Abstract
The karyotype of Litoria (L.) paraewingi (Watson et al., 1971) (Big River State Forest, Victoria) is described here for the first time. It is prepared following tissue culture of toe clipping macerates, cryopreservation, reculture and conventional 4’,6-diamidino-2-phenylindole (DAPI) staining. The L.paraewingi karyotype is then compared to similarly processed IUCN (International Union for the Conservation of Nature) least concern members L.ewingii (Duméril et Bibron, 1841) (southern Victoria) and L.jervisiensis (Duméril et Bibron, 1841) (Myall Lakes National Park, New South Wales), all members of the same L.ewingii complex/group. The L.paraewingi diploid number is 2n = 26, the same as for the other two species. Litoriaparaewingi chromosomes 1, 2, 6 and 7 are submetacentric, chromosomes 3 and 5 are subtelocentric and the remainder are metacentric. No secondary constriction or putative nucleolus organiser region (NOR) was readily identifiable following conventional DAPI staining in any scored L.paraewingi metaphase spread. Conversely, a putative NOR was readily identifiable on the long arm of chromosome 1 in all examined metaphase spreads for the other two species. The karyotypes of L.ewingii and L.jervisiensis here further differ from L.paraewingi with chromosome 1 being metacentric and chromosomes 8 and 10 being submetacentric for both former species. The L.jervisiensis karyotype differs from those of L.ewingii and L.paraewingi by DAPI staining with: (i) apparent relative length inversion of subtelocentric chromosome 3 and metacentric chromosome 4 and (ii) chromosome 6 being metacentric rather than submetacentric. All three species have a highly conserved chromosome morphology with respect to chromosomes 2, 5, 7, 9, 11, 12 and 13. The greatest gross morphological difference karyotypically is observed between L.paraewingi and L.jervisiensis. These karyotype data support the previous phylogenetic separation of these three species based upon genetic compatibility and behavioural, biochemical and molecular genetic analyses.
Keywords: Cell culture, cryopreservation, karyotype, Plains brown tree frog
Introduction
The current large scale existential threat to over 40% of amphibian species globally is well documented, making amphibians the most endangered vertebrate taxonomic class (Luedtke et al. 2023). Habitat loss facilitating disease spread, pollutant introduction and species invasion means that for many of these species, animal husbandry, assisted reproductive technologies and cryobanking programs, whether alone or in combination, are suggested requirements for their long-term survival (Kouba et al. 2013; Gillespie et al. 2016; Lampert et al. 2022). Cryobanking initiatives for amphibian assisted reproduction technologies, however, are currently restricted to sperm cells, with methods for oocyte or embryo cryopreservation remaining challenging (Clulow and Clulow 2016; Gagarinskiy et al. 2023). Numerous publications describe somatic cell culture of amphibian somatic cells (Fukui et al. 2003; Ferris et al. 2010; Strauß et al. 2011; Mollard 2018; Mollard et al. 2018; Bui-Marinos et al. 2022; Douglas et al. 2023). It is envisioned that cryopreservation of amphibian somatic cells will provide a necessary immediate resource for longer term genetic conservation initiatives including induced pluripotent stem cell technologies for cloning and gamete production (Kouba et al. 2013; Clulow and Clulow 2016; Codner et al. 2016; Oikawa et al. 2022). In respect to mouse ES cells, maintenance of > 50% euploid karyotype is essential for successful cloning outcomes and by proxy gamete production (Kusakabe et al. 2001; Olifent et al. 2002; Bonnet-Garnier et al. 2015). Cryobanking initiatives of somatic cells aimed at longer term conservation outcomes, therefore, must include steps to ensure recovery of karyotypically normal cells.
A generic level classification of taxa within the Australo-Papuan hyloid family Pelodryadidae has remained problematic largely due to the lack of a comprehensively sampled and well resolved phylogeny for these frogs. The family comprises 232 species split roughly half in Australia and half in Melanesia and eastern Indonesia and contributes 28% of anuran species diversity in the region. Molecular phylogenetic analysis indicates Pelodryadidae diverged approximately 50 million to 100 million years ago while the Australian/ New Guinean land mass and Antarctica were separating (Duellman et al. 2016; Feng et al. 2017; Brennan et al. 2023). Three genera, Litoria (Tschudi et Agassiz, 1838), Cyclorana (Steindachner, 1867) and Nyctimystes (Stejneger, 1916) have been used to taxonomically allocate diversity within the Pelodryadidae, but the description of possible new genera remains the subject of debate (Wells and Wellington 1985; Duellman et al. 2016; Frétey and Dubois 2016). The Australian species of this family are found in all major habitats of the continent (Vidal‐García and Keogh 2015). Despite this ecological breadth, morphological and life history variations are recognised to show a strong association with ecological specialisations. As such, previously applied informal sub-familial classification as species groups accommodating this variation remain widely recognised (Tyler and Davies 1978). One of the well characterised species groups is the Litoria (L.) ewingii group, which comprises nine species (Parkin et al. 2024): L.callicelis (Parkin et al., 2024), L.ewingii (Duméril et Bibron, 1841), L.jervisiensis (Duméril et Bibron, 1841), L.littlejohni (White et al., 1994), L.paraewingi (Watson et al., 1971), L.revelata (Ingram et al., 1982), L.sibilis (Parkin et al., 2024), L.watsoni (Mahony et al., 2020) and L.verreauxii (Duméril, 1853). Assignment to this complex is based upon a range of methods, including: genetic compatibility, mating call comparisons, biochemical analyses and most recently, detailed morphological, mitochondrial genetic and small nucleotide polymorphism (SNP) analyses (Mahony et al. 2020; Parkin et al. 2024).
