Abstract
Objective:
To evaluate trends and reproductive outcomes of gestational surrogacy in the United States.
Design:
Retrospective cohort study.
Setting:
Infertility clinics.
Patient(s):
IVF cycles transferring at least one embryo.
Intervention(s):
Use of a gestational carrier.
Main Outcome Measure(s):
Trends in gestational carrier cycles during 1999–2013, overall and for non-U.S. residents; reproductive outcomes for gestational carrier and nongestational carrier cycles during 2009–2013, stratified by the use of donor or nondonor oocytes.
Result(s):
Of 2,071,984 assisted reproductive technology (ART) cycles performed during 1999–2013, 30,927 (1.9%) used a gestational carrier. The number of gestational carrier cycles increased from 727 (1.0%) in 1999 to 3,432 (2.5%) in 2013. Among gestational carrier cycles, the proportion with non-U.S. residents declined during 1999–2005 (9.5% to 3.0%) but increased during 2006–2013 (6.3% to 18.5%). Gestational carrier cycles using nondonor oocytes had higher rates of implantation (adjusted risk ratio [aRR], 1.22; 95% confidence interval [CI], 1.17–1.26), clinical pregnancy (aRR, 1.14; 95% CI, 1.10–1.19), live birth (aRR, 1.17; 95% CI, 1.12–1.21), and preterm delivery (aRR, 1.14; 95% CI, 1.05–1.23) compared with nongestational carrier cycles. When using donor oocytes, multiple birth rates were higher among gestational carrier compared with nongestational carrier cycles (aRR, 1.13; 95% CI, 1.08–1.19).
Conclusion(s):
Use of gestational carriers increased during 1999–2013. Gestational carrier cycles had higher rates of ART success than nongestational carrier cycles, but multiple birth and preterm delivery rates were also higher. These risks may be mitigated by transferring fewer embryos given the higher success rates among gestational carrier cycles.
Keywords: Gestational carrier, surrogacy, in vitro fertilization (IVF), reproductive outcomes, multiple birth
Agestational carrier is a woman who bears a genetically unrelated child for another individual or couple (the intended parent[s]), usually through IVF, an assisted reproductive technology (ART) procedure involving the fertilization of oocytes outside the body and transferring the resulting embryo(s) into a woman’s uterus (1). The first reported successful pregnancy using a gestational carrier was in 1985 and has enabled those who cannot carry a pregnancy to have genetically related children (2). Since then, there has been growing interest in this form of ART. Little is known about the use of gestational carriers in the United States, the patients opting for gestational surrogacy, and the perinatal outcomes of these pregnancies compared with other ART cycles. Studies examining gestational carriers have been limited by small sample sizes or lack of appropriate comparison groups or have been conducted outside the United States (3–17).
Information on success rates and pregnancy outcomes of ART cycles using gestational carriers can help both intended parents and gestational carriers make informed decisions. Additionally, identifying current national estimates and trends for the use of gestational carriers can help inform policy makers in the realm of increasingly complex legal issues surrounding gestational surrogacy (18). The objectives of this study were to evaluate trends in ART cycles using a gestational carrier during 1999–2013 and to determine patient characteristics, ART treatment factors, and reproductive outcomes of gestational carrier cycles compared with cycles not using a gestational carrier.
MATERIALS AND METHODS
We used data from the Centers for Disease Control and Prevention’s (CDC) National ART Surveillance System (NASS). All U.S. fertility clinics performing ART are required to report annual data on all ART procedures to the CDC (19). The CDC estimates that NASS captures information on over 95% of all ART procedures performed in the United States (20). Typically, less than 5% of data have been shown to be inaccurately collected or entered according to the annual validation of 7%–10% of clinics (20). NASS collects cycle-specific information, and patients are not linked across multiple cycles. The unit of analysis for the current study was an ART cycle.
A gestational carrier was defined as a woman who gestates an embryo that did not develop from her oocyte, with the expectation of returning the infant to its intended parent(s). An intended parent was defined as the individual who was contracting with the gestational carrier and planning to be the social and legal parent of the child and may or may not be genetically related to the child (1).
We included all IVF cycles initiated between January 1, 1999, and December 31, 2013, where at least one embryo was transferred. We excluded ART cycles that were performed only for research purposes or for banking (ART cycles that are performed with the intention to freeze eggs or embryos for later use). Finally, cycles that had missing information on the above exclusion criteria were also excluded.
To explore trends in the use of gestational carriers, the number and percent of all IVF cycles using gestational carriers that resulted in transfer were plotted against the study year. The number and percent of all initiated cycles using gestational carriers regardless of whether they proceeded to ET were also plotted. To examine whether trends were a result of changes in the number of clinics performing gestational carrier cycles over time, the number and percent of clinics among all reporting clinics performing one or more gestational carrier cycles were plotted against study year. Given that many countries restrict gestational surrogacy (21), we examined trends in gestational carrier cycles among patients who were not residents of the United States, but using U.S. ART clinics, by restricting the study population to gestational carrier cycles and calculating the percent of these cycles with the intended parent reported to be a non-U.S. resident. Trends among non-U.S. residents were tested for two different periods, 1999–2005 and 2006–2013, owing to a change in trend in 2005. Statistically significant trends were determined using the Poisson regression.
We restricted all further analysis to the most recent years of data available, 2009–2013, to account for ART practice trends. We compared patient demographic characteristics and ART treatment factors for gestational carrier cycles and cycles not using a gestational carrier (nongestational carrier cycles). Infertility diagnoses were not mutually exclusive. Additionally, for infertility diagnosis designated as “other,” we examined free text entries for gestational carrier cycles and categorized them into non–mutually exclusive groups.
