Abstract
Dantrolene is an inhibitor of intracellular Ca2+ release from skeletal muscle SR (sarcoplasmic reticulum). Direct photoaffinity labelling experiments using [3H]azidodantrolene and synthetic domain peptides have demonstrated that this drug targets amino acids 590–609 [termed DP1 (domain peptide 1)] of RyR1 (ryanodine receptor 1), the skeletal muscle RyR isoform. Although the identical sequence exists in the cardiac isoform, RyR2 (residues 601–620), specific labelling of RyR2 by dantrolene has not been demonstrated, even though some functional studies show protective effects of dantrolene on heart function. Here we test whether dantrolene-active domains exist within RyR2 and if so, whether this domain can be modulated. We show that elongated DP1 sequences from RyR1 (DP1-2s; residues 590–628) and RyR2 (DP1-2c; residues 601–639) can be specifically photolabelled by [3H]azidodantrolene. Monoclonal anti-RyR1 antibody, whose epitope is the DP1 region, can recognize RyR1 but not RyR2 in Western blot and immunoprecipitation assays, yet it recognizes both DP1-2c and DP1-2s. This suggests that although the RyR2 sequence has an intrinsic capacity to bind dantrolene in vitro, this site may be poorly accessible in the native channel protein. To examine whether it is possible to modulate this site, we measured binding of [3H]dantrolene to cardiac SR as a function of free Ca2+. We found that ≥10 mM EGTA increased [3H]dantrolene binding to RyR2 by ∼2-fold. The data suggest that the dantrolene-binding site on RyR2 is conformationally sensitive. This site may be a potential therapeutic target in cardiovascular diseases sensitive to dysfunctional intracellular Ca2+ release.
Keywords: [3H]azidodantrolene, calcium, dantrolene, malignant hyperthermia, photoaffinity labelling, ryanodine receptor
Abbreviations: anti-C-term antibody, anti-(rabbit RyR1 C-terminus) antibody; DP1, domain peptide 1; mAb, monoclonal antibody; MH, malignant hyperthermia; RyR, ryanodine receptor; SR, sarcoplasmic reticulum
INTRODUCTION
The RyRs (ryanodine receptors) constitute a major class of intracellular Ca2+-release channels that generally respond to increases in cytoplasmic Ca2+ levels and release Ca2+ from internal stores by a mechanism termed Ca2+-induced Ca2+ release [1,2]. In skeletal muscle, however, the RyR responds by the mechanism of voltage-induced Ca2+ release, in which the sarcolemmal L-type, voltage-dependent Ca2+ channel responds to depolarization by directly contacting the RyR, inducing it to open and release Ca2+ [3]. Three distinct isoforms (RyR1, RyR2 and RyR3) of this channel have been identified in mammalian tissues by cDNA cloning studies [4]. RyR1 and RyR2 are abundantly expressed in skeletal and cardiac muscle cells respectively. RyR3 is expressed predominantly in smooth muscle and epithelial cells, and at low levels in a wide variety of cell types, including neurons, skeletal muscle and cardiac muscle [5].
RyR1 and RyR2 share more than 60% amino acid sequence identity, and respond similarly to a number of pharmacological and physiological effectors, such as Ca2+, Mg2+, ATP, caffeine, ryanodine and Ruthenium Red [6]. Among their differences, however, is their response to the drug dantrolene, an intracellular skeletal muscle relaxant. Dantrolene brings about its muscle relaxant effect by inhibiting Ca2+ release from the SR (sarcoplasmic reticulum) in skeletal muscle [7,8]. It is used clinically to treat MH (malignant hyperthermia), a pharmacogenetic disorder of skeletal muscle characterized by an uncontrolled release of Ca2+ from the SR in response to volatile anaesthetics, resulting in hypercontracture and hypermetabolism [9,10]. This drug is being investigated for potential use in other clinically relevant systems of intracellular hypercalcaemia. For example, dantrolene has been reported to be neuroprotective in some models of neuronal cell death [11,12].
In vitro muscle contractility assays and ligand binding studies with native and recombinant RyR proteins suggest that dantrolene interacts specifically with RyR1, not RyR2 [8,13,14]. Direct photoaffinity labelling experiments using [3H]azidodantrolene, a photoactivatable analogue of dantrolene [15], have demonstrated that while RyR1 is specifically photolabelled, RyR2 is a poor target of this drug [16]. However, there is accumulating evidence from studies on isolated cardiac myocytes and heart preparations demonstrating that dantrolene appears to affect RyR2-mediated, Ca2+-dependent processes, particularly under conditions of stress, i.e. ischaemia/reperfusion injury, hyperthyroid cardiomyopathy and doxorubicin toxicity [17–21]. These results suggest that the lack of effect of dantrolene on RyR2 could be due either to an inaccessible dantrolene-binding site in the ‘unstressed’ conformation or the possibility that accessory proteins play a major role in dantrolene's interaction with RyRs.
