Abstract
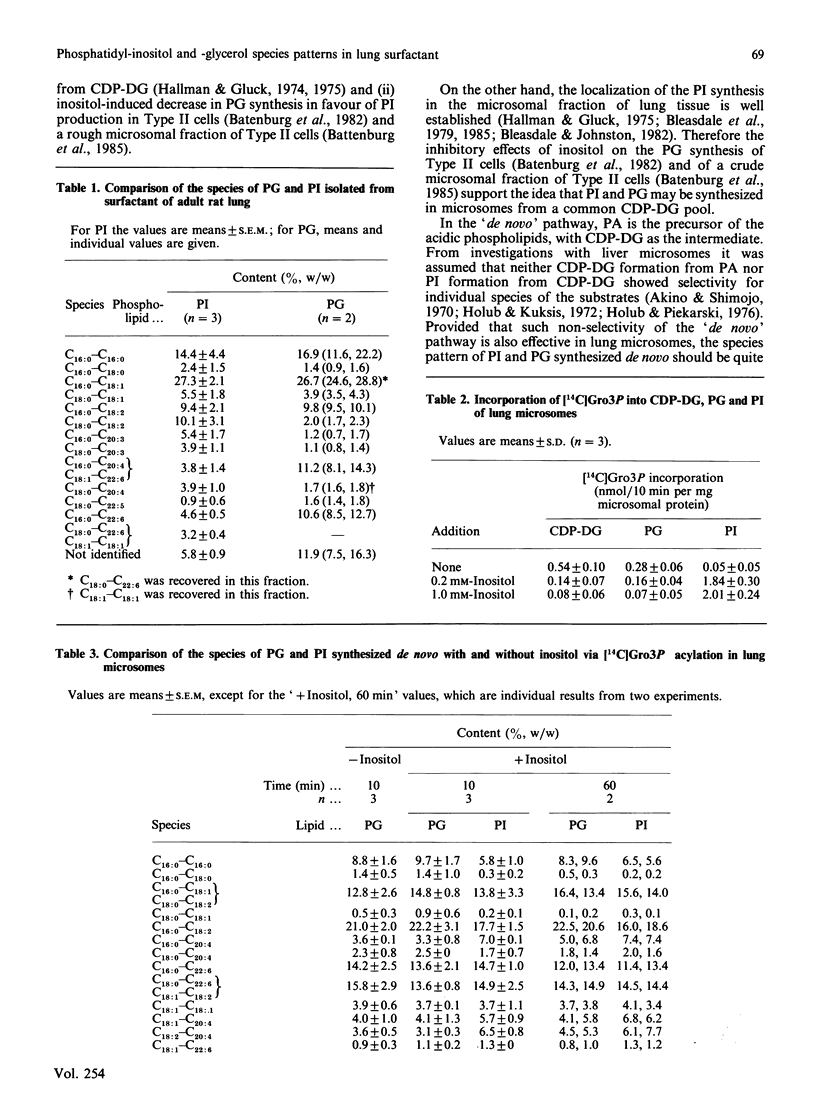
1. Phosphatidylinositol (PI) is a minor component of lung surfactant which may be able to replace the functionally important phosphatidylglycerol (PG) [Beppu, Clements & Goerke (1983) J. Appl. Physiol. 55, 496-502] without disturbing lung function. The dipalmitoyl species is one of the main species for both PI (14.4%) and PG (16.9%). Besides the C16:0--C16:0 species, the C16:0--C18:0, C16:0--C18:1, C16:0--C18:2 and C18:0--C18:1 species showed comparable proportions in the PG and PI fractions. These similarities of the species patterns and the acidic character of both phospholipids could explain why surfactant PG may be replaced by PI. 2. PI and PG were radiolabelled by incubation of microsomal fractions with [14C]glycerol 3-phosphate (Gro3P). For 11 out of 14 molecular species of PI and PG we measured comparable proportions of radioactivity. The radioactivity of these 11 species accounted together for more than 80% of the total. The addition of inositol to the incubation system decreased the incorporation in vitro of Gro3P into PG and CDP-DG (diacylglycerol) of lung microsomes (microsomal fractions), but did not change the distribution of radioactivity among the molecular species of PG. These results supported the idea that both acidic surfactant phospholipids may be synthesized de novo from a common CDP-DG pool in lung microsomes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akino T., Shimojo T. On the metabolic heterogeneity of rat liver phosphatidylinositol. Biochim Biophys Acta. 1970 Jul 14;210(2):343–346. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Batenburg J. J., Klazinga W., van Golde L. M. Regulation and location of phosphatidylglycerol and phosphatidylinositol synthesis in type II cells isolated from fetal rat lung. Biochim Biophys Acta. 1985 Jan 9;833(1):17–24. doi: 10.1016/0005-2760(85)90248-6. [DOI] [PubMed] [Google Scholar]
- Batenburg J. J., Klazinga W., van Golde L. M. Regulation of phosphatidylglycerol and phosphatidylinositol synthesis in alveolar type II cells isolated from adult rat lung. FEBS Lett. 1982 Oct 18;147(2):171–174. doi: 10.1016/0014-5793(82)81035-1. [DOI] [PubMed] [Google Scholar]
- Beppu O. S., Clements J. A., Goerke J. Phosphatidylglycerol-deficient lung surfactant has normal properties. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):496–502. doi: 10.1152/jappl.1983.55.2.496. [DOI] [PubMed] [Google Scholar]
- Bleasdale J. E., Johnston J. M. CMP-dependent incorporation of [14C]Glycerol 3-phosphate into phosphatidylglycerol and phosphatidylglycerol phosphate by rabbit lung microsomes. Biochim Biophys Acta. 1982 Mar 12;710(3):377–390. doi: 10.1016/0005-2760(82)90121-7. [DOI] [PubMed] [Google Scholar]
