Abstract
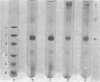
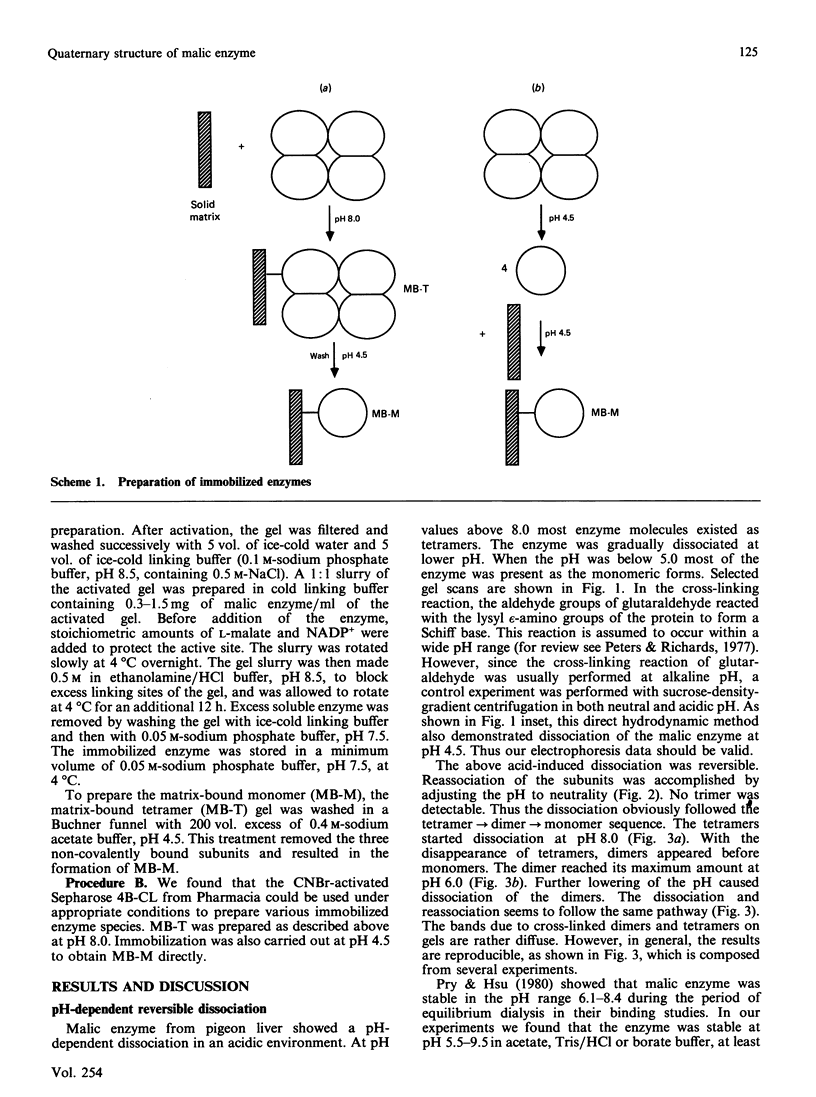
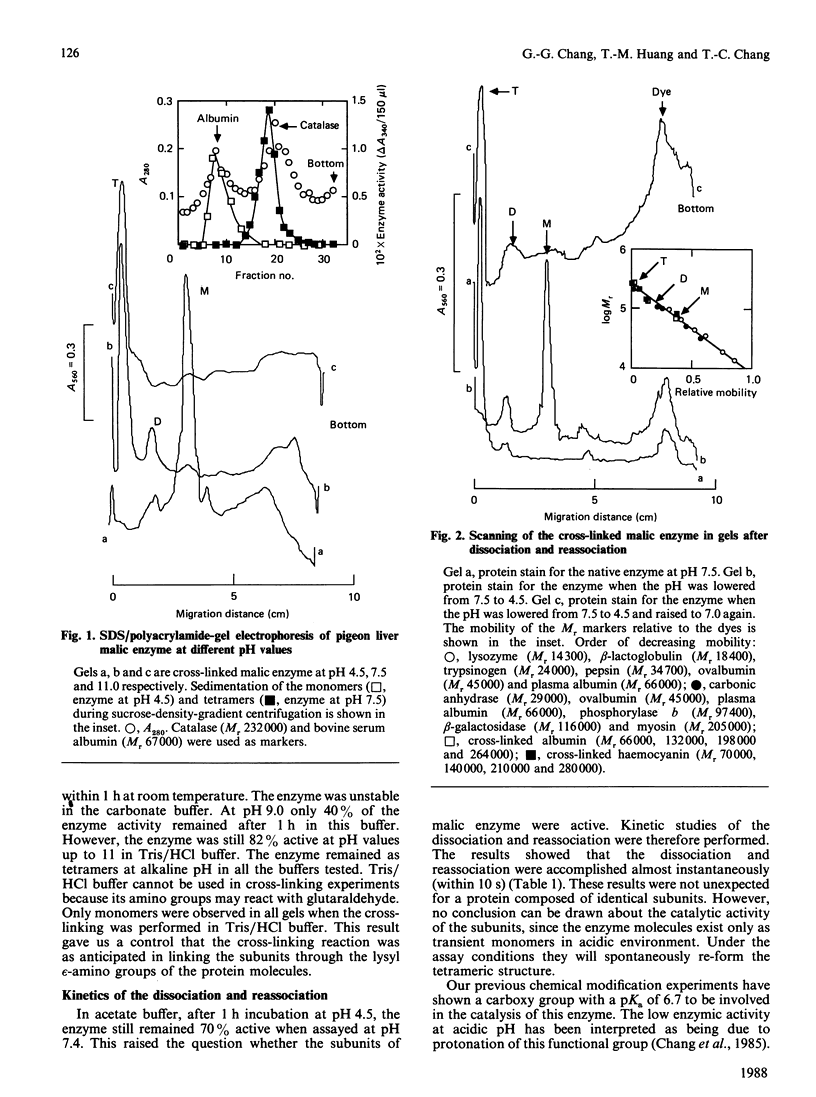
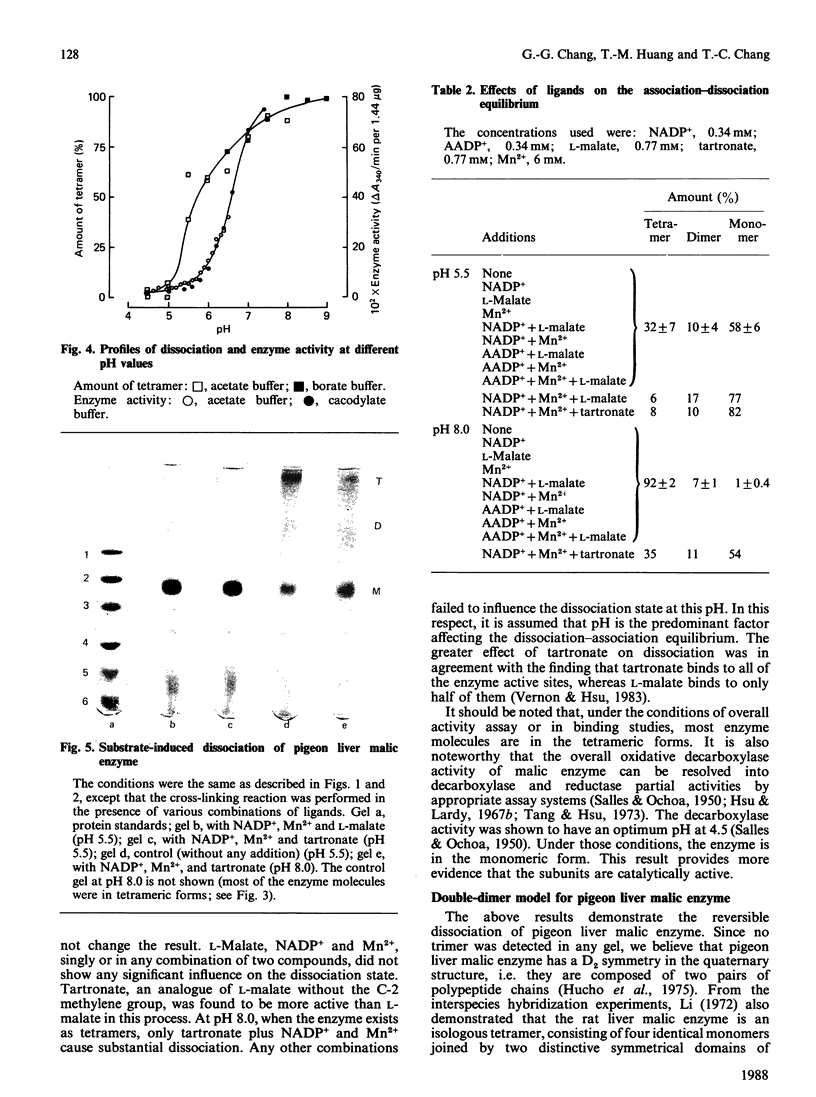
The pH-induced reversible dissociation of pigeon liver malic enzyme (EC 1.1.1.40) was studied by combined use of chemical cross-linking and SDS/polyacrylamide-gel electrophoresis. The tetrameric enzyme showed a pH-dependent dissociation in an acidic environment. At pH values above 8.0 most molecules existed as tetramers. The enzyme was gradually dissociated at lower pH. When the pH was below 5.0 most of the enzyme was present as the monomeric forms. Reassociation of the subunits was accomplished by adjusting the pH to neutrality. The dissociation and reassociation were almost instantaneous. No trimer was detected. The pigeon liver malic enzyme was thus shown to have a double-dimer quaternary structure with D2 symmetry. In the presence of substrates, the monomer-dimer-tetramer equilibrium favours the direction of dissociation. Tartronate, an L-malate analogue, was found to be more effective than L-malate in this process. When the monomeric forms were immobilized, the enzyme subunits were found to be fully active in catalysis. A possible arrangement of the four identical subunits of the enzyme molecule is proposed to account for the results obtained in this investigation. The origin of the half-of-the-sites reactivity of pigeon liver malic enzyme is also discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asryants R. A., Duszenkova I. V., Nagradova N. K. Determination of Sepharose-bound protein with Coomassie brilliant blue G-250. Anal Biochem. 1985 Dec;151(2):571–574. doi: 10.1016/0003-2697(85)90222-2. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Thomas D. H., Barton C. H., Rice D. W., Bailey E. Crystallization of an NADP+-dependent malic enzyme from rat liver. J Mol Biol. 1987 Jan 5;193(1):233–235. doi: 10.1016/0022-2836(87)90643-7. [DOI] [PubMed] [Google Scholar]
- Carvajal N., Martinez J., Fernandez M. Immobilised monomers of human liver arginase. Biochim Biophys Acta. 1977 Mar 15;481(1):177–183. doi: 10.1016/0005-2744(77)90149-8. [DOI] [PubMed] [Google Scholar]
