Abstract
Systemic lupus erythematosus (SLE) is a multi-system chronic autoimmune disease with a complex occurrence and development process, associated with immune disorders, uncertain prognosis, and treatment modalities which vary by patient and disease activity. At present, the clinical treatment of SLE mainly focuses on hormones and immunosuppressants. In recent years, the research on new treatment strategies for SLE has been booming, and strong preclinical results and clinical research have promoted the development of numerous drugs (such as rituximab and orencia), but numerous of these drugs have failed to achieve effectiveness in clinical trials, and there are some adverse reactions. Recent evidence suggests that resveratrol (RSV) has the effect of ameliorating immune disorders by inhibiting overactivation of immune cells. In the present review, advances in research on the protective effects and potential mechanisms of RSV against SLE are summarized and the potential potency of RSV and its use as a promising therapeutic option for the treatment of SLE are highlighted.
Keywords: systemic lupus erythematosus, resveratrol, autoimmune disease, immune cell, treatment prospect
1. Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that affects multiple organs and is more pronounced in female patients, with a female to male ratio of ~10:1 (1). The incidence ranges from 0.3–31.5 cases per 100,000 individuals per year and has increased over the past 40 years, probably due to recognition of milder cases (2). Global adjusted prevalence rates approach or exceed 50-100 cases per 100,000 adults (1). The pathogenesis of SLE is closely related to the overactivation of different immune cells (such as T cells, B cells and monocytes) (3), and the complex interaction with cytokines can lead to various clinical manifestations and even threaten life. This clinical heterogeneity is most likely caused by complex immune dysregulation, such as loss of immune tolerance to autoantigens and production of multiple autoantibodies (4). The immune complex formed by the combination of autoantibody and intracellular autoantigen is deposited in various tissues and organs. As the disease progresses, these immune complexes cause tissue damage and various diseases or manifestations, such as facial rashes, joint pain, nephritis and cardiovascular disease (2,5).
The complexity of SLE's clinical features indicates that SLE has multiple subtypes and a potentially unique combination of disease pathways, genes and environmental factors. Therefore, management and treatment options for SLE remain challenging for clinicians. Treatment is mainly based on non-steroidal anti-inflammatory drugs, corticosteroids, antimalarial drugs, immunosuppressants (such as cyclophosphamide, tacrolimus and mycophenolate) and biologics (6–8). Surprisingly, only three drug treatments related to SLE have been approved by the Food and Drug Administration in the past decade (corticosteroids, hydroxychloroquine and beliuzumab). However, traditional treatments can have a variety of side effects and ideal treatment options are few. The anti-inflammatory effects of these drugs are often accompanied by some adverse reactions, including eye lesions, severe osteoporosis, cardiovascular injury, severe infection, malignant tumors (2,9,10), and in severe cases, other organ damage and even death. In addition, patients with SLE are occassionally resistant to these drugs (11), therefore it is urgent to find a drug with significant efficacy and few side effects.
Resveratrol (RSV) is a natural plant antitoxin found in grapes, mulberries, peanuts, rhubarb and other plants (8). RSV exists in two isomeric forms, cis-trans and trans-trans, but the trans-form is the main form, which has the most effective therapeutic benefits due to its lower steric hindrance of the side chain and becomes the more biologically active form due to its higher stability (12–14). Regarding the immune system, RSV has been proposed for numerous years as an immunomodulator capable of regulating innate and adaptive immune responses by interacting with a variety of molecular pathways, such as macrophages, T cells and B cells. RSV can also participate in the inhibitory function of CD4+ CD25+ regulatory cell subsets (15,16) and affect B cell proliferation and autoantibody production (17,18). Its role as an immunomodulator has been demonstrated in various animal models and different cell lines. In addition, RSV has been reported to slow the progression of autoimmune diseases, such as rheumatoid arthritis (RA), psoriasis (PsO) and inflammatory bowel disease (IBD) (19). In addition, RSV exerts anti-inflammatory effects by inhibiting the activation of NF-κB in immune cells and reducing the levels of tumor necrosis factor-α (TNF-α), interleukin-1β, IL-6, transforming growth factor-β (TGF-β) and TNF (20,21). It suggests that RSV can be used as a potential treatment for SLE.
An increasing number of studies is currently focused on exploring new drugs and therapies, and certain natural products have been found to offer significant therapeutic promise in the treatment of SLE. In the present review, the authors focused on the protective effects and potential mechanisms of RSV against SLE. At the same time, the present review summarizes the progress of RSV in the treatment of SLE and its associated adverse reactions, providing new insights into the treatment options for SLE.
2. Related pathogenesis of SLE
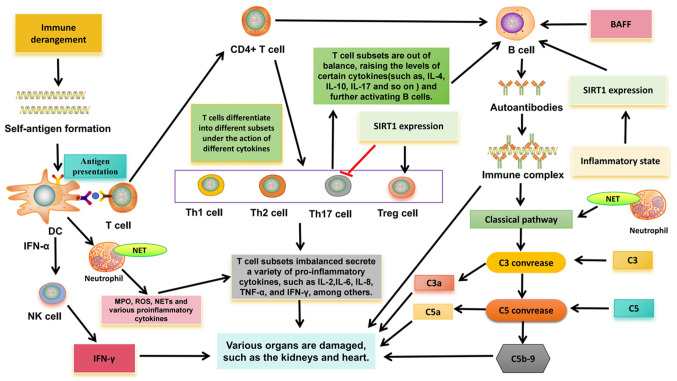
The pathogenesis of SLE is closely related to the overactivation of different immune cells (Fig. 1). In innate immunity, type I interferon (IFN)-α gene expression was detected in peripheral blood mononuclear cells in >50% of patients with SLE (22,23). There is a close relationship between IFN and SLE, especially IFN-α. IFN-α can promote the transformation of monocytes into dendritic cells (DC), which recognize antigens and continuously produce IFN-α, thus further activating lymphocytes and natural killer (NK) cells, thereby breaking autoimmune tolerance (24). In SLE, the increase of apoptotic rate or the clearance of obstacles may increase the autoantigen-antibody complexes, which are endogenous IFN inducers and can continue to produce IFN, forming a vicious cycle (24). In addition, IFN-α can promote the activation of helper T cells (Th), improve the ability of DC antigen presentation, and then induce the production of interleukin-1, IL-2, IL-4 and other cytokines, promote Th17 differentiation, activate B cells and produce autoantibodies (24).