Despite the in depth molecular analysis underpinning critical phylogenetic assignment within this complex, 2n = 26 karyotypes have been described in the literature for only L.ewingii, L.jervisiensis, L.littlejohni and L.verreauxii (Woodruff 1972; White et al. 1994; Schmid et al. 2018). Confirmation of diploid number, position of nucleolus organiser regions (NORs), centromeric positions and relative chromosomal length in karyograms of individual representatives of this complex remain wanting.
Here somatic cells from L.paraewingi, L.ewingii and L.jervisiensis were cultured and cryopreserved in liquid nitrogen (LN2) as a resource to safeguard against possible future existential threats. The previously undescribed karyotype of L.paraewingi is compared to that of L.ewingii and L.jervisiensis following recovery from cryopreservation. All three karyotypes show a 2n = 26 karyotype, yet also differ in several key respects. Most notably, the morphologies of chromosomes 1, 8 and 10 are common to L.ewingii and L.jervisiensis but not to L.paraewingi. A secondary restriction and potential NOR are identified on the long arms of chromosome 1 of both L.ewingii and L.jervisiensis, but not L.paraewingi. The obscure L.paraewingi secondary restriction perhaps more closely relates to the more obscure NOR of L.littlejohni which is located subterminally on the long arm of chromosome 11 and where satellites are not always observed (White et al. 1994). Karyotypes prepared from the cryobanking of cells from these three species reinforce their phylogenetic separation and provide assurance of relevantly cryopreserved cell types for any required future conservation initiative.
Methods
Ethics
This research was conducted in compliance with the EU Directive 2010/63/EU for animal experiments and according to The Declaration of Helsinki World Medical Association Code of Ethics. Prior to experimentation, all required Australian State governmental and institutional ethics, licenses and permissions were provided (Richard Mollard, Victorian Department of Environment, Land, Water & Planning Permit number 10008085). The L.ewingii specimen was collected from southern Victoria by Richard Mollard under an Animal Ethics Committee Notification of Scavenged Animal Tissue, University of Melbourne. The L.jervisiensis specimen was collected by Michael Mahony under the New South Wales National Parks Scientific Licence SL00190. The L.paraewingi specimen was collected from Big River State Forest, Victoria, Australia by Matthew West under the Victoria Wildlife Research Permit No. 10009587).
Tissue culture and cryopreservation
Toe clippings were obtained from deceased and unsexed L.ewingii and L.jervisiensis and a male L.paraewingi. Culture, cryopreservation, thawing and DAPI karyotyping were performed according to previously described methods (Mollard 2018; Mollard et al. 2018; Mollard and Mahony 2023). Chromosomes were arranged in size by descending order, with the largest chromosome designated chromosome 1 (King et al. 1990). Centromeric position and relative lengths were determined using Image J software with the Levan plugin (Levan et al. 1964). Metacentric, submetacentric and subtelocentric chromosomal morphologies were defined as long arm to short arm ratios of 1–1.69, 1.7–2.99 and 3–6.99, respectively (Levan et al. 1964). Four metaphase spreads each from L.ewingii and L.paraewingi and eight metaphase spreads from L.jervisiensis were arranged in descending order of size for putative NOR assignment, centromeric location and relative length measurements to chromosome 1, not including any secondary constrictions. An extra four L.jervisiensis metaphase spreads were scored to accurately compare relative lengths of chromosomes 3 and 4. Images were captured at 1000 × under oil immersion with an Olympus BX60 microscope, colour CCD Leica DFC425C camera, EL-6000 Leica light source and Leica LAS-AF and QCapture Pro7 Version 7.0.5 Build 4325 software (QImaging Inc, USA).
