by hematologists.
Table 1. The clinical presentations of EHPVO patients treated by splenectomy for refractory esophagogastric varices.
| Case | Age | Sex | Indication for splenectomy | Prophylactic or bleeding varices | JAK2V617 mutation | MPN |
|---|---|---|---|---|---|---|
| 1 | 44 | M | EGV | Bleeding | - | |
| 2 | 42 | F | EGV | Prophylactic | + | MPN-U |
| 3 | 32 | M | EGV | Bleeding | - | |
| 4 | 47 | F | EGV | Bleeding | + | ET |
| 5 | 27 | F | EGV | Bleeding | - | |
| 6 | 33 | F | GV | Prophylactic | - | |
| 7 | 64 | F | EGV | Bleeding | + | PV |
| 8 | 17 | F | EGV | Bleeding | - |
EGV: esophagogastric varices, ET: essential thrombocythemia, GV: gastric varices, MPN: myeloproliferative neoplasm, MPN-U: myeloproliferative neoplasm, unclassifiable, PV: polycythemia vera.
Figure 1 shows a comparison of the serum platelet counts of EHPVO patients with or without MPN before and after splenectomy at 1 week and 3 weeks. The perioperative serum platelet counts for EHPVO without MPN were 10.5, 35.4, and 36.6 (x104/ μL) preoperatively, after 1 week and after 3 weeks, respectively. Further, the perioperative serum platelet counts for EHPVO with MPN were 34.2, 86.4, and 137.0 (x104/μL) preoperatively, after 1 week, and after 3 weeks, respectively. The distribution of preoperative platelet counts in EHPVO patients demonstrated thrombocytopenia without MPN and normal to slightly high platelet count with MPN. The serum platelet trends of both groups were postoperatively increasing; however, splenectomy in MPN patients caused prominent elevation. Two of 3 MPN cases showed thrombocytosis of more than 120 × 104/μL 3 weeks after splenectomy, and exceeded 190 × 104/ μL during perioperative periods. The postoperative thrombocytosis of all 3 MPN cases was treated by hematological cytoreduction therapy including hydroxyurea with/without anagrelide by hematologists. All 3 MPN patients also continued anti-platelet agents under appropriate additional endoscopic treatment for esophageal varices. In 3 of 5 patients without MPN, splenectomy contributed to normalization of platelet counts after 3 weeks. The interaction of splenectomy and MPN in perioperative serum platelet counts was investigated using two-way ANOVA. Splenectomy and MPN showed positive interaction in postoperative thrombocytosis with statistical significance (F = 4.14, p = 0.033).
Figure 1.
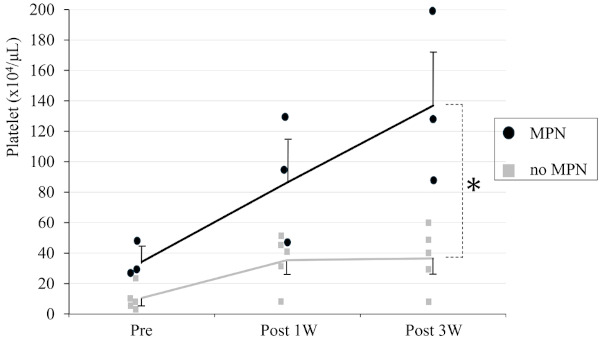
Comparison of serum platelet counts of EHPVO patients with or without MPN before and after splenectomy at 1 week and 3 weeks. Serum platelet counts increased to a greater degree in EHPVO with MPN than without MPN. Splenectomy and MPN showed positive interaction in platelet count trends with statical significance (*: F = 4.14, p = 0.033).