For nongestational carrier cycles, the patient was defined by reporting clinics as the woman undergoing the IVF cycle. For gestational carrier cycles, clinics defined the intended parent as the patient. However, in cases of male-male couples or single males using gestational carriers, clinics defined the gestational carrier as the patient and demographic information reported pertained to the carrier.
ART treatment factors included fresh versus frozen/thawed ET, donor versus nondonor oocytes, assisted hatching, intracytoplasmic sperm injection, preimplantation genetic diagnosis, stage of ET (day 2/3 or day 5/6 typically corresponding to cleavage- or blastocyst-stage embryos, respectively, or other), number of embryos transferred, elective single ET (the transfer of only one embryo when more than one embryo is available), and number of supernumerary embryos cryopreserved. Donor oocytes were retrieved from a donor and not derived from the gestational carrier or the intended parent. Nondonor oocytes were retrieved from the intended parent. The amount of missing data was less than 1% for all variables except for gestational carrier age (34.2%), donor age (56.2%), race/ethnicity (35.4%), U.S. residency status (2.7%), and the use of elective single ET (6.5%).
We compared the distribution of demographic characteristics and ART treatment factors between gestational carrier and nongestational carrier cycles using two-tailed χ2 tests with a significance level of P<.05. We assessed the rates of the following reproductive outcomes among gestational carrier and nongestational carrier cycles: among all ET procedures we calculated implantation (the maximum number of fetal heartbeats seen on ultrasound or infants born, whichever is greater, divided by the number of embryos transferred, multiplied by 100), clinical intrauterine pregnancy, and live-birth rates; among all clinical pregnancies we calculated miscarriage rates; and among all live births, we calculated multiple live-birth, preterm delivery, and low birth weight rates. We used log-binomial regression models with generalized estimating equations for correlated outcomes within clinics to calculate unadjusted and adjusted risk ratios (aRRs) and 95% confidence intervals (CIs) for the association between reproductive outcomes and use of a gestational carrier. All models were restricted to fresh cycles because many ART treatment variables that are associated with outcomes were not available for frozen cycles (e.g., day of embryo transfer). Because ART outcomes are improved with the use of donor oocytes (22, 23), we stratified our analysis by nondonor and donor oocyte cycles. Analysis of preterm delivery and low birthweight were also stratified by plurality. Data were analyzed using SAS 9.3. This research was approved by the Institutional Review Board at CDC.
RESULTS
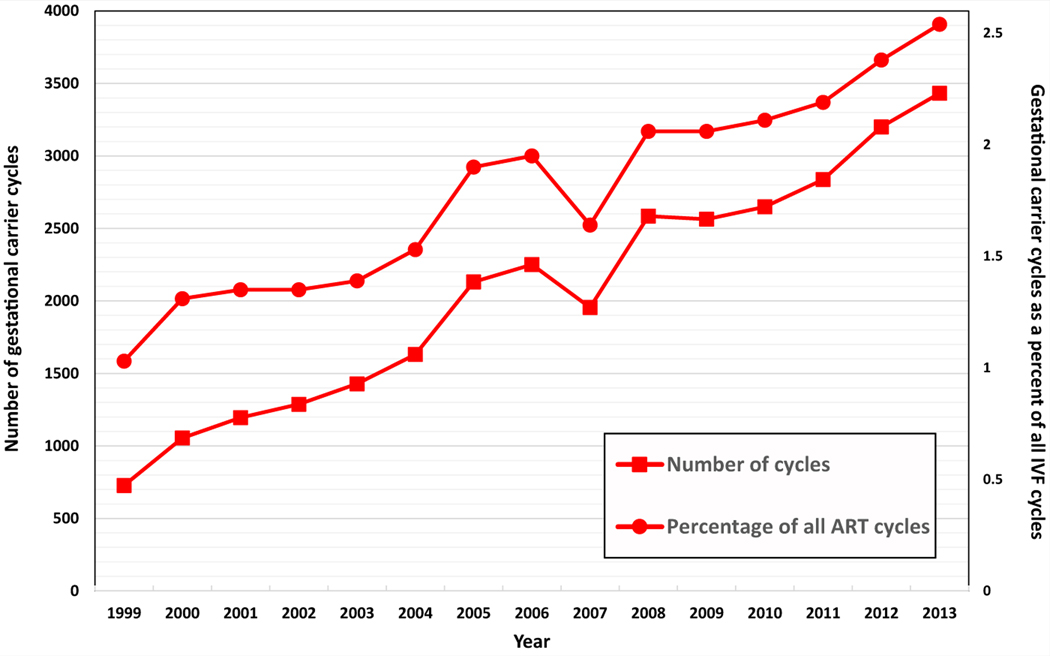
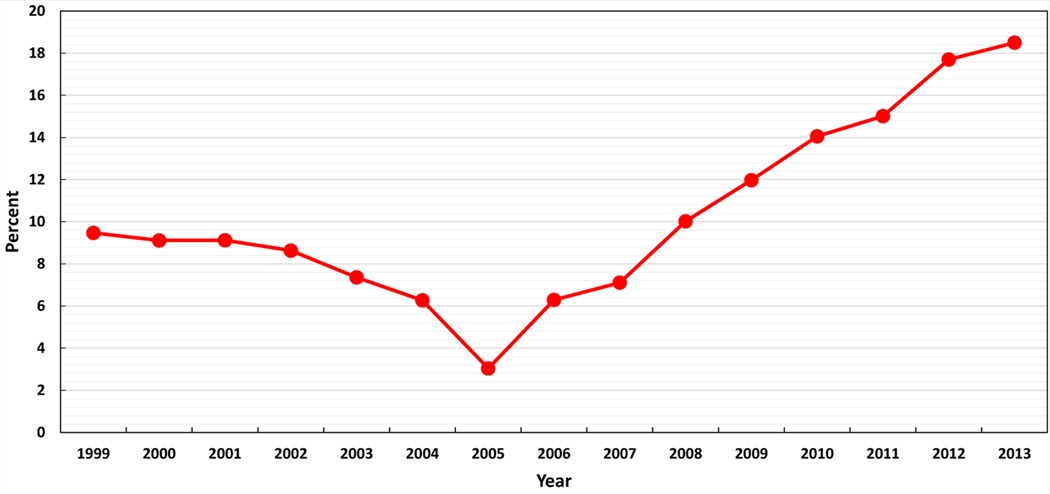
A total of 2,071,984 ART cycles were performed between 1999 and 2013. After applying our exclusion criteria, there were 1,664,844 cycles, of which 30,927 (1.9%) used a gestational carrier. Gestational carrier cycles resulted in 13,380 deliveries, of which 8,581 (64%) were singleton, 4,566 (34%) were twins, and 233 (2%) were triplet or greater, resulting in 18,400 infants, with 9,819 of these infants (53.4%) from multiple gestations. While gestational carrier cycles that resulted in ET in the United States increased from 727 (1.0%) in 1999 to 3,432 (2.5%) in 2013 (P for trend <.001; Fig. 1), there was an apparent decrease in 2007, followed by an increase thereafter. A similar increase was seen among all initiated gestational carrier cycles (Supplemental Fig. 1). The number of clinics performing one or more gestational carrier cycles among all reporting clinics in the United States increased from 167 (45.1%) in 1999 to 324 (69.4%) in 2013 (P for trend <.001, Supplemental Fig. 2). Figure 2 depicts the percent of non-U.S. intended parents among gestational carrier cycles by year. Although the proportion of non-U.S. residents among gestational carrier cycles decreased from 9.5% (n = 68) in 1999 to 3.0% (n = 59) in 2005 (P<.04), this proportion increased from 6.3% (n = 138) in 2006 to 18.5% (n = 619) in 2013 (P<.001).
