Abstract
OBJECTIVE:
This study aimed to elucidate the association between IV contrast media CT and acute kidney injury (AKI) and in-hospital mortality among patients requiring emergency admission.
DESIGN:
In this retrospective observational study, we examined AKI within 48 hours after CT, renal replacement therapy (RRT) dependence at discharge, and in-hospital mortality in patients undergoing contrast-enhanced CT or nonenhanced CT. We performed 1:1 propensity score matching to adjust for confounders in the association between IV contrast media use and outcomes. Subgroup analyses were performed according to age, sex, diagnosis at admission, ICU admission, and preexisting chronic kidney disease (CKD).
SETTING AND PATIENTS:
This study used the Medical Data Vision database between 2008 and 2019. This database is Japan’s largest commercially available hospital-based claims database, covering about 45% of acute-care hospitals in Japan, and it also records laboratory results.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The study included 144,149 patients with (49,057) and without (95,092) contrast media exposure, from which 43,367 propensity score-matched pairs were generated. Between the propensity score-matched groups of overall patients, exposure to contrast media showed no significant risk of AKI (4.6% vs. 5.1%; odds ratio [OR], 0.899; 95% CI, 0.845–0.958) or significant risk of RRT dependence (0.6% vs. 0.4%; OR, 1.297; 95% CI, 1.070–1.574) and significant benefit for in-hospital mortality (5.4% vs. 6.5%; OR, 0.821; 95% CI, 0.775–0.869). In subgroup analyses regarding preexisting CKD, exposure to contrast media was a significant risk for AKI in patients with CKD but not in those without CKD.
CONCLUSIONS:
In this large-scale observational study, IV contrast media was not associated with an increased risk of AKI but concurrently showed beneficial effects on in-hospital mortality among patients requiring emergency admission.
Keywords: acute kidney injury, contrast media, contrast-induced nephropathy, emergency department, postcontrast acute kidney injury
KEY POINTS
Question: How does the risk of contrast-enhanced CT relate to kidney dysfunction and mortality compared with nonenhanced CT in patients requiring emergency admission?
Findings: In this large retrospective study of 144,149 emergently admitted patients in a Japanese database who underwent CT scans, exposure to IV contrast media was not associated with an increased risk of acute kidney injury. However, it was significantly associated with lower hospital mortality compared with nonexposure, even after adjustment for important confounders.
Meaning: Our findings suggest that contrast-enhanced CT may pose a limited risk for renal impairment in many emergency department patients.
Contrast-enhanced CT plays a crucial role in the prompt diagnosis of life-threatening conditions such as bleeding in trauma (1), infections in sepsis (2, 3), and critical diagnoses like aortic dissection (4, 5). Timely and accurate diagnosis is mandatory in emergency settings, where patients often face potentially fatal situations. However, the use of contrast media has been associated with nephrotoxicity, including acute kidney injury (AKI) and death (6, 7). Some studies have revealed the mechanisms by which contrast agents cause renal injury (8, 9). Two pathways have been suggested: direct damage to renal tubular epithelial cells by contrast agents and indirect damage via renal ischemia due to intrarenal vasoconstriction and reduced renal glomerular blood flow (8, 9). Clinical studies have shown that contrast media administration causes contrasted-induced nephropathy (CIN), especially in high-risk patients, who are the elderly (10), have chronic kidney disease (CKD) (10–12), diabetes mellitus (10, 13, 14), chronic heart failure (10, 14), anemia (14), and take nephrotoxic drugs (14). The occurrence rate of contrast-induced AKI reportedly varies widely, ranging from 3.3% to 14.5% (15–17).
However, it has been suggested that the perceived risk of AKI associated with contrast media use may have been overestimated in previous reports due to methodological limitations such as the absence of adequate control groups (18, 19). Recent observational studies and meta-analyses reported that exposure to contrast media was not significantly associated with the incidence of AKI (20–23). Also, studies in patients with renal impairment have indicated no significant difference in AKI incidence between patients who underwent contrast-enhanced CT and those who did not (24). Similar findings extend to critically ill patients who had a 20–50% chance of developing AKI regardless of contrast media use in ICUs (19). In addition, AKI and mortality do not increase with contrast media use, even in sepsis patients who were introduced to renal replacement therapy (RRT) at an early stage (25).
Despite these findings across various conditions, little research has been conducted on patients visiting emergency departments. Existing clinical evidence in this area is limited due to small-scale studies, particularly with almost no research focusing specifically on patients requiring emergency admission. Therefore, we aimed to elucidate the potential risks associated with contrast-enhanced CT, including AKI, induction of RRT, and in-hospital mortality, using comprehensive clinical big data from patients requiring emergency admission.