Cells were processed in culture from toe clippings of L.ewingii, L.paraewingi and L.jervisiensis (representative species images shown in Fig. 1). Following 15, 15 and 3 month LN2 cryopreservation periods, respectively, L.ewingii, L.paraewingi and L.jervisiensis cells were thawed into 24 well plates and passaged for alternate karyotyping and recryopreservation.
Figure 1.
L.ewingii; photographed by Matthew West at Merri Creek, Australia, 2020. L.paraewingi; photographed by Stephen Mahony at Wangaratta, Victoria, Australia, 2017. L.jervisiensis; photographed by Stephen Mahony at Mungo Brush Park Myall Lakes National Park, New South Wales, Australia, 2021.
Results
Of the first 23 L.ewingii metaphases spreads scored, 16 (70%) showed a 2n = 26 chromosome count, with the remaining metaphase spreads showing 22 chromosomes (number of spreads = 2), 24 chromosomes (number of spreads = 2) and 25 chromosomes (number of spreads = 3) chromosomes. Of the first 15 L.paraewingi metaphase spreads, 13 (87%) showed a 2n = 26 chromosome count with the remaining showing either 23 or 25 chromosomes. Of the first 71 L.jervisiensis metaphase spreads scored, 67 (94%) showed a 2n = 26 chromosome count, with the remaining showing either 16, 21, 24 or 25 chromosomes. A higher number of L.jervisiensis metaphase spreads were prepared to accurately resolve this species’ unique chromosomal relative length order as outlined below. Reconstruction of the anomalous karyotypes did not reveal obvious aneuploidies such as trisomies or chromosomal pair loss or repeated aneuploidies. Diversion from the 2n = 26 count is most likely technical, therefore, attributable to loss of individual chromosomes during cell dropping and spreading for preparation of DAPI staining and scoring.
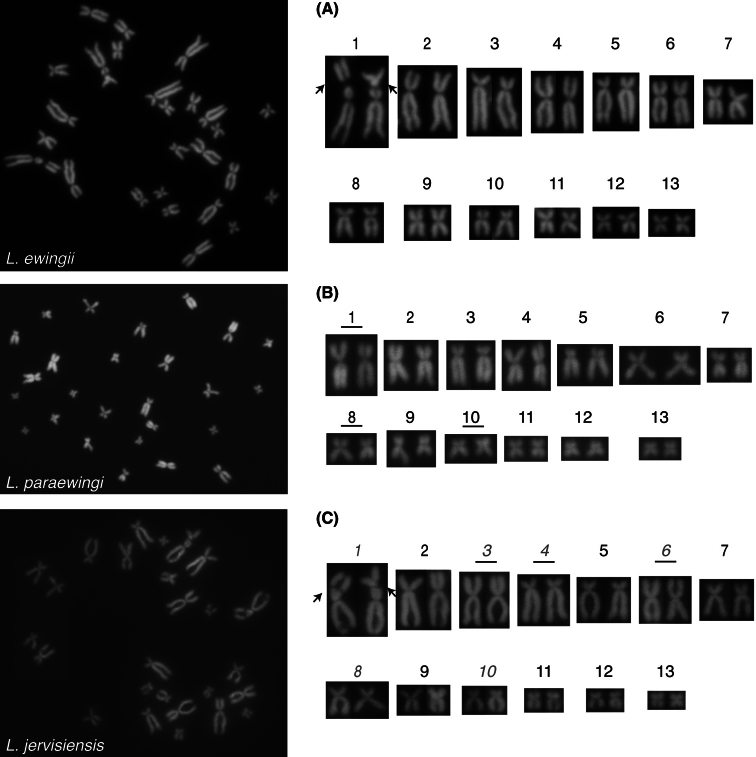
For L.ewingii, chromosomes 2, 6, 7, 8 and 10 are submetacentric, chromo- somes 3 and 5 are subtelocentric and chromosomes 1, 4, 9, 11, 12, and 13 are metacentric (Table 1, Figs 2, 3A–C). A DAPI negative region was apparent on the long arms of chromosome 1 of all scored L.ewingii metaphase spreads and presumed to represent an NOR (Figs 2, 3A–C.) For L.paraewingi chromosomes 1, 2, 6 and 7 are metacentric, chromosomes 3 and 5 are subtelocentric and chromosomes 4, 8, 9, 10, 11, 12 and 13 are metacentric (Table 1, Figs 2, 4A–C). No DAPI negative chro- mosomal region was apparent in any of the 15 L.paraewingi metaphase spreads (Figs 2, 4A–C). For L.jervisiensis, chromosomes 2, 7, 8 and 10 are submetacentric, chromosomes 4 and 5 are subtelocentric and chromosomes 1, 3, 9, 11, 12, and 13 are metacentric (Table 1, Figs 2, 5A–C). A DAPI negative region was apparent on the long arms of chromosome 1 of all scored L.jervisiensis metaphase spreads and presumed to represent an NOR (Figs 2, 5A–C).