FIGURE 1.
Number and percent of gestational carrier cycles, United States, 1999–2013. P<.001.
FIGURE 2.
Percent of gestational carrier ART cycles where intended parent was a non-U.S. resident, United States, 1999–2013. P=.04 (1999–2005); P<.001 (2006–2013).
All further analyses were restricted to cycles performed during 2009–2013 (n = 648,457). During this time, there were 14,682 (2.3%) gestational carrier cycles (Table 1). Compared with nongestational carrier cycles, a greater proportion of intended parents in gestational carrier cycles were 44 years or older (23.5% vs. 6.7%). In contrast, the majority of gestational carriers were younger than 35 years. Among gestational carrier cycles, intended parents were more likely to be non-U.S. residents compared with patients from nongestational carrier cycles (15.7% vs. 1.8%). Gestational carrier cycles also had a higher proportion with two or more prior ART cycles, prior spontaneous abortions, pregnancies, and live births among intended parents compared with nongestational carrier cycles.
TABLE 1.
Population characteristics and ART treatment factors of gestational carrier cycles and nongestational carrier cycles, United States, 2009–2013.
| Variable | Gestational carrier cycles, n (%) | Nongestational carrier cycles, n (%) | P value (χ2) |
|---|---|---|---|
| No. of cycles | 14,682 (2.3) | 633,775 (97.7) | |
| Patient factorsa | |||
| Age, y | |||
| <30 | 987 (6.7) | 71,463 (11.3) | < .001 |
| 30–34 | 2,783 (19.0) | 189,600 (29.9) | |
| 35–37 | 2,508 (17.1) | 130,082 (20.5) | |
| 38–40 | 2,663 (18.1) | 119,347(18.8) | |
| 41–43 | 2,295 (15.6) | 80,745 (12.7) | |
| 44+ | 3,443 (23.5) | 42,538 (6.7) | |
| Age of gestational carrier, y | NA | NA | |
| <30 | 3,655 (24.9) | ||
| 30–34 | 4,902 (33.4) | ||
| 35–37 | 906 (6.2) | ||
| 38–40 | 105 (0.7) | ||
| 41–43 | 65 (0.4) | ||
| 44+ | 27 (0.2) | ||
| Missing | 5,022 (34.2) | ||
| Age of donor, yb | .08 | ||
| <35 | 2,901 (39.4) | 34,051 (43.4) | |
| 35+ | 40 (0.5) | 627 (0.8) | |
| Missing | 4,422 (60.1) | 43,845 (55.8) | |
| Race/ethnicity | < .001 | ||
| White (non-Hispanic) | 7,092 (48.3) | 287,710 (45.4) | |
| Black (non-Hispanic) | 378 (2.6) | 28,308 (4.5) | |
| Asian | 1,191 (8.1) | 55,994 (8.8) | |
| Hispanic | 850 (5.6) | 36,473 (5.8) | |
| Other | 6 (0.04) | 1,047 (0.2) | |
| Missing/unknown | 5,165 (35.2) | 224,243 (35.4) | |
| Residency of intended parent | < .001 | ||
| U.S. | 11,876 (84.3) | 606,480 (98.2) | |
| Non-U.S. | 2,216 (15.7) | 11,102(1.8) | |
| Prior ART cycles | < .001 | ||
| 0 | 4,920 (33.6) | 259,935 (41.1) | |
| 1 | 3,206 (21.9) | 164,099 (25.9) | |
| 2+ | 6,527 (44.5) | 209,204 (33.0) | |
| Prior spontaneous abortionsc | < .001 | ||
| 0 | 9,957 (67.8) | 423,359 (66.8) | |
| 1 | 2,384 (16.2) | 133,227 (21.0) | |
| 2+ | 2,340 (15.9) | 77,182 (12.2) | |
| Prior pregnancies | < .001 | ||
| 0 | 6,039 (41.5) | 245,150 (38.8) | |
| 1 | 2,969 (20.4) | 179,587 (28.4) | |
| 2+ | 5,537 (38.1) | 206,763 (32.7) | |
| Prior live births | < .001 | ||
| 0 | 9,569 (65.9) | 418,440 (66.4) | |
| 1 | 3,027 (20.9) | 158,824 (25.2) | |
| 2+ | 1,918 (13.2) | 52,833 (8.4) | |
| Infertility diagnosisd | |||
| Male factor | 1,712 (11.7) | 224,276 (35.4) | < .001 |