METHODS
Data Source
We performed a retrospective observational cohort study using the Medical Data Vision (MDV) database, Japan’s largest commercially available hospital‐based claims database. As of April 1, 2022, the MDV database contained over 35 million inpatients and outpatients treated at any of the 793 hospitals, covering about 45% of acute-care hospitals in Japan (26). This database is composed of administrative claims data originating from Japan’s healthcare system and includes data on each patient’s age, sex, admission and discharge dates, discharge status, primary diagnoses, comorbidities at admission, postadmission complication diagnoses, types of surgical procedures coded with the original Japanese operation codes, daily records of procedures, diagnostic examinations, drug administrations, and results of laboratory tests. The diagnoses in this database are recorded using the International Classification of Diseases, 10th revision (ICD-10) codes. From the original MDV database, we extracted the clinical data of 534,739 patients who required emergency admissions to 42 hospitals that recorded laboratory data from April 2008 to December 2019.
This study was conducted in compliance with the principles of the Declaration of Helsinki of 1975 and according to the ethical standard of our institutional committee (the institutional review board [IRB] for Clinical Research of Osaka Medical Center) and the Strengthening the Reporting of Observational Studies in Epidemiology guideline. Because of the anonymous nature of the data, the requirement for informed consent from patients was waived according to our institutional committee (IRB number S201916015, study title: “Evaluation of clinical issues in emergency and intensive care medicine using big data,” approved on July 7, 2020).
Study Participants and Exposure Variable
Patients were eligible for this study if they met the following criteria: patients 1) required emergency admission, 2) received a CT scan with or without IV contrast media at admission, and 3) were older than 18 years at the time of admission. We excluded patients who underwent coronary angiography or percutaneous coronary intervention at admission, who had end-stage renal disease based on the ICD-10 code of N18.0 or N18.5 at admission, who died within 24 hours after admission, and who did not have laboratory test results for serum creatinine level at admission and within 48 hours after admission. We also excluded data from the second and subsequent hospitalizations of patients who were hospitalized multiple times during the study period and used only the data from their first hospitalization. Patients who received a CT scan with IV contrast media were classified into the enhanced CT group, and those who received a CT scan without IV contrast media were classified into the nonenhanced CT group based on the procedure code. Only low-osmolar contrast media were used for contrast-enhanced CT in this study.
Definition of Outcomes
The primary outcome was the incidence of AKI. In this study, we evaluated three different definitions of AKI. AKI (stages 1–3) was defined as an increase in serum creatine level of more than 0.3 mg/dL or a rise of at least 1.5 times from the result of the baseline serum creatinine level within 48 hours after admission, following the criteria of the Kidney Disease Improving Global Outcomes (KDIGO). AKI stage 3 was defined as an increase in serum creatinine level of more than 4.0 mg/dL or a rise of more than 3.0 times from the result of the baseline creatinine level within 48 hours of admission and the use of RRT within 48 hours of admission, following stage 3 of the KDIGO criteria. CIN was diagnosed based on serum creatine level when serum creatinine increases by more than 0.5 mg/dL or rises to at least 1.25 times from the baseline creatinine level within 72 hours of contrast-enhanced CT scan. The secondary outcomes included RRT during hospitalization, RRT dependence at discharge, and in-hospital mortality. RRT during hospitalization was defined as receiving RRT at least once during hospitalization. RRT dependence was defined as receiving RRT within 2 days before discharge. In Japan, patients dependent on RRT at discharge and who require outpatient RRT after discharge are scheduled to undergo RRT thrice weekly as conventional intermittent dialysis (27). Therefore, the definition of RRT dependence as the requirement for RRT “within 2 days before discharge” appears reasonable (22).
Data Collection
We collected the following data: age, sex, height, weight, hospital volume, comorbidities, laboratory tests, Charlson Comorbidity Index (CCI) (28), use of nephrotoxic medications, primary diagnoses at admission, ICU admission, and emergency operation. We used predefined ICD-10 coding algorithms for evaluating CCI (29). Based on the ICD-10 coding, we identified a recorded diagnosis of congestive heart failure, diabetic nephropathy, CKD, end-stage renal disease, and hypertension as a complication disease, and trauma, macrovascular disease, cerebrovascular disease, myocardial infarction, and sepsis as the primary diagnosis. We defined emergency operation as any surgery using general anesthesia within 2 days of admission. We identified the following treatments performed at admission: vasoactive agents, including dopamine, dobutamine, norepinephrine, epinephrine, or vasopressin, and nephrotoxic medications, such as nonsteroidal anti-inflammatory drugs and diuretics. Details on the definition of the ICD-10 codes used in this study are provided in Supplemental Table 1 (http://links.lww.com/CCX/B386).
Statistical Analysis
Due to the nature of a retrospective study, it was assumed that an imbalance existed among the covariates in the enhanced CT group and the nonenhanced CT group at baseline. Propensity score matching was thus performed between each group (30). The propensity scores were determined using logistic regression with 19 variables assumed to be associated with IV contrast media used as covariates (Supplemental Table 2, http://links.lww.com/CCX/B386). We chose these covariates to calculate the propensity scores based on the risk factors of contrast-inducing AKI and the clinical importance of enhanced CT (31). C-statistics were evaluated as a measure of discrimination. The distribution of propensity scores for each group was compared graphically to assess the overlap assumption. We conducted a one-to-one analysis using nearest-neighbor matching based on the estimated propensity scores for each patient. A match occurred when a patient in the enhanced CT group had an estimated score within 0.2 sds of a patient in the nonenhanced CT group. Standardized mean difference was calculated for the balance assessment between each group before and after matching, and we considered values less than 0.1 acceptable (32).