Table 1.
Centromeric position (morphology) and relative lengths of chromosomes following DAPI staining of metaphase spreads. Measurements were taken from four L.ewingii, four L.paraewingi and eight L.jervisiensis metaphase spreads. Long arm to short arm ratios (A.R) and relative lengths (R.L.) are provided as average plus or minus standard deviation for all scored metaphase spreads of that species. R.L. is to chromosome 1, designated as length = 1. Chromosomal morphologies (Morph) in cells with light grey shading represent those differing to L.ewingii. Italicised chromosomal morphologies represent L.jervisiensis morphologies differing to those of L.paraewingi.
| Litoriaewingii Chromosome Number | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| A.R | 1.33 ± 0.12 | 1.86 ± 0.26 | 3.38 ± 0.73 | 1.32 ± 0.10 | 3.35 ± 0.42 | 1.86 ± 0.25 | 1.92 ± 0.36 |
| Morph | Metacentric | Submetacentric | Subtelocentric | Metacentric | Subtelocentric | Submetacentric | Submetacentric |
| R.L. | 1 | 0.771 | 0.7198 | 0.6833 | 0.5977 | 0.5418 | 0.4185 |
| 8 | 9 | 10 | 11 | 12 | 13 | ||
| A.R. | 1.78 ± 0.58 | 1.59 ± 0.42 | 2.06 ± 0.50 | 1.30 ± 0.19 | 1.33 ± 0.15 | 1.21 ± 0.16 | |
| Morph | Submetacentric | Metacentric | Submetacentric | Metacentric | Metacentric | Metacentric | |
| R.L. | 0.4039 | 0.3533 | 0.3435 | 0.2797 | 0.2785 | 0.2539 | |
| Litoriaparaewingi Chromosome Number | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| A.R. | 1.81 ± 0.26 | 1.87 ± 0.33 | 3.78 ± 0.72 | 1.41 ± 0.30 | 3.43 ± 0.41 | 1.97 ± 0.44 | 1.97 ± 0.38 |
| Morph | Submetacentric | Submetacentric | Subtelocentric | Metacentric | Subtelocentric | Submetacentric | Submetacentric |
| R.L. | 1 | 0.8937 | 0.8609 | 0.7374 | 0.6351 | 0.6192 | 0.5297 |
| 8 | 9 | 10 | 11 | 12 | 13 | ||
| A.R. | 1.52 ± 0.24 | 1.54 ± 0.24 | 1.55 ± 0.35 | 1.46 ± 0.38 | 1.36 ± 0.20 | 1.45 ± 0.28 | |
| Morph | Metacentric | Metacentric | Metacentric | Metacentric | Metacentric | Metacentric | |
| R.L. | 0.4585 | 0.4108 | 0.3643 | 0.2909 | 0.2532 | 0.1966 | |
| Litoriajervisiensis Chromosome Number | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| A.R. | 1.12 ± 0.09 | 2.27 ± 0.18 | 1.41 ± 0.14 | 3.93 ± 0.48 | 3.69 ± 0.62 | 1.36 ± 0.14 | 2.24 ± 0.29 |
| Morph | Metacentric | Submetacentric | Metacentric | Subtelocentric | Subtelocentric | Metacentric | Submetacentric |
| R.L. | 1 | 0.7927 | 0.7116 | 0.7098 | 0.6112 | 0.5978 | 0.5022 |
| 8 | 9 | 10 | 11 | 12 | 13 | ||
| A.R. | 1.92 ± 0.32 | 1.24 ± 0.16 | 2.25 ± 0.56 | 1.53 ± 0.32 | 1.59 ± 0.36 | 1.24 ± 0.21 | |
| Morph | Submetacentric | Metacentric | Submetacentric | Metacentric | Metacentric | Metacentric | |
| R.L. | 0.4187 | 0.3439 | 0.3087 | 0.2348 | 0.2067 | 0.1769 | |
Figure 2.
Representative metaphrase spreads of cryopreserved, thawed and cultured cells AL.ewingiiBL.paraewingiCL.jervisiensis. Arrows indicate DAPI negative regions, or presumptive NORs. No DAPI negative regions were apparent in the L.paraewingi metaphase spreads. As per Table 1, underlined numbers represent those morphologies differing to L.ewingii and italicised (in red) numbers represent L.jervisiensis morphologies differing to those of L.paraewingi.
Figure 3.
Metaphrase spreads of cryopreserved, thawed and cultured cells from L.ewingii. A–C three individual metaphase spreads. Arrows indicate DAPI negative regions, or presumptive NORs.
Figure 4.
