Highlights
-
•
Allergic reactions to paclitaxel can be effectively managed by switching to nab-paclitaxel.
-
•
No allergic reactions were noted in 43 patients with endometrial or ovarian cancer following the transition to nab-paclitaxel.
-
•
Transition to nab-paclitaxel after paclitaxel allergy maybe preferable over transition to docetaxel or desensitization.
Keywords: Nab-paclitaxel, Paclitaxel, Allergic reactions, Chemotherapy, Ovarian cancer, Endometrial cancer
Abstract
The goal of this study was to assess the safety of nab-paclitaxel in patients with ovarian cancer or endometrial cancer who had an allergic reaction to paclitaxel. We performed a retrospective review of patients with endometrial cancer or ovarian cancer with an allergic reaction to paclitaxel who were subsequently treated with nab-paclitaxel at the Mayo Clinic Florida from January 2016 to June 2023. A total of 43 patients with ovarian cancer (31) or endometrial cancer (12) and a paclitaxel allergic reaction were identified. All patients were pre-medicated against allergic reactions prior to paclitaxel and subsequent nab-paclitaxel. Allergic reactions to paclitaxel were mild in fourteen patients (33%), moderate in twenty-five patients (58%) and severe in four (9%) patients. None of the 43 patients had an allergic reaction to subsequent nab-paclitaxel. Our data suggests that the administration of nab-paclitaxel to endometrial cancer or ovarian cancer patients with allergic reactions to paclitaxel is safe and should be considered a preferable treatment option in this clinical situation.
1. Introduction
Gynecological cancers are the third leading cause of cancer in US women, following breast cancer and lung cancer (Siegel et al., 2024). Paclitaxel in combination with carboplatin is the standard first line treatment for most metastatic ovarian and endometrial cancers (Ledermann, 2018). Hypersensitive reactions to paclitaxel infusions are relatively common (varying from 2-42 %) and vary from severe anaphylactic shock to mild skin rashes (Haine et al. 2022). These hypersensitive reactions are believed to be secondary to the Cremophor which is used as a solvent to paclitaxel. Different approaches have been used to manage hypersensitivity reactions to paclitaxel including increased doses of steroids, anti-histaminics, a trial of docetaxel and desensitization procedures (Haine et al. 2022). Nab-paclitaxel has also been successfully used in patients with allergic reactions to paclitaxel as it is dissolved in protein albumin nanoparticles and does not require Cremophor (Maurer et al. 2017). In this study, we report a single institution's experience using nab-paclitaxel in patients with paclitaxel allergies.
2. Methods
A retrospective review of medical records of consecutive patients with endometrial cancer or ovarian cancer and a history of an allergic reaction to paclitaxel seen at the Mayo Clinic in Florida from January 1, 2016, through June 30, 2023, was performed. Patients were identified by doing a search of the electronic medical records for diagnosis of paclitaxel allergy and manually reviewing identified records for subsequent treatments. All patients with endometrial or ovarian cancer treated at Mayo Clinic in Florida during the study period were cared for by authors of the manuscript (S.K. and G.C.O.) and all of them were subsequently treated with nab-paclitaxel. Demographic data on the patients, number of cycles of treatment and information on the severity of the observed allergic reactions were collected. Hypersensitivity reactions (HSR) were classified according to Brown’s classification (Brown, 2004). IRB approval was obtained (Mayo Clinic IRB17-006677).
3. Results
Forty-five patients were identified. Among these 45 patients, two were excluded from the analysis due to lack of specific information about the allergic reactions. Demographics of the study population are included in Table 1.
Table 1.
Demographic data.