We performed propensity score matching for the subgroups of age (≥ 65 yr old vs. < 65 yr old), sex (male vs. female), diagnosis at admission (trauma vs. sepsis vs. cerebrovascular disease vs. macrovascular disease), preexisting CKD, ICU admission (ICU vs. general ward), and admission patient’s condition with or without organ dysfunction.
We also performed a sensitivity analysis using logistic regression analysis. In total, 21 independent variables were included, of which 19 were the same factors as in the propensity score matching, and the newly added variables were enhanced CT and an interaction term between CKD and enhanced CT. The final model estimated the relative risk of event occurrence for each variable by calculating the odds ratio (OR) and 95% CI.
Continuous variables are reported as means with sd and categorical variables as numbers with percentages. We analyzed continuous outcomes using the t-test and categorical outcomes using the Fisher exact test and the chi-square test. All statistical inferences were two-sided; a p value less than 0.05 indicated statistical significance. Data were analyzed using R software, version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
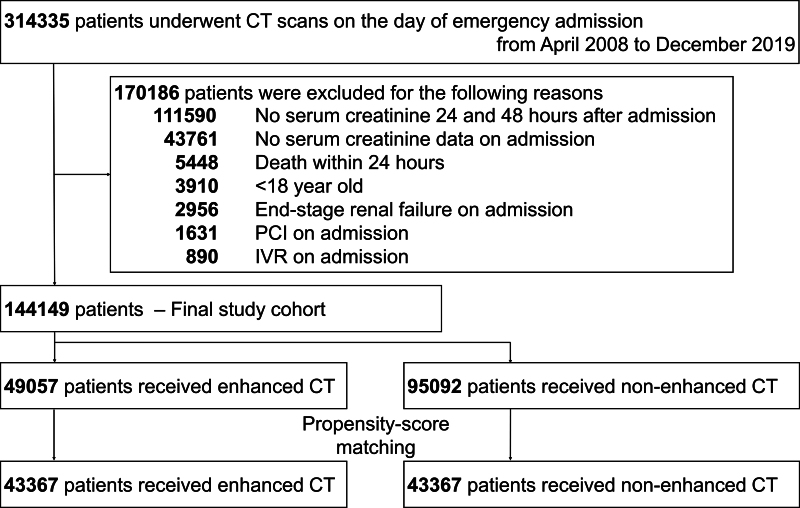
We identified 314,335 patients who fulfilled the inclusion criteria and subsequently excluded 170,186 patients who met the exclusion criteria. The remaining 144,149 eligible patients were divided into the enhanced CT group (n = 49,057) and the nonenhanced CT group (n = 95,092). Supplemental Figure 1 (http://links.lww.com/CCX/B386) shows the distributions of propensity scores in the two groups. The C-statistic for the fit of the propensity scores was 0.727 (95% CI, 0.724–0.729). After matching, 43,367 propensity score-matched pairs were generated (Fig. 1).
Figure 1.
Flowchart for patient selection. IVR = interventional radiology, PCI = percutaneous coronary intervention.
Table 1 shows the baseline characteristics of the two groups before and after propensity score matching. In the unmatched groups, patients in the enhanced CT group tended to be younger, have lower rates for several comorbidities and better renal function, and be more often diagnosed as having macrovascular disease than those in the nonenhanced CT group. Also, the enhanced CT group received more intensive treatment, including catecholamines, ICU admission, and emergency operation, than the nonenhanced CT group. After propensity score matching, the two groups had no significant differences in inpatient characteristics.
TABLE 1.
