Abstract
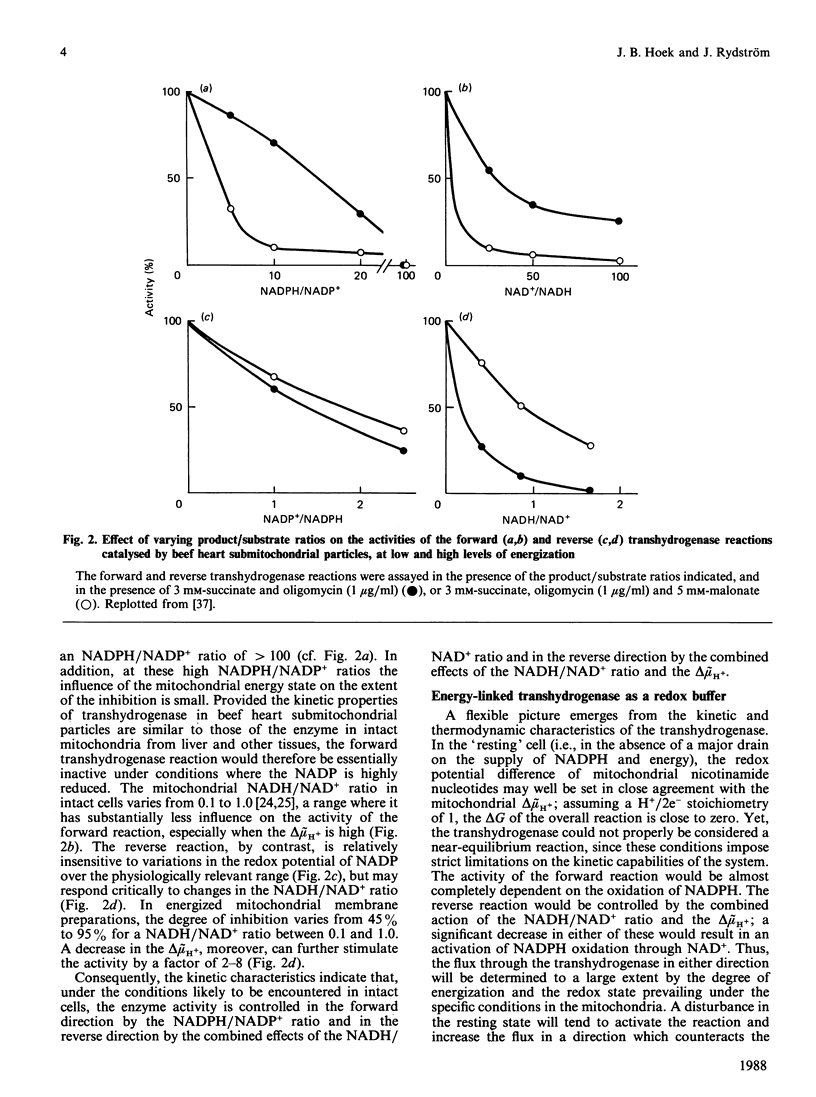
From the foregoing considerations, the energy-linked transhydrogenase reaction emerges as a powerful and flexible element in the network of redox and energy interrelationships that integrate mitochondrial and cytosolic metabolism. Its thermodynamic features make it possible for the reaction to respond readily to challenges, either on the side of NADPH utilization or on the side of energy depletion. Yet, the kinetic features are designed to prevent a wasteful input of energy when other sources of reducing equivalents to NADP are available, or to deplete the redox potential of NADPH in other than emergency conditions. By virtue of these characteristics, the energy-linked transhydrogenase can act as an effective buffer system, guarding against an excessive depletion of NADPH, preventing uncontrolled changes in key metabolites associated with NADP-dependent enzymes and calling on the supply of reducing equivalents from NAD-linked substrates only under conditions of high demand for NADPH. At the same time, it can provide an emergency protection against a depletion of energy, especially in situations of anoxia where a supply of reducing equivalents through NADP-linked substrates can be maintained. The flexibility of this design makes it possible that the functions of the energy-linked transhydrogenase vary from one tissue to another and are readily adjustable to different metabolic conditions.
