Abstract
Introduction:
We investigated outcomes and prognostic factors for patients treated for cutaneous angiosarcoma (CA).
Methods:
We conducted a retrospective review of patients treated for CA of the face and scalp from 1962–2019. All received definitive treatment with surgery, radiation (RT), or a combination (S-XRT). The Kaplan-Meier method was used to estimate outcomes. Multivariable analyses were conducted using the Cox proportional hazards model.
Results:
For the 143 patients evaluated median follow-up was 33 months. Five-year LC was 51% and worse in patients with tumors >5 cm, multifocal tumors, those treated pre-2000, and with single modality therapy (SMT). These remained associated with worse LC on multivariable analysis. The 5-yr DSS for the cohort was 56%. Tumor size >5 cm, non-scalp primary site, treatment pre-2000, and SMT were associated with worse DSS.
Conclusion:
Large or multifocal tumors are negative prognostic factors in patients with head and neck CA. S-XRT improved outcomes.
INTRODUCTION:
Angiosarcomas are rare vascular tumors of mesenchymal origin that account for 1–2% of soft tissue sarcomas (STS) (1, 2). Cutaneous angiosarcoma (CA) is the most common type of angiosarcoma, with the head and neck being the most common anatomic location (3). Outcomes are poor, with the estimated 5-year overall survival for CA arising from the head and neck ranging from 10% to 45% (3–8). Diagnosis is often late, as initially patients may assume that they have a bruise or benign skin finding (5, 9, 10) or the primary tumor is obscured by hair on the scalp. In the context of disease being typically bulky and widely infiltrative at diagnosis, negative-margin surgical resection (R0) is often difficult to achieve (4). Multifocal disease is also common at presentation (6, 8), which further complicates local management. Additionally, nodal and distant metastases occur frequently, with lung and bone being most common (11–13).
Due to its rarity, there are no large prospective studies examining the optimal treatment for CA. However, prior retrospective studies have reported that aggressive local therapy consisting of surgical resection and adjuvant radiation therapy (RT) provides the best outcomes in patients with locoregionally confined disease at diagnosis (6–8, 11). Patients with unresectable disease at diagnosis have historically experienced overall poor outcomes (5). For these patients, definitive RT provides a degree of local control in lieu of surgery, but it remains unclear whether RT offers sufficiently durable local control (6, 8, 14, 15). Due to its propensity for distant metastases, the use of systemic therapy (e.g., chemotherapy or immunotherapy), in addition to local treatment, is often indicated for eligible patients. To date, there have been mixed results as to the utility of chemotherapy, and whether it improves disease-specific outcomes (6, 11, 16), but this is largely due to the rarity of the disease making it difficult to conduct clinical trials. In the unresectable setting, paclitaxel is well tolerated with good efficacy, and more recently a phase II study of oraxol demonstrated impressive efficacy with 0% of patients having progressive disease, and 22% with a complete response (CR) in non-metastatic patients (17). The goal of this study is to retrospectively analyze long-term outcomes in patients treated with curative intent for CA of the head and neck.
METHODS:
After obtaining IRB approval, we performed a retrospective review of all patients treated at our tertiary cancer center for non-metastatic CA of the face and scalp between 1962 and 2019. Patients were eligible if they received definitive local treatment either with surgery, RT, or combined modality therapy (S-XRT), consisting of surgery and RT. We identified 143 patients who met these criteria and abstracted, from the medical record, clinical characteristics, treatment course, and outcomes. At diagnosis all patients underwent physical examination, routine blood tests, and appropriate imaging before treatment initiation, typically consisting of a CT Head and Neck with contrast to delineate the extent of primary disease, and a CT Chest or chest radiograph (older treatment era) to rule out the presence of lung metastases. A histopathologic diagnosis of angiosarcoma was confirmed in each case through review of the slides by an expert sarcoma pathologist at our institution.
Statistical analysis and follow-up
Follow-up time for survival analyses was computed from the last local therapy date: last RT date for those treated with definitive RT or post-operative RT or the surgical date for those patients treated with preoperative RT or surgery alone. The Kaplan-Meier method was used to calculate actuarial curves for local control (LC), distant metastasis-free survival (DMFS), disease-free survival (DFS), disease-specific survival (DSS), overall survival (OS) and development of treatment-related complications (18). The log-rank statistic was used for univariable analyses. Covariates examined included: age, gender, tumor size, primary site location (scalp versus face/other), multifocality at presentation, nodal disease at presentation, local therapy approach, surgical resection margin for those undergoing surgery, treatment era (pre-2000 and after 2000) and receipt of chemotherapy. Local control was defined as any skin recurrence in the head and neck. Surgical and RT-related toxicities were retrospectively categorized into mild (requiring no treatment, noted by provider or patient during follow up), moderate (requiring medical management), or severe (requiring surgical management or hospitalization). Differences between demographic and treatment variables were analyzed using chi-square test or Fisher’s exact test as appropriate (19). Multivariable analyses were conducted using Cox proportional hazards model. The analyses were conducted using Python version 3.7 (Python Software Foundation).