Baseline Characteristics of Patients in Unmatched and 1:1 Propensity Score-Matched Groups
| Characteristics | Unmatched Groups | Matched Groups | ||||
|---|---|---|---|---|---|---|
| Nonenhanced CT | Enhanced CT | SMD | Nonenhanced CT | Enhanced CT | SMD | |
| Patient number | 95,092 | 49,057 | 43,367 | 43,367 | ||
| Age, yr | 73.29 (16.65) | 65.62 (18.07) | 0.441 | 66.41 (18.84) | 66.91 (17.33) | 0.028 |
| Sex, female | 44,867 (47.2) | 21,951 (44.7) | 0.049 | 19,377 (44.7) | 19,510 (45.0) | 0.006 |
| Hospital volume | 0.201 | 0.033 | ||||
| 199 beds | 4,233 (4.5) | 1,179 (2.4) | 1,124 (2.6) | 987 (2.3) | ||
| 200–499 beds | 66,081 (69.5) | 31,087 (63.4) | 27,716 (63.9) | 28,340 (65.3) | ||
| 500 beds | 24,778 (26.1) | 16,791 (34.2) | 14,527 (33.5) | 14,040 (32.4) | ||
| Comorbidities | ||||||
| Congestive heart failure | 17,664 (18.6) | 4663 (9.5) | 0.263 | 3,661 (8.4) | 4,080 (9.4) | 0.034 |
| Chronic kidney disease | 5,210 (5.5) | 639 (1.3) | 0.232 | 512 (1.2) | 590 (1.4) | 0.016 |
| Hypertension | 37,444 (39.4) | 17,033 (34.7) | 0.097 | 14,488 (33.4) | 14,844 (34.2) | 0.017 |
| Diabetic nephropathy | 1,394 (1.5) | 249 (0.5) | 0.097 | 191 (0.4) | 242 (0.6) | 0.017 |
| Myocardial infarction | 5,118 (5.4) | 1,949 (4.0) | 0.067 | 1,765 (4.1) | 1,799 (4.1) | 0.004 |
| Laboratory test results at admission | ||||||
| Glomerular filtration rate, mL/min/1.73 m2 | 60.98 (34.19) | 73.99 (27.39) | 0.420 | 73.20 (38.95) | 72.18 (24.96) | 0.031 |
| Creatinine, mg/dL | 1.20 (1.26) | 0.84 (0.55) | 0.370 | 0.96 (0.91) | 0.85 (0.57) | 0.138 |
| Hemoglobin < 11 g/dL | 25,096 (26.4) | 9,840 (20.1) | 0.150 | 8,664 (20.0) | 8,936 (20.6) | 0.016 |
| Charlson Comorbidity Index | 2.40 (2.49) | 2.36 (2.68) | 0.014 | 2.11 (2.41) | 2.43 (2.73) | 0.124 |
| Nephrotoxic medication | ||||||
| Nonsteroidal anti-inflammatory drugs | 23,616 (24.8) | 16,317 (33.3) | 0.186 | 12,906 (29.8) | 13,180 (30.4) | 0.014 |
| Catecholamine | 1,756 (1.8) | 1,404 (2.9) | 0.067 | 981 (2.3) | 1,046 (2.4) | 0.010 |
| Diuretic medication | 14,607 (15.4) | 4,840 (9.9) | 0.166 | 3,561 (8.2) | 3,996 (9.2) | 0.036 |
| Antibiotic (aminoglycoside) | 31,639 (33.3) | 12,027 (24.5) | 0.039 | 422 (1.0) | 634 (1.5) | 0.045 |
| Antibiotic (anti-methicillin-resistant Staphylococcus aureus drug) | 889 (0.9) | 661 (1.3) | 0.014 | 567 (1.3) | 498 (1.1) | 0.014 |
| Diagnosis at admission | ||||||
| Trauma | 12,561 (13.2) | 3,155 (6.4) | 0.229 | 3,110 (7.2) | 3,127 (7.2) | 0.002 |
| Sepsis | 9,084 (9.6) | 2,845 (5.8) | 0.141 | 2,584 (6.0) | 2,741 (6.3) | 0.015 |
| Cerebrovascular disease | 9,659 (10.2) | 4,326 (8.8) | 0.046 | 3,742 (8.6) | 4,017 (9.3) | 0.022 |
| Macrovascular disease | 441 (0.5) | 2,772 (5.7) | 0.305 | 437 (1.0) | 673 (1.6) | 0.048 |
| Organ dysfunction | 31,639 (33.3) | 12,027 (24.5) | 0.194 | 12,280 (28.3) | 10,757 (24.8) | 0.080 |
| ICU admission | 5,133 (5.4) | 4,734 (9.6) | 0.162 | 3,002 (6.9) | 3,230 (7.4) | 0.020 |
| Emergency operation | 7,453 (7.8) | 9,573 (19.5) | 0.345 | 5,794 (13.4) | 6,662 (15.4) | 0.057 |
SMD = standardized mean difference.
Continuous variables are presented as means with sd, and categorical variables are presented as numbers with percentages.
Effect of Contrast Media on AKI and Mortality
After propensity score matching, there was a significantly lower risk of AKI (stages 1–3, stage 3) and CIN associated with IV contrast media administration (Table 2). The incidence of RRT during hospitalization and RRT dependence at discharge were significantly higher, and in-hospital mortality was significantly lower in the enhanced CT group (Table 3).
TABLE 2.
Risk of Acute Kidney Injury in the Propensity Score-Matched Groups
| Primary Outcomes | Nonenhanced CT, n (%) | Enhanced CT, n (%) | OR | 95% CI | p |
|---|---|---|---|---|---|
| AKI stages 1–3 | 2199 (5.1) | 1989 (4.6) | 0.899 | 0.845–0.958 | 0.001 |
| AKI stage 3 | 427 (1.0) | 493 (1.1) | 1.156 | 1.013–1.320 | 0.031 |
| Contrast-induced nephropathy | 3889 (9.0) | 3669 (8.5) | 0.938 | 0.895–0.984 | 0.008 |
AKI = acute kidney injury, OR = odds ratio.