We then examined preoperative spleen volume and perioperative platelet increasing degree with or without MPN, in EHPVO patients. The median splenic volume determined using 3-dimensional CT volumetry Synapse Vincent® (FUJI FILM Medical, Tokyo, Figure 2A and 2B) was 788.4 (IQR, 622.9-1019.1; range, 187.3- 1719.2) cm3 and the mean splenic volume was 841.2 ± 420.4 cm3. The median SpVI as splenic volume (cm3) / body surface area (m2) was 540.5 (IQR, 367.5-660.8; range, 118.6-859.6) cm3/ m2. Figure 2C demonstrated the distribution between the SpVI and PER in all 8 EHPVO patients with splenectomy. With or without MPN in EHPVO patients, SpVI was 572.9 ± 73.6 and 488.0 ± 269.3, respectively, and no significant difference was noted (Figure 2D). We also examined the PER calculated as 3-week postoperative platelet counts (/μL) / preoperative platelet counts (/μL) with and without MPN in EHPVO patients. The PER was 4.32 ± 1.85 and 4.79 ± 3.22, with or without MPN in EHPVO patients, respectively, and there were also no significant differences (Figure 2E).
Figure 2.
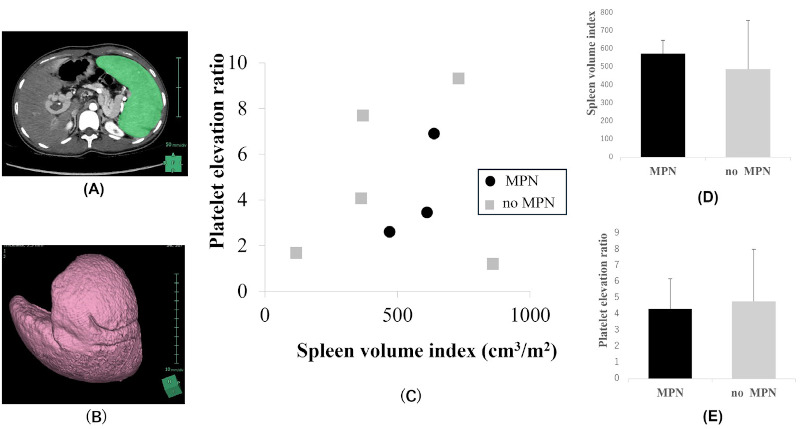
(A, B) Measurement of splenic volume. Splenic volume was automatically measured by 3-dimensional computed tomography volumetry using Synapse Vincent® (FUJI FILM Medical, Tokyo); (C) The distribution of the SpVI (Splenic volume index) and PER (Platelet elevation ratio) in all 8 EHPVO patients with splenectomy; (D, E) With or without MPN in EHVPO patients, SpVI and PER showed no difference.
Figure 3 shows resected spleen weighing 574 grams (Figure 3A) and pathological assessment in case 4. Histological examination of the resected spleen demonstrated splenic sinus congestion and decreased white pulp (Figure 3B) in all the 8 EHPVO patients, the 5 without MPN and the 3 with MPN. Furthermore, 2 of 3 EHPVO patients with MPN revealed local splenic extramedullary hematopoiesis (EMH), as shown Fig Figure 3C. Immunohistochemical examination of case 4 revealed CD41-positive megakaryocytes (Figure 3D, white arrowhead), CD71-positive erythroid lineage cells (Figure 3E) and myeloperoxidase-positive myeloid lineage cells (Figure 3F) in spleen. In cases of EHPVO with MPN, not only portal hypertensive change of spleen, but also EMH in spleen might be associated with the pathogenesis of splenomegaly.
Figure 3.