RESULTS
Patients
Among 143 patients evaluated, the median age at diagnosis was 70 years (IQR 64–76 years). The median follow-up time for those alive at last follow-up was 33 months (IQR 16–58). Most patients (n=100, 70%) were treated after 2000, and the majority were male (n=109, 76%). The distribution of primary lesions was as follows: 87 (61%) scalp, 53 (37%) face/forehead, and 3 (2%) other sites (1 ear, and 2 peri-parotid skin/soft tissue). Maximum tumor dimension was documented in 124 patients and was median 3.0 cm (IQR 1.5–5.0). Fifty-nine (41%) patients had a multifocal tumor at the primary site, and 20 (14%) had nodal disease at diagnosis (Table 1).
Table 1:
Demographics and patient characteristics of patients treated with definitive intent for locoregionally confined angiosarcoma of the head and neck
| Characteristic | Number of patients (%) |
|---|---|
| Age | |
| ≤70 | 72 (50.3%) |
| >70 | 71 (49.7%) |
| Gender | |
| Male | 109 (76.2%) |
| Female | 34 (23.8%) |
| Tumor size | |
| < 5cm | 90 (72.5%) * |
| ≥ 5cm | 34 (27.4%) * |
| Site | |
| Scalp | 87 (60.8%) |
| Face/other | 56 (39.2%) |
| Multifocal at presentation | 59 (41.2%) |
| Nodal disease at diagnosis | 20 (14.0%) |
| Local Therapy | |
| Surgery alone | 24 (16.8%) |
| Radiation Alone | 50 (35.0%) |
| Surgery + Radiation (S-XRT) | 69 (48.2%) |
| Positive/Uncertain Resection Margin | 42 (45.2%) ** |
| Neoadjuvant Chemotherapy | 99 (69.2%) |
| Adjuvant chemotherapy | 48 (33.6%) |
| Received IMRT | 34 (28.6%) ⱡ |
| Total Scalp RT | 21 (30.0%) ⱡ ⱡ |
| Treatment era | |
| Pre 2000. | 41 (29%) |
| 2000 or after | 102 (72%) |
Tumor size was not available for 19 patients.
Only includes the 93 pts who received surgery (with or without RT) for primary local therapy
Only includes 119 patients who received radiation
Includes 70 patients for whom information was available
Treatment
As our center is a sarcoma care referral center, many patients had already received some angiosarcoma-directed treatment prior to presentation. Thirty-nine (27%) patients had undergone a wide local excision or excisional biopsy prior to presentation. Most patients presented with gross disease (n=117), with the remaining patients having undergone an excisional procedure at an outside facility. Based on multidisciplinary evaluation, including physical examination, review of any outside operative and pathology reports, communication with the referring surgeon, and imaging review, definitive local treatment consisted of surgery (either wide local excision or excisional biopsy) and RT (S-XRT) for 69 patients (48%), RT alone for gross disease for 50 patients (35%), and surgery alone for 24 patients (17%) (SMT, n=74). Of the 93 patients who underwent surgery as part of their curative treatment, 42 (45%) had positive resection margins and 51 (55%) had negative margins. Among the 69 patients who received S-XRT, 31 patients (45%) had positive/uncertain resection margins, and 38 patients (55%) had negative margins. Among the 24 patients treated with surgery alone, 11 patients (46%) had positive/uncertain resection margins and 13 (54%) had negative margins. There was no significant difference in the proportion of patients with positive/uncertain margins who had surgery alone compared to those who got S-XRT (p=0.87). Seventy patients (75%) had wound closure with a vascularized tissue transfer, and/or split thickness skin grafting, or complex closure with the assistance of a plastics/reconstructive surgical specialist.
RT was delivered with photons or electrons using techniques and modality appropriate for the site and distribution of the tumor, with 34 (29%) of patients receiving IMRT. Tissue-equivalent bolus material was used to ensure adequate skin dose. The median RT dose for patients treated with S-XRT was 60 Gy (range 50–70 Gy) with median 2 Gy/fraction. The median RT dose for patients treated with RT alone was also 60 Gy (range 35–75 Gy) with median 2 Gy/fraction. Elective total scalp RT was delivered to 21 patients prior to 2010 after which this practice was discontinued due to a prior analysis showing no benefit (6). Regional lymphatics in addition to primary site RT were treated in 29 patients (24%). Of the 50 patients who received RT alone to their primary site, 84% (n=42) experienced clinical complete response, 12% (n=6) patients had partial response, and 4% (n=2) patients experienced progressive disease after RT.
Most patients (n=114, 80%) received chemotherapy: 66 (46%) neoadjuvantly, 15 (10%) adjuvantly, and 33 (23%) both neoadjuvantly and adjuvantly. The type of chemotherapy was not available for all patients. However, most received a taxane-based regimen (n=67, 59%). In patients who received neoadjuvant chemotherapy, 22 (15%) had a complete response, 64 (45%) had a partial response, 4 (3%) had no response, and 9 (6%) had progressive disease.