TABLE 3.
Risk of Secondary Patient-Centered Outcomes in the Propensity Score-Matched Groups
| Secondary outcomes | Nonenhanced CT, n (%) | Enhanced CT, n (%) | OR | 95% CI | p |
|---|---|---|---|---|---|
| RRT during hospitalization | 408 (0.9) | 543 (1.5) | 1.335 | 1.171–1.523 | < 0.001 |
| RRT dependence | 193 (0.4) | 250 (0.6) | 1.297 | 1.070–1.574 | 0.008 |
| In-hospital mortality | 2812 (6.5) | 2335 (5.4) | 0.821 | 0.775–0.869 | < 0.001 |
RRT = renal replacement therapy, OR = odds ratio.
Effect of Contrast Media by Subgroups
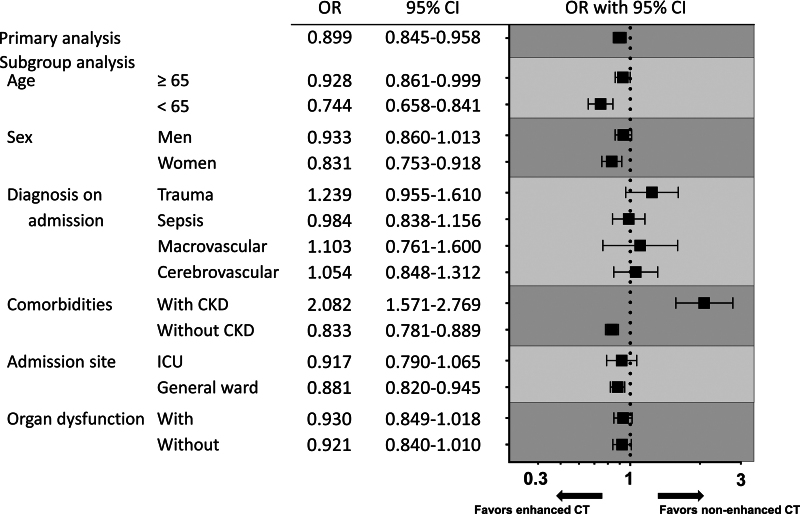
All outcomes in the subgroups using propensity score matching are shown in Supplemental Tables 3–8 (http://links.lww.com/CCX/B386). The risk of AKI stages 1–3 associated with enhanced CT by the clinically relevant subgroups is summarized in Figure 2. There were significant associations between the incidence of AKI stages 1–3 and enhanced CT in the subsets of patients with CKD (OR, 2.082; 95% CI, 1.571–2.769), and in-hospital mortality showed no significant difference between the groups. Among patients without CKD, the risk of AKI stages 1–3 was significantly lower (OR, 0.833; 95% CI, 0.781–0.889), and in-hospital mortality was also significantly lower (OR, 0.796; 95% CI, 0.751–0.843) in the enhanced CT group (Supplemental Table 6, http://links.lww.com/CCX/B386). According to the diagnostic disease, the risk of AKI stages 1–3 in each disease showed no significant difference between the groups, whereas the in-hospital mortality in trauma patients was significantly higher in the enhanced CT group (OR, 1.530; 95% CI, 1.162–2.021). However, among the patients with sepsis, there was no significant difference in the risk of AKI stages 1–3, whereas the in-hospital mortality was significantly lower in the enhanced CT group (OR, 0.796; 95% CI, 0.675–0.938) (Supplemental Table 5, http://links.lww.com/CCX/B386). Among patients with organ dysfunction, the risk of AKI stages 1–3 and in-hospital mortality did not show statistical significance, but a trend toward lower incidence in the enhanced CT group was revealed (Supplemental Table 8, http://links.lww.com/CCX/B386). A similar trend was observed among the patients without organ dysfunction, with a significant difference noted in in-hospital mortality (OR, 0.834; 95% CI, 0.765–0.910).
Figure 2.
Association between IV contrast media administration and the risk of acute kidney injury (AKI) in several subsets based on patient characteristics. The risk of AKI between: 1) overall, 2) elderly patients (65 yr and older) and younger patients (younger than 65 yr), 3) men and women, 4) primary diagnoses such as trauma, sepsis, macrovascular disease, and cerebrovascular disease, 5) patients with chronic kidney disease (CKD) and without CKD, 6) admission to ICU and general ward, and 7) patients with organ dysfunction and without organ dysfunction was evaluated by propensity score-matched analysis. OR = odds ratio.
Results of Sensitivity Analysis
All outcomes in the multiple logistic regression analysis are shown in Supplemental Table 9 (http://links.lww.com/CCX/B386). Sensitivity analyses confirmed that there were no significant changes in the adjusted ORs for all outcomes. Additionally, the results for the interaction term of CKD and enhanced CT were consistent with those obtained from the subgroup analysis based on CKD.