Metaphrase spreads of cryopreserved, thawed and cultured cells from L.paraewingi. A–C three individual metaphase spreads. No DAPI negative regions, or presumptive NORs, were apparent.
Figure 5.
Metaphrase spreads of cryopreserved, thawed and cultured cells from L.jervisiensis. A–C three individual metaphase spreads. Arrows indicate DAPI negative regions, or presumptive NORs.
Discussion
Somatic cells from L.paraewingi, L.ewingii and L.jervisiensis were successfully cryobanked in this study with respect to demonstrating recovery of karyotypically normal cells following freeze-thaw cycles. Karyotypes of all three species showed common morphologies for chromosomes 2, 5, 7, 9, 11, 12 and 13, but also unique morphologies. For example, L.paraewingi chromosomes 1, 8 and 10 differed morphologically to those of L.ewingii and L.jervisiensis. The L.jervisiensis karyotype differed from those of L.ewingii and L.paraewingi with respect to an apparent inverted relative length assignment for its metacentric chromosome 3 and subtelocentric chromosome 4. Furthermore, a secondary restriction was discernible on the long arms of chromosome 1 for L.ewingii and L.jervisiensis but not for L.paraewingi. The greatest number of chromosome morphological differences was observed between L.paraewingi and L.jervisiensis.
L.paraewingi is considered a cryptic species due to its high holotypic similarity to L.ewingii, with differentiation based upon detailed call analysis, genetic compatibility and molecular taxonomic analysis (Watson et al. 1971; Mahony et al. 2020). Here, by DAPI staining, L.paraewingi chromosomes 1, 8 and 10 showed marked divergence from that of L.ewingii, providing a further cytological differentiation of these species. The obvious L.ewingii and L.jervisiensis secondary constriction discernible on the long arms of all chromosome 1 metaphase spreads following DAPI staining is similar to that described previously for L.verreauxii, (Schmid et al. 2018). Apparent absence of a DAPI negative region, or secondary restriction, from the karyotype of L.paraewingi may be a more similar observation to that reported for L.littlejohni (White et al. 1994). For L.littlejohni an NOR was discernible sub-terminally on the long arms of chromosome 11 most notably in silver nitrate (Ag-NO3) stained chromosomes, with satellites not always observable with conventional aceto-orcein staining. Confirmation of an L.paraewingi secondary restriction or NOR location, therefore, may be better resolved in the future by alternative staining techniques such as Ag-NO3 staining or 18S or 28S rDNA FISH if also located more terminally without obvious satellites (White et al. 1994; Zaleśna et al. 2017).
Conclusion
In conclusion, the karyotypes of L.paraewingi, L.ewingii and L.jervisiensis demonstrate a high level of morphological conservation yet also many unique attributes. These data support the phylogenetic separation of these species based upon previous behavioural, genetic compatibility, biochemical and molecular analyses (Mahony et al. 2020).
Competing interests
Richard Mollard has registered a company called Amphicell Pty Ltd (www.amphicell.com). Amphicell Pty Ltd received no funding for this work and privately provided the materials to execute the experimental procedures described in this study.
Acknowledgments
Tissues used in these studies were supplied from programs supported by Earthwatch Australia (Michael Mahony), and Zoos Victoria (Matthew West). We thank Stephen Mahony for provision of and permissions to use images of L.paraewingi and L.jervisiensis. The authors are also grateful to Jean-Pierre Scheerlinck, Vern Bowles and Charlie Pagel for granting access to tissue culture and microscopy facilities at the University of Melbourne’s Veterinary School
Citation
Mollard R, Mahony M, West M (2024) Karyotypic description and comparison of Litoria (L.) paraewingi (Watson et al., 1971), L. ewingii (Duméril et Bibron, 1841) and L. jervisiensis (Duméril et Bibron, 1841) (Amphibia, Anura). Comparative Cytogenetics 18: 161–174. https://doi.org/10.3897/compcytogen.17.129133