Full text
PDF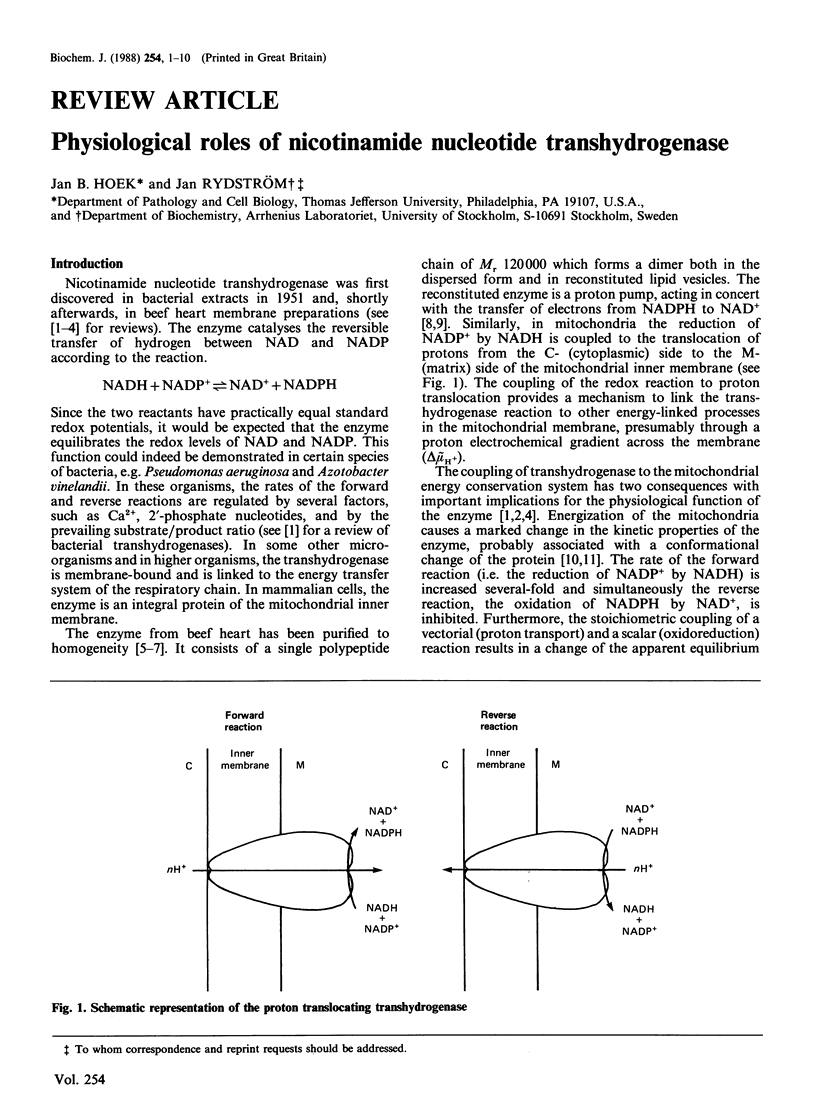








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. M., Fisher R. R. Purification and partial characterization of bovine heart mitochondrial pyridine dinucleotide transhydrogenase. Arch Biochem Biophys. 1978 Apr 15;187(1):180–190. doi: 10.1016/0003-9861(78)90021-8. [DOI] [PubMed] [Google Scholar]
- Andersson B. S., Aw T. Y., Jones D. P. Mitochondrial transmembrane potential and pH gradient during anoxia. Am J Physiol. 1987 Apr;252(4 Pt 1):C349–C355. doi: 10.1152/ajpcell.1987.252.4.C349. [DOI] [PubMed] [Google Scholar]
- Aw T. Y., Andersson B. S., Jones D. P. Mitochondrial transmembrane ion distribution during anoxia. Am J Physiol. 1987 Apr;252(4 Pt 1):C356–C361. doi: 10.1152/ajpcell.1987.252.4.C356. [DOI] [PubMed] [Google Scholar]
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Beavis A. D., Lehninger A. L. Determination of the upper and lower limits of the mechanistic stoichiometry of incompletely coupled fluxes. Stoichiometry of incompletely coupled reactions. Eur J Biochem. 1986 Jul 15;158(2):307–314. doi: 10.1111/j.1432-1033.1986.tb09752.x. [DOI] [PubMed] [Google Scholar]
- Belinsky S. A., Kauffman F. C., Thurman R. G. Reducing equivalents for mixed function oxidation in periportal and pericentral regions of the liver lobule in perfused livers from normal and phenobarbital-treated rats. Mol Pharmacol. 1984 Nov;26(3):574–581. [PubMed] [Google Scholar]
- Bellomo G., Martino A., Richelmi P., Moore G. A., Jewell S. A., Orrenius S. Pyridine-nucleotide oxidation, Ca2+ cycling and membrane damage during tert-butyl hydroperoxide metabolism by rat-liver mitochondria. Eur J Biochem. 1984 Apr 2;140(1):1–6. doi: 10.1111/j.1432-1033.1984.tb08058.x. [DOI] [PubMed] [Google Scholar]
- Corrigall J., Tselentis B. S., Mowbray J. The efficiency of oxidative phosphorylation and the rapid control by thyroid hormone of nicotinamide nucleotide reduction and transhydrogenation in intact rat liver mitochondria. Eur J Biochem. 1984 Jun 1;141(2):435–440. doi: 10.1111/j.1432-1033.1984.tb08210.x. [DOI] [PubMed] [Google Scholar]
- Crompton M., Costi A., Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987 Aug 1;245(3):915–918. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cruz A. T., Rydström J., Ernster L. Steady-state kinetics of mitochondrial nicotinamide nucleotide transhydrogenase. 1. The nonenergy-linked reaction. Eur J Biochem. 1971 Nov 11;23(2):203–211. doi: 10.1111/j.1432-1033.1971.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Dontsov A. E., Grinius L. L., Jasaitis A. A., Severina I. I., Skulachev V. P. A study on the mechanism of energy coupling in the redox chain. I. Transhydrogenase: the fourth site of the redox chain energy coupling. J Bioenerg. 1972 Jun;3(3):277–303. doi: 10.1007/BF01515975. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., DANIELSON L., LJUNGGREN M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962 Apr 9;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- Earle S. R., Anderson W. M., Fisher R. R. Evidence that reconstituted bovine heart mitochondrial transhydrogenase functions as a proton pump. FEBS Lett. 1978 Jul 1;91(1):21–24. doi: 10.1016/0014-5793(78)80008-8. [DOI] [PubMed] [Google Scholar]
