Summary
Providing treatment to patients with cancer, even during the coronavirus disease (COVID-19) pandemic, is essential. In collaboration with infectious disease specialists, we established guidelines for the management of patients with cancer receiving ambulatory treatment during the pandemic on April 8, 2020. This study examined the practice and management of ambulatory chemotherapy under emergency conditions. Following the guidelines, our Breast and Medical oncology department developed a chemotherapy strategy for the phases. Additionally, to distinguish fever during chemotherapy, we developed a flow chart for fever. As part of a fact-finding survey, the status of outpatient chemotherapy was investigated: (1) whether there was any change in the number of chemotherapies before and after the declaration of a state of emergency by the Tokyo Metropolitan Government and (2) the frequency and severity of febrile neutropenia (FN) cases. Compared to before the first declaration of the state of emergency, the number of chemotherapies decreased except after the declaration, but no decrease was observed during the rest of the period; no difference was observed in the frequency or severity of FN outbreaks or in the use of pegfilgrastim for primary prevention before and after the epidemic. With appropriate treatment guidelines, routine chemotherapy can be performed in an outpatient setting during an outbreak.
Keywords: COVID-19, coronavirus, cancer, chemotherapy, breast cancer, outpatient
Introduction
It is important to provide medical care without stopping cancer screening and treatment during an unknown infectious disease pandemic, such as COVID-19. However, there were no treatment guidelines at the beginning of the pandemic, which caused confusion among healthcare providers. Many information and states (1-3) from not only Japan but also other countries were submitted. Regardless of the source of information, the basic policy was the same: patients who should avoid treatment interruptions should continue chemotherapy during the COVID-19 pandemic, while patients with stable disease who could continue treatment without face-to-face consultations to reduce the frequency of visits to the hospital.
We established guidelines for the management of patients with cancer receiving ambulatory treatment during the pandemic in April 2020. This study examined the practice and management of ambulatory chemotherapy under emergency conditions.
Activities in our department since the COVID-19 pandemic
Development of treatment guidelines
At the beginning of the COVID-19 pandemic, our outpatient treatment center was divided into three phases according to the number of patients requiring oxygen administration and the number of patients on ventilators. Treatment strategies were established for each phase (Supplemental Table S1, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=88). In Phase 1, the usual medical care was continued; in Phase 2, the usual medical care was reduced to 60%-80%; in Phase 3, the usual medical care was discontinued, and the Ambulatory Treatment Center (ATC) was closed. In addition, the department established guidelines for chemotherapy (Supplemental Table S2, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=88) according to these phases. During preoperative and postoperative chemotherapy, patients with metastatic breast cancer continued to receive treatment according to the schedule. In contrast, patients with relatively stable diseases visited the hospital less frequently to prevent infection.
Creation of a flow chart for responding to febrile illness
Depending on the causative microorganism, FN is a highly lethal adverse event in cancer chemotherapy (4). Therefore, identifying fever during treatment is important. Our department prepared a "Fever Handling Flow" (Figure 1) for outpatient chemotherapy classified according to the FN risk of the regimen. Patients receiving high-risk FN regimens (Supplemental Table S3, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=88) were prescribed an antibiotic (LVFX: levofloxacin) at initial administration to manage their FN at home.
Figure 1.