Outcomes
Survival
Fifty-nine patients (41%) died of angiosarcoma. The actuarial 5-yr OS was 45% (95% CI 35–54%). Median OS was 52 months. The 5-yr DSS was 56% (95% CI 46–65), and the 5-yr DFS was 38% (95% CI: 29–47%)] (Figure 1). Among those who experienced any relapse, median time to relapse was 10 months (mos) (IQR: 4–18). Receipt of SMT (compared to S-XRT) was associated with poorer DSS on both univariate (Table 2) and multivariable analyses (Table 3); additionally, the following remained significant on multivariable analyses: size >5cm (HR 2.79 [1.49 – 5.21], p=0.01) treatment pre-2000 (HR 2.98 [1.56 – 5.68], p<0.05), and SMT (HR 2.08 [1.07 – 4.04], p=0.03). In addition, having the primary site of disease on a location other than the scalp was associated with improved DSS (HR 0.54 [0.29 – 0.99], p=0.046).
Figure 1:
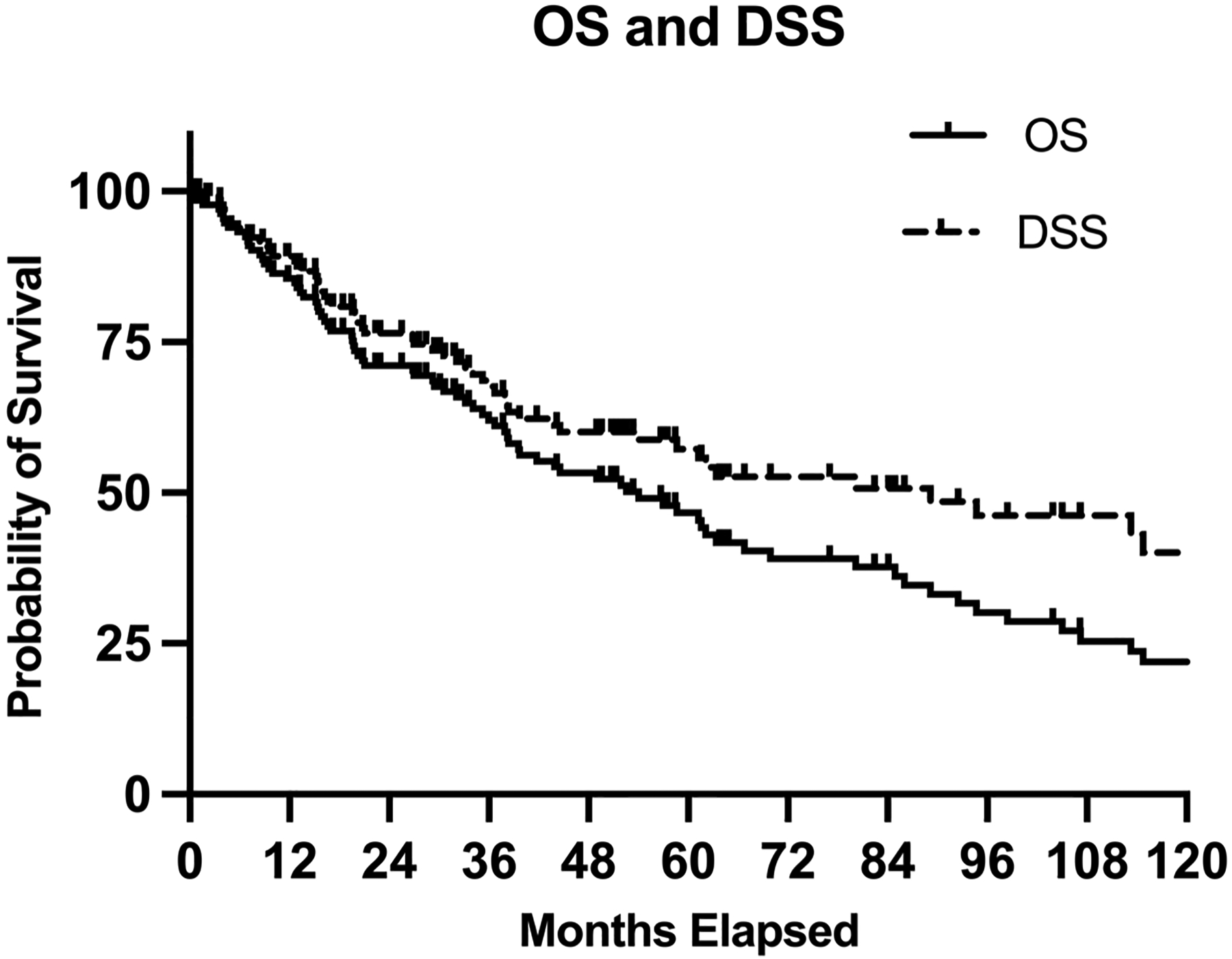
Overall survival (OS) and disease-specific survival (DSS) in entire cohort of patients undergoing definitive treatment for cutaneous head and neck angiosarcoma.