| Tubal factore | 720 (4.9) | 92,896 (14.7) | < .001 |
| Endometriosis | 676 (4.6) | 63,269 (10.0) | < .001 |
| Uterine factor | 3,907 (26.6) | 30,825 (4.9) | < .001 |
| Ovulatory disorderf | 717 (4.9) | 94,004 (14.8) | < .001 |
| Diminished ovarian reserve | 4,617 (31.5) | 175,268 (27.7) | < .001 |
| Unexplained | 732 (5.0) | 82,833 (13.1) | < .001 |
| Other | 6,842 (46.6)g | 84,252 (13.3) | < .001 |
| ART treatment factors | |||
| Cycle type | |||
| Fresh | 7,539 (51.4) | 444,084 (70.1) | < .001 |
| Frozen-thawed | 7,143 (48.7) | 189,691 (29.9) | |
| Nondonor oocyte | 7,319 (49.9) | 555,252 (87.6) | < .001 |
| Donor oocyte | 7,363 (50.2) | 78,523 (12.4) | |
| Use of assisted hatching | .06 | ||
| No | 8,453 (57.6) | 359,885 (56.8) | |
| Yes | 6,229 (42.4) | 273,890 (43.2) | |
| Use of intracytoplasmic sperm injectionh | < .001 | ||
| No | 1,582 (21.0) | 108,231 (24.4) | |
| Yes | 5,951 (79.0) | 334,983 (75.6) | |
| Use of preimplantation genetic diagnosish | < .001 | ||
| No | 6,639 (88.5) | 423,100 (95.8) | |
| Yes | 862 (11.5) | 18,612 (4.2) | |
| Day of ETh | |||
| Day 2/3 | 2,490 (33.0) | 225,321 (50.7) | < .001 |
| Day 5/6 | 4,737 (62.8) | 208,138 (46.9) | |
| Other | 312 (4.1) | 10,624 (2.4) | |
| No. of embryos transferred | < .001 | ||
| 1 | 3,149 (21.5) | 140,456 (22.2) | |
| 2 | 8,865 (60.4) | 346,207 (54.6) | |
| 3 | 1,995 (13.6) | 104,909 (16.6) | |
| 4+ | 672 (4.6) | 43,167 (6.7) | |
| Elective single ETi | < .001 | ||
| No | 11,940 (81.3) | 505,229 (79.7) | |
| Yes | 2,281 (15.5) | 86,537 (13.7) | |
| Missing | 461 (3.1) | 42,009 (6.6) | |
| No. of supernumerary embryos cryopreserved | |||
| 0 | 9,798 (67.2) | 436,726 (69.3) | < .001 |
| 1–2 | 1,509 (10.4) | 78,938 (12.5) | |
| 3–5 | 1,645 (11.3) | 68,638 (10.9) | |
| 6+ | 1,621 (11.1) | 46,169 (7.3) |
Note: NA = not applicable.
For gestational carrier cycles, the patient is the intended parent.
Only if donor oocyte used.
Pregnancy loss at <20 weeks’ gestation.
Categories are not mutually exclusive.
Includes hydrosalpinx, tubal ligation (not reversed), and other tubal disease (not hydrosalpinx).
Includes polycystic ovary syndrome.
Only 701 (10.3%) had a free text diagnosis entered, with most (n = 359, 47.3%) only noting the use of a gestational carrier.
Restricted to fresh cycles because variable not collected for frozen cycles.
Defined as the transfer of only one embryo when more than one high-quality embryo is available.
Infertility diagnosis varied between gestational and nongestational carrier cycles. Close to half (46.6%) of gestational carrier cycles had “other” reported for infertility diagnosis. However, of these 6,842 cycles, only 701 (10.3%) had a free text diagnosis entered, with most (n = 359, 47.3%) only noting the use of a gestational carrier, 11.6% reporting other nonspecific reasons (i.e., family balancing, previous failed ART cycles), 10.5% reporting male same-sex couples or absence of a female partner, 9.5% reporting advanced maternal age, 9.4% reporting medical conditions that make pregnancy unsafe (i.e., kidney disease, cardiac disease), 6.3% reporting reasons compatible with uterine factor infertility (i.e., hysterectomy, Asherman’s syndrome), 2.9% reporting recurrent pregnancy loss, 1.7% reporting a history of pregnancy complications (i.e., Hemolysis, Elevated Liver enzymes, Low Platelet count [HELLP] syndrome), and 0.8% reporting genetic issues. Diminished ovarian reserve (31.5%) and uterine factor infertility (26.6%) were the second most common infertility diagnoses reported among gestational carrier cycles. The most common infertility diagnoses reported among nongestational carrier cycles were male factor (35.4%) and diminished ovarian reserve (27.7%).