- Earle S. R., Fisher R. R. A direct demonstration of proton translocation coupled to transhydrogenation in reconstituted vesicles. J Biol Chem. 1980 Feb 10;255(3):827–830. [PubMed] [Google Scholar]
- Eklöw L., Moldéus P., Orrenius S. Oxidation of glutathione during hydroperoxide metabolism. A study using isolated hepatocytes and the glutathione reductase inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea. Eur J Biochem. 1984 Feb 1;138(3):459–463. doi: 10.1111/j.1432-1033.1984.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Epps D. E., Palmer J. W., Schmid H. H., Pfeiffer D. R. Inhibition of permeability-dependent Ca2+ release from mitochondria by N-acylethanolamines, a class of lipids synthesized in ischemic heart tissue. J Biol Chem. 1982 Feb 10;257(3):1383–1391. [PubMed] [Google Scholar]
- Eytan G. D., Persson B., Ekebacke A., Rydström J. Energy-linked nicotinamide-nucleotide transhydrogenase. Characterization of reconstituted ATP-driven transhydrogenase from beef heart mitochondria. J Biol Chem. 1987 Apr 15;262(11):5008–5014. [PubMed] [Google Scholar]
- Ferguson S. J., Sorgato M. C. Proton electrochemical gradients and energy-transduction processes. Annu Rev Biochem. 1982;51:185–217. doi: 10.1146/annurev.bi.51.070182.001153. [DOI] [PubMed] [Google Scholar]
- GOEBELL H., KLINGENBERG M. DPN-SPEZIFISCHE ISOCITRAT-DEHYDROGENASE DER MITOCHONDRIEN. I. KINETISCHE EIGENSSCHAFTEN, VORKOMMEN UND FUNKTION DER DPN-SPEZIFISCHEN ISOCITRAT-DEHYDROGENASE. Biochem Z. 1964 Sep 28;340:441–464. [PubMed] [Google Scholar]
- Hoek J. B., Ernster L., de Haan E. J., Tager J. M. The nicotinamide nucleotide specificity of glutamate dehydrogenase in intact rat-liver mitochondria. Biochim Biophys Acta. 1974 Mar 26;333(3):546–559. doi: 10.1016/0005-2728(74)90138-8. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- Hoek J. B., Rydström J., Ernster L. The NAD-linked isocitrate dehydrogenase activity in rat-liver mitochondria. Biochim Biophys Acta. 1973 Jun 28;305(3):669–674. doi: 10.1016/0005-2728(73)90090-x. [DOI] [PubMed] [Google Scholar]
- Höjeberg B., Rydström J. Purification and molecular properties of reconstitutively active nicotinamide nucleotide transhydrogenase from beef heart mitochondria. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1183–1190. doi: 10.1016/0006-291x(77)91418-8. [DOI] [PubMed] [Google Scholar]
- JARABAK J., ADAMS J. A., WILLIAMS-ASHMAN H. G., TALALAY P. Purification of a 17beta-hydroxysteroid dehydrogenase of human placenta and studies on its transhydrogenase function. J Biol Chem. 1962 Feb;237:345–357. [PubMed] [Google Scholar]