Table 2:
Univariate analysis of disease-specific survival and overall survival
| Characteristic | 5-yr DSS % |
p-value | 5-yr OS % |
p-value |
|---|---|---|---|---|
| Entire Cohort | 56% (95% CI: 46–65%) | 45% (95% CI: 35–54%) | ||
| Age | 0.19 | 0.01 | ||
| ≤70 | 56% | 49% | ||
| >70 | 58% | 40% | ||
| Gender | 0.53 | 0.72 | ||
| Male | 56% | 43% | ||
| Female | 58% | 49% | ||
| Tumor size | <0.005 | 0.01 | ||
| < 5cm | 62% | 49% | ||
| ≥ 5cm | 36% | 31% | ||
| Site | 0.07 | 0.13 | ||
| Scalp | 51% | 42% | ||
| Face/other | 65% | 49% | ||
| Multifocal at presentation | 0.01 | 0.01 | ||
| No | 66% | 52% | ||
| Yes | 42% | 34% | ||
| Nodal disease at diagnosis | 0.73 | 0.32 | ||
| No | 57% | 44% | ||
| Yes | 56% | 53% | ||
| Local Therapy | 0.01 | 0.11 | ||
| Single Modality | 45% | 38% | ||
| S-XRT | 69% | 52% | ||
| Surgical Resection Margin | 0.62 | 0.83 | ||
| Positive/uncertain | 66% | 50% | ||
| Negative | 53% | 43% | ||
| Neoadjuvant Chemotherapy | 0.54 | 0.36 | ||
| No | 53% | 41% | ||
| Yes | 57% | 46% | ||
| Adjuvant chemotherapy | 0.88 | 0.77 | ||
| No | 63% | 46% | ||
| Yes | 47% | 43% | ||
| Treatment era | ||||
| Pre 2000 | 42% | 0.01 | 34% | 0.19 |
| 2000 or after | 62% | 49% |
Red indicates significant value with p<0.05
Table 3.
Multivariable analysis of disease-specific survival, and overall survival
| Characteristic | DSS HR | 95% CI | OS HR | 95% CI |
|---|---|---|---|---|
| Age >70 | - | - | 1.76 | 1.10 – 2.79 |
| Tumor size ≥ 5cm | 2.79 | 1.49 – 5.21 | 2.00 | 1.21 – 3.31 |
| Site | ||||
| Scalp | Ref | - | - | |
| Face/other | 0.54 | 0.29 – 0.99 | - | - |
| Multifocality | 1.73 | 0.98 – 3.07 | 1.59 | 1.02 – 2.50 |
| Local Therapy | ||||
| Combined Modality | Ref | - | - | |
| Single Modality | 2.08 | 1.07 – 4.04 | - | - |
| Treatment Era | ||||
| 2000 or after | Ref | - | - | |
| Pre-2000 | 2.98 | 1.56 – 5.68 | - | - |
Patterns of angiosarcoma recurrence
Eighty-three patients (58%) experienced disease recurrence, of which 61 (43%) experienced local recurrence. The median time to local recurrence was 11.6 months (IQR: 5–18.5). Five-year actuarial LC was 51% (95% CI 40–60%) (Table 4). Twenty-five patients (17%) experienced nodal relapse with an actuarial 5-yr nodal relapse rate of 23% (95% CI: 15–33%). Nine of these 25 patients had nodal disease at diagnosis, and had nodal dissection, radiation therapy targeting their nodes, or both. Forty-four patients (31%) developed distant metastases (DM). The interval to development of DM was a median 15.6 months (IQR: 7.7–36 mos). Of these, the first site of distant metastatic involvement was the lung in 26 patients, bone in 6 patients, liver in 6 patients, and other sites in 6 patients. The 5-yr actuarial rate of DM was 36% (95% CI: 28–47%). The only variable significant for the development of distant metastases on multivariable analysis was the presence of multifocal disease at diagnosis (HR 2.33 [1.28 – 4.22], p=0.005) (Table 5). Tumor size ≥5 cm, presence of multifocal tumor, treatment pre-2000, and treatment with SMT versus S-XRT was adversely predictive of disease relapse to any site (≥ 5cm HR 2.29 [95% CI 1.38 – 3.82], p<0.005; multifocality HR 2.20 [1.36 – 3.54], p<0.005; pre-2000 HR 2.02 [1.18 – 3.44], p=0.01; SMT HR 1.92 [1.15 – 3.18], p=0.01) (Table 5).