Gestational carrier cycles had a higher proportion of frozen/thawed cycles compared with nongestational carrier cycles (48.7% vs. 29.9%). More than half (50.2%) of gestational carrier cycles used donor oocytes, compared with only 12.4% among nongestational carrier cycles. The use of preimplantation genetic diagnosis was higher among gestational carrier cycles compared with nongestational carrier cycles (11.5% vs. 4.2%). Additionally, day 5/6 ETs were most common among gestational carrier cycles (62.8%), while day 2/3 ETs were most common among nongestational carrier cycles (50.7%). The transfer of two embryos was also more common among gestational carrier cycles than among nongestational carrier cycles (60.4% vs. 54.6%), and a higher percentage of gestational carrier cycles had six or more embryos cryopreserved (11.1% vs. 7.3%).
Among cycles using fresh, nondonor oocytes, gestational carrier cycles had higher rates of the following reproductive outcomes compared with nongestational carrier cycles (Table 2): implantation (aRR, 1.22; 95% CI, 1.17–1.26), clinical pregnancy (aRR, 1.14; 95% CI, 1.10–1.19), live birth (aRR, 1.17; 95% CI, 1.12–1.21), and preterm delivery (aRR, 1.14; 95% CI, 1.05–1.23). When the risk for preterm delivery was stratified by plurality, multiple births accounted for the increased risk of preterm delivery (singletons: aRR, 1.01; 95% CI, 0.87–1.18; multiples: aRR, 1.12; 95% CI, 1.05–1.20). There was no difference in the risk of miscarriage (aRR, 0.99; 95% CI, 0.90–1.09), multiple live birth (aRR, 1.07; 95% CI, 1.00–1.15), or low birth weight (aRR, 0.93; 95% CI, 0.85–1.01). When the risk for low birth weight was stratified by plurality, however, gestational carrier cycles conferred a protective effect among singleton births but not among multiple births (singletons: aRR, 0.71; 95% CI, 0.57–0.88; multiples: aRR, 0.93; 95% CI, 0.87–1.00).
TABLE 2.
Reproductive outcomes for gestational carrier and nongestational carrier cycles using fresh nondonor or fresh donor oocytes, United States, 2009–2013.
| Fresh nondonor oocytes | ||||||
|---|---|---|---|---|---|---|
| Gestational carrier | Nongestational carrier | |||||
| Variable | n | % | n | % | RR (95% CI) | aRRa (95% CI) |
| Among transfers | ||||||
| Implantation rateb | 2,462 | 30.3 | 224,974 | 25.9 | 1.17 (1.11–1.22) | 1.22 (1.17–1.26) |
| Clinical pregnancy | 1,918 | 51.8 | 178,557 | 44.7 | 1.16 (1.12–1.20) | 1.14 (1.10–1.19) |
| Live births | 1,537 | 41.5 | 145,963 | 36.5 | 1.14 (1.09–1.18) | 1.17 (1.12–1.21) |
| Among pregnancies, miscarriage | 347 | 18.2 | 28,729 | 16.2 | 1.13 (1.02–1.24) | 0.99 (0.90–1.09) |
| Among live births | ||||||
| Multiple live birth | 466 | 30.3 | 41,939 | 28.7 | 1.06 (0.98–1.14) | 1.07 (1.00–1.15) |
| Preterm delivery | 472 | 30.8 | 37,899 | 26.0 | 1.18 (1.10–1.28) | 1.14 (1.05–1.23) |
| Singletons | 150 | 14.0 | 12,513 | 12.0 | 1.16 (1.00–1.35) | 1.01 (0.87–1.18) |
| Multiples | 322 | 69.1 | 25,386 | 60.6 | 1.14 (1.07–1.21) | 1.12 (1.05–1.20) |
| Low birth weight (in any infant) | 383 | 25.6 | 38,704 | 27.0 | 0.95 (0.87–1.03) | 0.93 (0.85–1.01) |
| Singletons | 79 | 7.6 | 9,698 | 9.5 | 0.80 (0.65–0.99) | 0.71 (0.57–0.88) |
| Multiples | 304 | 66.8 | 29,006 | 70.7 | 0.94 (0.88–1.01) | 0.93 (0.87–1.00) |
| Fresh donor oocytes | ||||||
| n | % | N | % | RR (95% CI) | aRRc (95% CI) | |
| Among transfers | ||||||
| Implantation rateb | 3,825 | 53.3 | 38,450 | 47.4 | 1.12 (1.07–1.18) | 1.11 (1.07–1.15) |
| Clinical pregnancy | 2,669 | 69.7 | 28,898 | 65.0 | 1.07 (1.04–1.10) | 1.05 (1.03–1.08) |
| Live births | 2,320 | 60.5 | 24,537 | 55.2 | 1.10 (1.06–1.13) | 1.08 (1.05–1.11) |
| Among pregnancies, miscarriage | 303 | 11.4 | 3,857 | 13.4 | 0.85 (0.76–0.96) | 0.87 (0.77–0.97) |
| Among live births | ||||||
| Multiple live birth | 987 | 42.5 | 8,615 | 35.1 | 1.21 (1.14–1.29) | 1.13 (1.08–1.19) |
| Preterm delivery | 757 | 32.7 | 8,080 | 33.0 | 0.99 (0.92–1.06) | 0.96 (0.89–1.02) |
| Singletons | 181 | 13.6 | 2,532 | 15.9 | 0.85 (0.72–1.00) | 0.84 (0.71–0.98) |
| Multiples | 576 | 58.6 | 5,548 | 64.6 | 0.91 (0.85–0.97) | 0.91 (0.86–0.97) |
| Low birth weight (in any infant) | 642 | 29.8 | 7,707 | 32.2 | 0.92 (0.86–0.99) | 0.89 (0.83–0.95) |
| Singletons | 106 | 8.5 | 1,752 | 11.3 | 0.75 (0.61–0.93) | 0.75 (0.60–0.93) |
| Multiples | 536 | 59.0 | 5,955 | 71.0 | 0.83 (0.78–0.89) | 0.84 (0.79–0.90) |
Models were adjusted for patient age, number of prior ART cycles, number of prior spontaneous abortions, number of prior live births, infertility diagnosis, use of assisted hatching, use of intracytoplasmic sperm injection, use of preimplantation genetic diagnosis, day of ET, number of embryos transferred, and number of embryos cryopreserved.