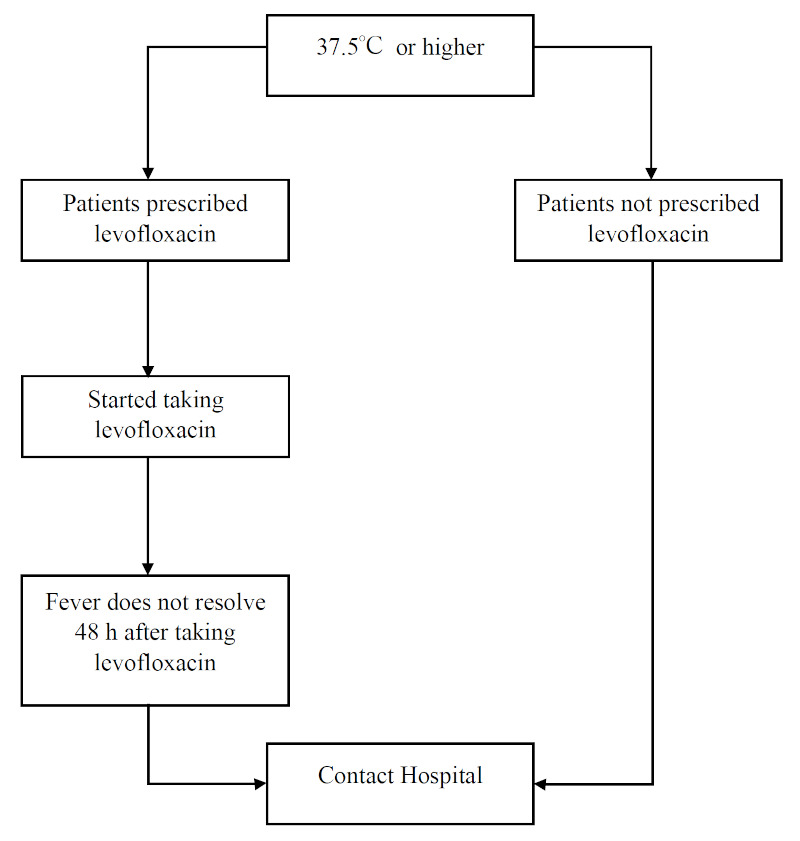
Flow chart for handling fevers established by our department.
Creation of COVID-19 vaccination procedures in patients with cancer
Patients with cancer are at a high risk of severe disease when they contract COVID-19 (5), and patients with cancer under treatment were identified as priority candidates for COVID-19 vaccination (6). Various guidelines (2,7-9) reported that COVID-19 vaccination should be considered prospectively, so to differentiate between vaccine- and treatment-induced fever, our department developed a procedure for COVID-19 vaccination in patients with cancer (Supplemental Table S4, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=88).
Research on outpatient chemotherapy during the COVID-19 pandemic
Evaluation of outpatient chemotherapy performance
We evaluated the number of chemotherapies administered at the ATC of the Department of Breast and Medical Oncology at the National Center for Global Health and Medicine, Tokyo, Japan. We evaluated for 30 days before and after the first (April 7, 2020), second (January 8, 2021), third (April 25, 2021), and fourth (July 12, 2021) declarations of emergency in Tokyo. The first period (Period 1; P1) is from March 8, 2020, to May 6, 2020. The second period (Period 2; P2) is from December 9, 2020, to February 6, 2021. The third period (Period 3; P3) is from March 26, 2021, to May 24, 2021, and the fourth period (Period 4; P4) is from June 12, 2021, to August 10, 2021. We evaluated the type of cancer, stage of disease, number of chemotherapies, regimens, and changes in treatment strategy (changes in regimen and discontinuation) related to the pandemic, presence of fever, and COVID-19. The exclusion criteria were as follows: i) patients who did not receive chemotherapy within 60 days before the emergency declaration, ii) Patients who participated in a clinical trial. All information was retrieved from electronic medical records.
Research regarding whether the frequency of FN occurrence changed before and after the COVID-19 pandemic in patients on high-risk FN regimens
The differentiation of fever during treatment is important. The target period was February 1, 2019, to January 31, 2020 (Period 5; P5) before the pandemic and February 1, 2020, to January 31, 2021 (Period 6; P6) after the pandemic. In patients with breast cancer on preoperative and postoperative chemotherapy who received a high-risk FN regimen (Supplemental Table S3, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=88), we retrospectively investigated whether there were changes in the number of FN cases, response to FN (use of prophylactic antimicrobial or pegfilgrastim [Peg-G-CSF] primary prevention for FN), frequency and severity of FN, and number of patients with COVID-19.
These studies were performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of the National Center for Global Health and Medicine, Tokyo (Date: March 20, 2020; No. NCGM-G-003481-01).