Table 4:
Univariate analysis of local control, distant-metastasis free survival, and disease-free survival
| Characteristic | 5-yr LC % |
p-value | 5-yr DMFS % |
p-value | 5-yr DFS % |
p-value |
|---|---|---|---|---|---|---|
| Entire Cohort | 51% (95% CI: 40–60%) | 64% (95% CI: 53–72%) | 38% (95% CI: 29–47%) | |||
| Age | 0.29 | 0.94 | 0.65 | |||
| ≤70 | 54% | 62% | 39% | |||
| >70 | 48% | 67% | 38% | |||
| Gender | 0.44 | 0.45 | 0.92 | |||
| Male | 53% | 60% | 38% | |||
| Female | 43% | 77% | 39% | |||
| Tumor size | 0.01 | 0.07 | <0.005 | |||
| < 5cm | 59% | 67% | 45% | |||
| ≥ 5cm | 19% | 49% | 14% | |||
| Site | 0.37 | 0.11 | 0.44 | |||
| Scalp | 58% | 61% | 39% | |||
| Face/other | 41% | 67% | 38% | |||
| Multifocal at presentation | <0.005 | <0.005 | <0.005 | |||
| No | 59% | 70% | 47% | |||
| Yes | 35% | 53% | 23% | |||
| Nodal disease at diagnosis | 0.30 | 0.28 | *** | |||
| No | 49% | 62% | 57% | |||
| Yes | 63% | 77% | 56% | |||
| Local Therapy | <0.005 | 0.30 | <0.005 | |||
| Single Modality | 29% | 57% | 24% | |||
| S-XRT | 75% | 70% | 54% | |||
| Surgical Resection Margin** | 0.30 | 0.98 | 0.72 | |||
| Positive/uncertain | 62% | 65% | 42% | |||
| Negative | 46% | 63% | 36% | |||
| Neoadjuvant Chemotherapy | 0.23 | 0.34 | 0.69 | |||
| No | 43% | 72% | 38% | |||
| Yes | 53% | 60% | 38% | |||
| Adjuvant chemotherapy | 0.29 | 0.09 | 0.43 | |||
| No | 49% | 71% | 42% | |||
| Yes | 53% | 53% | 28% | |||
| Treatment era | <0.005 | 0.25 | 0.03 | |||
| Pre 2000 | 37% | 55% | 30% | |||
| 2000 or after | 56% | 67% | 41% |
Red indicates significant value with p<0.05
Only includes the 93 pts who received surgery (with or without RT) for primary local therapy
S-XRT (Surgery + Radiation)
Table 5:
Multivariable analysis of local control, distant-metastasis free survival, and disease-free survival
| Characteristic | LC HR | 95% CI | DMFS HR | 95% CI | DFS HR | 95% CI |
|---|---|---|---|---|---|---|
| Age >70 | - | - | - | - | - | - |
| Tumor size ≥ 5cm | 2.49 | 1.37 – 4.54 | - | - | 2.29 | 1.38–3.82 |
| Site | ||||||
| Scalp | - | - | - | - | - | - |
| Face/other | - | - | - | - | - | - |
| Mulifocal | 1.92 | 1.09 – 3.38 | 2.33 | 1.28–4.22 | 2.20 | 1.36–3.54 |
| Local Therapy | ||||||
| Combined Modality | Ref | - | - | Ref | ||
| Single Modality | 3.07 | 1.60 – 5.89 | - | - | 1.92 | 1.15–3.18 |
| Treatment Era | ||||||
| 2000 or after | Ref | - | - | Ref | ||
| Pre-2000 | 2.10 | 1.15 – 3.84 | - | - | 2.02 | 1.18–3.44 |
Local recurrence predictors and primary local therapy
On univariable analysis, tumor size <5cm was significantly associated with better 5-yr LC (59% vs 19%, p=0.01) (Table 4). Multifocal lesions at presentation (35% vs 59%, p<0.005) were associated with poorer 5-yr LC. Surgical resection margin was not associated with 5-yr LC (p=0.30). SMT was associated with a significantly poorer 5-yr LC when compared to S-XRT (29% vs 75%, p<0.005) (Figure 2). The use of IMRT in those that did receive RT was not significantly associated with improved 5-yr LC when compared to those treated with non-IMRT techniques (72% vs 52%, p=0.10). However, treatment pre-2000 was associated with decreased 5-yr LC (37% vs 56%, p<0.005). Neither neoadjuvant nor adjuvant chemotherapy had an impact on 5-yr LC (p=0.23, p=0.29; respectively). On multivariable analysis, size >5cm (HR 2.49 [95% CI 1.37 – 4.54], p<0.005), multifocal primary tumor (HR 1.92 [1.09 – 3.38], p=0.02), treatment pre-2000 (HR 2.10 [1.15 – 3.84], p=0.02), and SMT (HR 3.07 [1.60 – 5.89], p<0.005) all remained significantly associated with poorer LC (Table 5). Of the 119 pts who received RT either alone or in combination with surgery, 45 patients experienced a local recurrence. 25 of those were within the irradiated field, 9 were at the field edge, and 11 were outside the RT field.