- Jocelyn P. C. The reduction of diamide by rat liver mitochondria and the role of glutathione. Biochem J. 1978 Dec 15;176(3):649–664. doi: 10.1042/bj1760649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D. W., Brierley G. P. The redox state of pyridine nucleotides controls permeability of uncoupled mitochondria to K+. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1372–1377. doi: 10.1016/0006-291x(82)91265-7. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Hydrogen transfer in mitochondria. 3. Energetic aspects of the NH3-incorporation into amino acids. Biochem Z. 1965 Dec 31;343(4):479–503. [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- Kulinsky V. I., Trufanova L. V., Medvedev A. E. Catecholamine control of enzymes involved in isocitrate oxidation of rat liver mitochondria. FEBS Lett. 1984 Nov 5;177(1):143–145. doi: 10.1016/0014-5793(84)80999-0. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Lee C., Ernster L. Studies of the energy-transfer system of submitochondrial particles. I. Competition between oxidative phosphorylation and the energy-linked nicotinamide-adenine dinucleotide transhydrogenase reaction. Eur J Biochem. 1968 Feb;3(4):385–390. doi: 10.1111/j.1432-1033.1967.tb19541.x. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Vercesi A., Bababunmi E. A. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev A. E., Trufanova L. V., Kulinskii V. I. Reguliatsiia aktivnosti mitokhondrial'noi transgidrogenazy katekholaminami. Biokhimiia. 1986 Jul;51(7):1165–1173. [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982 Apr 10;257(7):3747–3753. [PubMed] [Google Scholar]
- Mitchell R., West I. C., Moody A. J., Mitchell P. Measurement of the proton-motive stoichiometry of the respiratory chain of rat liver mitochondria: the effect of N-ethylmaleimide. Biochim Biophys Acta. 1986 Apr 24;849(2):229–235. doi: 10.1016/0005-2728(86)90029-0. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Grundin R., Vadi H., Orrenius S. A study of drug metabolism linked to cytochrome P-450 in isolated rat-liver cells. Eur J Biochem. 1974 Jul 15;46(2):351–360. doi: 10.1111/j.1432-1033.1974.tb03627.x. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. The proton-translocating nicotinamide-adenine dinucleotide (phosphate) transhydrogenase of rat liver mitochondria. Biochem J. 1973 Mar;132(3):571–585. doi: 10.1042/bj1320571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG RAND P. B. Th control of isocitrate oxidation by rat liver mitochondria. Biochem J. 1969 Sep;114(2):215–225. doi: 10.1042/bj1140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Baldi C., Svensson S. A., Bellomo G., Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem. 1986 Nov 5;261(31):14628–14635. [PubMed] [Google Scholar]
- Nicotera P., Moore M., Mirabelli F., Bellomo G., Orrenius S. Inhibition of hepatocyte plasma membrane Ca2+-ATPase activity by menadione metabolism and its restoration by thiols. FEBS Lett. 1985 Feb 11;181(1):149–153. doi: 10.1016/0014-5793(85)81131-5. [DOI] [PubMed] [Google Scholar]