Evaluation of outpatient chemotherapy performance
The number of eligible patients was 131, 133, 150, and 157 in P1 to P4, respectively (Table 1). The number of chemotherapy sessions was 303, 319, 376, and 409 in P1 to P4, respectively, decreasing after the declaration (112 times) compared with before the declaration of emergency status (191 times) in P1. The number of patients for whom treatment was (or could have been) changed was 27, two, three, and eight in P1 to P4, respectively. Fever was observed in eight, nine, six, and 20 patients (including 10 with adverse reactions to the COVID-19 vaccine) from P1 to P4. None, one, one, and two patients with COVID-19 infection in P1 to P4 and one patient in P3 had moderate or severe COVID-19 symptoms and received hormone therapy.
Table 1. Chemotherapy-related outpatient treatment implementation status.
| (A) | ||||
|---|---|---|---|---|
| Characteristics | P1 (n = 131) | P2 (n = 133) | P3 (n = 150) | P4 (n = 157) |
| Age, median [interquartile range], years | 58 [28–88] | 57 [29–89] | 55 [29–89] | 56 [29–89] |
| Type of Cancer | ||||
| Breast cancer | 122 (93%) | 129 (97%) | 145 (97%) | 152 (97%) |
| Gynecologic cancer | 7 (5.3%) | 4 (3.0%) | 5 (3.0%) | 5 (3.0%) |
| Others | 2 (1.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Stages of disease | ||||
| NAC or adjuvant | 43 (33%) | 35 (26%) | 48 (32%) | 56 (36%) |
| Palliative | 88 (67%) | 98 (74%) | 102 (68%) | 101 (64%) |
| Number of chemotherapeutic regimens | 137 | 140 | 164 | 167 |
| NAC or Adjuvant | 48 (35%) | 39 (28%) | 57 (35%) | 59 (35%) |
| Palliative | 89 (65%) | 101 (72%) | 107 (65%) | 108 (65%) |
| Number of chemotherapeutic times | 303 | 319 | 376 | 409 |
| Percentage of chemotherapeutic times※ | 62% | 58% | 61% | 61% |
| Before the declaration | 191 times (39%) | 157 times (28%) | 194 times (32%) | 192 times (28%) |
| NAC or Adjuvant | 72 (15%) | 49 (9%) | 69 (11%) | 83 (12%) |
| Palliative | 119 (24%) | 108 (20%) | 125 (20%) | 109 (16%) |
| After the declaration | 112 times (23%) | 162 times (29%) | 182 times (30%) | 217 times (32%) |
| NAC or Adjuvant | 50 (10%) | 60 (11%) | 64 (10%) | 94 (14%) |
| Palliative | 62 (13%) | 102 (18%) | 118 (19%) | 123 (18%) |
|
| ||||
| (B) | ||||
|
| ||||
| Characteristics | P1 (n = 131) | P2 ( n = 133) | ||
| Number of patients who changed (or may have changed) treatment※ | 27 (21%) | 2 (1.5%) | ||
| NAC or Adjuvant | Palliative | NAC or Adjuvant | Palliative | |
| 9 (6.9%) | 18 (14%) | 0 (0%) | 2 (1.5%) | |
| Change of regimens | 0 (0%) | 3 (2.3%) | 0 (0%) | 0 (0%) |
| Extention of interval | 2 (1.5%) | 4 (3.1%) | 0 (0%) | 2 (1.5%) |
| Skip of therapy | 1 (0.76%) | 4 (3.1%) | 0 (0%) | 0 (0%) |
| Postponement of treatment initiation | 3 (2.3%) | 6 (4.6%) | 0 (0%) | 0 (0%) |
| Prescription by telephone | 3 (2.3%) | 2 (1.5%) | 0 (0%) | 0 (0%) |
| Others | 3 (2.3%) | 1 (0.76%) | 0 (0%) | 0 (0%) |
| Number of patients with fever | 8 (6.1%) | 9 (6.8%) | ||
| Number of patients with COVID-19 | 0 (0%) | 1 (0.75%) | ||
|
| ||||
| (C) | ||||
|
| ||||
| Characteristics | P3 (n = 150) | P4 ( n = 157) | ||
| Number of patients who changed (or may have changed) treatment※ | 3 (2.0%) | 8 (5.1%) | ||
| NAC or Adjuvant | Palliative | NAC or Adjuvant | Palliative | |
| 0 (0%) | 3 (2.0%) | 1 (0.64%) | 7 (4.5%) | |
| Change of regimens | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Extension of interval | 0 (0%) | 2 (1.3%) | 0 (0%) | 1 (0.64%) |
| Skip of therapy | 0 (0%) | 1 (0.67%) | 0 (0%) | 6 (3.8%) |
| Postponement of treatment initiation | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Prescription by telephone | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Others | 0 (0%) | 0 (0%) | 1 (0.64%) | 0 (0%) |
| Number of patients with fever | 6 (4.0%) | 20 (13%) | ||
| Number of patients with COVID-19 | 1 (0.67%) | 1 (1.3%) | ||
In the case of oral medications, we counted the number of prescriptions as the number of chemotherapies. The percentage of chemotherapeutic times was calculated based on the total number of patients and the number of chemotherapeutic times. ※The total does not add up because of overlapping element. Abbreviation: NAC, Neoadjuvant chemotherapy. COVID-19, Coronavirus disease 2019.