Figure 2:
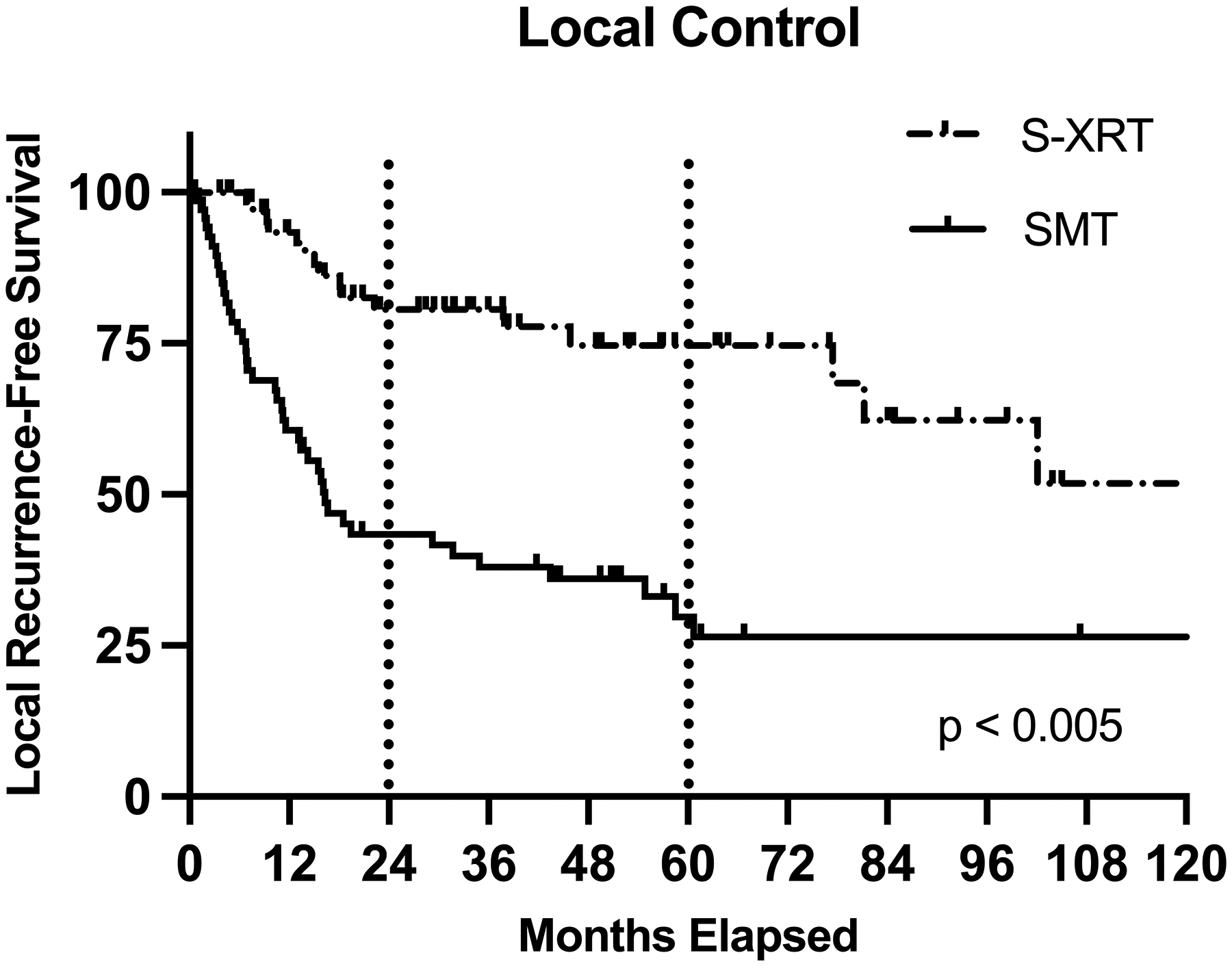
Comparing local control (LC) in patients with cutaneous head and neck angiosarcoma who received combined modality therapy (S-XRT) versus single modality therapy (SMT)
Toxicities:
Twelve patients (8%) experienced a surgical complication at median 3 months (IQR: 0.7–5.3 mos) after surgery. One patient’s surgical complication was categorized as mild, one was moderate, and 10 patients required reoperation or hospitalization for a severe surgical complication. Seven of these events were due to flap/graft failure after surgery. Two patients had severe post-operative infections, and one patient experienced unexpected nerve damage after surgery. Thirty-three patients (23%) experienced a complication from RT at a median 9.1 months from end of RT (IQR: 5.5–17.4 mos). Fourteen had mild RT related complications, while 11 complications were moderate, and 8 patients had severe complications. Thirteen patients experienced an ocular complication (primarily permanent dry eye), 5 patients experienced soft tissue fibrosis, 3 had soft tissue necrosis, 3 had osteoradionecrosis, 2 had xerostomia, and 7 had other complications including non-healing wounds, mild edema, neuropathy, pituitary dysfunction and intermittent nosebleeds.
DISCUSSION:
To our knowledge, this is the largest single-institution experience examining patterns of care and outcomes for patients treated curatively for CA of the head and neck. Our data suggests that treatment with surgery followed by RT provides the best approach to optimize LC and DFS. Consistent with our prior report, large tumors (>5cm) and primary site multifocality at presentation were both associated with poor disease control and survival, regardless of local treatment approach.