Calculated as the maximum of the number of fetal heartbeats or infants born divided by the number of embryos transferred, multiplied by 100.
Models were adjusted for use of assisted hatching, use of intracytoplasmic sperm injection, use of preimplantation genetic diagnosis, day of ET, number of embryos transferred, and number of embryos cryopreserved.
Among cycles using fresh, donor oocytes (Table 2), adjusted analyses similarly demonstrated higher rates of reproductive outcomes among gestational carrier cycles compared with nongestational carrier cycles as seen among fresh, nondonor cycles; however, there was no difference for the risk of preterm delivery (aRR, 0.96; 95% CI, 0.89–1.02), although stratification by plurality suggests a lower risk for preterm delivery when using gestational carriers among singleton deliveries (aRR, 0.84; 95% CI, 0.71–0.98) and, to a lesser degree, multiple deliveries (aRR, 0.91; 95% CI, 0.86–0.97). Additionally, among live births, the risk for multiple birth was higher (aRR, 1.13; 95% CI, 1.08–1.19), and among pregnancies, the risk for miscarriage was lower (aRR, 0.87; 95% CI, 0.77–0.97) among gestational carrier cycles compared with nongestational carrier cycles. Overall, aRRs for implantation, clinical pregnancy, and live-birth rates were attenuated for cycles using fresh donor oocytes cycles versus cycles using fresh nondonor oocytes. Additionally, gestational carrier cycles using donor oocytes had a lower risk of low birth weight compared with nongestational carrier cycles (aRR, 0.89; 95% CI. 0.83–0.95) among both singleton and multiple births (singletons: aRR, 0.75; 95% CI, 0.60–0.93; multiples: aRR, 0.84; 95% CI, 0.79–0.90). Adjusted RRs were similar when adjusting for donor age (data not shown).
DISCUSSION
Our study, using national data, revealed an increase in the number of gestational carrier cycles during 1999–2013. We found that the number of IVF cycles using gestational carriers in the United States has more than quadrupled since 1999 and accounted for over 18,000 infants born. The reasons for this increase are unclear but may be due to the growing number of states with court cases that have established some legal framework for gestational surrogacy (24), an increasing number of clinics that are performing gestational carrier cycles, and greater awareness and acceptance of the practice. The rapidly rising number of patients who are not U.S. residents using gestational carriers in the United States is also striking and may be due to the fact that the United States is one of the few industrialized countries that does not federally prohibit compensated gestational surrogacy, although regulations do vary by state (25, 26).
We also found that, among ETs, gestational carrier cycles had higher rates of implantation, pregnancy, and live birth compared with nongestational carrier cycles; associations with gestational carrier status were slightly higher when non-donor oocytes were used even after adjusting for patient age. Higher rates of ART success associated with gestational carrier cycles in our study are likely due to several factors. Women serving as gestational carriers were younger than patients not using gestational carriers, with the majority of gestational carriers being less than 35 years old. Younger maternal age is associated with improved ART outcomes (27–29). Additionally, the American Society for Reproductive Medicine guidelines recommend that gestational carriers have had at least one prior, full-term, uncomplicated pregnancy (30). Demonstrating previous reproductive success may improve the chances of pregnancy and live birth in ART cycles using gestational carriers. Women who have had a successful pregnancy are also likely to be healthier and have other favorable patient characteristics, such as normal body mass index and nutritional status, that may improve reproductive outcomes compared with their infertile counterparts (31).
The higher rates of implantation among gestational carrier cycles combined with the frequent transfer of two or more embryos in these cycles contribute to the higher risk detected for multiple live birth. Almost 80% of cycles involved the transfer two or more embryos, and less than 20% opted for elective single ET. Multifetal pregnancies are associated with elevated risks to mothers, including increased risk of hypertensive disorders, hemorrhage, cesarean delivery, and peripartum hysterectomy (32–34). We were unable to assess adverse maternal pregnancy outcomes among our study population because NASS does not currently collect this information.
Oocyte source also plays an important role in reproductive outcomes. The magnitude of the effect estimates for implantation, clinical pregnancy, and live birth among gestational carrier cycles compared with nongestational carrier cycles in our study was somewhat higher with nondonor oocytes, likely because donor oocytes independently improve ART outcomes (23). A significantly higher risk of multiple births among gestational carriers, however, was detected only for cycles using donor oocytes, likely due to overall higher implantation rates among donor cycles. Additionally, sample sizes were smaller among gestational carrier cycles using nondonor oocytes than among those using donor oocytes, which may have limited our power to detect significant differences in multiple live-birth rates among nondonor cycles. The risk of preterm delivery was 14% higher among gestational carrier births using nondonor oocytes; but when using donor oocytes, the increased risk of multiple birth in gestational carrier cycles did not seem to confer an increased risk of preterm delivery and was associated with an 11% decreased risk of low birth weight compared with nongestational carrier births. The use of donor oocytes has been associated with improved rates of the birth of term, healthy weight infants (22).