- Chan W. W. Immobilized subunits. Methods Enzymol. 1976;44:491–503. doi: 10.1016/s0076-6879(76)44035-1. [DOI] [PubMed] [Google Scholar]
- Chang G. G., Chang T. C., Huang T. M. Involvement of lysine residue in the nucleotide binding of pigeon liver malic enzyme: modification with affinity label periodate-oxidized NADP. Int J Biochem. 1982;14(7):621–627. doi: 10.1016/0020-711x(82)90046-5. [DOI] [PubMed] [Google Scholar]
- Chang G. G., Hsu R. Y. Mechanism of pigeon liver malic enzyme: kinetics, specificity, and half-site stoichiometry of the alkylation of a cysteinyl residue by the substrate-inhibitor bromopyruvate. Biochemistry. 1977 Jan 25;16(2):311–320. doi: 10.1021/bi00621a024. [DOI] [PubMed] [Google Scholar]
- Chang G. G., Huang T. M., Wuu J. A. Mechanism of pigeon liver malic enzyme: modification of essential carboxyl groups. Proc Natl Sci Counc Repub China B. 1985 Jan;9(1):56–66. [PubMed] [Google Scholar]
- Chang J. T., Chang G. G. Purification of pigeon liver malic enzyme by affinity chromatography. Anal Biochem. 1982 Apr;121(2):366–369. doi: 10.1016/0003-2697(82)90494-8. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Koshland D. E., Jr The influence of binding domains on the nature of subunit interactions in oligomeric proteins. Application to unusual kinetic and binding patterns. J Biol Chem. 1970 Dec 10;245(23):6241–6250. [PubMed] [Google Scholar]
- Degani C., Degani Y. Further evidence for nonsymmetric subunit association and intersubunit cooperativity in creatine kinase. Subunit-selective modifications by 2,4-dinitrophenylthiocyanate. J Biol Chem. 1980 Sep 10;255(17):8221–8228. [PubMed] [Google Scholar]
- Degani Y., Degani C. Subunit-selective chemical modifications of creatine kinase. Evidence for asymmetrical association of the subunits. Biochemistry. 1979 Dec 25;18(26):5917–5923. doi: 10.1021/bi00593a024. [DOI] [PubMed] [Google Scholar]