Figure 1.
Related pathogenesis of systemic lupus erythematosus. DC, dendritic cell; IL, interleukin; MPO, myeloperoxidase; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon gamma; ROS, reactive oxygen species; IFN, interferon; SIRT1, Sirtuin-1; BAFF, B-cell activating factor from the tumor necrosis factor family; C, complement; NK cell, natural killer cell; Th, T-helper; Treg, regulatory T cell; NET, neutrophil extracellular trap.
In addition, in SLE, overactivation of neutrophils can release tissue damage factors, such as myeloperoxidase (MPO), reactive oxygen species (ROS) and a large number of cytokines, resulting in immune regulation disorders and further tissue damage (25). Especially neutrophil extracellular traps (NETs). Obstruction of clearance and/or excessive formation of NET can externalize self-antigens, thus inducing IFN synthesis and endothelial damage, which is related to the pathogenesis of SLE (26). NET fragments are taken up by plasmacytoid dendritic cells and presented to autoreactive B cells and T cells, thereby producing more IFN-α and autoantibodies (27). NETs bind to complement 1q, activate the classical pathway of complement, consume a large amount of complement (C3 and C4) and activate other inflammatory complement fragments (C3a and C5a), and activated complement fragments can inhibit the degradation of NETs and further aggravate autoimmunity (24).
In addition, new evidence suggests that NK cells may be involved in SLE. A number of studies have found that the number of circulating NK cells in patients with SLE is reduced, which is not only related to clinical manifestations and disease activity, but also related to increased serum IFN-α level (28–30). In addition, previous studies have shown that NK cells can promote the production of IFN-α by DC (30,31), and the immune complex mediation of the production of IFN-α by DC is a characteristic of SLE; thus, there may be an association between the two. However, the interaction between NK cells and DC in SLE needs to be further explored. In addition to reduced numbers, NK cell production of IFN-γ to various stimuli is significantly increased in patients with active SLE, and NK cell-derived IFN-γ has been shown to associate with serum IFN-α levels (32). In mouse animal models, sustained high levels of IFN-γ in serum can trigger SLE like syndrome, and the expression of IFN-γ is related to the formation of anti-double stranded-DNA (anti-DS-DNA) antibodies and anti-Smith antibodies (33,34), indicating that IFN-γ is the main effector molecule in the pathogenesis of SLE. It also suggests that NK cells may play an important role in the pathogenesis of SLE.
In adaptive immunity, T cells play an important role in the destruction of immune tolerance in SLE. Autoreactive T cells are present in both human and mouse SLE, indicating an imbalance in T cell subsets, that is, between pathogenic T cells and regulatory T (Treg) cells (35). The imbalance of Th1/Th2 cells is considered to be closely related to the occurrence and development of SLE. At present, most scholars consider that SLE is characterized by decreased Th1 function and hyper Th2 function. Cytokines secreted by Th1 cells (TNF-α, IL-2 and IFN-γ) are involved in the activation of macrophages and CD8+ T cells. Th2 cytokines (such as IL-4 and IL-10) can cause excessive activation of B cells, produce autoantibodies and cause tissue damage (24,36). In addition, the imbalance of Th17 cells and Treg cells is also associated with SLE. Previous studies have shown that in SLE, Th17 cells enter inflammatory tissues, such as the kidney, and promote inflammation by increasing the production of cytokines (IL-17), which in turn can activate the production of B-cell antibodies (24). Numerous studies have demonstrated a reduction in the number and function of Tregs, as well as an increase in Th17 cells, in patients with SLE (37,38), indicating a disruption in the dynamic balance between Th17 cells and Treg cells. Sirtuin-1 (SIRT1), an NAD+-dependent histone deacetylase, has been shown to be a key regulator of various physiological processes, including cell differentiation, immune response and more. Recent studies have provided evidence that SIRT1 may be a regulatory element in the immune system, and the imbalance of T cell subsets caused by changes in its function may be related to the development of SLE (39). For example, the downregulation of SIRT1 expression can promote the activation of CD4+ T cells and the secretion of IFN-γ, indicating that the downregulation of SIRT1 expression can promote T cells to differentiate Th1 and produce IFN-γ (40). Aromatics receptor (AhR) is a transcription factor involved in autoimmune diseases, and this ligand is required for CD4+ T cells to differentiate and mature into Th17 or Tregs cells. Activation of AhR in peripheral blood is associated with lupus activity (41). SIRT1 activation has been shown to reverse AhR-induced imbalances between Th17 and Treg populations and to upregulate IL-17A and IL-22 levels in CD4+ T cells (42,43). In T cells, FOXP3 can be directly deacylated by SIRT1, thereby inhibiting proliferation of Tregs (39). Downregulation of SIRT1 expression can inhibit Th17 cell differentiation (39). Therefore, SIRT1 is important in maintaining the balance between Treg cells and Th17 cells, and may be involved in the pathogenesis of SLE, but there is no direct evidence to clarify it.