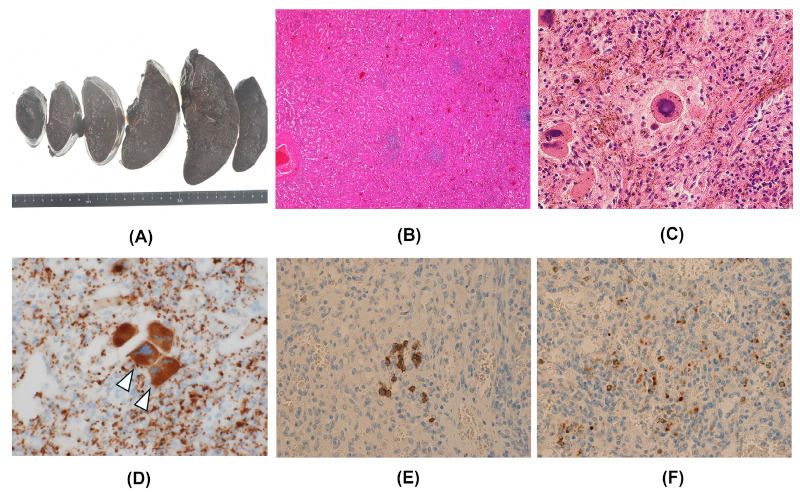
Macroscopic (A) and histological (B-F) findings of the resected spleen in case 4. (A) Photograph of resected spleen weighing 574 gram; (B, C) Histological examination demonstrated splenic sinus congestion and decreased white pulp, with local extramedullary hematopoiesis (B: hematoxylin-eosin, x 40, C: hematoxylin-eosin, x 400); (D, E, F) Immunohistochemical studies revealed CD41-positive megakaryocytes (D, white arrowhead, x 400), CD71-positive erythroid lineage cells (E, x 200), and myeloperoxidase-positive myeloid lineage cells (F, x 200) in the spleen.
MPN is reported to be the most significant factor for EHPVO (3-9). The original cause of EHPVO is based on thrombotic tendency; therefore, the association between MPN and increased risk of thrombosis should be considered in screening for EHPVO (15). However, awareness of the association between EHPVO and MPN among gastroenterologists is generally insufficient.
A significant relationship between EHPVO and MPN was proven by the high frequency of a clonal mutation of JAK2V617F, and which should be utilized as a diagnostic tool to detect latent MPN in EHPVO patients (9). Screening for JAK2V617F mutation in patients with splanchnic vein thrombosis including EHPVO is extremely important, and can assist in both the diagnosis and treatment of MPN. The JAK2V617F mutation is present in more than 90% of patients with polycythemia vera and in approximately 60% of patients with essential thrombocythemia and myelofibrosis (16,17). JAK2 tyrosine kinase activates a cytokine-independent JAK-STAT pathway, causing proliferation of mature myeloid cells. The JAK2V617F mutation was shown to be an independent risk factor for splanchnic vein thrombosis (3,18-20). None of the risk factors could be identified in a third of patients with EHPVO; however, more than half of these go on to develop an overt MPN during follow up (8,20,21). Patients with EHPVO should be screened for the JAK2V617F mutation to avoid overlooking a potential underlying persistent thrombophilia due to MPN (9).
We reported that hypersplenic hematologic changes of thrombo-leukocytopenia in EHPVO patients might be veiled by MPN-induced proliferation of blood cells (9). In this finding, we suspected that hypersplenism as a comorbidity with MPN might be associated with latent MPN, and that the spleen may have contributed to the normalized blood counts in latent MPN. These splenectomies with MPN led to the strong suspicion that extreme thrombocytosis in postoperative asplenia was veiled by preoperative EHPVO-induced hypersplenism. This phenomenon may also explain the spleen function's contribution to a condition of latent MPN including masked polycythemia vera.