Using synthetic domain peptides as potential conformational mimics of RyR1-active domains, we previously identified the RyR1 sequence comprising residues 590–609 [DP1 (domain peptide 1)] as the dantrolene-binding site by the following criteria: (1) DP1 is specifically photolabelled by the photoaffinity analogue of dantrolene, [3H]azidodantrolene; (2) DP1 is recognized by anti-RyR1 mAb (monoclonal antibody; IgM) raised against RyR1; and (3) RyR1 photolabelling by [3H]azidodantrolene is specifically inhibited by anti-RyR1 mAb [22]. Anti-DP1 antibodies enhance [3H]ryanodine binding to skeletal SR, sensitize such SR to Ca2+ release, and enhance accessibility to a macromolecular fluorescence quencher, demonstrating that the dantrolene-binding sequence is in a conformationally significant region of RyR1 and is involved in the regulation of channel activity [23]. Significantly, this sequence is identical among the three known mammalian isoforms of RyR.
In the present study, we examine whether anti-RyR1 mAb can be used to detect a dantrolene-responsive site on RyR2 and whether a synthetic peptide containing the dantrolene-binding sequence elongated into the RyR2 sequence still maintains a conformation that permits dantrolene binding. The implications of these experiments for therapeutic potential are discussed.
EXPERIMENTAL
Materials
Azumolene sodium·2H2O was generously donated by Proctor & Gamble (Norwich, NY, U.S.A.). [3H]Azidodantrolene was synthesized, purified and characterized as described in [15], and specific radioactivity was determined to be 28 Ci/mmol. Anti-(rabbit RyR1) mAb (IgM; clone XA7) and polyclonal anti-C-term [anti-(rabbit RyR1 C-terminus)] antibodies (raised against a synthetic C-terminal peptide corresponding to amino acids 5023–5037 of RyR1) were gifts from Dr K. P. Campbell (University of Iowa, Iowa City, IA, U.S.A.). Rabbit fast-twitch skeletal muscle was supplied by Dr H. Weiss (UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ, U.S.A.). Protein A–agarose beads, alkaline phosphate-conjugated secondary antibodies, as well as all other reagents and chemicals of high-purity grade, were obtained from Sigma.
[3H]Azidodantrolene photolabelling of peptides
RyR domain peptides DP1-2s (skeletal RyR sequence), DP1-2c (cardiac RyR sequence) and DP3 were synthesized and purified as described [25]. Synthetic peptides (12.5 μM) were photolabelled with 50–100 nM [3H]azidodantrolene in binding buffer (20 mM Pipes, pH 7) containing 10 μg of BSA, in the absence (T; total binding) or presence (N; non-specific binding) of azumolene (150–300 μM), as described previously [22]. Following photolabelling, samples were fractionated by SDS/PAGE on a 20% (w/v) polyacrylamide gel [26], and then electroblotted on to PVDF membranes (Sequi-Blot; Bio-Rad) [27]. Autoradiography was performed on these membranes as described [16].
[3H]Dantrolene binding assay
Crude SR vesicles were prepared from rabbit fast-twitch skeletal muscle as described in [24] and kept at 80 °C until use. Pig cardiac SR membranes were kindly supplied by Dr B. Fruen (University of Minnesota, Minneapolis, MN, U.S.A.). Binding of [3H]dantrolene (10.2 Ci/mmol) to cardiac muscle SR was assayed as described [16]. Briefly, purified pig cardiac SR protein (50–100 μg) was incubated in triplicate with 200 nM [3H]dantrolene with or without 150 μM azumolene in the presence or absence of appropriate concentrations of EGTA for 90 min at 37 °C in binding buffer (20 mM Na-Pipes, pH 7, 150 mM KCl, 0.5 mM adenosine 5′-[β,γ-methylene]triphosphate, 0.5 mM MgCl2), before bound ligand was separated from free ligand by rapid filtration through Whatman GF/C glass filters. Bound radioactivity was determined by liquid scintillation counting.
Immunoprecipitation and Western blotting of RyR proteins
Solubilized skeletal and cardiac SR proteins (100 μg) were immunoprecipitated with mAb (1:200 dilution) essentially as described [16] and adapted from Zhang et al. [28]. Non-immune IgM was used as negative control. Immunoprecipitated proteins were eluted from Protein A–agarose beads by the addition of 80 μl of 5×Laemmli sample buffer followed by incubation at room temperature for 30 min. The eluted proteins were resolved by SDS/5%-PAGE and electroblotted on to PVDF membranes.
