Abstract
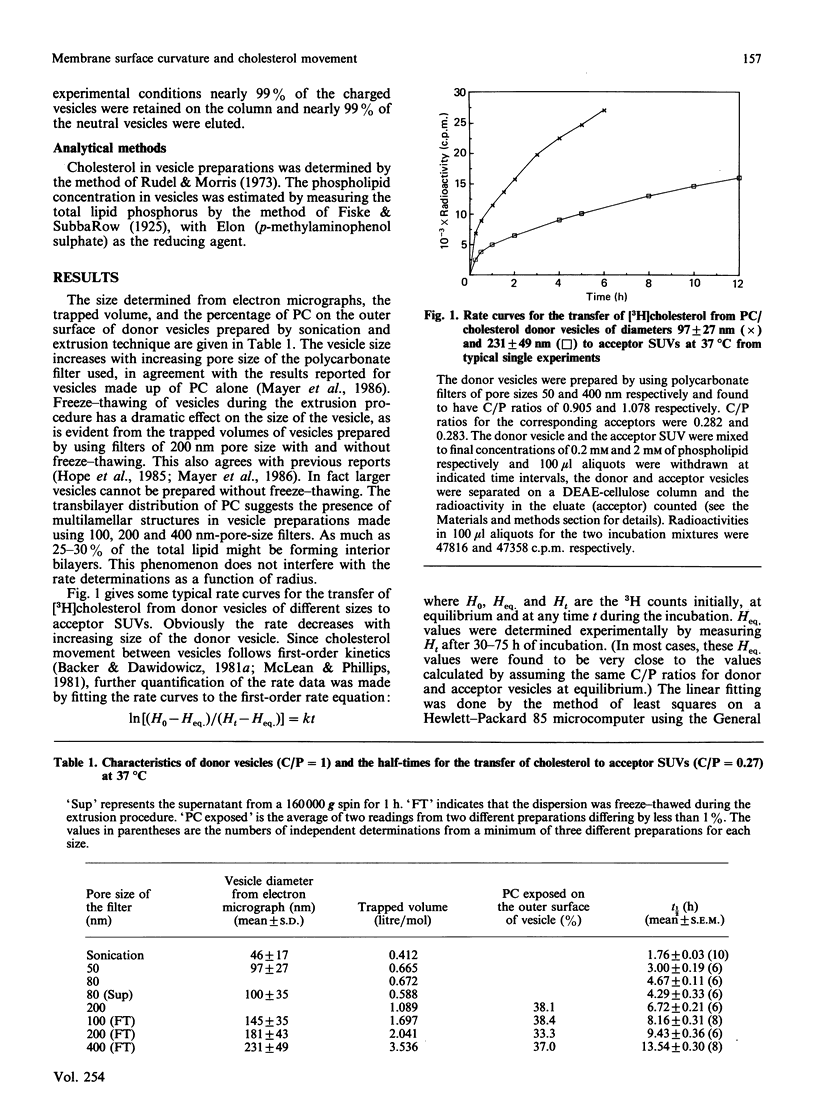
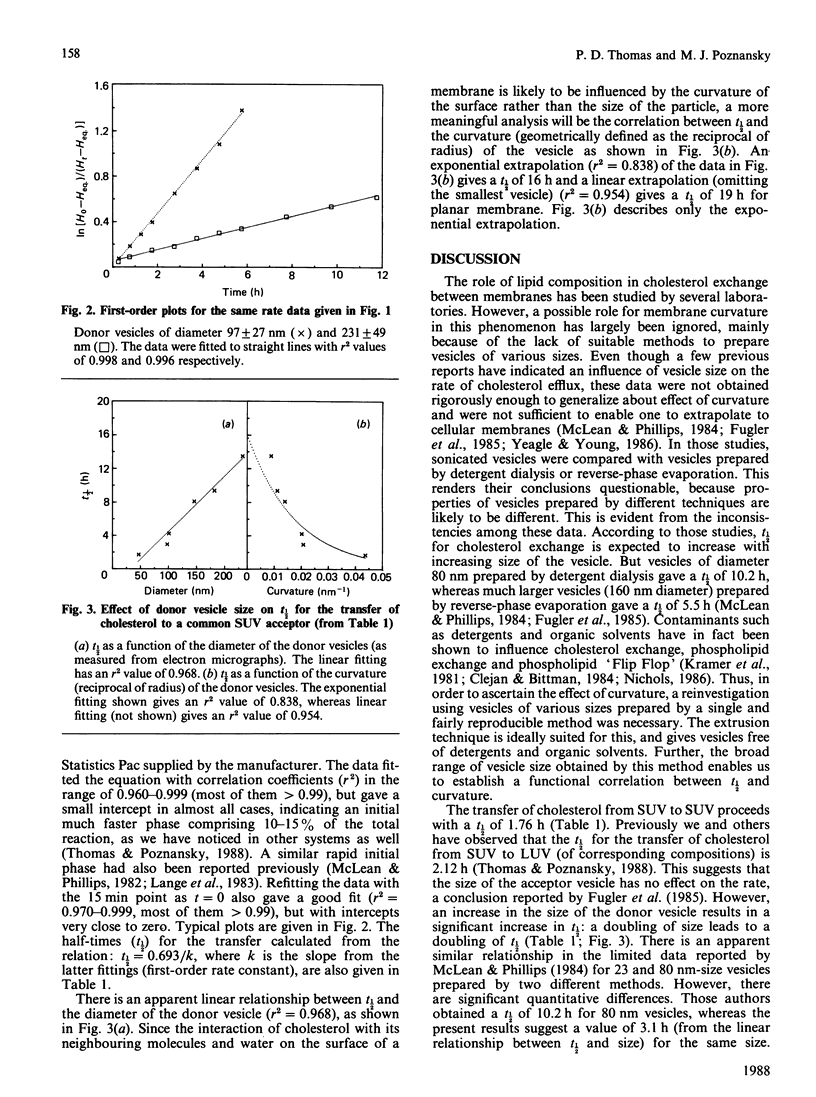
The effect of surface curvature on the spontaneous movement of cholesterol between membranes was investigated by measuring the rates of cholesterol transfer from donor vesicles of various sizes to a common acceptor vesicle. Donor vesicles of size in the range 40-240 nm were prepared by extruding multilamellar dispersions through polycarbonate filters of different pore sizes under pressure. The smallest donor vesicle and the acceptor vesicles were obtained by the normal sonication procedures. The rate of cholesterol transfer, as measured by the movement of [3H]cholesterol, decreases with increasing size of the donor vesicle in an almost linear fashion. The extrapolation of the results gave a half-time (t1/2) of 16-20 h of the desorption of cholesterol from a planar bilayer, and this can be considered as a reference value for most cellular membranes which are characterized by very low curvatures. Our earlier studies have shown that the t1/2 for cholesterol efflux is influenced by the presence of gangliosides and phosphatidylethanolamine, and the asymmetric distribution of these lipids in the plasma membrane could partially account for the large difference in the rates of cholesterol movement from the two sides of the plasma membrane. The small differences in rates arising from asymmetric distribution will be magnified by the longer t1/2 obtained here for membranes of low curvatures, so that the large difference in rates might be a coupled effect of lipid asymmetry and low curvature of the plasma membrane. This, in turn, may have a role in maintaining the large differences in cholesterol/phospholipid molar ratios observed between plasma membrane and intracellular membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Dawidowicz E. A. Mechanism of cholesterol exchange between phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3805–3810. doi: 10.1021/bi00516a021. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Dawidowicz E. A. Transmembrane movement of cholesterol in small unilamellar vesicles detected by cholesterol oxidase. J Biol Chem. 1981 Jan 25;256(2):586–588. [PubMed] [Google Scholar]
- Brockerhoff H. Model of interaction of polar lipids, cholesterol, and proteins in biological membranes. Lipids. 1974 Sep;9(9):645–650. doi: 10.1007/BF02532169. [DOI] [PubMed] [Google Scholar]