Thrombot ic complicat ion is very ser ious because EHPVO patients often suffer from vascular disease, including myocardial or cerebral infarction. Furthermore, we have experienced cases of myocardial and cerebral infarction in EHPVO patients in their 30s (9). Owing to the thrombotic tendency, prophylactic thrombotic agents should be considered in EHPVO; however, the majority of symptomatic EHPVO patients experience intestinal variceal rupture. The dilemma between thrombosis and hemorrhage in EHPVO is the most difficult for general gastroenterologists (9,22). Prognosis in EHPVO patients is strongly associated with the control of esophagogastric variceal bleeding. EHPVO patients that are appropriately controlled for portal hypertensive complication showed favorable prognosis with a survival rate of 69-86% at 10 years (23). For eradication of the refractory esophagogastric varices of EHPVO with MPN, we experienced successful cases of combination therapy of Hassab's operation and subsequent endoscopic variceal ligation (20,23). Hassab's operation includes splenectomy with devascularization of the upper half of the stomach and distal esophagus (24,25). However, portal thrombus after splenectomy was a major postoperative complication in portal hypertension. In MPN cases, splenectomy induces excessive thrombocytosis, and thrombocytosis with reduced portal vein velocity is associated with the formation of further portal vein thrombosis causing hepatic failure or disappointing portal hypertension. Splenectomy in cases of hypersplenism with large splenic vein diameter is thought to further promote the progression of portal vein thrombus, and early antithrombotic administration should be considered in these situations (23,26). Contrary to thrombotic complications, extreme thrombocytosis is sometimes characterized by severe bleeding tendency with decrease or abnormality of von Willebrand factor as a consequence of the precipitous increase in platelets, referred to as acquired von Willebrand factor syndrome (27-29). All 3 MPN cases with splenectomy demonstrated excessive thrombocytosis and required strict cytoreduction treatment for platelet count to address concern about subsequent coagulopathy by acquired von Willebrand factor syndrome. In our cases, the timely introduction of cytoreduction therapy for extreme thrombocytosis by hematologists was thought to be possible because of a preoperative diagnosis of MPN and constant perioperative collaboration with hematologists. If hematological disease including MPN is associated with EHPVO, collaboration between gastroenterologists and hematologists is essential for better treatment and prognosis.
EMH is reportedly associated with many diseases, including chronic anemia, sickle cell disease, thalassemia, spherocytosis, and hematological neoplasms (27). EMH is generally a compensatory response occurring secondary to inadequate bone marrow function. Few papers on splenic EMH with MPN have been published, and less frequent cases of splenic EMH associated with MPN under hypersplenic condition of EHPVO have rarely been reported.
Further detailed data on EHPVO with MPN in a large series must be accumulated; however, we hope that our results will contribute to the clarification of pathogenesis and better treatment for the intractable conditions.
Gastroenterologists sometimes experience EHPVO patients requiring splenectomy for complications of portal hypertension, including hemorrhagic esophagogastric varices. It is important to consider underlying MPN based on perioperative hematological change and histological character of resected spleen. Otherwise, the gastroenterologist may overlook the underlying MPN causing EHPVO due to feigned normalization of platelet counts by the conflicting effects of hypersplenic hematopenia by EHPVO and blood cell proliferation by MPN. Based on cases of EHPVO with MPN, our team considers screening for JAK2V617F mutation to be essential for all patients with suspected EHPVO.
In conclusion, in EHPVO patients with MPN, hypersplenism causes feigned normalization of platelet count by masking MPN-induced thrombocytosis; however, splenectomy unveils postoperative thrombocytosis. Spleen in EHPVO with MPN also participates in extramedullary hematopoiesis.