A large number of autoreactive B cells in patients with SLE produce multiple autoantibodies, which are associated with the pathogenesis. Through classical T-B cell interactions, B cells are activated, and activated B cells interact with CD4+ T cells through TCR and co-stimulators. Activated B cells can secrete cytokines IL-6, TNF, IFN-γ and IL-10 (44). Activated CD4+ T cells produce IL-21 and T follicular helper (Tfh) cells in response to IFN-γ. Tfh cells promote the proliferation and differentiation of B cells through the production of IL-21 and produce IgG and IgA (24). A number of studies have revealed that the increase of Tfh cells is not only related to SLE disease activity, but also positively associated with the level of autoantibody titers (45,46). In addition, B cell activating factor (BAFF) is also involved in T-B cell interactions. Overexpression of BAFF may lead to defects in the survival rate of autoreactive B cells, and at the same time promote the proliferation of B cells to continuously form autoantibodies, such as anti-DS-DNA antibodies, and various serum immunoglobulins (IgM, IgA, IgE and IgG), while immune complexes are deposited in kidney tissue (47). Regarding SIRT1, SIRT1 deficiency was found to enhance T cell-dependent antibody response. Thus, the differentiation and activation of B cells into plasma cells is inhibited, the secretion of pro-inflammatory cytokines of B cells is enhanced, and the production of autoantibodies is promoted (39), which may be a possible cause of autoimmune diseases. A previous study demonstrated that SIRT1 deficient mice develop lupus nephritis (48). In SLE, overexpression of SIRT1 can significantly improve the activity of B cells, inhibit cell apoptosis and upregulate pro-inflammatory cytokines (IL-1, IL-6 and TNF-α) by regulating the nuclear factor kappa-B pathway (49), indicating its potential function in the pathogenesis of SLE.
3. Effects of RSV on immune cells
RSV can enter cells via passive diffusion, mediated endocytosis, or via transporters to bind to specific receptors, such as the integrin receptor αvβ3 (50,51). It helps regulate innate and adaptive immunity, such as regulating the activity of mononuclear/macrophages, T cells, B cells and NK cells. At the same time, because RSV has the ability to activate SIRT1, it may be able to mitigate the progression of autoimmune diseases.
RSV and innate immunity
RSV revealed anti-inflammatory effects in monocytes/macrophages and DCs (52). Macrophages are derived from blood monocytes and are involved in innate and adaptive immunity. RSV controls macrophage overactivation by inhibiting lipopolysaccharide, toll-like receptor 4 signaling and other immune activators (17). For example, it inhibits the activation of NF-κB pathway, COX-2 pathway, and inflammatory body containing the pyridine domain 3 of the NLR family (17), thereby inhibiting the secretion of cytokines, such as TNF-α and IL-6. This is consistent with the findings of Schwager et al (53), in which the authors found that although RSV was found to enhance the expression of IL-1β and IL-6 in peripheral blood lymphocytes, it had the opposite effect in macrophages. In addition, it can also alter the expression of maturation markers on the DC surface and the production of pro-inflammatory cytokines such as IFN. Since RSV has numerous molecular targets, numerous of which are related to optimal maturation of DC, RSV appears to play a more effective immunosuppressive role during DC differentiation, e.g., DC can secrete more IL-10 and play an immunosuppressive role (54).
In addition, activation of neutrophils is associated with organ damage in SLE, such as accumulation in the kidney, which can cause kidney damage. A previous study revealed that oxidative stress may also be associated with glomerular damage caused by a range of pro-inflammatory mediators, including cytokines and chemical factors, leading to ROS production (55). RSV has a wide range of antioxidant and anti-inflammatory effects in numerous biological reactions (56–58). RSV can inhibit neutrophil activation, downregulate the release of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, IFN, MPO, reduce the release of NETs, control inflammatory response and play an important role in regulating renal blood flow and glomerular filtration function by upregulating nitric oxide levels (59–61). These results indicated that RSV had a protective effect on renal involvement in SLE.
Regarding NK cells, in inflammatory diseases, levels of inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-8 and IFN-γ are elevated, which in turn activate circulating white blood cells, such as NK cells (62). Secreted pro-inflammatory cytokines and activated immune cells cause damage to endothelial cells and can affect important organs (62). RSV can inhibit the aforementioned inflammatory cytokines, thereby reducing the activation of NK cells. In addition, previous studies have evaluated the properties of RSV on human NK cells. RSV was found to have a concentration-dependent biphasic effect on NK cells. At high concentrations (50 µM), RSV inhibited NK cell activity and promoted apoptosis, which may affect inflammatory signaling pathways (63). However, when the concentration range was reduced from 3.13 to 1.56 µM, RSV showed a positive effect on NK cells by increasing the cytotoxicity of NK cells through upregulation of IFN-γ expression (mRNA and protein levels) (63). Therefore, more studies are needed to confirm the relationship between RSV and NK cells.
RSV and adaptive immunity
RSV effectively controls inflammation by targeting T cells, regulating T cell differentiation and inhibiting the release of pro-inflammatory cytokines and other inflammatory mediators (17). RSV, as an excitant of SIRT1, can induce the deacetylation of STAT3 and inhibit its migration into the nucleus, thus interrupting the activation of retinoic acid orphan receptor γt and inhibiting the differentiation of T cells to Th1 (41). A previous study found that RSV can reduce the proliferation of CD4+ T cells and the expression levels of CD69 and CD71, thus triggering the apoptosis of CD4+ T cells, in addition, the ratio of CD4 IFN-γ+ Th1 cells and Th1/Th2 cells is reduced (64). RSV can regulate Th1/Th2 balance. In addition, Th17 cells are also one of the important T cell subsets targeted by RSV. One mode of action of RSV involves AhR activation, which inhibits Th17 cell activity (41). Another way is to activate SIRT1 and block the production of IL-17 (41). The aforementioned findings suggested that RSV can tilt the balance in favor of the anti-inflammatory Th2 and Treg cell subsets.
