Abstract
The active sites of actinidin (EC 3.4.22.14) and papain (EC 3.4.22.2) display different reactivity characteristics to probes targeted at the active-site cysteine residue despite the close structural similarity of their active sites. The calculated electrostatic fields in the active-site clefts of actinidin and papain differ significantly and may explain the reactivity characteristics of these enzymes. Calculation of electrostatic potential also focuses attention on the electrostatic properties that govern formation of the active-site thiolate-imidazolium ion-pair. These calculations will guide the modification of the pH-activity profile of the cysteine proteinases by site-directed mutagenesis.
Full text
PDF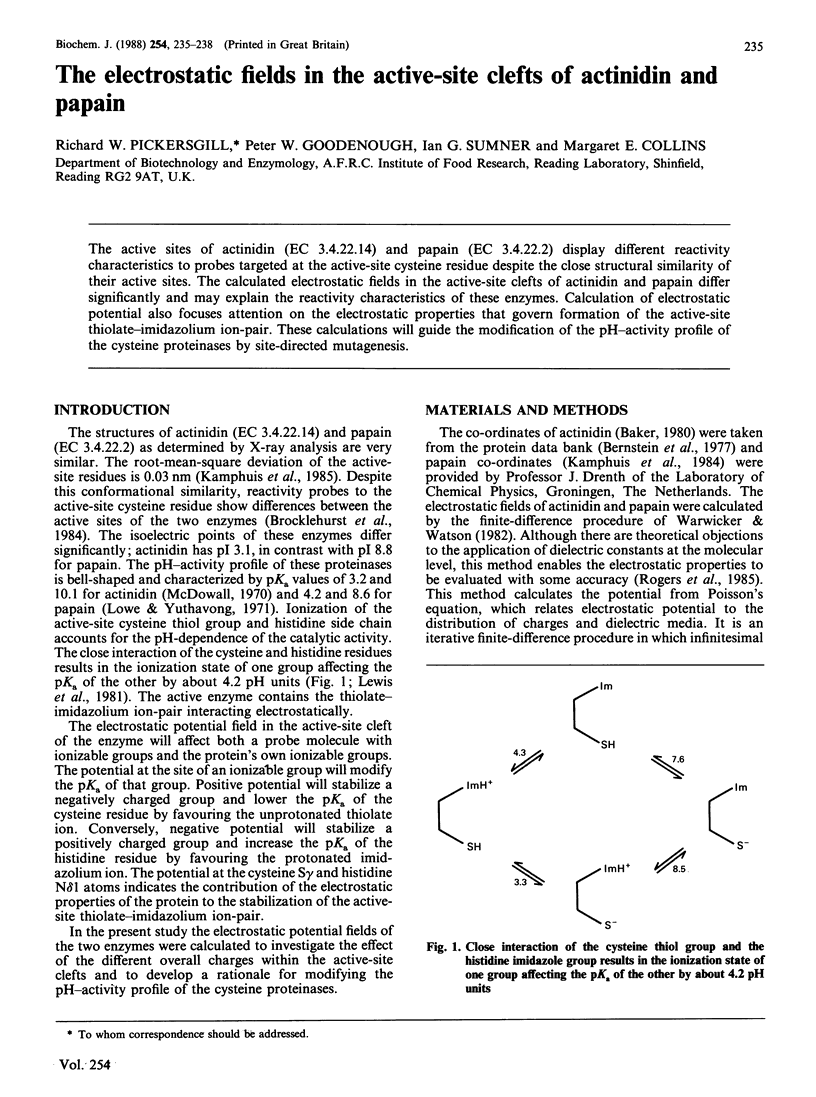

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E. N. Structure of actinidin, after refinement at 1.7 A resolution. J Mol Biol. 1980 Aug 25;141(4):441–484. doi: 10.1016/0022-2836(80)90255-7. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Mushiri S. M., Patel G., Willenbrock F. A marked gradation in active-centre properties in the cysteine proteinases revealed by neutral and anionic reactivity probes. Reactivity characteristics of the thiol groups of actinidin, ficin, papain and papaya peptidase A towards 4,4'-dipyridyl disulphide and 5,5'-dithiobis-(2-nitrobenzoate) dianion. Biochem J. 1983 Mar 1;209(3):873–879. doi: 10.1042/bj2090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Salih E., Lodwig T. S. Differences between the electric fields of the catalytic sites of papain and actinidin detected by using the thiol-located nitrobenzofurazan label as a spectroscopic reporter group. Biochem J. 1984 Jun 1;220(2):609–612. doi: 10.1042/bj2200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Kalk K. H., Swarte M. B., Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233–256. doi: 10.1016/0022-2836(84)90467-4. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Lewis S. D., Johnson F. A., Shafer J. A. Effect of cysteine-25 on the ionization of histidine-159 in papain as determined by proton nuclear magnetic resonance spectroscopy. Evidence for a his-159--Cys-25 ion pair and its possible role in catalysis. Biochemistry. 1981 Jan 6;20(1):48–51. doi: 10.1021/bi00504a009. [DOI] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. pH-dependence and structure-activity relationships in the papain-catalysed hydrolysis of anilides. Biochem J. 1971 Aug;124(1):117–122. doi: 10.1042/bj1240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowall M. A. Anionic proteinase from Actinidia chinensis. Preparation and properties of the crystalline enzyme. Eur J Biochem. 1970 Jun;14(2):214–221. doi: 10.1111/j.1432-1033.1970.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Rogers N. K., Moore G. R., Sternberg M. J. Electrostatic interactions in globular proteins: calculation of the pH dependence of the redox potential of cytochrome c551. J Mol Biol. 1985 Apr 20;182(4):613–616. doi: 10.1016/0022-2836(85)90248-7. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Watson H. C. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982 Jun 5;157(4):671–679. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]