- Frenkel R. Regulation and physiological functions of malic enzymes. Curr Top Cell Regul. 1975;9:157–181. doi: 10.1016/b978-0-12-152809-6.50012-3. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Levitzki A. Ligand competition curves as a diagnostic tool for delineating the nature of site-site interactions: theory. Eur J Biochem. 1979 Dec 17;102(2):449–465. doi: 10.1111/j.1432-1033.1979.tb04260.x. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Levitzki A. The sequential nature of the negative cooperativity in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1980 Nov;112(1):59–73. doi: 10.1111/j.1432-1033.1980.tb04987.x. [DOI] [PubMed] [Google Scholar]
- Hermann R., Rubolph R., Jaenicke R. Kinetics of in vitro reconstitution of oligomeric enzymes by cross-linking. Nature. 1979 Jan 18;277(5693):243–245. doi: 10.1038/277243a0. [DOI] [PubMed] [Google Scholar]
- Hsu R. Y., Lardy H. A. Pigeon liver malic enzyme. II. Isolation, crystallization, and some properties. J Biol Chem. 1967 Feb 10;242(3):520–526. [PubMed] [Google Scholar]
- Hsu R. Y., Lardy H. A. Pigeon liver malic enzyme. IV. Pyruvate reductase activity of the crystalline enzyme. Acta Biochim Pol. 1967;14(1):183–186. [PubMed] [Google Scholar]
- Hsu R. Y., Mildvan A. S., Chang G., Fung C. Mechanism of malic enzyme from pigeon liver. Magnetic resonance and kinetic studies of the role of Mn2+. J Biol Chem. 1976 Nov 10;251(21):6574–6583. [PubMed] [Google Scholar]
- Hsu R. Y. Pigeon liver malic enzyme. Mol Cell Biochem. 1982 Mar 5;43(1):3–26. doi: 10.1007/BF00229535. [DOI] [PubMed] [Google Scholar]
- Hsu R. Y., Pry R. A. Kinetic studies of the malic enzyme of pigeon liver. "Half-of-the-sites" behavior of the enzyme tetramer in catalysis and substrate inhibition. Biochemistry. 1980 Mar 4;19(5):962–968. doi: 10.1021/bi00546a021. [DOI] [PubMed] [Google Scholar]
- Huang C. Y., Rhee S. G., Chock P. B. Subunit cooperation and enzymatic catalysis. Annu Rev Biochem. 1982;51:935–971. doi: 10.1146/annurev.bi.51.070182.004443. [DOI] [PubMed] [Google Scholar]
- Hucho F., Müllner H., Sund H. Investigation of the symmetry of oligomeric enzymes with bifunctional reagents. Eur J Biochem. 1975 Nov 1;59(1):79–87. doi: 10.1111/j.1432-1033.1975.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Jurgensen S. R., Harrison J. H. Active subunits in hybrid-modified malate dehydrogenase. J Biol Chem. 1982 Jan 10;257(1):569–574. [PubMed] [Google Scholar]