Our findings corroborate smaller studies that have similarly reported that S-XRT should be the standard of care when feasible to improve local control, disease-free survival, and disease-specific survival (6, 11, 20, 21). In addition to S-XRT, size ≥5cm and multifocality at presentation were significantly associated with decreased LC, DSS, and OS. This was not surprising, as these factors have consistently been found to be poor prognostic variables in multiple prior studies (6, 12, 14, 20, 22–25). CA is a diffuse, infiltrative process (4, 8). Therefore, it is not surprising that our study and others have found the presence of larger tumors or multifocality at diagnosis to be associated with worse outcomes, as it is probably indicative of not just poorer LC, but a higher propensity for these tumors to spread both regionally and distantly. In addition, we found that patients with lesions on their face had improved DSS than those with lesions on their scalp at diagnosis, which makes intuitive sense as patients are likely to present sooner for cosmetic reasons.
There have been conflicting reports about the importance of obtaining negative surgical margins in the treatment of CA of the head and neck. In our series, we found that positive margins did not impact either LC or OS. Multiple other studies have reported this association and found that positive resection margin was not prognostic (26, 27), likely because RT is able compensate in this more radiosensitive sarcoma. Indeed, Pawlik et al. found that on pathologic review of surgical specimens skip lesions were common, thus negative margins are not necessarily truly negative (20). As such, wide margins on both surgical resection and in the post-operative radiation field are important. However, if negative margins cannot be “easily” achieved by surgery due to size of resection or nearby structures, an extensive surgery to “chase” a negative margin is not necessary; an extensive reconstructive procedure and prolonged wound healing is not desirable as the tumor can recur in the postoperative healing period before RT is initiated. The surgical goal should be to remove gross disease and plan a reconstruction that minimizes the time to RT. Our institutional practice is to aggressively irradiate these tumors postoperatively with large clinical treatment volumes (CTVs) of 5cm surrounding the gross tumor or operative bed to ensure we are treating the area at highest risk for microscopic disease. In the past, total scalp irradiation was thought to possibly improve outcomes (28) but this fell out of favor when data were unable to demonstrate a LC advantage and toxicities were high (6).
Interestingly, in our series we found that while S-XRT was superior to SMT, definitive RT alone still provided reasonably good outcomes. Other studies have shown that the addition of surgery to RT did not improve patient outcomes (14, 15). However, these were smaller studies with less than 30 patients. Most larger series have shown an improvement in LC and DFS with S-XRT, as demonstrated here (11, 20). Thus, while S-XRT is preferred, RT alone can be an acceptable option for patients who are not candidates for surgical resection. Treatment after the year 2000 was also associated with improved LC, DFS, and DSS. This is likely multifactorial due to better imaging and staging at diagnosis, improved surgical and radiation techniques, and more efficacious chemotherapy. It should be noted that the improvement seen in LC translated to both improvements in DFS and DSS. This highlights the importance of aggressive primary local therapy. Patients with CA likely benefit from referral to a high-volume sarcoma for assessment of the optimal multidisciplinary management approach (29).
While most patients received chemotherapy at some point in their management course, we did not observe any association between outcomes and its use in this study. Of course, the utility of chemotherapy cannot be ascertained upon a retrospective review, as there may be multiple confounding factors as to why patients did not receive chemotherapy, including the presence of comorbidities, selection bias, and multiple treatment eras with heterogeneous systemic therapy approaches encompassed in this study. Therefore, it is beyond the scope of this report to firmly draw conclusions regarding the role of modern chemotherapy for this disease.
While other studies have shown an improvement in overall survival with the addition of RT to surgery, we did not see that association (6, 20). This may reflect the longer follow-up in our study (33 months compared to 2.1 years and 18 months, respectively) and the fact that patients with this disease are often elderly and also experience a high propensity for metastatic relapse (6, 20). While we observed that DSS was improved with S-XRT, there was not a prolonged effect on OS in this elderly population.
Due to the retrospective nature of this investigation, there are many inherent limitations to this study. Chief among them is that there was likely selection bias in the determination of whether a patient received SMT versus S-XRT for local management of their CA. Specifically, patients with very large or unresectable tumors were most likely to be dispositioned to RT alone. In addition, we included patients treated over a 50-year time span, which encompasses many treatment eras, conferring much heterogeneity in the surgical and radiotherapeutic management of this complex and rare tumor over the past 5–6 decades.
In conclusion, our data continues to suggest that CA patients presenting with large, multifocal tumors are likely to have worse prognosis. However, to optimize outcomes patients should receive S-XRT when feasible. For patients whose disease is not amenable to surgery, RT alone provides reasonable local control.
Funding:
Supported in part by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest to report
Data Availability Statement:
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.