RSV has been shown to have inhibitory effects on B cells and plasma cells, as well as significant effects on the production of autoantibodies, which play a role in the pathogenesis of lupus (17). A previous study revealed that treatment of norphytane-induced lupus mice with RSV may inhibit CD4+ T cells by triggering SIRT1, controlling B cell proliferation and autoantibody production (64). In addition, RSV can also increase the expression of Fc γ receptor IIb (FCGR2B) in B cells of MRL/lpr mice, leading to apoptosis, and a large decrease in the activation and number of B cells/plasma cells in spleen and bone marrow, thereby reducing serum autoantibody titers (18). RSV can also promote autophagy and autophagy flux by inhibiting the Akt/mTOR pathway, thereby impeding the proliferation and survival of BAFF stimulated B cells (65). The aforementioned findings indicated that RSV may be a promising drug for the prevention and treatment of aggressive B-cell diseases and autoimmune diseases caused by BAFF.
4. RSV and autoimmune diseases
Autoimmune diseases are mainly caused by the disorder of immune cells in the body, which leads to excessive activation of immune cells and the production of a large number of inflammatory cytokines, such as TNF-α, IFN-γ and IL-1β and so on (19,66–75). A strong immune response can attack different organs or tissues at the same time, resulting in local or systemic immune responses that damage one or more body tissues or organs in the human body, including type 1 diabetes, autoimmune hepatitis, RA, amyotrophic lateral sclerosis, PsO and IBD (66–98). Natural products have been extensively studied in the treatment and prevention of various chronic diseases. RSV, a molecule derived from natural products, is a well-studied substance known for its effects and therapeutic effects on a variety of chronic diseases, such as antioxidant, anti-inflammatory and immune regulation (15–19). In addition to SLE, the autoimmune diseases that have been studied more can be observed in Table I.
Table I.
Role of RSV in different rheumatic diseases.
| Disease | Disease mechanism | The therapeutic mechanism of RSV | Dose of RSV | Efficacy |
|---|---|---|---|---|
| T1DM | Islet resident DC uptake of beta cell antigens, presenting to naïve T cells and promoting Th1 differentiation will activate B lymphocytes that will produce autoantibodies against beta cells. Th1 will also activate macrophage and neutrophil migrations to the islet that will promote beta cell destruction by increasing ROS (19,66–69). | RSV acts via SIRT1 to inhibit apoptotic cell injury during oxidative stress and increases antioxidant capacity by reducing ROS. In addition, it plays a role in restoring beta cells in the islets (19,70). | Orally 250 mg/kg, orubcutaneous injection 25 mg/kg or 10 mg/kg intraperitoneally (71,72). | Reversing higher stages of insulitis in the islets of Langerhans. Decrement in glycosuria (71,72). |
| IBD | The activation of neutrophils and macrophages in the epithelium leads to the production of inflammatory mediators, such as ROS and TNF-α. Antigen recognition by naïve T lymphocytes induces the differentiation to Th1 and Th17 profiles in Crohn's disease, and to Th2 and Th17 profiles in ulcerative colitis, with the release of inflammatory cytokines, especially TNF-α (19,71–75). | RSV is capable of acting on the inhibition of inflammatory cytokines and neutralizing ROS (19). | Orally 20 mg/kg or 500 mg/day (74,75). | NF-κB activity, plasma levels of inflammatory factors and highly sensitive C-reactive protein were reduced (76,77). |
| PsO | The immune complex activates resident DC cells and then releases IL-23 to activate T lymphocytes, promoting differentiation into Th17. IL-16 promotes differentiation into Th1. These cells produce three major cytokines, IL-17, IL-22 and IFN, which promote the proliferation of keratinocytes (78–80). | Inhibiting the production of IL-17 and directly inhibiting the proliferation of keratinocytes (19). | Orally 400 mg/kg/day (81). | Reduces the thickness of the animal's skin (81). |
| RA | T cells secrete cytokines to activate B cells, and autoantibody production increases. Th17 response increased with the increase of the pro-inflammatory cytokine IL-17. In addition, TNF-α and IL-1 production increased, stimulating synovial cells. Synovial fibroblasts at the site of inflammation increased COX2/PGE2 and decreased SIRT1. Macrophages increase the recruitment of neutrophils at the site of inflammation through increased ROS production and activation of MPO and NF-κB (19,82–85). | RSV is able to act by reducing the production of autoantibodies, Th17 population, oxidative stress and NF-κB activation and reduces COX2 and PGE2 expression and activates SIRT1 (86–89). | Orally 20 mg/kg (91). | Inhibits levels of pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, IL-1, MPO and IL-4. It also inhibited NF-κB activation. Reduce the production of autoantibodies (89,90). |
| ALS | TAR DNA-binding protein 43 accumulation in the motor neuron cytoplasm deregulates mitochondrial biogenesis and SOD1 function, increasing glutamate and free radicals in the cytosol. Microglia detect an abnormal cell and activate naïve T-cell differentiation in the Th1 pattern that releases cytokines (TNF-α, INF, IL-1, IL-2, IL-6, and IL-7) (91,92). | RSV acts by activating SIRT1 and regulates its substrate expression, increases the SOD1 useful life, reduces ROS, and acts in mitochondrial biogenesis as an antioxidant and antiapoptotic (93). | Orally high fat diet containing 4 g resveratrol per kg diet (93). | It can reduce motor neuron degeneration and delay muscle atrophy (19,93). |
| AIH | Dense lymphoplasmic inflammatory infiltration occurs in the portal vein bundle (94,95). Activation and clonal expansion of T cells lead to the release of autoantibodies and pro-inflammatory cytokines by B cells (96). | RSV against concanavalin-A-(ConA-) induced liver injury by significantly inhibiting IL-2, IL-6, TNF-α (97). | Orally 30 mg/kg (98). | Inflammatory cytokines and infiltration of macrophages, neutrophils and T cells in the mouse liver were significantly reduced, and ConA-mediated downregulation of SIRT1 in the liver was reversed (98). |
AIH, autoimmune hepatitis; ALS, amyotrophic lateral sclerosis; RA, rheumatoid arthritis; PsO, psoriasis; IBD, inflammatory bowel disease; T1DM, type 1 diabetes; RSV, resveratrol; DC, dendritic cell; IL, interleukin; Th, T-helper; ROS, reactive oxygen species; SIRT1, sirtuin-1; TNF-α, tumor necrosis factor alpha; IL, interleukin; IFN, interferon; COX, cyclooxygenase-2; PGE2, prostaglandin E2; MPO, myeloperoxidase; NF-κB, nuclear factor κ light chain enhancer of activated B cells; SOD1, superoxide dismutase 1; ConA, concanavalin A.