Funding:
None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Ageno W, Dentali F, Pomero F, Fenoglio L, Squizzato A, Pagani G, Re R, Bonzini M. Incidence rates and case fatality rates of portal vein thrombosis and Budd-Chiari Syndrome. Thromb Haemost. 2017; 117:794-800. [DOI] [PubMed] [Google Scholar]
- 2. Ohfuji S, Furuichi Y, Akahoshi T, Kage M, Obara K, Hashizume M, Matsuura T, Fukushima W, Nakamura Y. Japanese periodical nationwide epidemiologic survey of aberrant portal hemodynamics. Hepatol Res. 2019; 49:890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd- Chiari syndrome and portal vein thrombosis: A meta-analysis. Blood. 2012; 120:4921-4928. [DOI] [PubMed] [Google Scholar]
- 4. Denninger MH, Chaït Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J, Valla D. Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors. Hepatology. 2000; 31:587-591. [DOI] [PubMed] [Google Scholar]
- 5. Janssen HL, Wijnhoud A, Haagsma EB, van Uum SH, van Nieuwkerk CM, Adang RP, Chamuleau RA, van Hattum J, Vleggaar FP, Hansen BE, Rosendaal FR, van Hoek B. Extrahepatic portal vein thrombosis: Aetiology and determinants of survival. Gut. 2001; 49:720-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bayraktar Y, Harmanci O, Büyükasik Y, Shorbagi AI, Sungur AH, Boylu CA, Gürgey A, Balkanci F. JAK2V617F mutation in patients with portal vein thrombosis. Dig Dis Sci. 2008; 53:2778-2783. [DOI] [PubMed] [Google Scholar]
- 7. Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: A prospective multicenter follow-up study. Hepatology. 2010; 51:210-218. [DOI] [PubMed] [Google Scholar]
- 8. Valla D, Casadevall N, Huisse MG, Tulliez M, Grange JD, Muller O, Binda T, Varet B, Rueff B, Benhamou JP. Etiology of portal vein thrombosis in adults. A prospective evaluation of primary myeloproliferative disorders. Gastroenterology. 1988; 94:1063-1069. [DOI] [PubMed] [Google Scholar]
- 9. Shimizu T, Yoshida H, Taniai N, Yoshioka M, Kawano Y, Matsushita A, Ueda J, Iwai T, Murokawa T, Ono T, Hamaguchi A. Clinical features of extrahepatic portal vein obstruction: Myeloproliferative neoplasms eliminate hypersplenic hematologic changes in extrahepatic portal vein obstruction. Intractable Rare Dis Res. 2024; 13:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Titmarsh GJ, Duncombe AS, McMullin MF, O'Rorke M, Mesa R, De Vocht F, Horan S, Fritschi L, Clarke M, Anderson LA. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol. 2014; 89:581-587. [DOI] [PubMed] [Google Scholar]
- 11. Barbui T, Thiele J, Carobbio A, Gisslinger H, Finazzi G, Rumi E, Luigia Randi M, Vannucchi AM, Gisslinger B, Müllauer L, Ruggeri M, Rambaldi A, Tefferi A. Masked polycythemia vera diagnosed according to WHO and BCSH classification. Am J Hematol. 2014; 89:199-202. [DOI] [PubMed] [Google Scholar]
- 12. Sharma A, Keshava SN, Eapen A, Elias E, Eapen CE. An update on the management of Budd-Chiari syndrome. Dig Dis Sci. 2021; 66:1780-1790. [DOI] [PubMed] [Google Scholar]
- 13. Yoshida H, Shimizu T, Yoshioka M, Matsushita A, Kawano Y, Ueda J, Kawashima M, Taniai N, Mamada Y. The role of the spleen in portal hypertension. J Nippon Med Sch. 2023; 90:20-25. [DOI] [PubMed] [Google Scholar]
- 14. Maeda D, Sakane K, Kanzaki Y, Horai R, Akamatsu K, Tsuda K, Ito T, Sohmiya K, Hoshiga M. Splenic volume index determined using computed tomography upon admission is associated with readmission for heart failure among patients with acute decompensated heart failure. Int Heart J. 2021; 62:584-591. [DOI] [PubMed] [Google Scholar]
- 15. Patel RK, Lea NC, Heneghan MA, Westwood NB, Milojkovic D, Thanigaikumar M, Yallop D, Arya R, Pagliuca A, Gäken J, Wendon J, Heaton ND, Mufti GJ. Prevalence of the activating JAK2 tyrosine kinase mutation V617F in the Budd-Chiari syndrome. Gastroenterology. 2006; 130:2031-2038. [DOI] [PubMed] [Google Scholar]