- Bleasdale J. E., Tyler N. E., Snyder J. M. Subcellular sites of synthesis of phosphatidylglycerol and phosphatidylinositol in type II pneumonocytes. Lung. 1985;163(6):345–359. doi: 10.1007/BF02713835. [DOI] [PubMed] [Google Scholar]
- Bleasdale J. E., Wallis P., MacDonald P. C., Johnston J. M. Changes in CDP-diglyceride:inositol transferase activity during rabbit lung development. Pediatr Res. 1979 Oct;13(10):1182–1183. doi: 10.1203/00006450-197910000-00022. [DOI] [PubMed] [Google Scholar]
- Egberts J., Beintema-Dubbeldam A., de Boers A. Phosphatidylinositol and not phosphatidylglycerol is the important minor phospholipid in rhesus-monkey surfactant. Biochim Biophys Acta. 1987 May 13;919(1):90–92. doi: 10.1016/0005-2760(87)90221-9. [DOI] [PubMed] [Google Scholar]
- Egberts J., Gorree G. C., Reyngoud D. J. Inositol affects the intracellular turnover of pulmonary surfactant phospholipids in the rat. Respir Physiol. 1985 Dec;62(3):281–291. doi: 10.1016/0034-5687(85)90085-4. [DOI] [PubMed] [Google Scholar]
- Gluck L. Evaluating functional fetal maturation. Clin Obstet Gynecol. 1978 Jun;21(2):547–559. doi: 10.1097/00003081-197806000-00022. [DOI] [PubMed] [Google Scholar]
- Goerke J. Lung surfactant. Biochim Biophys Acta. 1974 Dec 16;344(3-4):241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Hallman M., Epstein B. L. Role of myo-inositol in the synthesis of phosphatidylglycerol and phosphatidylinositol in the lung. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1151–1159. doi: 10.1016/0006-291x(80)90407-6. [DOI] [PubMed] [Google Scholar]
- Hallman M., Feldman B. H., Kirkpatrick E., Gluck L. Absence of phosphatidylglycerol (PG) in respiratory distress syndrome in the newborn. Study of the minor surfactant phospholipids in newborns. Pediatr Res. 1977 Jun;11(6):714–720. doi: 10.1203/00006450-197706000-00003. [DOI] [PubMed] [Google Scholar]
- Hallman M., Gluck L. Phosphatidyl glycerol in lung surfactant. 1. Synthesis in rat lung microsomes. Biochem Biophys Res Commun. 1974 Sep 9;60(1):1–7. doi: 10.1016/0006-291x(74)90163-6. [DOI] [PubMed] [Google Scholar]
- Hallman M., Gluck L. Phosphatidylglycerol in lung surfactant. II. Subcellular distribution and mechanism of biosynthesis in vitro. Biochim Biophys Acta. 1975 Nov 21;409(2):172–191. doi: 10.1016/0005-2760(75)90152-6. [DOI] [PubMed] [Google Scholar]
- Hallman M., Gluck L. Phosphatidylglycerol in lung surfactant. III. Possible modifier of surfactant function. J Lipid Res. 1976 May;17(3):257–262. [PubMed] [Google Scholar]
- Hallman M., Slivka S., Wozniak P., Sills J. Perinatal development of myoinositol uptake into lung cells: surfactant phosphatidylglycerol and phosphatidylinositol synthesis in the rabbit. Pediatr Res. 1986 Feb;20(2):179–185. doi: 10.1203/00006450-198602000-00018. [DOI] [PubMed] [Google Scholar]