5. RSV and SLE
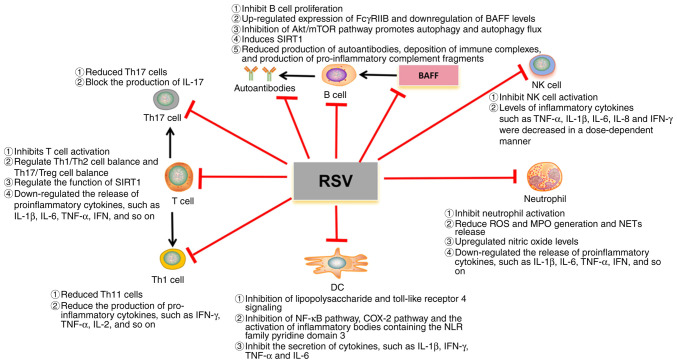
SLE can affect all organs of the body, such as the heart, lungs, kidneys and nervous system (1–3). Cardiovascular complications and kidney damage are common in patients with SLE, often leading to disability and death (1,2). RSV is used to treat SLE mainly through its effect on immune cells, the potential mechanism of which is illustrated in Fig. 2. At present, the existing studies mainly focus on the effects of RSV on kidney damage and cardiovascular complications in SLE, and a small number also involve the nervous system (97–100). Therefore, a corresponding discussion on this is included.
Figure 2.
Potential mechanism of RSV in SLE. RSV, resveratrol; DC, dendritic cell; IL, interleukin; MPO, myeloperoxidase; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon gamma; ROS, reactive oxygen species; IFN, interferon; SIRT1, Sirtuin-1; BAFF, B cell activating factor from the tumor necrosis factor family; C, complement; NK cell, natural killer cell; Th, T-helper; Treg, regulatory T cell; NET, neutrophil extracellular trap; COX, cyclooxygenase.
In vitro study
In vitro studies have shown that RSV can inhibit the activation of CD4+ T cells and B cells, thereby affecting the proliferation of B cells and antibody production (64). One of the functions of IL-10 is to inhibit excessive pro-inflammatory effects that may lead to tissue damage. It has been shown that IL-10 may play multiple and opposite roles in mouse lupus (97). IL-10 can promote the proliferation of autoreactive B cells, Ig class switching and antibody secretion in SLE, thereby promoting disease progression (98,99). A different study reported that monocytes in the blood of patients with active SLE can significantly reduce the level of IL-10 in the cell culture supernatant after co-culture with RSV (100). The inhibitory effect of RSV on IL-10 synthesis suggests that it may be useful in the treatment of SLE. In another study, it was found that the levels of ATP-binding box transporters A1 and G1 were significantly reduced in cells treated with plasma from 10% of patients with SLE, which are not only involved in reverse cholesterol transport, but also enhance cholesterol efflux from macrophages (101). When SLE plasma is co-incubated with RSV, efflux protein can be restored to the cellular level of healthy patients (102), indicating that RSV can affect cholesterol transport and reduce the level of oxidized low-density lipoprotein, thus having potential therapeutic activity for atherosclerotic cardiovascular disease in SLE.
Animal model
With regard to lupus nephritis, it has been revealed that different doses of RSV reduced proteinuria, renal immunoglobulin deposition, glomerulonephritis and serum Ig levels. One study found that in a mouse model of lupus BBA/C induced by prostin (102), the mice were injected with 0.5 ml of Pristine for 7 consecutive months, along with RSV treatment (50 and 75 mg/kg/day), and were assessed for autoantibody levels and kidney damage (64). The authors found that RSV can reduce urinary protein, reduce the deposition of IgM and IgG in the kidney and reduce the degree of renal histological injury. RSV has a protective effect on mice with lupus, especially on the kidney. In a recent study, MRL/lpr mice treated with RSV (50 mg/kg) for 8 weeks were found to have upregulated SIRT1 expression in the kidneys (103). Upregulation of SIRT1 interferes with NF-κB expression, transcription and expression of inflammatory cytokines (103). The second signal is blocked by SIRT1 regulating ROS production and TRPM2-mediated calcium perfusion to inhibit the NLRP3 inflammation, thereby slowing down the progression of lupus nephritis (103). This also suggests that SIRT1 may be a potential therapeutic target for RSV treatment of SLE. In addition, Pannu and Bhatnagar (104) studied the combined effects of RSV (25 and 50 mg/kg) and piperidine in a mouse model of lupus. The investigators revealed that the combination of RSV and piperidine successfully alleviated renal manifestations (decreased urinary protein and serum creatinine), and the combination of other medications reduced the dose of RSV. The authors also found that unlike RSV combined with piperine treatment, prophylactic treatment was more beneficial in reducing lupus-like manifestations such as lupus nephritis when RSV was used alone (105).