| Variable | Number (percentage) |
|---|---|
| Patients | 43 |
| Ovarian Cancer | 31 (72.09 %) |
| Endometrial Cancer | 12 (27.91 %) |
| Median age (in years, range) | 63 (31 – 88) |
| Race (self-reported) | |
| White | 39 (90.68 %) |
| African American | 1 (2.33 %) |
| Middle Eastern | 1 (2.33 %) |
| Asian | 1 (2.33 %) |
| Not listed | 1 (2.33 %) |
| Ovarian cancer | 31 |
| Staging | |
| Stage 1 | 0 (0%) |
| Stage 2 | 5 (16.13%) |
| Stage 3 | 14 (45.16%) |
| Stage 4 | 11 (35.48%) |
| Recurrent | 1 (3.23%) |
| Histology | |
| High Grade Serous | 25 (80.64%) |
| Low Grade Serous | 1 (3.23%) |
| Endometroid | 2 (6.45%) |
| Mixed | 3 (9.68%) |
| Clear cell | 0(0%) |
| Treatment | |
| Neo-Adjuvant | 20 (64.52%) |
| Adjuvant | 10 (32.26%) |
| Recurrent | 1 (3.23%) |
| Endometrial Cancer Staging |
12 |
| Staging | |
| Stage 1 | 4 (33.33%) |
| Stage 2 | 2 (16.67%) |
| Stage 3 | 4 (33.33%) |
| Stage 4 | 0 (0%) |
| Recurrent | 2 (16.67%) |
| Histology | |
| Endometroid | 6 (50.00%) |
| Carcinosarcoma | 4 (33.33%) |
| Serous | 2 (16.67%) |
| Treatment | |
| Neo-Adjuvant+Adjuvant | 1 (8.33%) |
| Adjuvant | 9 (75.00%) |
| Recurrent | 2 (16.67%) |
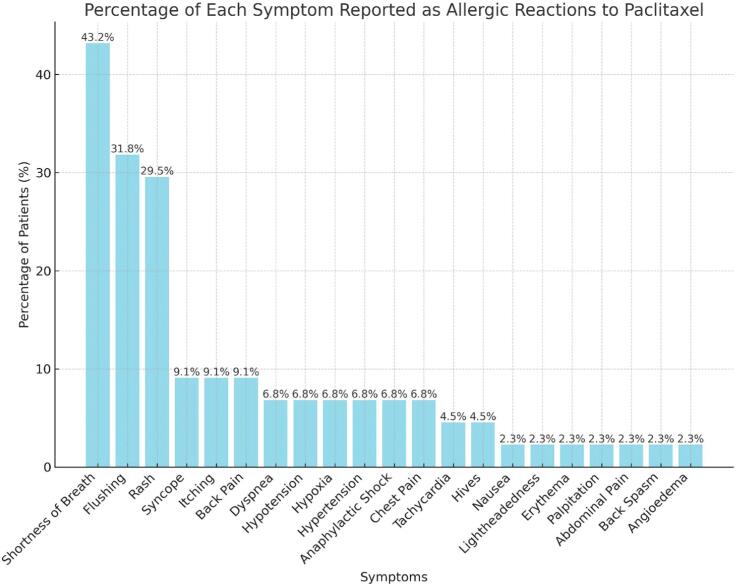
Prior to the treatment with paclitaxel or subsequent nab-paclitaxel, all the patients were pre-medicated with dexamethasone 12 mg IV, hydrocortisone 100 mg IV (for first 2 doses of paclitaxel), famotidine 20 mg IV, and diphenhydramine 50 mg IV. Most of the patients had a reaction to the first dose of paclitaxel (N=23) with 7 patients having the reaction after the second dose, 10 after the third dose and 3 to subsequent doses. The clinical presentation or symptoms reported during the allergic reaction to paclitaxel ranged from anaphylactic shock, skin rash/erythema, flushing, dyspnea, shortness of breath, hypoxia, hypotension, hypertension, chest pain, nausea, throat tightness, syncopal episodes, abdominal pressure, back pain/spasm, tachycardia, pruritus, hives, facial tingling, numbness, lightheadedness, and angioedema (Fig. 1). Fourteen patients (33 %) had mild reactions, twenty-five patients (58 %) had moderate reaction and four (9 %) patients had severe reactions, including two of them who needed hospital admission.
Fig. 1.
Observed manifestations of hypersensitivity reactions (HSR) to paclitaxel in the current series (more than one symptom was observed in some patients).
After these HSR, all patients were switched from paclitaxel to nab-paclitaxel and treated in the outpatient setting. All patients tolerated the nab-paclitaxel treatment without any allergic reactions. None of these 43 patients subsequently discontinued nab-paclitaxel due to allergic reactions. Patients received a median of 5 doses of nab-paclitaxel (range 1–19 doses). The dosing of nab-paclitaxel ranged from 50 mg/m2 weekly (n = 1), 60 mg/m2 weekly (n = 2), 80 mg/m2 weekly (n = 30) and 175 mg/m2 every 3 weeks (n = 10) in combination with carboplatin doses of AUC2 weekly or AUC5-6 every 3 weeks respectively or Gemcitabine 800 mg/m2 given weekly.
4. Discussion
Hypersensitivity reactions (HSR) to paclitaxel are a significant clinical problem, varying from 2- 42 %, and sometimes can be lethal (De Leon et al., 2013, Haine et al., 2022). These allergic reactions, predominantly type 1 HSR, are manifested by hypotension, tachycardia, flushing, respiratory distress, abdominal and chest pain, as well as dermal reactions (Haine et al. 2022). Of all 43 patients in the current series, as many as 9 % of them developed severe symptoms like hypotension, hypoxia, confusion, and anaphylactic edema to paclitaxel. Current preventive strategies for taxane-induced HSR include premedication with H1 and H2 receptor antagonists, and intravenous corticosteroids. For patients who develop severe HSR despite these measures, currently recommended options include desensitization protocols or the use of alternative taxanes like docetaxel (Haine et al. 2022). Cross sensitivity to docetaxel in patients with allergic reactions to paclitaxel are quite common, varying from 41 % to 90 % (Sanchez-Munoz et al., 2011, Dizon et al., 2006) and desensitization procedures require significant time commitment and frequently hospitalizations (De Leon et al. 2013) which makes the option of nab-paclitaxel a preferable and safer option in these cases.