Funding Statement
No supporting agencies
ORCID
Michael Mahony https://orcid.org/0000-0002-1042-0848
References
- Bonnet-Garnier A, Veillard AC, Bed’Hom B, Hayes H, Britton-Davidian J. (2015) Assessing the quality of donor cells: karyotyping methods. Methods in Molecular Biology 1222: 83–99. 10.1007/978-1-4939-1594-1_7 [DOI] [PubMed] [Google Scholar]
- Brennan IG, Lemmon AR, Moriarty Lemmon E, Hoskin CJ, Donnellan SC, Keogh JS. (2023) Populating a continent: Phylogenomics reveal the timing of australian frog diversification. Systematic Biology 73: 1–11. 10.1093/sysbio/syad048 [DOI] [PubMed] [Google Scholar]
- Bui-Marinos MP, Todd LA, Douglas AJ, Katzenback BA. (2022) So, you want to create a frog cell line? A guide to establishing frog skin cell lines from tissue explants. MethodsX 9: 101693. 10.1016/j.mex.2022.101693 [DOI] [PMC free article] [PubMed]
- Clulow J, Clulow S. (2016) Cryopreservation and other assisted reproductive technologies for the conservation of threatened amphibians and reptiles: bringing the ARTs up to speed. Reproduction Fertility & Development 28: 1116–1132. 10.1071/rd15466 [DOI] [PubMed] [Google Scholar]
- Codner GF, Lindner L, Caulder A, Wattenhofer-Donzé M, Radage A, Mertz A, Eisenmann B, Mianné J, Evans EP, Beechey CV, Fray MD, Birling MC, Hérault Y, Pavlovic G, Teboul L. (2016) Aneuploidy screening of embryonic stem cell clones by metaphase karyotyping and droplet digital polymerase chain reaction. BMC Molecular and Cellular Biology 17: 30. 10.1186/s12860-016-0108-6 [DOI] [PMC free article] [PubMed]
- Douglas AJ, Todd LA, Katzenback BA. (2023) The amphibian invitrome: Past, present, and future contributions to our understanding of amphibian immunity. Developmental & Comparative Immunology 142: 104644. 10.1016/j.dci.2023.104644 [DOI] [PubMed]
- Duellman WE, Marion AB, Hedges SB. (2016) Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa 4104: 1–109. 10.11646/zootaxa.4104.1.1 [DOI] [PubMed] [Google Scholar]
- Duméril AHA. (1853) Mémoire sur les batraciens anoures, de la famille des hylaeformes ou rainettes, comprenent la description d’un genre nouveau et de onze espèces nouvelles. Zoologie et Biologie Animale Paris 3: 135–179. https://www.biodiversitylibrary.org/part/22065 [Google Scholar]
- Duméril AMC, Bibron G. (1841) Erpétologie Genérale ou Histoire Naturelle Complète des Reptiles 6: https://www.biodiversitylibrary.org/bibliography/45973
- Feng YJ, Blackburn DC, Liang D, Hillis DM, Wake DB, Cannatella DC, Zhang P. (2017) Phylogenomics reveals rapid, simultaneous diversification of three major clades of Gondwanan frogs at the Cretaceous-Paleogene boundary. Proceedings of the National Academy of Sciences U S A 114: E5864–E5870. 10.1073/pnas.1704632114 [DOI] [PMC free article] [PubMed]
- Ferris DR, Satoh A, Mandefro B, Cummings GM, Gardiner DM, Rugg EL. (2010) Ex vivo generation of a functional and regenerative wound epithelium from axolotl (Ambystoma mexicanum) skin. Dev Growth Differ 52: 715–724. 10.1111/j.1440-169x.2010.01208.x [DOI] [PubMed] [Google Scholar]
- Frétey T, Dubois A. (2016) A new nomen for a subfamily of frogs (Amphibia, Anura). Dumerilia 6: 17–23. [Google Scholar]
- Fukui Y, Furue M, Myoishi Y, Sato JD, Okamoto T, Asashima M. (2003) Long-term culture of Xenopus presumptive ectoderm in a nutrient-supplemented culture medium. Development Growth and Differentiatoin 45: 499–506. 10.1111/j.1440-169X.2003.00717.x [DOI] [PubMed] [Google Scholar]
- Gagarinskiy E, Uteshev V, Fesenko Jr E. (2023) Prolonged hypothermic storage of oocytes of the European common frog Rana temporaria in a gas mixture of oxygen and carbon monoxide. PLoS ONE 18: e0288370. 10.1371/journal.pone.0288370 [DOI] [PMC free article] [PubMed]
- Gillespie GR, McNabb E, Gaborov R. (2016) The biology and status of the Large Brown Tree Frog Litorialittlejohni in Victoria. The Victorian Naturalist 133: 128. https://search.informit.org/doi/10.3316/informit.365260579800091