DISCUSSION
We used a nationwide, large-scale inpatient database in Japan to evaluate the risk of AKI (stages 1–3, stage 3, CIN) and clinical in-hospital outcomes between patients requiring emergency admissions who received contrast-enhanced CT or noncontrast-enhanced CT. To our knowledge, this study is the largest observational study regarding this clinically relevant research topic. The main finding of this study was that the use of contrast media did not show a significant difference in the incidence of AKI (stages 1–3, stage 3, CIN) among patients requiring emergency admission. Additionally, the in-hospital mortality in the enhanced CT group was significantly lower than in the nonenhanced CT group. These findings suggested that withholding enhanced CT may not be necessary when an accurate diagnosis is required to succeed in treating patients requiring emergency admission.
This study also elucidated the risk of mortality and revealed a low proportion of patients admitted to the ICU, which reflects that the patients were not critically ill. Consequently, the study included patients with an overall lower mortality. However, AKI is reportedly associated with risks for mortality and morbidity (33–35), but in patients who develop CIN, there is a debate about whether this form of AKI is associated with these clinically adverse outcomes (36). Recent studies found that an amount exceeding 100 mL of contrast medium used per patient was associated with in-hospital death (37) and that mortality following AKI was often due to the time required for recovery of renal function (38). The maximum serum creatinine level within 24 hours of AKI diagnosis was also an independent predictor of short-term prognosis (39). In the present study, which focused on patients diagnosed early after the administration of contrast media, the risk of death was observed to decrease, regardless of an increased risk of RRT. As this trend was also observed in subgroup analysis among more severe patients with organ dysfunction, using contrast media for accurate diagnosis at the time of admission is considered beneficial for improving patient outcomes.
Emergency patients are characterized by a variety of diseases and poor general health and often have multiple organ damage, including renal failure. It is hard for physicians to instantly ascertain a patient’s condition and clinical risk of CIN. We, therefore, performed a subgroup analysis of various diagnoses at admission. In trauma patients, the amount of blood transfusion (40), injury severity score, and transcatheter arterial embolization (41) are also known to be associated with the incidence of AKI. In the present study, in which we were unable to adjust for these factors, we found that the short-term prognosis in trauma patients deteriorated in the enhanced CT group compared with the nonenhanced CT group. In sepsis, AKI has generally been associated with high mortality (42, 43); however, assessment of sources of infection with enhanced CT is critical in the initial management of sepsis (44). Miyamoto et al (25) reported there to be no association between IV contrast media use and adverse in-hospital outcomes in patients with septic AKI requiring dialysis. Our findings also corroborated their findings.
Contrast media can cause potential toxicity to the kidney through direct toxicity on the renal tubules and indirect toxicity induced by renal ischemia due to vasospasm (8, 9). Patients with CKD are particularly susceptible to this adverse effect (45, 46). Observational studies showed that enhanced CT was associated with AKI in emergency patients with renal impairment (12, 47). In our results, the risks of RRT induction and RRT dependence were higher in the enhanced CT group. Among the patients who underwent RRT in the enhanced CT group, 27% had CKD, and of those who became RRT dependent, 40% had CKD. These proportions were approximately two to three times higher than those in the nonenhanced CT group. In the subgroup analysis, patients with CKD were at higher risk for AKI, RRT induction, and RRT dependence. Therefore, the use of contrast media in patients with CKD may pose more harm than benefit.
Limitations
Our study has several limitations. First, this was a retrospective observational study. Therefore, there may be potential unmeasured confounders between those patients with and without contrast media use, although we adjusted for differences in baseline characteristics using propensity score matching. For example, information on the site of the CT scan, periprocedural IV hydration, and vital signs was lacking in the dataset used in this study. However, we were not able to completely remove residual confounding due to unmeasurable confounding factors. Second, the accuracy of diagnoses recorded in administrative claims databases is typically lower than that recorded in prospective studies. Physicians often register diagnosis codes based on clinical judgment, and there are no established accurate criteria. Third, we considered only the risk of AKI within 48–72 hours and mortality during hospitalization. We were unable to assess long-term adverse events following hospital discharge because the dataset used in this study comprised only hospitalized patients. Finally, we were unable to collect information on the criteria for using contrast media as its use was determined by the treating medical team based on factors such as the patient’s condition and renal function. Therefore, we could not adjust for this factor.
CONCLUSIONS
Our study using a nationwide inpatient database suggested that the use of contrast-enhanced CT for diagnostic purposes may not be associated with a significantly increased risk of AKI and might be associated with a mortality benefit in patients requiring emergency admission. Even though the patient’s condition and renal function must be considered, contrast-enhanced CT may be considered necessary for accurate diagnosis and treatment in the emergency department.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Ethics approval and consent for each institution to participate in the study were approved by the institutional research ethics committee in accordance with local ethical regulations.
Informed consent was not required due to the observational and anonymous nature of data collection.