- Persson B., Enander K., Tang H. L., Rydström J. Energy-linked nicotinamide nucleotide transhydrogenase. Properties of proton-translocating mitochondrial transhydrogenase from beef heart purified by fast protein liquid chromatography. J Biol Chem. 1984 Jul 10;259(13):8626–8632. [PubMed] [Google Scholar]
- Persson B., Rydström J. Evidence for a role of a vicinal dithiol in catalysis and proton pumping in mitochondrial nicotinamide nucleotide transhydrogenase. Biochem Biophys Res Commun. 1987 Jan 30;142(2):573–578. doi: 10.1016/0006-291x(87)90312-3. [DOI] [PubMed] [Google Scholar]
- Petcu L. G., Plaut G. W. NADP-specific isocitrate dehydrogenase in regulation of urea synthesis in rat hepatocytes. Biochem J. 1980 Sep 15;190(3):581–592. doi: 10.1042/bj1900581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prpić V., Bygrave F. L. On the inter-relationship between glucagon action, the oxidation-reduction state of pyridine nucleotides, and calcium retention by rat liver mitochondria. J Biol Chem. 1980 Jul 10;255(13):6193–6199. [PubMed] [Google Scholar]
- Reynafarje B., Lehninger A. L. The K+/site and H+/site stoichiometry of mitochondrial electron transport. J Biol Chem. 1978 Sep 25;253(18):6331–6334. [PubMed] [Google Scholar]
- Riley W. W., Jr, Pfeiffer D. R. The effect of Ca2+ and acyl coenzyme A:lysophospholipid acyltransferase inhibitors on permeability properties of the liver mitochondrial inner membrane. J Biol Chem. 1986 Oct 25;261(30):14018–14024. [PubMed] [Google Scholar]
- Robillard G. T., Konings W. N. A hypothesis for the role of dithiol-disulfide interchange in solute transport and energy-transducing processes. Eur J Biochem. 1982 Oct;127(3):597–604. doi: 10.1111/j.1432-1033.1982.tb06914.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Rydström J., Da Cruz A. T., Ernster L. Steady-state kinetics of mitochondrial nicotinamide nucleotide transhydrogenase. 2. The energy-linked reaction. Eur J Biochem. 1971 Nov 11;23(2):212–219. doi: 10.1111/j.1432-1033.1971.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Rydström J., da Cruz A. T., Ernster L. Factors governing the kinetics and steady state of the mitochondrial nicotinamide nucleotide transhydrogenase system. Eur J Biochem. 1970 Nov;17(1):56–62. doi: 10.1111/j.1432-1033.1970.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Sies H., Akerboom T. P., Tager J. M. Mitochondrial and cytosolic NADPH systems and isocitrate dehydrogenase indicator metabolites during ureogensis from ammonia in isolated rat hepatocytes. Eur J Biochem. 1977 Jan;72(2):301–307. doi: 10.1111/j.1432-1033.1977.tb11253.x. [DOI] [PubMed] [Google Scholar]
- Sies H., Summer K. H., Bücher T. A process requiring mitochondrial NADPH: urea formation from ammonia. FEBS Lett. 1975 Jun 15;54(2):274–278. doi: 10.1016/0014-5793(75)80091-3. [DOI] [PubMed] [Google Scholar]