- Ingram GJ, Corben CJ, Hosmer W. (1982) Litoriarevelata: a new species of tree-frog from eastern Australia. Memoirs of the Queensland Museum 20: 635–637. https://www.biodiversitylibrary.org/part/218379 [Google Scholar]
- King M, Contreras N, Honeycutt R. (1990) Variation within and between nucleolar organizer regions in Australian hylid frogs (Anura) shown by 18S+ 28S in-situ hybridization. Genetica 80: 17–29. 10.1007/bf00120116 [DOI] [PubMed] [Google Scholar]
- Kouba AJ, Lloyd RE, Houck ML, Silla AJ, Calatayud N, Trudeau VL, Clulow J, Molinia F, Langhorne C, Vance C, Arregui L, Germano J, Lermen D, Della Togna G. (2013) Emerging trends for biobanking amphibian genetic resources: The hope, reality and challenges for the next decade. Biological Conservation 164: 12. 10.1016/j.biocon.2013.03.010 [DOI]
- Kusakabe H, Szczygiel MA, Whittingham DG, Yanagimachi R. (2001) Maintenance of genetic integrity in frozen and freeze-dried mouse spermatozoa. Proceedings of the National Academy of Sciences U S A 98: 13501–13506. 10.1073/pnas.241517598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert SS, Burger IJ, Julien AR, Gillis AB, Kouba AJ, Barber D, Kouba CK. (2022) Sperm Cryopreservation as a Tool for Amphibian Conservation: Production of F2 Generation Offspring from Cryo-Produced F1 Progeny. Animals (Basel) 13(1): 53. 10.3390/ani13010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan A, Fredga K, Sandberg AA. (1964) Nomenclature for Centromeric Position on Chromosomes. Hereditas 52: 201–220. 10.1111/j.1601-5223.1964.tb01953.x [DOI] [Google Scholar]
- Luedtke JA, Chanson J, Neam K, Hobin L, Maciel AO, Catenazzi A, Borzée A, Hamidy A, Aowphol A, Jean A, Sosa-Bartuano Á, Fong GA, de Silva A, Fouquet A, Angulo A, Kidov AA, Muñoz Saravia A, Diesmos AC, Tominaga A, Shrestha B, Gratwicke B, Tjaturadi B, Martínez Rivera CC, Vásquez Almazán CR, Señaris C, Chandramouli SR, Strüssmann C, Cortez Fernández CF, Azat C, Hoskin CJ, Hilton-Taylor C, Whyte DL, Gower DJ, Olson DH, Cisneros-Heredia DF, Santana DJ, Nagombi E, Najafi-Majd E, Quah ESH, Bolaños F, Xie F, Brusquetti F, Álvarez FS, Andreone F, Glaw F, Castañeda FE, Kraus F, Parra-Olea G, Chaves G, Medina-Rangel GF, González-Durán G, Ortega-Andrade HM, Machado IF, Das I, Dias IR, Urbina-Cardona JN, Crnobrnja-Isailović J, Yang JH, Jianping J, Wangyal JT, Rowley JJL, Measey J, Vasudevan K, Chan KO, Gururaja KV, Ovaska K, Warr LC, Canseco-Márquez L, Toledo LF, Díaz LM, Khan MMH, Meegaskumbura M, Acevedo ME, Napoli MF, Ponce MA, Vaira M, Lampo M, Yánez-Muñoz MH, Scherz MD, Rödel MO, Matsui M, Fildor M, Kusrini MD, Ahmed MF, Rais M, Kouamé NG, García N, Gonwouo NL, Burrowes PA, Imbun PY, Wagner P, Kok PJR, Joglar RL, Auguste RJ, Brandão RA, Ibáñez R, von May R, Hedges SB, Biju SD, Ganesh SR, Wren S, Das S, Flechas SV, Ashpole SL, Robleto-Hernández SJ, Loader SP, Incháustegui SJ, Garg S, Phimmachak S, Richards SJ, Slimani T, Osborne-Naikatini T, Abreu-Jardim TPF, Condez TH, De Carvalho TR, Cutajar TP, Pierson TW, Nguyen TQ, Kaya U, Yuan Z, Long B, Langhammer P, Stuart SN. (2023) Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 622: 308–314. 10.1038/s41586-023-06578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony M, Moses B, Mahony SV, Lemckert FL, Donnellan S. (2020) A new species of frog in the Litoriaewingii species group (Anura: Pelodryadidae) from south-eastern Australia. Zootaxa 4858: 201–230. 10.11646/zootaxa.4858.2.3 [DOI] [PubMed]
- Mollard R. (2018) Culture, cryobanking and passaging of karyotypically validated native Australian amphibian cells. Cryobiology 81: 201–205. 10.1016/j.cryobiol.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Mollard R, Mahony M. (2023) Cell culture and karyotypic description of Pseudophrynecoriacea (Keferstein, 1868) (Amphibia, Anura) from the New South Wales Central Coast. Comparative Cytogenetics 17: 263–272. 10.3897/compcytogen.17.113526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard R, Mahony M, Marantelli G, West M. (2018) The critically endangered species Litoria spenceri demonstrates subpopulation karyotype diversity. Amphibian & Reptile Conservation 12: 28–36 [e166]. http://amphibian-reptile-conservation.org/pdfs/Volume/Vol_12_no_2/ARC_12_2_[Special_Section]_28-36_e166_high_res.pdf