After the COVID-19 pandemic, the frequency of FN changed in patients on high-risk FN regimens
A total of 93 patients (64 in period 5 and 29 in period 6) were included in the study. The total number of regimens administered was 317 (P5: 226, P6: 91), the number of FN cases (including suspected FN) was 32 (10%, P5: 26 [12%], P6: six [6.6%]), and the number of FN cases requiring hospitalization was four (1.3%, P5: four [1.3%], P6: none [0%]). The number of chemotherapy regimens using Peg-G-CSF for primary prevention was eight (2.5%, P5: eight [2.5%], P6: none [0%]), and there was no significant difference between the periods. The number of FN (or suspected FN) cases was one patient whose regimen was pertuzumab plus trastuzumab plus docetaxel in P5 and one patient who received docetaxel plus cyclophosphamide in P6. All other patients' regimens were doxorubicin plus cyclophosphamide. The number of patients with FN treated with doxorubicin and cyclophosphamide was comparable to that reported in a previous study (9%) (10).
Discussion
According to the declarations, the number of chemotherapy sessions was affected in P1 but not in P2 to P4. This is consistent with the results of a previous study (11) and may be due to the pandemic, which caused a shortage of personal protective equipment (PPE) which are essential for healthcare providers when administering chemotherapy. In P2, with the first reason for this being P1, the number may have decreased owing to the lack of treatment guidelines, both institutional and professional. The fact that the number of chemotherapy treatments recovered after the treatment guidelines were established suggests that familiarity with the basic policy and the establishment of treatment guidelines at individual facilities may help healthcare providers provide medical care to patients without confusion during a pandemic. Second, PPE, such as masks and gowns, were in short supply, and the environment for medical care needed to be more conducive in P1. Third, after the first declaration, people were more cautious about the unknown virus, and patients might have restricted themselves from going out for hospital visits.
Of the patients who had COVID-19 during the study, only one had moderate or severe symptoms and was undergoing hormonal therapy for breast cancer. This patient did not meet any of these criteria that is reported in previous studies (12). It is also important to proceed with cancer treatment without unnecessary fear because COVID-19 does not necessarily cause severe disease even if the patient is immunosuppressed while receiving cytotoxic anticancer drugs.
This study found no differences in the frequency or severity of FN occurrence, preventive use of antimicrobials, or Peg-G-CSF in high-risk FN regimens by period. The frequency of FN with doxorubicin plus cyclophosphamide was similar to that reported previously. This suggests that FN treatment can be continued during the COVID-19 pandemic without special measures. Peg-G-CSF acts on neutrophil progenitor cells to promote neutrophil differentiation. Because lymphocyte counts have been reported to decrease during COVID-19 infection (13), Peg-G-CSF is unlikely to be useful for active use other than in identifying fever, even in light of its mechanism of action.
Our study had two limitations. First, because it was conducted at a single institution or department, it was limited to the target population and needed more generality. Second, the proportion of patients with COVID-19 among eligible patients during the study period was small. The number of positive COVID-19 cases in the country increased after the sixth wave (January 1, 2022). However, this period was outside the study's coverage period.