The incidence of allergic reactions to nab-paclitaxel are rare enough to not merit any allergic premedications in its current FDA approved indications, contrary to paclitaxel and docetaxel which require steroid premedications in order to prevent allergic reactions to its lipid solvents. Allergic reactions to nab-paclitaxel had been described in the literature, although usually these reactions are not severe, and therefore the absence of any allergic reactions in our series may had been in part due to the relatively small size of our cohort. For example, in a randomized phase 3 clinical trial of 460 patients with metastatic breast cancer, comparing nab-paclitaxel with paclitaxel, less than 1 % of patients receiving nab-paclitaxel had an allergic reaction, with none of the allergic reactions been classified as severe, compared to 2 % patients receiving paclitaxel (Gradishar et al, 2005). Additionally, it is possible that the use of premedications with steroids and H1 and H2 blockers prior to nab-paclitaxel in our series may had prevented allergic reactions and mitigated any cross sensitivity to nab-paclitaxel. We decided to use premedications in our patients in order to mitigate the possibility of cross sensitivity to nab-paclitaxel.
The majority of the patients in our study developed HSR during the initial cycles of paclitaxel. Upon switching to nab-paclitaxel, all patients tolerated the treatment well, including the four patients with severe HSR to paclitaxel, with no reports of allergic reactions, and none discontinued treatment due to subsequent allergic reactions. Our report is the largest single institution series on the use of nab-paclitaxel in patients with an allergic reaction to paclitaxel (Table 2). Previous reports were mostly case reports except for three series, one by Maurer et al. of 37 patients previously experiencing a HSR to paclitaxel, who had no immediate reactions to subsequent treatment with nab-paclitaxel (Maurer et al. 2017). Parisi et al reported a series of 10 patients with no reported reactions to nab-paclitaxel after an allergic reaction to paclitaxel (Parisi et al., 2020). Fader et al. reported a series of five patients treated successfully with nab-paclitaxel after discontinuation of paclitaxel (Fader and Rose, 2009). Our series adds important data supporting the use of nab-paclitaxel following a paclitaxel HSR.
Table 2.
Reports on nab-paclitaxel use after hypersensitivity reactions to paclitaxel.
| Reference | Number of patients | Taxane which caused hypersensitivity | Cycles after which HSR occurred | Premedication | Hypersensitivity caused by Nab-paclitaxel | Type of cancer |
|---|---|---|---|---|---|---|
| Maurer et al., 2017 | 37 | Paclitaxel and Docetaxel | 1––3 | yes | no | Ovarian, uterine and cervical |
| Parisi et al., 2020 | 10 | Paclitaxel | _ | yes | no | Ovarian |
| Pellegrino et al., 2017 | 1 | Docetaxel | 2 | yes | no | Breast |
| Kimura et al., 2013 | 1 | Docetaxel | 2 | yes | no | Breast |
| Fader and Rose, 2009 | 5 | Paclitaxel | 1 | yes | no | Ovarian, uterine, cervical |
| de Leon et al, 2013 | 1 | Paclitaxel and Docetaxel | 1 | yes | no | Ovarian |
| Micha et al., 2006 | 1 | Paclitaxel | 1 | yes | no | Ovarian |
5. Conclusions
This retrospective single institution experience shows that the use of nab-paclitaxel in patients with ovarian cancer or endometrial cancer who have experienced HSR to paclitaxel is safe and not associated with allergic reactions. Nab-paclitaxel should be considered a safe and acceptable treatment option in patients with paclitaxel induced HSR, with clear benefits over the alternative use of docetaxel or desensitization procedures.
CRediT authorship contribution statement
Swapna Kochuveettil: Writing – review & editing, Writing – original draft, Validation, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Roberto Angeli Morales: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation. Alicja Kaminska: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Gerardo Colon-Otero: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Acknowledgments
Funded by National Cancer Institute: [Grant Number CA15083];National Institutes ofHealth: [GrantNumber UL1 TR002377].
Authors contribution
Swapna Kochuveettil: Conceptualization; Data curation; Formal analysis; Methodology; Writing − original draft. Roberto Angeli Morales: Data curation; Formal analysis; Methodology; Writing − original draft. Alicja Kaminska: Formal analysis; Writing − original draft. Gerardo Colon-Otero: Conceptualization; Data curation; Formal analysis; Methodology; Writing − original draft; and Writing − review & editing.
IRB review concluded that written informed consent was not needed for this retrospective chart review that included only de-identified patient information.