Skeletal and cardiac SR proteins (100 μg), transferred on to PVDF membranes, were probed with either anti-RyR1 mAb (RyR1-specific) or polyclonal anti-C-term antibody. The latter antibody recognizes both RyR1 and RyR2. Immunoprecipitated skeletal and cardiac SR proteins were probed with anti-C-term antibody, while synthetic peptides (12.5 μM) were probed with anti-RyR1 mAb. Both primary antibodies were used at a dilution of 1:1000. Immunoreactive bands were visualized with 5-bromo-4-chloro-3-indolyl phosphate/p-Nitro Blue Tetrazolium, after incubation with alkaline phosphatase-conjugated secondary antibodies.
RESULTS AND DISCUSSION
Synthetic RyR domain peptides DP1-2c and DP1-2s contain a dantrolene-binding motif
In our earlier paper, we demonstrated that the DP1 sequence in RyR1 contains a dantrolene-binding site [22]. The identical sequence exists in RyR2, yet this isoform does not seem to be a target of dantrolene. This perplexing result could mean (a) that the conformation of DP1 in RyR2 is different from that in RyR1 due to conformational constraints imparted by sequence differences in an adjacent domain(s); or (b) that although DP1 in RyR2 is conformationally competent to bind dantrolene, access to the site is blocked by an associated molecule or post-translational modification.
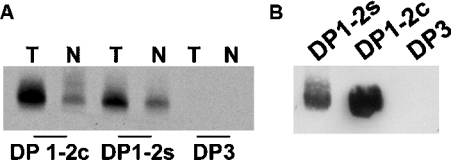
To test an approximation of the first possibility, we synthesized a 39-amino-acid RyR2 peptide containing DP1 elongated by the adjacent C-terminal 20 amino acids, i.e. domain 2. This peptide, designated DP1-2c (amino acids 601–639 of RyR2), is identical to the dantrolene-binding sequence DP1-2s (amino acids 590–628 of RyR1) except for four residues within domain 2 (Figure 1). We photolabelled both DP1-2 peptides with [3H]azidodantrolene in the absence or presence of excess azumolene and processed them for autoradiography. The autoradiograph in Figure 2(A) shows that [3H]azidodantrolene specifically labelled DP1-2c, as well as DP1-2s (n=5). Another RyR1 domain peptide, DP3 (corresponding to amino acids 324–351), used as a negative control in these experiments, was not labelled. These results demonstrate that a synthetic peptide with a RyR2 sequence has the capacity to specifically bind dantrolene. We can conclude that DP1-2s and DP1-2c have similar conformations in solution that allow dantrolene binding.
Figure 1. Synthetic RyR domain peptide sequences.
Amino acids that differ between DP1-2s and DP1-2c are in bold, and the italicized R at position 615 in DP1-2s is the site of the canonical R615C mutation conferring MH susceptibility. s, skeletal; c, cardiac.
Figure 2. Specific photolabelling and Western blotting of RyR domain peptides.
(A) DP1-2c, DP1-2s and DP3 were photolabelled with [3H]azidodantrolene in the absence (T) or presence (N) of azumolene, resolved by SDS/20%-PAGE, electroblotted on to a PVDF membrane and subjected to autoradiography. (B) Synthetic DP1-2c and DP1-2s, as well as DP3 (as a negative control), were resolved by SDS/20%-PAGE, transferred on to a PVDF membrane and probed with anti-RyR1 mAb. Similar results were obtained in five other experiments.
Anti-RyR1 mAb recognizes both DP1-2c and DP1-2s
We then sought to determine whether the DP1-2c sequence has a conformation similar to that of the native RyR channel protein. Anti-RyR1 mAb (clone XA7), produced against rabbit RyR1 [29], has been shown to be immunoreactive towards both the RyR1 monomer and the synthetic peptide DP1, and it inhibits [3H]azidodantrolene photolabelling of RyR1 in a concentration-dependent manner [16,22]. Since the DP1 region is common to both DP1-2s and DP1-2c, and the latter peptide is also specifically labelled by [3H]azidodantrolene, one would expect that mAb anti-RyR1 would recognize both of these peptides. Indeed, a Western blot of DP1-2s and DP1-2c, along with DP3 as negative control, confirmed that anti-RyR1 mAb recognizes both DP1-2s and DP1-2c peptides (Figure 2B; n=5).