- 16. James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur- Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005; 434:1144-1148. [DOI] [PubMed] [Google Scholar]
- 17. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR; Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005; 365:1054-1061. [DOI] [PubMed] [Google Scholar]
- 18. Primignani M, Barosi G, Bergamaschi G, Gianelli U, Fabris F, Reati R, Dell'Era A, Bucciarelli P, Mannucci PM. Role of the JAK2 mutation in the diagnosis of chronic myeloproliferative disorders in splanchnic vein thrombosis. Hepatology. 2006; 44:1528-1534. [DOI] [PubMed] [Google Scholar]
- 19. De Stefano V, Vannucchi AM, Ruggeri M, et al. Splanchnic vein thrombosis in myeloproliferative neoplasms: Risk factors for recurrences in a cohort of 181 patients. Blood Cancer J. 2016; 6:e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shimizu T, Yoshioka M, Matsushita A, Ueda J, Kawashima M, Ono T, Kawano Y, Yoshida H. Esophagogastric varix caused by extrahepatic portal vein obstruction with essential thrombocythemia: A case report. J Nippon Med Sch. Advance online publication. doi: 10.1272/jnms.JNMS.2024_91-601 [DOI] [PubMed] [Google Scholar]
- 21. Amarapurkar P, Bhatt N, Patel N, Amarapurkar D. Primary extrahepatic portal vein obstruction in adults: a single center experience. Indian J Gastroenterol. 2014; 33:19-22. [DOI] [PubMed] [Google Scholar]
- 22. Shimizu T, Yoshioka M, Ueda J, Kawashima M, Irie T, Kawano Y, Matsushita A, Taniai N, Mamada Y, Yoshida H. Stenting of inferior right hepatic vein in a patient with Budd-Chiari syndrome: A case report. J Nippon Med Sch. 2024; 91:119-123. [DOI] [PubMed] [Google Scholar]
- 23. Shimizu T, Yoshioka M, Kawano Y, et al. Modified Hassab's operation and endoscopic variceal ligation for esophagogastric varices caused by extrahepatic portal vein obstruction with JAK2V617F-mutated myeloproliferative neoplasm: A case report. Japanese Journal of Portal Hypertension. 2023; 29:227-234. [Google Scholar]
- 24. Hassab MA. Gastroesophageal decongestion and splenectomy. A method of prevention and treatment of bleeding from esophageal variceal associated with bilharzial hepatiiic fibrosis: Preliminary report. J Int Coll Surg. J Int Coll Surg. 1964; 41:232-248. [PubMed] [Google Scholar]
- 25. Yoshida H, Shimizu T, Yoshioka M, Taniai N. Management of portal hypertension based on portal hemodynamics. Hepatol Res. 2021; 51:251-262. [DOI] [PubMed] [Google Scholar]
- 26. Kawanaka H, Akahoshi T, Kinjo N, Konishi K, Yoshida D, Anegawa G, Yamaguchi S, Uehara H, Hashimoto N, Tsutsumi N, Tomikawa M, Maehara Y. Impact of antithrombin III concentrates on portal vein thrombosis after splenectomy in patients with liver cirrhosis and hypersplenism. Ann Surg. 2010; 251:76-83. [DOI] [PubMed] [Google Scholar]
- 27. Hosoda K, Shimizu A, Kubota K, Notake T, Sugenoya S, Yasukawa K, Hayashi H, Kobayashi R, Soejima Y. A focal extramedullary hematopoiesis of the spleen in a patient with essential thrombocythemia presenting with a complicated postoperative course: A case report. Surg Case Rep. 2021; 7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castaman G, Lattuada A, Ruggeri M, Tosetto A, Mannucci PM, Rodeghiero F. Platelet von Willebrand factor abnormalities in myeloproliferative syndromes. Am J Hematol. 1995; 49:289-293. [DOI] [PubMed] [Google Scholar]
- 29. Franchini M, Mannucci PM. Acquired von Willebrand syndrome: Focused for hematologists. Haematologica. 2020; 105:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]