Our study was subject to some limitations. NASS began collecting age of gestational carriers in 2007, and this is the only demographic information that is gathered on these women. Because NASS does not routinely collect information on whether a cycle is for a male-male couple or for a single male, we were only able to identify such cycles if this was mentioned in the free text field for infertility diagnosis. Therefore, we were unable to consistently distinguish gestational carrier cycles where demographic information collected pertained to the intended parent or to the gestational carrier. As a result, our findings may underestimate the difference in reproductive outcomes between gestational carrier and nongestational carrier cycles. Additionally, we have no way of knowing whether a gestational carrier is genetically related to a patient, which could also affect ART outcomes. NASS does not currently explicitly collect information on the indication for using a gestational carrier, and almost half of all gestational carrier cycles noted “other” as the reason for infertility. This makes differences in reproductive outcomes difficult to interpret as outcomes would likely differ based on indications for using a gestational carrier. Accordingly, NASS plans to collect information on gestational carrier indication in the future. Finally, given our large sample size, some of the statistically significant differences detected may not be clinically relevant. However, small improvements in outcomes such as live-birth rates can be substantial to patients.
Despite these limitations, our study adds much needed information to the limited existing data on the trends and outcomes of gestational surrogacy. A recent systematic review by Soderstrom-Anttila and colleagues including 55 studies that examined the medical and psychological outcomes of gestational carriers, intended parents, and babies rated all studies assessing reproductive and perinatal outcomes as low-quality evidence. The review revealed wide variability in results, and the investigators concluded that most studies suffer from “serious methodologic limitations,” given the small sample sizes and lack of appropriate comparison groups in most (3).
Although the use of gestational carriers was associated with improved implantation, pregnancy, and live-birth rates, there were concomitant increases in the risks for multiple birth and preterm delivery. The elevated risk of multiple birth among gestational carriers can potentially be mitigated by the transfer of fewer embryos given the higher chances of ART success among these cycles. Increasing the use of elective single ET among gestational carrier cycles may decrease neonatal and maternal morbidity risk. The health and future reproductive potential of gestational carriers warrants further study to protect the well-being of these women. With the dramatic increase of gestational carrier cycles in the United States, more detailed information on gestational carrier cycles may help better understand the risks and benefits of gestational surrogacy for intended parents, babies, and gestational carriers and may help inform U.S. policy decisions.
Supplementary Material
SUPPLEMENTAL FIGURE 2
Number and percent of reporting clinics performing gestational carrier cycles, United States, 1999–2013. P<.001.
SUPPLEMENTAL FIGURE 1
Number and percent of initiated gestational carrier cycles among all initiated cycles, United States, 1999–2013.
Acknowledgments:
The authors thank Richard B. Vaughn, Esq., and Aaron D. Levine, Ph.D., for their helpful review of this manuscript. Mr. Vaughn and Dr. Levine received no compensation for their contributions.
Footnotes
K.M.P. has nothing to disclose. S.L.B. has nothing to disclose. D.J.J. has nothing to disclose. D.M.K. has nothing to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/perkinsk-gestational-surrogacy-united-states/
REFERENCES
- 1.American Society for Reproductive Medicine. Consideration of the gestational carrier: a committee opinion. Fertil Steril 2013;99:1838–41. [DOI] [PubMed] [Google Scholar]
- 2.Utian WH, Sheean L, Goldfarb JM, Kiwi R. Successful pregnancy after in vitro fertilization and embryo transfer from an infertile woman to a surrogate. N Engl J Med 1985;313:1351–2. [DOI] [PubMed] [Google Scholar]
- 3.Soderstrom-Anttila V, Wennerholm UB, Loft A, Pinborg A, Aittomaki K, Romundstad LB, et al. Surrogacy: outcomes for surrogate mothers, children and the resulting families-a systematic review. Hum Reprod Update 2016; 22:260–76. [DOI] [PubMed] [Google Scholar]
- 4.Utian WH, Goldfarb JM, Kiwi R, Sheean LA, Auld H, Lisbona H. Preliminary experience with in vitro fertilization-surrogate gestational pregnancy. Fertil Steril 1989;52:633–8. [DOI] [PubMed] [Google Scholar]
- 5.Marrs RP, Ringler GE, Stein AL, Vargyas JM, Stone BA. The use of surrogate gestational carriers for assisted reproductive technologies. Am J Obstet Gynecol 1993;168:1858–61. discussion 61–3. [DOI] [PubMed] [Google Scholar]
- 6.Meniru GI, Craft IL. Experience with gestational surrogacy as a treatment for sterility resulting from hysterectomy. Hum Reprod 1997;12:51–4. [DOI] [PubMed] [Google Scholar]
- 7.Parkinson J, Tran C, Tan T, Nelson J, Batzofin J, Serafini P. Perinatal outcome after in-vitro fertilization-surrogacy. Hum Reprod 1999;14:671–6. [DOI] [PubMed] [Google Scholar]
- 8.Corson SL, Kelly M, Braverman AM, English ME. Gestational carrier pregnancy. Fertil Steril 1998;69:670–4. [DOI] [PubMed] [Google Scholar]