REFERENCES:
- 1.Costache M, Ene AM, Simionescu O, Sajin M. Histopathological diagnosis of cutaneous vascular sarcomas. Rom J Morphol Embryol. 2010;51(1):105–9. [PubMed] [Google Scholar]
- 2.Rouhani P, Fletcher CDM, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma incidence patterns in the U.S. Cancer. 2008;113(3):616–27. [DOI] [PubMed] [Google Scholar]
- 3.Albores-Saavedra J, Schwartz AM, Henson DE, Kostun L, Hart A, Angeles-Albores D, et al. Cutaneous angiosarcoma. Analysis of 434 cases from the Surveillance, Epidemiology, and End Results Program, 1973–2007. Annals of Diagnostic Pathology. 2011;15(2):93–7. [DOI] [PubMed] [Google Scholar]
- 4.Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Annals of Oncology. 2007;18(12):2030–6. [DOI] [PubMed] [Google Scholar]
- 5.Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59(5):1046–57. [DOI] [PubMed] [Google Scholar]
- 6.Guadagnolo BA, Zagars GK, Araujo D, Ravi V, Shellenberger TD, Sturgis EM. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head & Neck. 2011;33(5):661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SH, Hayden RE, Hinni ML, Wong WW, Foote RL, Milani S, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141(4):335–40. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa K, Takahashi K, Asato Y, Yamamoto Y, Taira K, Matori S, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85(1019):e1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44(3):1106–13. [DOI] [PubMed] [Google Scholar]
- 10.Wong A, Flores J. Cutaneous angiosarcoma of the head and neck resembling rosacea: A case report. SAGE Open Med Case Rep. 2020;8:2050313x20940419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer. 1996;77(11):2400–6. [DOI] [PubMed] [Google Scholar]
- 12.Morgan MB, Swann M, Somach S, Eng W, Smoller B. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50(6):867–74. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Tang M, Lv S, Wang H, Du W, Zhao X, et al. Prognostic usefulness of clinical features and pretreatment (18)F-FDG PET/CT metabolic parameters in patients with angiosarcoma. Quant Imaging Med Surg. 2022;12(5):2792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki R, Soejima T, Kishi K, Imajo Y, Hirota S, Kamikonya N, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52(4):1032–40. [DOI] [PubMed] [Google Scholar]
- 15.Ohguri T, Imada H, Nomoto S, Yahara K, Hisaoka M, Hashimoto H, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61(5):1446–53. [DOI] [PubMed] [Google Scholar]
- 16.Penel N, Lansiaux A, Adenis A. Angiosarcomas and taxanes. Curr Treat Options Oncol. 2007;8(6):428–34. [DOI] [PubMed] [Google Scholar]
- 17.Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26(32):5269–74. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 19.McDonald JH. Handbook of Biological statistics. 3 ed. Baltimore, Maryland: Sparky House Publishing; 2014. [Google Scholar]
- 20.Pawlik TM, Paulino AF, McGinn CJ, Baker LH, Cohen DS, Morris JS, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98(8):1716–26. [DOI] [PubMed] [Google Scholar]
- 21.Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24(1):25–31. [DOI] [PubMed] [Google Scholar]
- 22.Sharon CE, Straker RJ, Shannon AB, Shabason JE, Zhang PJL, Fraker DL, et al. Neoadjuvant radiation for cutaneous and soft tissue angiosarcoma. J Surg Oncol. 2022;125(3):509–15. [DOI] [PubMed] [Google Scholar]
- 23.Lee KC, Chuang SK, Philipone EM, Peters SM. Characteristics and Prognosis of Primary Head and Neck Angiosarcomas: A Surveillance, Epidemiology, and End Results Program (SEER) Analysis of 1250 Cases. Head Neck Pathol. 2019;13(3):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C, Wu SP, Hu K, Li Z, Schreiber D, Oliver J, et al. Patterns of Care and Survival of Cutaneous Angiosarcoma of the Head and Neck. Otolaryngol Head Neck Surg. 2020;162(6):881–7. [DOI] [PubMed] [Google Scholar]
- 25.Shin JY, Roh S-G, Lee N-H, Yang K-M. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: Systematic review and meta-analysis. Head & Neck. 2017;39(2):380–6. [DOI] [PubMed] [Google Scholar]
- 26.Ward JR, Feigenberg SJ, Mendenhall NP, Marcus RB Jr., Mendenhall WM. Radiation therapy for angiosarcoma. Head & Neck. 2003;25(10):873–8. [DOI] [PubMed] [Google Scholar]
- 27.Lydiatt WM, Shaha AR, Shah JP. Angiosarcoma of the head and neck. The American Journal of Surgery. 1994;168(5):451–4. [DOI] [PubMed] [Google Scholar]
- 28.Hata M, Wada H, Ogino I, Omura M, Koike I, Tayama Y, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190(10):899–904. [DOI] [PubMed] [Google Scholar]
- 29.Blay JY, Soibinet P, Penel N, Bompas E, Duffaud F, Stoeckle E, et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol. 2017;28(11):2852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.