- Holub B. J., Kuksis A. Further evidence for the interconversion of monophosphoinositides in vivo. Lipids. 1972 Jan;7(1):78–80. doi: 10.1007/BF02531275. [DOI] [PubMed] [Google Scholar]
- Holub B. J., Piekarski J. Biosynthesis of molecular species of CDP-diglyceride from endogenously-labeled phosphatidate in rat liver microsomes. Lipids. 1976 Apr;11(4):251–257. doi: 10.1007/BF02544050. [DOI] [PubMed] [Google Scholar]
- Jobe A., Ikegami M., Sarton-Miller I., Jones S., Yu G. Characterization of phospholipids and localization of some phospholipid synthetic and subcellular marker enzymes in subcellular fractions from rabbit lung. Biochim Biophys Acta. 1981 Oct 23;666(1):47–57. doi: 10.1016/0005-2760(81)90089-8. [DOI] [PubMed] [Google Scholar]
- Kato H., Ishidate K., Nakazawa Y. Developmental changes in molecular species of phosphatidic acid in rat lung and liver during the perinatal stage. Biochim Biophys Acta. 1984 Dec 6;796(3):262–268. doi: 10.1016/0005-2760(84)90126-7. [DOI] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972 Sep;223(3):715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- King R. J., MacBeth M. C. Interaction of the lipid and protein components of pulmonary surfactant. Role of phosphatidylglycerol and calcium. Biochim Biophys Acta. 1981 Oct 2;647(2):159–168. doi: 10.1016/0005-2736(81)90242-x. [DOI] [PubMed] [Google Scholar]
- King R. J. Pulmonary surfactant. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jul;53(1):1–8. doi: 10.1152/jappl.1982.53.1.1. [DOI] [PubMed] [Google Scholar]
- Krüger J., Rabe H., Reichmann G., Rüstow B. Separation and determination of diacylglycerols as their naphthylurethanes by high-performance liquid chromatography. J Chromatogr. 1984 May 11;307(2):387–392. doi: 10.1016/s0378-4347(00)84110-9. [DOI] [PubMed] [Google Scholar]
- Kulovich M. V., Hallman M. B., Gluck L. The lung profile. I. Normal pregnancy. Am J Obstet Gynecol. 1979 Sep 1;135(1):57–63. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lau M. J., Keough K. M. Lipid composition of lung and lung lavage fluid from map turtles (Malaclemys geographica) maintained at different environmental temperatures. Can J Biochem. 1981 Mar;59(3):208–219. doi: 10.1139/o81-029. [DOI] [PubMed] [Google Scholar]
- Liau D. F., Barrett C. R., Bell A. L., Cernansky G., Ryan S. F. Diphosphatidylglycerol in experimental acute alveolar injury in the dog. J Lipid Res. 1984 Jul;25(7):678–683. [PubMed] [Google Scholar]
- Mavis R. D., Vang M. J. Optimal assay and subcellular location of phosphatidylglycerol synthesis in lung. Biochim Biophys Acta. 1981 May 22;664(2):409–415. doi: 10.1016/0005-2760(81)90063-1. [DOI] [PubMed] [Google Scholar]
