Abstract
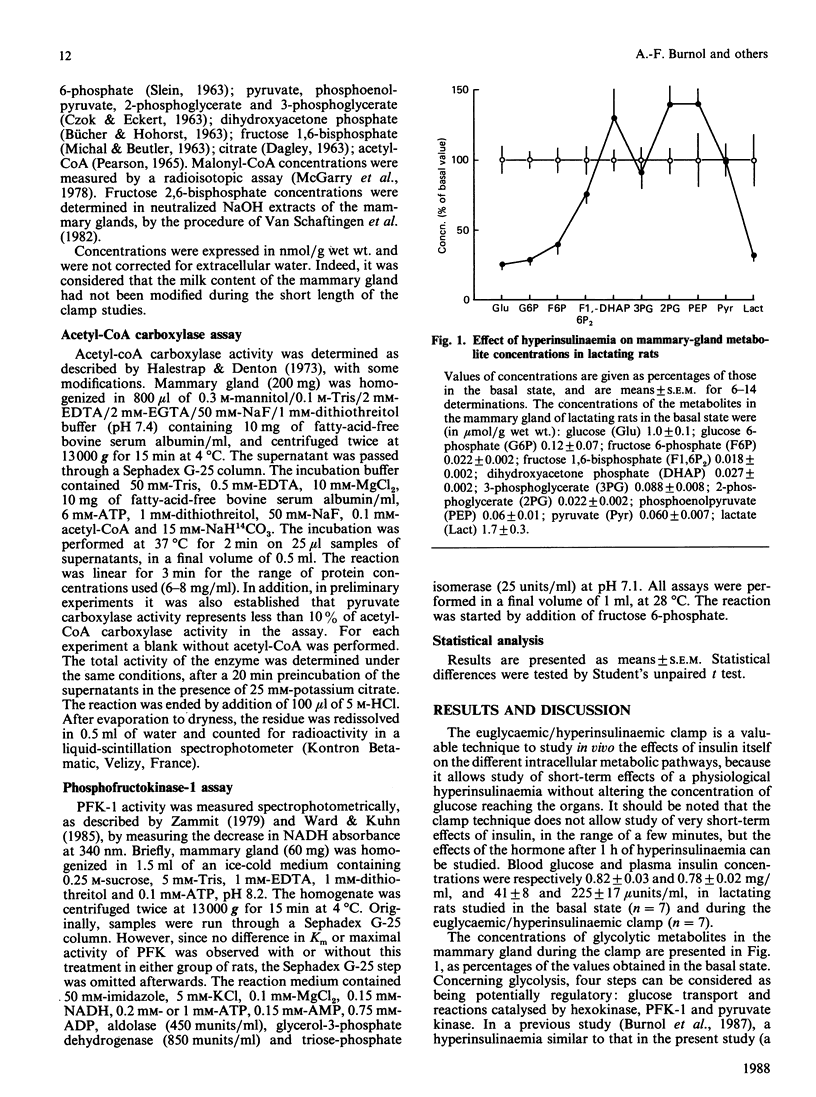
The effect of insulin on glucose metabolism in mammary gland was studied by the euglycaemic/hyperinsulinaemic-clamp technique. Measurement of metabolite concentrations and enzyme activities in the mammary gland suggests two sites of action of insulin: phosphofructokinase-1 and acetyl-coA carboxylase. The increase in phosphofructokinase-1 activity could be linked to the 2-fold increase in fructose 2,6-bisphosphate concentration, since no change in maximal activity and in sensitivity of the enzyme toward fructose 6-phosphate was detected in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter M. A., Coore H. G. The mode of regulation of pyruvate dehydrogenase of lactating rat mammary gland. Effects of starvation and insulin. Biochem J. 1978 Aug 15;174(2):553–561. doi: 10.1042/bj1740553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnol A. F., Ferre P., Leturque A., Girard J. Effect of insulin on in vivo glucose utilization in individual tissues of anesthetized lactating rats. Am J Physiol. 1987 Feb;252(2 Pt 1):E183–E188. doi: 10.1152/ajpendo.1987.252.2.E183. [DOI] [PubMed] [Google Scholar]
- Burnol A. F., Leturque A., Ferré P., Girard J. Glucose metabolism during lactation in the rat: quantitative and regulatory aspects. Am J Physiol. 1983 Oct;245(4):E351–E358. doi: 10.1152/ajpendo.1983.245.4.E351. [DOI] [PubMed] [Google Scholar]
- Carrick D. T., Kuhn N. J. Diurnal variation and response to food withdrawal of lactose synthesis in lactating rats. Biochem J. 1978 Jul 15;174(1):319–325. doi: 10.1042/bj1740319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Insulin and the regulation of adipose tissue acetyl-coenzyme A carboxylase. Biochem J. 1973 Mar;132(3):509–517. doi: 10.1042/bj1320509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Rider M. H. Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues. Biochem J. 1987 Jul 15;245(2):313–324. doi: 10.1042/bj2450313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. G., Ilic V., Williamson D. H. Regulation of lactating-rat mammary-gland lipogenesis by insulin and glucagon in vivo. The role and site of action of insulin in the transition to the starved state. Biochem J. 1984 Oct 15;223(2):345–351. doi: 10.1042/bj2230345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. G., Williamson D. H. Alterations in mammary-gland blood flow and glucose metabolism in the lactating rat induced by short-term starvation and refeeding. Biosci Rep. 1984 May;4(5):421–426. doi: 10.1007/BF01122507. [DOI] [PubMed] [Google Scholar]