- Jurgensen S. R., Wood D. C., Mahler J. C., Harrison J. H. The immobilization of mitochondrial malate dehydrogenase on Sepharose beads and the demonstration of catalytically active subunits. J Biol Chem. 1981 Mar 10;256(5):2383–2388. [PubMed] [Google Scholar]
- Kam P. L., Lin C. C., Chang G. G. Structural identity of the subunits of pigeon liver malic enzyme. Int J Pept Protein Res. 1987 Aug;30(2):217–221. doi: 10.1111/j.1399-3011.1987.tb03329.x. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Langerman N. R., Darnall D. W. Quaternary structure of proteins. Annu Rev Biochem. 1970;39:25–62. doi: 10.1146/annurev.bi.39.070170.000325. [DOI] [PubMed] [Google Scholar]
- Kochetov G. A., Solovieva O. N. Active subunits of transketolase from baker's yeast. Biochem Biophys Res Commun. 1978 Sep 29;84(2):515–519. doi: 10.1016/0006-291x(78)90199-7. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Chang G. G. Interactions of nicotinamide-adenine dinucleotide phosphate analogues and fragments with pigeon liver malic enzyme. Synergistic effect between the nicotinamide and adenine moieties. Biochem J. 1987 Jul 15;245(2):407–414. doi: 10.1042/bj2450407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr The role of negative cooperativity and half-of-the-sites reactivity in enzyme regulation. Curr Top Cell Regul. 1976;10:1–40. doi: 10.1016/b978-0-12-152810-2.50008-5. [DOI] [PubMed] [Google Scholar]
- Li J. J. NADP-malic enzyme. In vitro interspecies hybridization of rat and hamster liver enzymes. Evidence for an isologous tetrameric structure. Arch Biochem Biophys. 1972 Jun;150(2):812–814. doi: 10.1016/0003-9861(72)90104-x. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A., Morioka H., Tecce M. F., Nikodem V. M. Coding nucleotide sequence of rat liver malic enzyme mRNA. J Biol Chem. 1986 Jan 25;261(3):1183–1186. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Lehninger A. L. Purification, kinetic behavior, and regulation of NAD(P)+ malic enzyme of tumor mitochondria. J Biol Chem. 1984 May 25;259(10):6222–6227. [PubMed] [Google Scholar]
- Moreadith R. W., Lehninger A. L. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem. 1984 May 25;259(10):6215–6221. [PubMed] [Google Scholar]