Drs. Hisamune, Yamakawa, and Umemura designed the study. Drs. Hisamune, Umemura, and Ushio were involved in acquiring data. Drs. Hisamune, Yamakawa, Umemura, and Mochizuki were responsible for the statistical analysis. Drs. Hisamune and Yamakawa interpreted the data and wrote the first draft of the report. Drs. Umemura, Inokuchi, Doi, and Takasu revised the report critically for important intellectual content. All authors approved the final version of the article. The corresponding author confirms to have had full access to the data in the study and final responsibility for the decision to submit for publication.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Ryo Hisamune, Email: ryo.hisamune@ompu.ac.jp.
Yutaka Umemura, Email: plum00022@yahoo.co.jp.
Noritaka Ushio, Email: noritaka.ushio@ompu.ac.jp.
Katsunori Mochizuki, Email: katsunori.mochizuki@ompu.ac.jp.
Ryota Inokuchi, Email: inokuchir-icu@h.u-tokyo.ac.jp.
Kent Doi, Email: kentdoi@m.u-tokyo.ac.jp.
Akira Takasu, Email: akira.takasu@ompu.ac.jp.
REFERENCES
- 1.Adler C, Hangge PT, Albadawi H, et al. : Multi-detector computed tomography imaging techniques in arterial injuries. J Clin Med 2018; 7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. : Patterns of contrast enhancement in the brain and meninges. Radiographics 2007; 27:525–551 [DOI] [PubMed] [Google Scholar]
- 3.Serafino MD, Viscardi D, Lacobellis F, et al. : Computed tomography imaging of septic shock. Beyond the cause: The “CT hypoperfusion complex.” A pictorial essay. Insights Imaging 2021; 12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morello F, Santoro M, Fargion AT, et al. : Diagnosis and management of acute aortic syndromes in the emergency department. Intern Emerg Med 2021; 16:171–181 [DOI] [PubMed] [Google Scholar]
- 5.Hallinan J, Anil G: Multi-detector computed tomography in the diagnosis and management of acute aortic syndromes. World J Radiol 2014; 6:355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell AM, Kline JA, Jones AE, et al. : Major adverse events one year after acute kidney injury after contrast-enhanced computed tomography. Ann Emerg Med 2015; 66:267–274.e4 [DOI] [PubMed] [Google Scholar]
- 7.Cosa SG, Peixoto AJ, Garg AX, et al. : The prognostic importance of a small acute decrement in kidney function in hospitalized patients: A systematic review and meta-analysis. Am J Kidney Dis 2007; 50:712–720 [DOI] [PubMed] [Google Scholar]
- 8.Mehran R, Dangas GD, Weisbord SD: Contrast-associated acute kidney injury. N Engl J Med 2019; 380:2146–2155 [DOI] [PubMed] [Google Scholar]
- 9.Azzalini L, Spagnoli V, Ly HQ: Contrast-induced nephropathy: From pathophysiology to preventive strategies. Can J Cardiol 2016; 32:247–255 [DOI] [PubMed] [Google Scholar]
- 10.Maioli M, Toso A, Gallopin M, et al. : Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown) 2010; 11:444–449 [DOI] [PubMed] [Google Scholar]
- 11.Davenport A: Practical guidance for dialyzing a hemodialysis patient following acute brain injury. Hemodial Int 2008; 12:307–312 [DOI] [PubMed] [Google Scholar]
- 12.Su TH, Hsieh CH, Cham YL, et al. : Intravenous CT contrast media and acute kidney injury: A multicenter emergency department-based study. Radiology 2021; 301:571–581 [DOI] [PubMed] [Google Scholar]
- 13.Parfrey PS, Griffiths SM, Barrett BJ, et al. : Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med 1989; 320:143–149 [DOI] [PubMed] [Google Scholar]
- 14.Moore A, Dickeson E, Dillman JR, et al. : Incidence of nonconfounded post-computed tomography acute kidney injury in hospitalized patients with stable renal function receiving intravenous iodinated contrast material. Curr Probl Diagn Radiol 2014; 43:237–241 [DOI] [PubMed] [Google Scholar]
- 15.McCullough PA, Wolyn R, Rocher LL, et al. : Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med 1997; 103:368–375 [DOI] [PubMed] [Google Scholar]
- 16.Rihal CS, Textor SC, Grill DE, et al. : Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002; 105:2259–2264 [DOI] [PubMed] [Google Scholar]
- 17.Kooiman J, Pasha SM, Zondag W, et al. : Meta-analysis: Serum creatinine changes following contrast enhanced CT imaging. Eur J Radiol 2012; 81:2554–2561 [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm-Leen E, Montez-Rath ME, Chertow G: Estimating the risk of radiocontrast-associated nephropathy. J Am Soc Nephrol 2017; 28:653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald JS, McDonald RJ, Williamson EE, et al. : Post-contrast acute kidney injury in intensive care unit patients: A propensity score-adjusted study. Intensive Care Med 2017; 43:774–784 [DOI] [PubMed] [Google Scholar]
- 20.Aycock RD, Westafer LM, Boxen JL, et al. : Acute kidney injury after computed tomography: A meta-analysis. Ann Emerg Med 2018; 71:44–53.e4 [DOI] [PubMed] [Google Scholar]
- 21.Ehrmann S, Quartin A, Hobbs BP, et al. : Contrast-associated acute kidney injury in the critically ill: Systematic review and Bayesian meta-analysis. Intensive Care Med 2017; 43:785–794 [DOI] [PubMed] [Google Scholar]