- Kilgour E., Vernon R. G. Tissue-specific changes in the ability of insulin and noradrenaline to activate pyruvate dehydrogenase in vivo during lactation in the rat. Biochem J. 1987 Apr 1;243(1):69–74. doi: 10.1042/bj2430069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Stark M. J., Foster D. W. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978 Nov 25;253(22):8291–8293. [PubMed] [Google Scholar]
- McNeillie E. M., Zammit V. A. Regulation of acetyl-CoA carboxylase in rat mammary gland. Effects of starvation and of insulin and prolactin deficiency on the fraction of the enzyme in the active form in vivo. Biochem J. 1982 Apr 15;204(1):273–280. doi: 10.1042/bj2040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer S. W., Williamson D. H. The regulation of lipogenesis in vivo in the lactating mammary gland of the rat during the starved-refed transition. Studies wtih acarbose, a glucosidase inhibitor. Biochem J. 1987 Feb 15;242(1):235–243. doi: 10.1042/bj2420235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer S. W., Williamson D. H. Time course of changes in plasma glucose and insulin concentrations and mammary-gland lipogenesis during re-feeding of starved conscious lactating rats. Biochem J. 1986 Oct 15;239(2):489–492. doi: 10.1042/bj2390489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday M. R., Hardie D. G. The role of acetyl-CoA carboxylase phosphorylation in the control of mammary gland fatty acid synthesis during the starvation and re-feeding of lactating rats. Biochem J. 1986 Jul 1;237(1):85–91. doi: 10.1042/bj2370085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday M. R., Williamson D. H. Effects of starvation, insulin or prolactin deficiency on the activity of acetyl-CoA carboxylase in mammary gland and liver of lactating rats. FEBS Lett. 1982 Feb 22;138(2):285–288. doi: 10.1016/0014-5793(82)80462-6. [DOI] [PubMed] [Google Scholar]
- Threadgold L. C., Kuhn N. J. Monosaccharide transport in the mammary gland of the intact lactating rat. Biochem J. 1984 Feb 15;218(1):213–219. doi: 10.1042/bj2180213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Ward S., Kuhn N. J. Role of fructose 2,6-bisphosphate in mammary gland of fed, starved and re-fed lactating rats. Biochem J. 1985 Dec 15;232(3):931–934. doi: 10.1042/bj2320931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Ilic V., Jones R. G. Evidence that the stimulation of lipogenesis in the mammary glands of starved lactating rats re-fed with a chow diet is dependent on continued hepatic gluconeogenesis during the absorptive period. Effects of a gluconeogenic inhibitory, mercaptopicolinic acid, in vivo. Biochem J. 1985 Jun 15;228(3):727–733. doi: 10.1042/bj2280727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Effects of citrate on phosphofructokinase from lactating rat mammary gland acini. FEBS Lett. 1979 Dec 1;108(1):193–196. doi: 10.1016/0014-5793(79)81208-9. [DOI] [PubMed] [Google Scholar]