The COVID-19 pandemic has allowed healthcare providers to consider the actions that should be taken in the event of a pandemic. With limited information available, hospital guidelines were developed, and their implementation did not result in any serious problems. When a similar situation arises in the future, developing guidelines and triages within the information available at the time can help healthcare providers and patients make appropriate decisions. As the pandemic progresses, it is important to continue providing cancer treatment while considering the risks and benefits for individual patients.
Acknowledgements
We thank Masayo Kawamura, Department of Breast and Medical Oncology, National Center for Global Health and Medicine, for her assistance with these procedures.
Funding:
None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. American Society of Clinical Oncology. Cancer Treatment & Supportive Care. https://old-prod.asco.org/covid-resources/patient-care-info/cancer-treatment-supportive-care (accessed June 29, 2023).
- 2. Joint Collaboration Committee of three cancer-related societies (Japanese Cancer Association, Japanese Association for Cancer Therapy, and Japanese Society of Clinical Oncology) Working Group for Countermeasures against coronavirus (COVID-19) Japan Society of Clinical Oncology, Japanese Cancer Association, and the Japanese Society of Medical Oncology. Cancer care and new coronavirus infection: Q&A for healthcare professionals - Revised 3rd ed. Japanese Society of Medical Oncology. https://www.jsmo.or.jp/news/coronavirus-information/qa_vaccinel_3gakkai.html (accessed August 26, 2023). (in Japanese) .
- 3. Cooksley T, Font C, Scotte F, Escalante C, Johnson L, Anderson R, Rapoport B. Emerging challenges in the evaluation of fever in cancer patients at risk of febrile neutropenia in the era of COVID-19: A MASCC position paper. Support Care Cancer. 2021; 29:1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Japanese Society of Clinical Oncology: Guidelines for the treatment of febrile neutropenia (FN), (Revised 2nd Edition, Japanese Society of Clinical Oncology). Nankodo, Tokyo, 2017; pp.6-9.(in Japanese) [Google Scholar]
- 5. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020; 21:335-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ministry of Health, Labour and Welfare. Risk factors for severe disease. Guideline for the Treatment of New Coronavirus Infections (COVID-19), Version 8. 0. https://www.mhlw.go.jp/content/000936655.pdf (accessed June 29, 2023). (in Japanese) .
- 7. Nat ional Comp r eh en s iv e Can ce r Net w o r k. Recommendations of the National Comprehensive Cancer Network® (NCCN®) COVID-19 Vaccination Advisory Committee. NCCN: Cancer and COVID-19 Vaccination. https://www.eviq.org.au/getmedia/6de8cf1f-54d5-4c5d-9045-f3b5ff384bbc/2021-covid-19-vaccination-guidance-v8-0.pdf.aspx (accessed June 29, 2023).
- 8. American Society Of Clinical Oncology. ASCO Coronavirus Resources. 2020. https://www.asco.org/asco-coronavirus-information. (accessed June 30, 2024).
- 9. European Society for Medical Oncology. ESMO Statements on vaccination against COVID-19 in people with cancer. https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination (accessed June 29, 2023).
- 10. Biganzoli L, Cufer T, Bruning P, Coleman R, Duchateau L, Calvert AH, Gamucci T, Twelves C, Fargeot P, Epelbaum R, Lohrisch C, Piccart MJ. Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. J Clin Oncol. 2002; 20:3114-3121. [DOI] [PubMed] [Google Scholar]
- 11. Clark JJ, Dwyer D, Pinwill N, Clark P, Johnson P, Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: A retrospective analysis. Lancet Oncol. 2021; 22:66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharafeldin N, Bates B, Song Q, Madhira V, Yan Y, Dong S, Lee E, Kuhrt N, Shao YR, Liu F, Bergquist T, Guinney J, Su J, Topaloglu U. Outcomes of COVID-19 in patients with cancer: Report from the National COVID Cohort Collaborative (N3C). J Clin Oncol. 2021; 39:2232-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang W, Berube J, McNamara M, Saksena S, Hartman M, Arshad T, Bornheimer SJ, O'Gorman M. Lymphocyte subset counts in COVID-19 patients: A meta-analysis. Cytometry A. 2020; 97:772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]