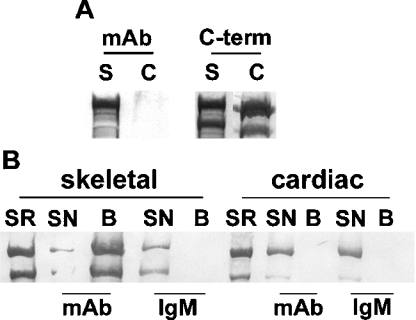
Next we resolved cardiac and skeletal SR proteins by SDS/5%-PAGE, electroblotted them on to a PVDF membrane and probed the membrane with anti-RyR1 mAb (n=3). The result, presented in Figure 3(A) (left panel), shows that the mAb was immunoreactive towards the skeletal (S) but not the cardiac (C) RyR isoform. An identical blot probed with the anti-C-term antibody, which recognizes both isoforms, demonstrated that this was not due to a lack of RyR2 in the sample (Figure 3A, right panel). In addition, the blot probed with anti-RyR1 mAb (Figure 3A, left panel) was also probed with anti-C-term to confirm the presence of RyR2 on this blot as well (results not shown). These results demonstrate that only RyR1, but not RyR2, is recognized by anti-RyR1 mAb.
Figure 3. Western blotting and immunoprecipitation of skeletal and cardiac RyRs with anti-RyR1 mAb.
(A) Western blot of skeletal (s) and cardiac (c) SR proteins probed with anti-C-term antibody and anti-RyR1 mAb. Note that anti-RyR1 mAb recognizes RyR1, but not RyR2. (B) Immunoprecipitation of skeletal and cardiac RyRs by anti-RyR1 mAb or non-immune IgM. Immunoprecipitated proteins were blotted on to PVDF and probed with anti-C-term antibody. SR denotes SR before solubilization; SN, supernatant fraction that did not bind to Protein A–agarose beads; B, proteins associated with Protein A–agarose beads.
The above results suggest that the epitope of the mAb on RyR2 remains inaccessible even after denaturation prior to gel electrophoresis and transfer. Both DP1-2 peptides, on the other hand, were denatured similarly prior to Western blotting, yet the antibody recognized both peptides. One possible explanation for these results is that RyR1 and RyR2 maintain a different conformational structure surrounding this epitope even after treatment with SDS. If this is true, it would suggest that the DP1 epitope is buried in RyR2, while in RyR1 it is more exposed. With the peptides, however, the absence of long-range contributions to structure and conformation renders the epitope accessible to antibody. A second, less plausible, explanation is that SDS/PAGE somehow destroys the epitope exclusively in RyR2, and not in RyR1, making the former unrecognizable to anti-RyR1 mAb. Nonetheless, we decided to test whether, under solution conditions, anti-RyR1 mAb would recognize the DP1 sequence on RyR2 in SR.
Immunoprecipitation of skeletal and cardiac SR proteins solubilized with 3% CHAPS was performed with anti-RyR1 mAb and Protein A–agarose beads. The bead-associated proteins were resolved by SDS/PAGE, transferred on to PVDF membranes, and probed with anti-C-term antibody to detect the presence of RyR isoforms. The Western blot (Figure 3B) demonstrates that, in the skeletal muscle preparation, RyR1 protein recognized by anti-C-term antibody was present primarily in the bead-associated fraction (B), while no detectable anti-C-term-antibody-reactive RyR2 protein from cardiac SR was associated with the bead fraction, despite the presence of substantial RyR2 in the supernatant. Non-specific IgM did not immunoprecipitate either RyR1 or RyR2, demonstrating that the effect of anti-RyR1 mAb (IgM) was specific. These results demonstrate the immunoprecipitation of RyR1, but not RyR2, by anti-RyR1 mAb.
Decreasing the free Ca2+ concentration enhances the interactions of dantrolene with RyR2 and RyR1
The lack of interaction of anti-RyR1 mAb with RyR2 supports our contention that, although the DP1 sequence is present in this isoform, the DP1 epitope, as it is present in RyR1, is absent. We wished to determine whether a dantrolene-sensitive conformation of RyR2 could be obtained. Since Ca2+ is known to regulate RyR activity both directly and indirectly, we reasoned that varying the Ca2+ concentration might modify the accessibility of RyR2 to dantrolene. To test this, we measured [3H]dantrolene binding to cardiac SR as a function of the concentration of EGTA. Table 1 shows that specific [3H]dantrolene binding to cardiac SR was enhanced approx. 2-fold in the presence of high concentrations of EGTA (≥10 mM; n=6). Lower concentrations of EGTA had no effect on baseline radioligand binding (results not shown). These results are not unique to RyR2. Despite the order-of-magnitude greater [3H]dantrolene binding to skeletal over cardiac SR, we again found enhancement of specific [3H]dantrolene binding as a result of treatment with similarly high concentrations of EGTA (Table 1; n=3).
Table 1. [3H]Dantrolene binding to cardiac and skeletal SR.