With regard to lupus cardiovascular disease, in vivo studies revealed that RSV has a therapeutic effect on atherosclerotic cardiovascular disease in SLE due to its antioxidant properties (101,106). These results were confirmed in vivo in a double-knockout ApoE−/− FAS−/− SLE mouse model (106). The authors found that mice in the RSV-treated group had fewer atherosclerotic plaques than those in the untreated group (not statistically significant), and that 43% of the RSV-treated animals had no plaques (106). In a different study, the authors evaluated the progression of aortic atherosclerosis in mice with SLE associated atherosclerosis after 10 weeks of oral treatment with RSV 0.3–0.4 mg/day in the treatment group (101). RSV was found to counteract the effect of SLE on atherosclerosis by preventing lipid excess by increasing cholesterol efflux (101). These findings suggest that RSV therapy may provide a novel approach to reduce the atherosclerotic cardiovascular effects of SLE.
In addition, a recent study also found that in lupus mice treated with water containing 0.01% RSV, RSV increased the level of SIRT1 in the hippocampus and decreased the level of vascular endothelial growth factor and C-X3-C motif chemokine ligand 1 (CX3CL1) by activating adenosine A2A receptors, but it was not statistically significant. It also showed a tendency to improve motor coordination in arteriosclerosis-prone lupus mice (107). This finding indicated that RSV may be a potential therapeutic candidate for the regulation of cognitive dysfunction in neuropsychiatric lupus, especially in motor disorders.
6. RSV bioavailability and associated toxicity
The main problem RSV faces in the treatment of diseases is its low bioavailability. In order to fully study the bioavailability of RSV, two maximum spikes in plasma levels occurred in healthy subjects after oral RSV: The first peak occurred 30-60 min after ingestion and the second peak occurred 6 h later (108). It was found that the RSV dose was 25 mg/day and the plasma peak concentration was 10 ng/ml (109). However, a previous study of high doses (500 mg/day) also demonstrated low plasma concentrations of ~71.2 ng/ml (110). After ingestion of RSV, although it is well absorbed orally and has a bioavailability of ~70%, the bioavailability of RSV itself is close to zero due to extensive metabolism in the liver and intestine, including glucoaldehyde and sulfation, which produce metabolites with low bioavailability (108). In addition, the low water solubility of RSV (~0.03 mg/ml) is another significant problem which severely affects the absorption and bioavailability of the compound (111). The solubility of RSV is strongly influenced by pH and temperature (112). A previous study revealed that RSV solubility is 64 µg/ml at pH 1.2, but becomes 61 and 50 µg/ml at pH 6.8 and above pH 7.4, respectively (112). RSV, when dissolved in water, is stable only at room or body temperature and under acidic conditions (113). Therefore, further research is needed to overcome these problems. In addition, certain factors may also influence the bioavailability and physiological response to RSV, such as variability of the human gut microbiome, genetic polymorphisms, age, sex, ethnicity, diet and exercise habits (113).
Regarding toxicity, in animal models, extensive studies using RSV supplements or for a range of diseases have shown that there are some side effects of the treatment, mainly that high doses may lead to cardiac inflammation, renal tubule dilation, nipple necrosis, acute inflammation of the pelvic region and severe kidney disease, leading to death (114). For instance, one study found that when animals were treated with successive doses of 0, 300, 1,000 and 3,000 mg/kg/day, no adverse reactions occurred at doses up to 300 mg/kg/day, while 1,000 and 3,000 mg/kg/day caused nephrotoxicity along with abnormal expression of liver genes. Serum liver enzyme and bilirubin levels were significantly increased (114). In addition, some studies have also observed different degrees of dehydration, dyspnea and other symptoms in animals (114–116). In mice with renal fibrosis, low-dose RSV (≤25 mg/kg) can partially improve renal function in mice with renal injury due to unilateral ureteral obstruction (UUO). Large doses of RSV (≥50 mg/kg) aggravate renal fibrosis, indicating that large doses of RSV lose its anti-fibrotic effect. Notably, mice with UUO-induced kidney damage were more susceptible to high-dose RSV-induced kidney damage than normal mice (117).
In human trials, RSV is generally well tolerated and a dose of 450 mg per day of RSV is safe for a person of 60 kg (118). However, some side effects have been reported, including cardiac and renal toxicity and gastrointestinal problems (119,120). Previous studies have found that high doses of RSV (1,000 mg/day) can increase biomarkers of cardiovascular disease risk such as oxidized low-density lipoprotein, soluble E-selectin 1, soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1 (121). RSV appears to have a negative effect on metabolic status, endothelial health, inflammation and cardiovascular markers in human patients (122). In a recent meta-analysis, patients experienced adverse reactions after treatment with high doses of RSV (123), with a maximum dose of 1,000 mg per day, 3 times per day. Adverse events occurred in 3 cases. These adverse events include mild elevation of alanine aminotransferase, diarrhea, mild indigestion, mild hypoglycemia and infection (patients with mild cellulitis at the biopsy site) (124,125). Although RSV is generally safe, there have been incidents of adverse effects; therefore, more animal and related human studies are needed to confirm its efficacy and safety.