- Mort J. S., Chong D. K., Chan W. W. Continuous spectrophotometric assay of a sepharose-bound enzyme and its use to study kinetics of coupling of the enzyme to sepharose. Anal Biochem. 1973 Mar;52(1):162–168. doi: 10.1016/0003-2697(73)90341-2. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Pry T. A., Hsu R. Y. Equilibrium substrate binding studies of the malic enzyme of pigeon liver. Equivalence of nucleotide sites and anticooperativity associated with the binding of L-malate to the enzyme-manganese(II)-reduced nicotinamide adenine dinucleotide phosphate ternary complex. Biochemistry. 1980 Mar 4;19(5):951–962. doi: 10.1021/bi00546a020. [DOI] [PubMed] [Google Scholar]
- Pry T. A., Hsu R. Y. Mechanism of pigeon liver malic enzyme. Reactivity of class II sulfhydryl groups as a conformational probe for the "half-of-the-sites" reactivity of the enzyme with bromopyruvate. Biochemistry. 1978 Sep 19;17(19):4024–4029. doi: 10.1021/bi00612a023. [DOI] [PubMed] [Google Scholar]
- RUTTER W. J., LARDY H. A. Purification and properties of pigeon liver malic enzyme. J Biol Chem. 1958 Aug;233(2):374–382. [PubMed] [Google Scholar]
- Reynolds C. H., Hsu R. Y., Matthews B., Pry T. A., Dalziel K. Transient kinetic studies of malic enzyme. A conformational change associated with substrate inhibition by malate. Arch Biochem Biophys. 1978 Aug;189(2):309–316. doi: 10.1016/0003-9861(78)90217-5. [DOI] [PubMed] [Google Scholar]
- Tang C. L., Hsu R. Y. Reduction of alpha-oxo carboxylic acids by pigeon liver 'malic' enzyme. Biochem J. 1973 Oct;135(2):287–291. doi: 10.1042/bj1350287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEIGA SALLES J. B., OCHOA S. Biosynthesis of dicarboxylic acids by carbon dioxide fixation. II. Further study of the properties of the "malic" enzyme of pigeon liver. J Biol Chem. 1950 Dec;187(2):849–861. [PubMed] [Google Scholar]
- Vernon C. M., Hsu R. Y. Pigeon liver malic enzyme: involvement of an arginyl residue at the binding site for malate and its analogs. Arch Biochem Biophys. 1983 Aug;225(1):296–305. doi: 10.1016/0003-9861(83)90033-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]