- Clejan S., Bittman R. Kinetics of cholesterol and phospholipid exchange between Mycoplasma gallisepticum cells and lipid vesicles. Alterations in membrane cholesterol and protein content. J Biol Chem. 1984 Jan 10;259(1):441–448. [PubMed] [Google Scholar]
- Enoch H. G., Strittmatter P. Formation and properties of 1000-A-diameter, single-bilayer phospholipid vesicles. Proc Natl Acad Sci U S A. 1979 Jan;76(1):145–149. doi: 10.1073/pnas.76.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugler L., Clejan S., Bittman R. Movement of cholesterol between vesicles prepared with different phospholipids or sizes. J Biol Chem. 1985 Apr 10;260(7):4098–4102. [PubMed] [Google Scholar]
- Green C. The movement of cholesterol within cells. Biochem Soc Trans. 1983 Dec;11(6):637–639. doi: 10.1042/bst0110637. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Hasselbach H. J., Semenza G. Rapid transmembrane movement of phosphatidylcholine in small unilamellar lipid vesicles formed by detergent removal. Biochim Biophys Acta. 1981 Apr 22;643(1):233–242. doi: 10.1016/0005-2736(81)90236-4. [DOI] [PubMed] [Google Scholar]
- Lange Y., Molinaro A. L., Chauncey T. R., Steck T. L. On the mechanism of transfer of cholesterol between human erythrocytes and plasma. J Biol Chem. 1983 Jun 10;258(11):6920–6926. [PubMed] [Google Scholar]
- Lundberg B., Suominen L. Accumulation and mobilization of cholesteryl esters in cultured human fibroblasts exposed to free cholesterol-rich phospholipid vesicles. Atherosclerosis. 1985 Sep;56(3):345–358. doi: 10.1016/0021-9150(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Magot T., Frein Y., Giraud F., Cheruy A. The measurement in vivo of the rate of unesterified cholesterol exchange between rat plasma and erythrocytes. Biochim Biophys Acta. 1985 May 17;834(3):331–335. doi: 10.1016/0005-2760(85)90006-2. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta. 1986 Jun 13;858(1):161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Phillips M. C. Cholesterol desorption from clusters of phosphatidylcholine and cholesterol in unilamellar vesicle bilayers during lipid transfer or exchange. Biochemistry. 1982 Aug 17;21(17):4053–4059. doi: 10.1021/bi00260a022. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Phillips M. C. Cholesterol transfer from small and large unilamellar vesicles. Biochim Biophys Acta. 1984 Sep 19;776(1):21–26. doi: 10.1016/0005-2736(84)90246-3. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Phillips M. C. Mechanism of cholesterol and phosphatidylcholine exchange or transfer between unilamellar vesicles. Biochemistry. 1981 May 12;20(10):2893–2900. doi: 10.1021/bi00513a028. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Inoue K., Nojima S. Transfer of cholesterol between liposomal membranes. Biochim Biophys Acta. 1979 May 17;553(2):307–319. doi: 10.1016/0005-2736(79)90234-7. [DOI] [PubMed] [Google Scholar]
- Nichols J. W. Low concentrations of bile salts increase the rate of spontaneous phospholipid transfer between vesicles. Biochemistry. 1986 Aug 12;25(16):4596–4601. doi: 10.1021/bi00364a021. [DOI] [PubMed] [Google Scholar]
- Poznansky M. J., Czekanski S. Cholesterol movement between human skin fibroblasts and phosphatidylcholine vesicles. Biochim Biophys Acta. 1982 Feb 23;685(2):182–190. doi: 10.1016/0005-2736(82)90096-7. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Poznansky M. J. The effect of non-receptor-mediated uptake of cholesterol on intracellular cholesterol metabolism in human skin fibroblasts. Biochem J. 1985 Dec 1;232(2):553–557. doi: 10.1042/bj2320553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Slotte J. P., Lundberg B. Transfer of [3H]cholesterol between lipid vesicles and rat arterial smooth muscle cells in vitro. Biochim Biophys Acta. 1983 Mar 1;750(3):434–439. doi: 10.1016/0005-2760(83)90182-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. D., Poznansky M. J. Cholesterol transfer between lipid vesicles. Effect of phospholipids and gangliosides. Biochem J. 1988 Apr 1;251(1):55–61. doi: 10.1042/bj2510055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg B. W., Silbert D. F. Sterol partitioning among intracellular membranes. Testing a model for cellular sterol distribution. J Biol Chem. 1983 Feb 25;258(4):2284–2289. [PubMed] [Google Scholar]
- Westerman J., Kamp H. H., Wirtz K. W. Phosphatidylcholine transfer protein from bovine liver. Methods Enzymol. 1983;98:581–586. doi: 10.1016/0076-6879(83)98185-5. [DOI] [PubMed] [Google Scholar]
- Wong M., Anthony F. H., Tillack T. W., Thompson T. E. Fusion of dipalmitoylphosphatidylcholine vesicles at 4 degrees C. Biochemistry. 1982 Aug 17;21(17):4126–4132. doi: 10.1021/bi00260a032. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Young J. E. Factors contributing to the distribution of cholesterol among phospholipid vesicles. J Biol Chem. 1986 Jun 25;261(18):8175–8181. [PubMed] [Google Scholar]