- Oikawa M, Kobayashi H, Sanbo M, Mizuno N, Iwatsuki K, Takashima T, Yamauchi K, Yoshida F, Yamamoto T, Shinohara T, Nakauchi H, Kurimoto K, Hirabayashi M, Kobayashi T. (2022) Functional primordial germ cell-like cells from pluripotent stem cells in rats. Science 376: 176–179. 10.1126/science.abl4412 [DOI] [PubMed] [Google Scholar]
- Olifent M, Miller AL, Beaton A, L’Huillier P, Wells DN, Laible G. (2002) Karyotyping as an important screen for suitable donor cells to generate cloned and cloned transgenic animals by nuclear transfer. Proceedings of the New Zealand Society of Animal Production. New Zealand Society of Animal Production, Palmerston North, 199–201. https://www.nzsap.org/system/files/proceedings/karyotyping-important-screen-suitable-donor-cells-generate-cloned-and-cloned-transgenic-animals.pdf
- Parkin T, Rowley JJL, Elliott-Tate J, Mahony MJ, Sumner J, Melville J, Donnellan SC. (2024) Systematic assessment of the brown tree frog (Anura: Pelodryadidae: Litoriaewingii) reveals two endemic species in South Australia. Zootaxa 5406: 1–36. 10.11646/zootaxa.5406.1.1 [DOI] [PubMed] [Google Scholar]
- Schmid M, Steinlein C, Haaf T, Feightinger W, Guttenbach M, Bogart JP, Gruber SL, Kasahara S, Kakampuy W, Del Pino EM, Carrillo AB, Romero-Carvajal A, Mahony M, King M, Duellman WE, Hedges SB. (2018) The Arboranan Frogs: Results and Discussion. Cytogenetics & Genome Research 155: 55–221. 10.1159/000489839 [DOI] [PubMed] [Google Scholar]
- Steindachner F. (1867) Reise der österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859 unter den Befehlen des Commodore B. von Wüllerstorf-Urbair. In: Muller F. (Ed.) Zoologischer Theil Ambibien. Kaiserlich-Königliche Hof- und Staatsdruckerei, Wien, Vol.5: 1–70.
- Stejneger L. (1916) Notes on amphisbaenian nomenclature. Proceedings of the Biological Society of Washington 29: 85–85. [Google Scholar]
- Strauß S, Ziegler T, Allmeling C, Reimers K, Frank-Klein N, Seuntjens R, Vogt PM. (2011) In vitro culture of skin cells from biopsies from the Critically Endangered Chinese giant salamander, Andrias davidianus (Blanchard, 1871) (Amphibia, Caudata, Cryptobranchidae). Amphibian & Reptile Conservation 5: 51–63. https://biostor.org/reference/170463 [Google Scholar]
- Tschudi JJ. (1838) Classification der Batrachier: mit Berucksichtigung der fossilen Thiere dieser Abtheilung der Reptilien. Neuchâtel (Petitpierre), 99 pp. 10.5962/bhl.title.4883 [DOI]
- Tyler M, Davies M. (1978) Species-groups within the Australopapuan hulid frog genus Litoria Tschudi. Australian Journal of Zoology Supplementary Series 26: 1–47. 10.1071/AJZS063 [DOI] [Google Scholar]
- Vidal‐García M, Keogh JS. (2015) Convergent evolution across the Australian continent: ecotype diversification drives morphological convergence in two distantly related clades of Australian frogs. Journal of Evolutionary Biology 28: 2136–2151. 10.1111/jeb.12746 [DOI] [PubMed] [Google Scholar]
- Watson G, Loftus-Hills J, Littlejohn M. (1971) The Litoria ewingi complex (Anura: Hylidae) in south-eastern Australia I A new species from Victoria. Australian Journal of Zoology 19: 401–416. 10.1071/ZO9710401 [DOI] [Google Scholar]
- Wells RW, Wellington CR. (1985) A classification of the Amphibia and Reptilia of Australia. Australian Journal of Herpetology (Supplemental Series) 1: 1–61 [Google Scholar]
- White AW, Whitford RW, Mahoney MJ. (1994) A new species of Litoria (Anura: Hylidae) from eastern Australia. Proceedings of the Linnean Society of New South Wales 114: 3–10. 10.11646/zootaxa.4858.2.3 [DOI] [Google Scholar]
- Woodruff DS. (1972) Australian Anuran Chromosome Number. Herpetological Review 4: 208. 10.1159/000490328 [DOI]
- Zaleśna A, Florek M, Rybacki M, Ogielska M. (2017) Variability of NOR patterns in European water frogs of different genome composition and ploidy level. Comparative Cytogenetics 11: 249–266. 10.3897/compcytogen.v11i2.10804 [DOI] [PMC free article] [PubMed] [Google Scholar]