- Sluse Francis E., Meijer Alfred J., Tager Joseph M. Anion translocators in rat-heart mitochondria. FEBS Lett. 1971 Oct 15;18(1):149–153. doi: 10.1016/0014-5793(71)80432-5. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Plaut G. W. Activities of NAD-specific and NADP-specific isocitrate dehydrogenases in rat-liver mitochondria. Studies with D-threo-alpha-methylisocitrate. Eur J Biochem. 1979 Jun;97(1):283–295. doi: 10.1111/j.1432-1033.1979.tb13113.x. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Hoek J. B., Farber J. L. Calcium-dependent and calcium-independent mechanisms of irreversible cell injury in cultured hepatocytes. J Biol Chem. 1986 Mar 5;261(7):3006–3012. [PubMed] [Google Scholar]
- Strzelecki T., Thomas J. A., Koch C. D., LaNoue K. F. The effect of hormones on proton compartmentation in hepatocytes. J Biol Chem. 1984 Apr 10;259(7):4122–4129. [PubMed] [Google Scholar]
- TAGER J. M. SYNTHESIS OF GLUTAMATE FROM ALPHA-OXOGLUTARATE AND AMMONIA IN RAT-LIVER MITOCHONDRIA. III. MALATE AS HYDROGEN DONOR. Biochim Biophys Acta. 1963 Oct 1;77:258–265. doi: 10.1016/0006-3002(63)90497-9. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Titheradge M. A., Binder S. B., Yamazaki R. K., Haynes R. C., Jr Glucagon treatment stimulates the metabolism of hepatic submitochondrial particles. J Biol Chem. 1978 May 25;253(10):3357–3360. [PubMed] [Google Scholar]
- Uhr M. L., Thompson V. W., Cleland W. W. The kinetics of pig heart triphosphopyridine nucleotide-isocitrate dehydrogenase. I. Initial velocity, substrate and product inhibition, and isotope exchange studies. J Biol Chem. 1974 May 10;249(9):2920–2927. [PubMed] [Google Scholar]
- Vercesi A. E. The participation of NADP, the transmembrane potential and the energy-linked NAD(P) transhydrogenase in the process of Ca2+ efflux from rat liver mitochondria. Arch Biochem Biophys. 1987 Jan;252(1):171–178. doi: 10.1016/0003-9861(87)90021-x. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Meijer A. J., Groen A. K., Tager J. M. Bicarbonate and the pathway of glutamate oxidation in isolated rat-liver mitochondria. Eur J Biochem. 1983 Jun 1;133(1):245–254. doi: 10.1111/j.1432-1033.1983.tb07455.x. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., van Doorn H. E., Tager J. M. The energy-linked transhydrogenase in rat liver in relation to the reductive carboxylation of 2-oxoglutarate. Eur J Biochem. 1981 Jun 1;116(3):609–614. doi: 10.1111/j.1432-1033.1981.tb05379.x. [DOI] [PubMed] [Google Scholar]
- Weigl K., Sies H. Drug oxidations dependent on cytochrome P-450 in isolated hepatocytes. The role of the tricarboxylates and the aminotransferases in NADPH supply. Eur J Biochem. 1977 Jul 15;77(2):401–408. doi: 10.1111/j.1432-1033.1977.tb11680.x. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Hoek J. B. Role of calcium in the hormonal regulation of liver metabolism. Biochim Biophys Acta. 1981 Dec 30;639(3-4):243–295. doi: 10.1016/0304-4173(81)90012-4. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., Nieuwenhuis F. J., van Dam K. On the reversibility of the energy-linked transhydrogenase. Biochim Biophys Acta. 1971 Apr 6;234(1):173–176. doi: 10.1016/0005-2728(71)90143-5. [DOI] [PubMed] [Google Scholar]