- 9.Brinsden PR. Gestational surrogacy. Hum Reprod Update 2003;9:483–91. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb JM, Austin C, Peskin B, Lisbona H, Desai N, de Mola JR. Fifteen years experience with an in-vitro fertilization surrogate gestational pregnancy programme. Hum Reprod 2000;15:1075–8. [DOI] [PubMed] [Google Scholar]
- 11.Soderstrom-Anttila V, Blomqvist T, Foudila T, Hippelainen M, Kurunmaki H, Siegberg R, et al. Experience of in vitro fertilization surrogacy in Finland. Acta Obstet Gynecol Scand 2002;81:747–52. [PubMed] [Google Scholar]
- 12.Duffy DA, Nulsen JC, Maier DB, Engmann L, Schmidt D, Benadiva CA. Obstetrical complications in gestational carrier pregnancies. Fertil Steril 2005;83:749–54. [DOI] [PubMed] [Google Scholar]
- 13.Raziel A, Schachter M, Strassburger D, Komarovsky D, Ron-El R, Friedler S. Eight years’ experience with an IVF surrogate gestational pregnancy programme. Reprod Biomed Online 2005;11:254–8. [DOI] [PubMed] [Google Scholar]
- 14.Smotrich DB, Ross RJ, Arnold LL, Batzofin D. Gestational surrogacy—ART’s stepchild. Fertil Steril 2008;90:S387–8. [Google Scholar]
- 15.Dermout S, van de Wiel H, Heintz P, Jansen K, Ankum W. Non-commercial surrogacy: an account of patient management in the first Dutch Centre for IVF Surrogacy, from 1997 to 2004. Hum Reprod 2010;25: 443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Check JH, Katsoff B, Brasile D, Wilson C, Summers-Chase D. Comparison of pregnancy outcome following frozen embryo transfer (ET) in a gestational carrier program according to source of the oocytes. Clin Exp Obstet Gynecol 2011;38:26–7. [PubMed] [Google Scholar]
- 17.Dar S, Lazer T, Swanson S, Silverman J, Wasser C, Moskovtsev SI, et al. Assisted reproduction involving gestational surrogacy: an analysis of the medical, psychosocial and legal issues: experience from a large surrogacy program. Hum Reprod 2015;30:345–52. [DOI] [PubMed] [Google Scholar]
- 18.James S, Chilvers R, Havemann D, Phelps JY. Avoiding legal pitfalls in surrogacy arrangements. Reprod Biomed Online 2010;21:862–7. [DOI] [PubMed] [Google Scholar]
- 19.Fertility Clinic Success Rate and Certification Act of 1992 PL-, 1063 Stat 146–3152. [Google Scholar]
- 20.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2012 Assisted Reproductive Technology Fertility Clinic Success Rates Report. Atlanta (GA): Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 21.Bromfield N, Rotabi K. Global surrogacy, exploitation, human rights and international private law: a pragmatic stance and policy recommendations. Glob Soc Welf 2014;1:123–35. [Google Scholar]
- 22.Kawwass JF, Monsour M, Crawford S, Kissin DM, Session DR, Kulkarni AD, et al. Trends and outcomes for donor oocyte cycles in the United States, 2000–2010. JAMA 2013;310:2426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh JS, Steward RG, Dude AM, Shah AA, Goldfarb JM, Muasher SJ. Pregnancy rates in donor oocyte cycles compared to similar autologous in vitro fertilization cycles: an analysis of 26,457 fresh cycles from the Society for Assisted Reproductive Technology. Fertil Steril 2014;102: 399–404. [DOI] [PubMed] [Google Scholar]
- 24.Creative Family Connections. Surrogacy Law by State; 2015. Available at: http://creativefamilyconnections.com/surrogacy-law-by-state/#. Accessed November 13, 2015. [Google Scholar]
- 25.Armour KL. An overview of surrogacy around the world: trends, questions and ethical issues. Nurs Women’s Health 2012;16:231–6. [DOI] [PubMed] [Google Scholar]
- 26.Burrell C, Edozien LC. Surrogacy in modern obstetric practice. Semin Fetal Neonatal Med 2014;19:272–8. [DOI] [PubMed] [Google Scholar]
- 27.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med 2009;360:236–43. [DOI] [PubMed] [Google Scholar]
- 28.Schieve LA, Peterson HB, Meikle SF, Jeng G, Danel I, Burnett NM, et al. Live-birth rates and multiple-birth risk using in vitro fertilization. JAMA 1999;282: 1832–8. [DOI] [PubMed] [Google Scholar]
- 29.Pantos K, Athanasiou V, Stefanidis K, Stavrou D, Vaxevanoglou T, Chronopoulou M. Influence of advanced age on the blastocyst development rate and pregnancy rate in assisted reproductive technology. Fertil Steril 1999;71:1144–6. [DOI] [PubMed] [Google Scholar]
- 30.Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the Society for Assisted Reproductive Technology. Recommendations for practices utilizing gestational carriers: a committee opinion. Fertil Steril 2015;103:e1–8. [DOI] [PubMed] [Google Scholar]
- 31.Jack BW, Culpepper L. Preconception care. Risk reduction and health promotion in preparation for pregnancy. JAMA 1990;264:1147–9. [DOI] [PubMed] [Google Scholar]
- 32.Conde-Agudelo A, Belizan JM, Lindmark G. Maternal morbidity and mortality associated with multiple gestations. Obstet Gynecol 2000;95: 899–904. [PubMed] [Google Scholar]
- 33.Spiliopoulos M, Kareti A, Jain NJ, Kruse LK, Hanlon A, Dandolu V. Risk of peripartum hysterectomy by mode of delivery and prior obstetric history: data from a population-based study. Arch Gynecol Obstet 2011;283: 1261–8. [DOI] [PubMed] [Google Scholar]
- 34.Francois K, Ortiz J, Harris C, Foley MR, Elliott JP. Is peripartum hysterectomy more common in multiple gestations? Obstet Gynecol 2005;105: 1369–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 2
Number and percent of reporting clinics performing gestational carrier cycles, United States, 1999–2013. P<.001.
SUPPLEMENTAL FIGURE 1
Number and percent of initiated gestational carrier cycles among all initiated cycles, United States, 1999–2013.