Cardiac or skeletal SR was incubated in triplicate with 200 nM [3H]dantrolene with or without 150 μM azumolene in the presence or absence of EGTA (10–40 mM), for 90 min at 37 °C in buffer containing 0.5 mM adenosine 5′-[β,γ-methylene]triphosphate, and bound radioactivity was separated from free by rapid filtration. Specific binding was calculated as total bound minus that bound in the presence of excess azumolene. Since radioligand binding was virtually equivalent in the EGTA concentration range 10–40 mM, results in the presence of EGTA are presented as pooled data.
| [3H]Dantrolene bound (pmol/mg of protein) | |||
|---|---|---|---|
| −EGTA | +EGTA | P | |
| RyR2 (n=6) | 1.13±0.19 | 2.21±0.30 | <10−5 |
| RyR1 (n=3) | 40.2±2.1 | 55.3±4.6 | <10−3 |
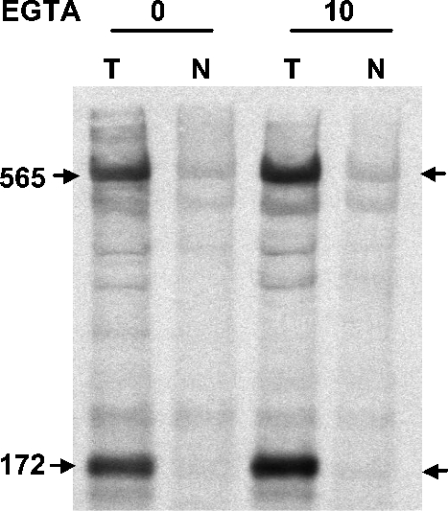
Furthermore, photoaffinity labelling of RyR1 with [3H]azidodantrolene in the presence or absence of 10 mM EGTA demonstrated enhanced specific photolabelling of both the 565 kDa monomer and its 172 kDa n-calpain fragment (Figure 4; n=3). Similar photoaffinity labelling experiments with cardiac SR were unsuccessful in revealing enhanced signals in the presence of high concentrations of EGTA due to the 10-fold lower ligand-binding affinity and the low efficiency of photolabelling [15,16].
Figure 4. Enhancement of specific [3H]azidodantrolene photolabelling of RyR1 in the presence of EGTA.
Skeletal muscle SR was photolabelled with 200 nM [3H]azidodantrolene in the absence (T) or presence (N) of 150 μM azumolene in binding buffer containing 0.5 mM adenosine 5′-[β,γ-methylene]triphosphate, in the absence or presence of 10 mM EGTA. The protein was resolved by SDS/5%-PAGE, electroblotted on to PVDF membranes and subjected to autoradiography. Values are in kDa, and arrows point to the bands of specific photolabelling.
It is tempting to speculate that the enhanced interactions of dantrolene with both RyR1 and RyR2 in the presence of high EGTA concentrations result not only from the decrease in extraluminal Ca2+, but also from the drastic decrease in the intraluminal Ca2+ concentration that must accompany the high solution concentrations of EGTA. There is evidence that calsequestrin, the primary calcium-binding protein of SR, binds directly to the RyR and forms a quaternary complex along with triadin and junctin. Modulation of this complex has been shown to effect not only SR Ca2+ release, but also store-operated Ca2+ entry, a plasma membrane process that responds to low SR Ca2+ stores by allowing Ca2+ to enter the cell [30,31]. We suspect that there is a relationship between the conformational interactions of RyRs and a calsequestrin-triggered complex that influences the ability of dantrolene to bind.
Our results suggest that interactions of RyR2 with dantrolene are susceptible to environmental modulation. Although the high concentrations of EGTA used in these experiments were greater than those normally necessary to modulate solution Ca2+ conditions, we have shown previously that inhibition of the SR membrane-resident, Ca2+-dependent, n-calpain (CALP3) cleavage of RyR1 in vitro does not occur until 10 mM EGTA is present [16]. The in vitro EGTA sensitivity of [3H]dantrolene binding to RyR2, therefore, demonstrates that it is possible to conformationally modulate this isoform so as to allow dantrolene binding, but does not inform us with regard to a potential mechanism of in vivo regulation of this site. Of necessity, in vivo mechanisms would have to involve modulation of this site in the presence of increased myoplasmic Ca2+ levels, rather than the decreased levels used herein, as the physiological studies that have demonstrated an effect of dantrolene on cardiac tissue have done so particularly during experimental pretreatment for physiological stresses associated with enhanced intracellular Ca2+ release: hyperthyroid cardiomyopathy [32], heart failure [33], myocardial stunning and ischaemia [17,34,35], post-infarction contractility and responsiveness to isoprenaline (isoproterenol) [36], and endotoxin- or thermal injury-related suppression of myocardial function [20,21]. All of these are chronic physiological stressors, the effects of which on a dantrolene–RyR2 interaction may involve post-translational modification of the channel and/or result from gene expression of a channel-interacting protein(s). Taken together, the evidence suggests that, under certain pathophysiological conditions, RyR2 may be capable of interacting with dantrolene, i.e. accessibility to the putative dantrolene-binding site on RyR2 may be conformationally regulated. This suggestion may have profound consequences for clinical cardiac pharmacotherapy.