Contributor Information
Swapna Kochuveettil, Email: Kochuveettil.Swapna@mayo.edu.
Roberto Angeli Morales, Email: roberto.angeli@upr.edu.
Alicja Kaminska, Email: Kaminska.AlicjaAleksandra@mayo.edu.
Gerardo Colon-Otero, Email: gcolonotero@mayo.edu.
References
- Brown S.G.A. Clinical Features and Severity Grading of Anaphylaxis. J. Allergy Clin. Immunol. 2004;114(2):371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Dizon D., Schwartz J., Rojan A., Miller J., Pires L., Disilvestro P., Gordinier M.E., Moore R., Granai S.O., Legare R.D. Cross-sensitivity between paclitaxel and docetaxel in a women's cancers program. Gynecol Oncol. 2006 Jan;100(1):149–151. doi: 10.1016/j.ygyno.2005.08.004. Epub 2005 Sep 28. [DOI] [PubMed] [Google Scholar]
- Fader A.N., Rose P.G. Abraxane for the Treatment of Gynecologic Cancer Patients With Severe Hypersensitivity Reactions to Paclitaxel. Int. J. Gynecol. Cancer. 2009;19(7):1281–1283. doi: 10.1111/IGC.0b013e3181a38e2f. [DOI] [PubMed] [Google Scholar]
- Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, and O'Shaughnessy J: Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil–Based Paclitaxel in Women With Breast Cancer. Journal of Clinical Oncology Volume 23, Number 31 https://doi.org/10.1200/JCO.2005.04.937. [DOI] [PubMed]
- Haine A.I., Cornelia M.A., Notenboom W., Liong V.P., Tan R.R., van der Deure W.M. Ranitidine and the Incidence of Hypersensitivity Reactions to Paclitaxel: A Retrospective Cohort Study. Pharmacol. Res. Perspect. 2022;10(4):e00985. doi: 10.1002/prp2.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Tanaka S., Iwamoto M., Fujioka H., Takahashi Y., Sato N., Terasawa R., Tominaga T., Ikari A., Uchiyama K. Safety of Nanoparticle Albumin-Bound Paclitaxel Administered to Breast Cancer Patients with Clinical Contraindications to Paclitaxel or Docetaxel: Four Case Reports. Oncol. Lett. 2013;6(4):881–884. doi: 10.3892/ol.2013.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledermann J.A. First-Line Treatment of Ovarian Cancer: Questions and Controversies to Address. Therapeutic Advances in Medical Oncology. 2018;10(January) doi: 10.1177/1758835918768232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon D.e., Maria C.B., Bolla S., Greene B., Hutchinson L., Del Priore G. Successful Treatment with Nab-Paclitaxel after Hypersensitivity Reaction to Paclitaxel and Docetaxel. Gynecologic Oncology Case Reports. 2013;5(August):70–71. doi: 10.1016/j.gynor.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer K., Michener C.a., Mahdi H., Rose P.G. Universal Tolerance of Nab-Paclitaxel for Gynecologic Malignancies in Patients with Prior Taxane Hypersensitivity Reactions. J. Gynecol. Oncol. 2017;28(4):e38. doi: 10.3802/jgo.2017.28.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha J.P., Goldstein B.H., Birk C.L., Rettenmaier M.A., Brown J.V. Abraxane in the Treatment of Ovarian Cancer: The Absence of Hypersensitivity Reactions. Gynecol. Oncol. 2006;100(2):437–448. doi: 10.1016/j.ygyno.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Parisi A., Palluzzi E., Cortellini A., Sidoni T., Cocciolone V., Lanfiuti Baldi P., Porzio G., Ficorella C., Cannita K. First-line carboplatin/nab-paclitaxel in advanced ovarian cancer patients, after hypersensitivity reaction to solvent-based taxanes: a single-institution experience. Clin Transl Oncol. 2020;22(1):158–162. doi: 10.1007/s12094-019-02122-x. Epub 2019 Apr 30. [DOI] [PubMed] [Google Scholar]
- Pellegrino B., Boggiani D., Tommasi C., Palli D., Musolino A. Nab-Paclitaxel after Docetaxel Hypersensitivity Reaction: Case Report and Literature Review. Acta Bio-Medica: Atenei Parmensis. 2017;88(3):329–333. doi: 10.23750/abm.v88i3.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Munoz A., Jimenez B., Garcia-Tapiador A., Romero-Garcia G., Medina L., Navarro V., Gonzalez-Sanchez A., Alba E. Cross sensitivity between taxanes in patients with breast cancer. Clin Transl Oncol. 2011 Dec;13(12):904–906. doi: 10.1007/s12094-011-0753-3. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics 2024. Ca: A Cancer J. Clin. 2024;17 doi: 10.3322/caac.21820. January 2024. [DOI] [Google Scholar]