7. Conclusions
SLE is an autoimmune disease that can accumulate in all organs of the body, therefore it is one of the most important factors that directly affect the quality of life of patients. The pathogenesis of SLE is very complex and is associated with autoimmune disorders, but the exact mechanism remains unclear. Glucocorticoids and immunosuppressants are still the treatment of choice, but long-term use of these drugs can lead to numerous unavoidable side effects. Bioactive natural ingredients derived from natural herbs may provide additional benefits in the prevention and treatment of SLE and represent an important source of new drug screening and development. RSV has a powerful anti-inflammatory effect by inhibiting the overactivation of immune cells, reducing the level of procytokines and the production of autoantibodies. Therefore, RSV is a potential and beneficial candidate for the treatment of SLE.
However, the current oral bioavailability of RSV is very low, with a maximum oral bioavailability of only 20%. Therefore, targeted delivery of RSV to desired tissues or increased stability of RSV in vivo through the development of sustained-release systems are critical to improving bioavailability. With respect to RSV for SLE, the dosage used in different studies has varied, thus the drug dosage remains unclear. If future studies can further explore the effect of different drug doses of RSV on treatment and the deeper mechanism, it will open a new window for the treatment of SLE. However, further studies in animals and humans are still needed to widely evaluate the biological activity, efficacy, safety and appropriate dosage of RSV, so as to provide a new way of thinking for clinicians to treat SLE.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RXH and YTY wrote the manuscript. XCH, DLM and RJH acquired and interpreted the data. YY, YJH, XZ and JYL conceptualised and designed the study. CCW and XXH modified the manuscript. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gergianaki I, Fanouriakis A, Repa A, Tzanakakis M, Adamichou C, Pompieri A, Spirou G, Bertsias A, Kabouraki E, Tzanakis I, et al. Epidemiology and burden of systemic lupus erythematosus in a Southern European population: Data from the community-based lupus registry of Crete, Greece. Ann Rheum Dis. 2017;76:1992–2000. doi: 10.1136/annrheumdis-2017-211206. [DOI] [PubMed] [Google Scholar]
- 2.Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80:14–25. doi: 10.1136/annrheumdis-2020-218272. [DOI] [PubMed] [Google Scholar]
- 3.Fang Q, Li T, Chen P, Wu Y, Wang T, Mo L, Ou J, Nandakumar KS. Comparative Analysis on Abnormal methylome of differentially expressed genes and disease pathways in the immune cells of RA and SLE. Front Immunol. 2021;12:668007. doi: 10.3389/fimmu.2021.668007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, Martín J, Fairfax BP, Knight JC, Chen L, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47:1457–1464. doi: 10.1038/ng.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fava A, Petri M. Systemic lupus erythematosus: Diagnosis and clinical management. J Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 7.Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, Vidal X, Mitjavila F, Castro Salomó A, Cuquet Pedragosa J, Ortiz-Santamaria V, Mauri Plana M, Cortés-Hernández J. Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: A randomised clinical trial. Ann Rheum Dis. 2017;76:1575–1582. doi: 10.1136/annrheumdis-2016-210882. [DOI] [PubMed] [Google Scholar]
- 8.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 9.Balasubramanian A, Wade SW, Adler RA, Lin CJF, Maricic M, O'Malley CD, Saag K, Curtis JR. Glucocorticoid exposure and fracture risk in patients with new-onset rheumatoid arthritis. Osteoporos Int. 2016;27:3239–3249. doi: 10.1007/s00198-016-3646-z. [DOI] [PubMed] [Google Scholar]
- 10.Singh BK, Singh S. Systemic lupus erythematosus and infections. Reumatismo. 2020;72:154–169. doi: 10.4081/reumatismo.2020.1303. [DOI] [PubMed] [Google Scholar]
- 11.Shi H, Gudjonsson JE, Kahlenberg JM. Treatment of cutaneous lupus erythematosus: Current approaches and future strategies. Curr Opin Rheumatol. 2020;32:208–214. doi: 10.1097/BOR.0000000000000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardile V, Chillemi R, Lombardo L, Sciuto S, Spatafora C, Tringali C. Antiproliferative activity of methylated analogues of E- and Z-resveratrol. Z Naturforsch C J Biosci. 2007;62:189–195. doi: 10.1515/znc-2007-3-406. [DOI] [PubMed] [Google Scholar]
- 13.Weiskirchen S, Weiskirchen R. Resveratrol: How much wine do you have to drink to stay healthy? Adv Nutr. 2016;7:706–718. doi: 10.3945/an.115.011627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raj P, Thandapilly SJ, Wigle J, Zieroth S, Netticadan T. A comprehensive analysis of the efficacy of resveratrol in atherosclerotic cardiovascular disease, myocardial infarction and heart failure. Molecules. 2021;26:6600. doi: 10.3390/molecules26216600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Švajger U, Jeras M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int Rev Immunol. 2012;31:202–222. doi: 10.3109/08830185.2012.665108. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Paik JH, Cho D, Cho JA, Kim CW. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int Immunopharmacol. 2008;8:542–547. doi: 10.1016/j.intimp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11:946. doi: 10.3390/nu11050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhou JP, Chen SJ, Huang HY, Lin WW, Huang DY, Tzeng SJ. Upregulation of FcγRIIB by resveratrol via NF-κB activation reduces B-cell numbers and ameliorates lupus. Exp Mol Med. 2017;49:e381. doi: 10.1038/emm.