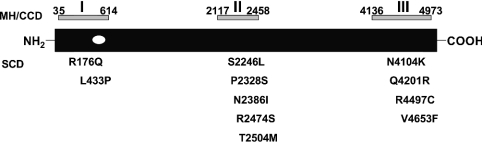
Aberrant regulation of myocardial intracellular Ca2+ is common in pathophysiological states such as myocardial ischaemia/stunning and heart failure, and some rare syndromes such as Long QT Syndrome, and has been linked to the development of cardiac arrhythmias [37–40]. Recently, arrhythmogenic right ventricular dysplasia type II (ARVD II) and catecholaminergic (familial) polymorphic ventricular tachycardia [C(F)PVT], two genetic syndromes leading to sudden cardiac death, have been traced to three regions of RyR2 that are related to the mutationally ‘hot’ regions of RyR1 associated with MH and the rare myopathy central core disease, both of which are associated with leaky myocardial SR (Figure 5) [41–44]. Ca2+ leakage from myocardial SR via dysfunctional RyR2, therefore, is suggested as a proximal cause of lethal ventricular arrhythmias. The dantrolene-binding site on RyR1 lies within the first of the mutationally hot regions associated with dysregulated Ca2+ release, and we have presented evidence that a modulatable putative site exists in the equivalent region of RyR2. The development of RyR2-specific dantrolene derivatives as an entirely new class of anti-arrhythmics would present us with potential new agents for a novel target in the treatment of lethal ventricular arrhythmias.
Figure 5. Localization of the dantrolene-binding site within the ‘hot spots’ of RyR mutations associated with MH/central core disease and inherited ventricular tachycardias resulting in sudden cardiac death.
The positions of the three MH/central core disease (CCD) and homologous sudden cardiac death (SCD) mutation domains are indicated above and below the canonical RyR protein respectively. The numbers denote the boundary amino acids identified as likely causative mutations in each domain. The individual amino acids in the SCD mutation domains are denoted below the RyR protein. The position of the dantrolene-binding site, represented as a clear ellipse within the first mutation-rich domain, suggests it as a potential therapeutic target in RyR2.
Conclusions
In summary, our results demonstrate that a putative dantrolene-binding site exists on RyR2. The data suggest, however, that the native conformation of RyR2 restricts access of the drug to its binding site. We hypothesize that conditions that expose this site would result in dantrolene suppressing RyR2-dependent Ca2+ release. We propose that it may be possible to design dantrolene derivatives that interact preferentially with RyR2 for potential use as anti-arrhythmic agents. Further studies are required to elucidate the cellular and molecular mechanisms regulating the potential interaction of dantrolene with RyR2.
Acknowledgments
This work was supported by NIH grants R01 AR45593 (J.P.), RO1 AG-15556 and RO1 HL-69000 (J.M.), and R01 AR16922 RO1 and HL072841 (N.I.); NSF grant CHE-9502149; grants from the Petroleum Research Fund and the Charles and Johanna Busch Memorial Fund (L.S.J.); and NIH/NCRR Program Project Grant P41 RR01237 (P.G.W.).