- Montfoort A., van Golde L. M., van Deenen L. L. Molecular species of lecithins from various animal tissues. Biochim Biophys Acta. 1971 Mar 16;231(2):335–342. doi: 10.1016/0005-2760(71)90147-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Horrocks L. A. Separation of alkenylacyl, alkylacyl, and diacyl analogues and their molecular species by high performance liquid chromatography. J Lipid Res. 1983 Sep;24(9):1268–1275. [PubMed] [Google Scholar]
- Quirk J. G., Bleasdale J. E., MacDonald P. C., Johnston J. M. A role for cytidine monophosphate in the regulation of the glycerophospholipid composition of surfactant in developing lung. Biochem Biophys Res Commun. 1980 Aug 14;95(3):985–992. doi: 10.1016/0006-291x(80)91570-3. [DOI] [PubMed] [Google Scholar]
- Rooney S. A., Page-Roberts B. A., Motoyama E. K. Role of lamellar inclusions in surfactant production: studies on phospholipid composition and biosynthesis in rat and rabbit lung subcellular fractions. J Lipid Res. 1975 Nov;16(6):418–425. [PubMed] [Google Scholar]
- Rüstow B., Kunze D., Rabe H., Reichmann G. The molecular species of phosphatidic acid, diacylglycerol and phosphatidylcholine synthesized from sn-glycerol 3-phosphate in rat lung microsomes. Biochim Biophys Acta. 1985 Jul 31;835(3):465–476. doi: 10.1016/0005-2760(85)90116-x. [DOI] [PubMed] [Google Scholar]
- Sanders R. L., Longmore W. J. Phosphatidyglycerol in rat lung. II. Comparison of occurrence, composition, and metabolism in surfactant and residual lung fractions. Biochemistry. 1975 Feb 25;14(4):835–840. doi: 10.1021/bi00675a030. [DOI] [PubMed] [Google Scholar]
- Schlame M., Rüstow B., Kunze D., Rabe H., Reichmann G. Phosphatidylglycerol of rat lung. Intracellular sites of formation de novo and acyl species pattern in mitochondria, microsomes and surfactant. Biochem J. 1986 Nov 15;240(1):247–252. doi: 10.1042/bj2400247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. Effect of protein, cholesterol, and phosphatidylglycerol on the surface activity of the lipid-protein complex reconstituted from pig pulmonary surfactant. J Lipid Res. 1982 Jan;23(1):62–69. [PubMed] [Google Scholar]
- Thompson W., MacDonald G. Cytidine diphosphate diglyceride of bovine brain. Positional distribution of fatty acids and analysis of major molecular species. Eur J Biochem. 1976 May 17;65(1):107–111. doi: 10.1111/j.1432-1033.1976.tb10394.x. [DOI] [PubMed] [Google Scholar]
- Thompson W., MacDonald G. Isolation and characterization of cytidine diphosphate diglyceride from beef liver. J Biol Chem. 1975 Sep 10;250(17):6779–6785. [PubMed] [Google Scholar]
- Van Heusden G. P., Ruestow B., Van der Mast M. A., Van den Bosch H. Synthesis of disaturated phosphatidylcholine by cholinephosphotransferase in rat lung microsomes. Biochim Biophys Acta. 1981 Dec 23;666(3):313–321. doi: 10.1016/0005-2760(81)90289-7. [DOI] [PubMed] [Google Scholar]