- 22.Dekkers IA, van der Molen AJ: Propensity score matching as a substitute for randomized controlled trials on acute kidney injury after contrast media administration: A systematic review. AJR Am J Roentgenol 2018; 211:822–826 [DOI] [PubMed] [Google Scholar]
- 23.Williams LS, Walker GR, Loewenherz JW, et al. : Association of contrast and acute kidney injury in the critically ill: A propensity-matched study. Chest 2020; 157:866–876 [DOI] [PubMed] [Google Scholar]
- 24.Murakami R, Hayashi H, Sugizaki K, et al. : Contrast-induced nephropathy in patients with renal insufficiency undergoing contrast-enhanced MDCT. Eur Radiol 2012; 22:2147–2152 [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto Y, Iwagami M, Aso S, et al. : Association between intravenous contrast media exposure and non-recovery from dialysis-requiring septic acute kidney injury: A nationwide observational study. Intensive Care Med 2021; 45:1570–1579 [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Ono Y, Qiao Q, et al. : Trends in heart failure prevalence in Japan 2014–2019: A report from healthcare administration databases. ESC Heart Fail 2023; 10:1996–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe Y, Kawanishi H, Suzuki K, et al. ; “Maintenance Hemodialysis: Hemodialysis Prescriptions” Guideline Working Group, Japanese Society for Dialysis Therapy: Japanese society for dialysis therapy clinical guideline for “maintenance hemodialysis: Hemodialysis prescriptions.” Ther Apher Dial 2015; 19:67–92 [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum PR, Rubin DB: Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985; 39:33–38 [Google Scholar]
- 31.Brookhart MA, Schneeweiss S, Rothman KJ, et al. : Variable selection for propensity score models. Am J Epidemiol 2006; 163:1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coca SG, Yusuf B, Shlipak M, et al. : Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 2009; 53:961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HE, Muntner P, Chertow GM, et al. : Acute kidney injury and mortality in hospitalized patients. Am J Nephrol 2012; 35:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spinbartl K, Kellum JA: AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int 20122012; 81:819–825 [DOI] [PubMed] [Google Scholar]
- 36.Rudnick MR, Leonberg-Yoo AK, Litt HI, et al. : The controversy of contrast-induced nephropathy with intravenous contrast: What is the risk? Am J Kidney Dis 2019; 75:105–113 [DOI] [PubMed] [Google Scholar]
- 37.Chua HR, Low S, Murali TM, et al. : Cumulative iodinated contrast exposure for computed tomography during acute kidney injury and major adverse kidney events. Eur Radiol 2020; 31:3258–3266 [DOI] [PubMed] [Google Scholar]
- 38.Forni LG, Darmon M, Ostermann M, et al. : Renal recovery after acute kidney injury. Intensive Care Med 2017; 43:855–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Lu Y, Li S, et al. : Predicting renal function recovery and short-term reversibility among acute kidney injury patients in the ICU: Comparison of machine learning methods and conventional regression. Ren Fail 2022; 44:1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh TM, Tsai TH, Liu YW, et al. : Risk factors for contrast-induced nephropathy and their association with mortality in patients with blunt splenic injuries. Int J Surg 2016; 35:69–75 [DOI] [PubMed] [Google Scholar]
- 41.Schnider A, Gallaher J, Purcell LN, et al. : Risk of acute kidney injury requiring hemodialysis after contrast-enhanced imaging after traumatic injury: A National Trauma Databank analysis. Surgery 2022; 171:1085–1091 [DOI] [PubMed] [Google Scholar]
- 42.Bellomo R, Kellum JA, Ronco C, et al. : Acute kidney injury in sepsis. Intensive Care Med 2017; 43:816–828 [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto Y, Iwagami M, Aso S, et al. : Temporal change in characteristics and outcomes of acute kidney injury on renal replacement therapy in intensive care units: Analysis of a nationwide administrative database in Japan, 2007–2016. Crit Care 2019; 23:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cecconi M, Evans L, Levy M, et al. : Sepsis and septic shock. Lancet 2018; 392:75–87 [DOI] [PubMed] [Google Scholar]
- 45.Hoste EA, Kellum JA, Selby NA, et al. : Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018; 14:607–625 [DOI] [PubMed] [Google Scholar]
- 46.De Simone B, Ansaloni L, Sartelli M, et al. : Is the risk of contrast-induced nephropathy a real contraindication to perform intravenous contrast enhanced computed tomography for non-traumatic acute abdomen in emergency surgery department? Acta Biomed 2018; 89(9-S):158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kene M, Arasu VA, Mahapatra AK, et al. : Acute kidney injury after CT in emergency patients with chronic kidney disease: A propensity score-matched analysis. West J Emerg Med 2021; 22:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.