References
- 1.Endo M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Fabiato A., Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann. N.Y. Acad. Sci. 1978;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- 3.Franzini-Armstrong C., Protasi F., Ramesh V. Shape, size, and distribution of Ca2+ release units and couplons in skeletal and cardiac muscles. Biophys. J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- 5.Giannini G., Conti A., Mammarella S., Scrobogna M., Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J. Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutko J. L., Airey J. A. Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiol. Rev. 1996;76:1027–1071. doi: 10.1152/physrev.1996.76.4.1027. [DOI] [PubMed] [Google Scholar]
- 7.Francis K. T. The effect of dantrolene sodium on the efflux of Ca45 from rat heavy sarcoplasmic reticulum. Res. Commun. Chem. Pathol. Pharmacol. 1978;21:573–576. [PubMed] [Google Scholar]
- 8.Fruen B. R., Mickelson J. R., Louis C. F. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J. Biol. Chem. 1997;272:26965–26971. doi: 10.1074/jbc.272.43.26965. [DOI] [PubMed] [Google Scholar]
- 9.Loke J. C., MacLennan D. H. Bayesian modeling of muscle biopsy contracture testing for malignant hyperthermia. Anesthesiology. 1998;88:589–600. doi: 10.1097/00000542-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Ward A., Chaffman M. O., Sorkin E. M. Dantrolene: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neurolept malignant syndrome, and an update of its use in muscle spasticity. Drugs. 1986;32:130–168. doi: 10.2165/00003495-198632020-00003. [DOI] [PubMed] [Google Scholar]
- 11.Wei H., Perry D. C. Dantrolene is cytoprotective in two models of neuronal cell death. J. Neurochem. 1996;67:2390–2398. doi: 10.1046/j.1471-4159.1996.67062390.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama R., Yano T., Ushijima K., Abe E., Terasaki H. Effects of dantrolene on extracellular glutamate concentration and neuronal death in the rat hippocampal CA1 region subjected to transient ischemia. Anesthesiology. 2002;96:705–710. doi: 10.1097/00000542-200203000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Fratea S., Langeron O., Lecarpentier Y., Coriat P., Riou B. In vitro effects of dantrolene on rat myocardium. Anesthesiology. 1997;86:205–215. doi: 10.1097/00000542-199701000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Zhao F., Li P., Chen S. R., Louis C. F., Fruen B. R. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J. Biol. Chem. 2001;276:13810–13816. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]
- 15.Palnitkar S. S., Bin B., Jimenez L. S., Morimoto H., Williams P. G., Paul-Pletzer K., Parness J. [3H]Azidodantrolene: synthesis and use in identification of a putative skeletal muscle dantrolene binding site in sarcoplasmic reticulum. J. Med. Chem. 1999;42:1872–1880. doi: 10.1021/jm9805079. [DOI] [PubMed] [Google Scholar]
- 16.Paul-Pletzer K., Palnitkar S. S., Jimenez L. S., Morimoto H., Parness J. The skeletal muscle ryanodine receptor identified as a molecular target of [3H]azidodantrolene by photoaffinity labeling. Biochemistry. 2001;40:531–542. doi: 10.1021/bi001502s. [DOI] [PubMed] [Google Scholar]
- 17.Yu G., Zucchi R., Ronca-Testoni S., Ronca G. Protection of ischemic rat heart by dantrolene, an antagonist of the sarcoplasmic reticulum calcium release channel. Basic Res. Cardiol. 2000;95:137–143. doi: 10.1007/s003950050175. [DOI] [PubMed] [Google Scholar]
- 18.Wheatley A. M., Butkow N. The effect of external calcium concentration on the negative inotropic action of dantrolene in isolated hyperthyroid and euthyroid heart. Pharmacol. Res. 1991;24:65–74. doi: 10.1016/1043-6618(91)90066-7. [DOI] [PubMed] [Google Scholar]
- 19.Tian Q., Katz A. M., Kim D. H. Effects of azumolene on doxorubicin-induced Ca2+ release from skeletal and cardiac muscle sarcoplasmic reticulum. Biochim. Biophys. Acta. 1991;1094:27–34. doi: 10.1016/0167-4889(91)90022-p. [DOI] [PubMed] [Google Scholar]
- 20.Horton J. W., White D. J., Maass D., Sanders B., Thompson M., Giroir B. Calcium antagonists improve cardiac mechanical performance after thermal trauma. J. Surg. Res. 1999;87:39–50. doi: 10.1006/jsre.1999.5726. [DOI] [PubMed] [Google Scholar]
- 21.Thompson M., Kliewer A., Maass D., Becker L., White D. J., Bryant D., Arteaga G., Horton J., Giroir B. P. Increased cardiomyocyte intracellular calcium during endotoxin-induced cardiac dysfunction in guinea pigs. Pediatr. Res. 2000;47:669–676. doi: 10.1203/00006450-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Paul-Pletzer K., Yamamoto T., Bhat M. B., Ma J., Ikemoto N., Jimenez L. S., Morimoto H., Williams P. G., Parness J. Identification of the dantrolene binding sequence on the skeletal muscle ryanodine receptor. J. Biol. Chem. 2002;277:34918–34923. doi: 10.1074/jbc.M205487200. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S., Yamamoto T., Parness J., Ikemoto N. Antibody probe study of Ca2+ channel regulation by interdomain interaction within the ryanodine receptor. Biochem. J. 2004;380:561–569. doi: 10.1042/BJ20040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkes M. J., Diaz Munoz M., Hamilton S. L. A procedure for purification of the ryanodine receptor from skeletal muscle. Membr. Biochem. 1989;8:133–145. doi: 10.3109/09687688909025827. [DOI] [PubMed] [Google Scholar]
- 25.El Hayek R., Saiki Y., Yamamoto T., Ikemoto N. A postulated role of the near amino-terminal domain of the ryanodine receptor in the regulation of the sarcoplasmic reticulum Ca2+ channel. J. Biol. Chem. 1999;274:33341–33347. doi: 10.1074/jbc.274.47.33341. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Kelley J., Schmeisser G., Kobayashi Y. M., Jones L. R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- 29.Campbell K. P., Knudson C. M., Imagawa T., Leung A. T., Sutko J. L., Kahl S. D., Raab C. R., Madson L. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J. Biol. Chem. 1987;262:6460–6463. [PubMed] [Google Scholar]
- 30.Herzog A., Szegedi C., Jona I., Herberg F. W., Varsanyi M. Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca2+ release channel/ryanodine receptor with calsequestrin. FEBS Lett. 2000;472:73–77. doi: 10.1016/s0014-5793(00)01431-9. [DOI] [PubMed] [Google Scholar]
- 31.Shin D. W., Pan Z., Kim E. K., Lee J. M., Bhat M. B., Parness J., Kim D. H., Ma J. A retrograde signal from calsequestrin for the regulation of store-operated Ca2+ entry in skeletal muscle. J. Biol. Chem. 2003;278:3286–3292. doi: 10.1074/jbc.M209045200. [DOI] [PubMed] [Google Scholar]
- 32.Butkow N., Wheatley A. M., Lippe I. T., Marcus R. H., Rosendorff C. The role of calcium in the enhanced myocardial contractility of the hyperthyroid rat heart. Basic Res. Cardiol. 1990;85:297–306. doi: 10.1007/BF01907118. [DOI] [PubMed] [Google Scholar]
- 33.Meissner A., Min J. Y., Haake N., Hirt S., Simon R. Dantrolene sodium improves the force-frequency relationship and beta-adregenic responsiveness in failing human myocardium. Eur. J. Heart Failure. 1999;1:177–186. doi: 10.1016/s1388-9842(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell M. B., Winter C. B., Banerjee A., Harken A. H. Inhibition of sarcoplasmic reticulum calcium release reduces myocardial stunning. J. Surg. Res. 1993;54:411–417. doi: 10.1006/jsre.1993.1065. [DOI] [PubMed] [Google Scholar]
- 35.Zucchi R., Ronca F., Ronca-Testoni S. Modulation of sarcoplasmic reticulum function: a new strategy in cardioprotection? Pharmacol. Ther. 2001;89:47–65. doi: 10.1016/s0163-7258(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 36.Min J. Y., Meissner A., Feng X., Wang J., Malek S., Wang J. F., Simon R., Morgan J. P. Dantrolene: effects on abnormal intracellular Ca2+ handling and inotropy in postinfarcted rat myocardium. Eur. J. Pharmacol. 2003;471:41–47. doi: 10.1016/s0014-2999(03)01816-8. [DOI] [PubMed] [Google Scholar]
- 37.Shorofsky S. R., Balke C. W. Calcium currents and arrhythmias: insights from molecular biology. Am. J. Med. 2001;110:127–140. doi: 10.1016/s0002-9343(00)00586-6. [DOI] [PubMed] [Google Scholar]
- 38.Pogwizd S. M., Bers D. M. Na/Ca exchange in heart failure: contractile dysfunction and arrhythmogenesis. Ann. N.Y. Acad. Sci. 2002;976:454–465. doi: 10.1111/j.1749-6632.2002.tb04775.x. [DOI] [PubMed] [Google Scholar]
- 39.Walker M. L., Rosenbaum D. S. Repolarization alternans: implications for the mechanism and prevention of sudden cardiac death. Cardiovasc. Res. 2003;57:599–614. doi: 10.1016/s0008-6363(02)00737-x. [DOI] [PubMed] [Google Scholar]
- 40.Choi B. R., Burton F., Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J. Physiol. (Cambridge, U.K.) 2002;543:615–631. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marks A. R. Clinical implications of cardiac ryanodine receptor/calcium release channel mutations linked to sudden cardiac death. Circulation. 2002;106:8–10. doi: 10.1161/01.cir.0000021746.82888.83. [DOI] [PubMed] [Google Scholar]
- 42.Allen P. D. Leaky “feet” and sudden death. Circ. Res. 2002;91:181–182. doi: 10.1161/01.res.0000030194.38795.86. [DOI] [PubMed] [Google Scholar]
- 43.George C. H., Higgs G. V., Lai F. A. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ. Res. 2003;93:531–540. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 44.Wehrens X. H., Lehnart S. E., Huang F., Vest J. A., Reiken S. R., Mohler P. J., Sun J., Guatimosim S., Song L. S., Rosemblit N., et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]