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira ALB, Monteiro VVS, Navegantes-Lima KC, Reis JF, Gomes RS, Rodrigues DVS, Gaspar SLF, Monteiro MC. Resveratrol role in autoimmune disease-a mini-review. Nutrients. 2017;9:1306. doi: 10.3390/nu9121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu Y, Yan M, Xie C, Hu J, Zeng X, Hu Q. Polydatin relieves paraquat-induced human MRC-5 fibroblast injury through inhibiting the activation of the NLRP3 inflammasome. Ann Transl Med. 2020;8:765. doi: 10.21037/atm-20-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10:1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han GM, Chen SL, Shen N, Ye S, Bao CD, Gu YY. Analysis of gene expression profiles in human systemic lupus erythematosus using oligonucleotide microarray. Genes Immun. 2003;4:177–186. doi: 10.1038/sj.gene.6363966. [DOI] [PubMed] [Google Scholar]
- 23.Ishii T, Onda H, Tanigawa A, Ohshima S, Fujiwara H, Mima T, Katada Y, Deguchi H, Suemura M, Miyake T, et al. Isolation and expression profiling of genes upregulated in the peripheral blood cells of systemic lupus erythematosus patients. DNA Res. 2005;12:429–439. doi: 10.1093/dnares/dsi020. [DOI] [PubMed] [Google Scholar]
- 24.Pan L, Lu MP, Wang JH, Xu M, Yang SR. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Pediatr. 2020;16:19–30. doi: 10.1007/s12519-019-00229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol. 2015;27:448–453. doi: 10.1097/BOR.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 26.Berthelot JM, Le Goff B, Neel A, Maugars Y, Hamidou M. NETosis: At the crossroads of rheumatoid arthritis, lupus, and vasculitis. Joint Bone Spine. 2017;84:255–262. doi: 10.1016/j.jbspin.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Fresneda Alarcon M, McLaren Z, Wright HL. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: Same Foe Different M.O. Front immunol. 2021;12:649693. doi: 10.3389/fimmu.2021.649693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park YW, Kee SJ, Cho YN, Lee EH, Lee HY, Kim EM, Shin MH, Park JJ, Kim TJ, Lee SS, et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1753–1763. doi: 10.1002/art.24556. [DOI] [PubMed] [Google Scholar]
- 29.Riccieri V, Spadaro A, Parisi G, Taccari E, Moretti T, Bernardini G, Favaroni M, Strom R. Down-regulation of natural killer cells and of gamma/delta T cells in systemic lupus erythematosus. Does it correlate to autoimmunity and to laboratory indices of disease activity? Lupus. 2000;9:333–337. doi: 10.1191/096120300678828460. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z, Fu B, Zheng SG, Li X, Sun R, Tian Z, Wei H. Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J Immunol. 2011;186:3421–3431. doi: 10.4049/jimmunol.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eloranta ML, Lövgren T, Finke D, Mathsson L, Rönnelid J, Kastner B, Alm GV, Rönnblom L. Regulation of the interferon-alpha production induced by RNA-containing immune complexes in plasmacytoid dendritic cells. Arthritis Rheum. 2009;60:2418–2427. doi: 10.1002/art.24686. [DOI] [PubMed] [Google Scholar]
- 32.Hervier B, Beziat V, Haroche J, Mathian A, Lebon P, Ghillani-Dalbin P, Musset L, Debré P, Amoura Z, Vieillard V. Phenotype and function of natural killer cells in systemic lupus erythematosus: Excess interferon-γ production in patients with active disease. Arthritis Rheum. 2011;63:1698–1706. doi: 10.1002/art.30313. [DOI] [PubMed] [Google Scholar]
- 33.Hodge DL, Berthet C, Coppola V, Kastenmüller W, Buschman MD, Schaughency PM, Shirota H, Scarzello AJ, Subleski JJ, Anver MR, et al. IFN-gamma AU-rich element removal promotes chronic IFN-gamma expression and autoimmunity in mice. J Autoimmun. 2014;53:33–45. doi: 10.1016/j.jaut.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Liu J, Hao S, Wu P, Zhang X, Xiao Y, Jiang G, Huang X. Higher activation of the interferon-gamma signaling pathway in systemic lupus erythematosus patients with a high type I IFN score: Relation to disease activity. Clin Rheumatol. 2018;37:2675–2684. doi: 10.1007/s10067-018-4138-7. [DOI] [PubMed] [Google Scholar]
- 35.Nandakumar KS, Nündel K. Editorial: Systemic lupus erythematosus-predisposition factors, pathogenesis, diagnosis, treatment and disease models. Front Immunol. 2022;13:1118180. doi: 10.3389/fimmu.2022.1118180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz DA, Gray JD, Behrendsen SC, Kubin M, Rengaraju M, Ohtsuka K, Trinchieri G. Decreased production of interleukin-12 and other Th1-type cytokines in patients with recent-onset systemic lupus erythematosus. Arthritis Rheum. 1998;41:838–844. doi: 10.1002/1529-0131(199805)41:5<838::AID-ART10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Lee HY, Hong YK, Yun HJ, Kim YM, Kim JR, Yoo WH. Altered frequency and migration capacity of CD4+CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology (Oxford) 2008;47:789–794. doi: 10.1093/rheumatology/ken108. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Yu J, Tao X, Cai L, Wang J, Zheng SG. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol. 2010;29:1251–1258. doi: 10.1007/s10067-010-1510-7. [DOI] [PubMed] [Google Scholar]
- 39.Qiu Y, Zhou X, Liu Y, Tan S, Li Y. The role of sirtuin-1 in immune response and systemic lupus erythematosus. Front Immunol. 2021;12:632383. doi: 10.3389/fimmu.2021.632383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Zhang XL, Zhang GH, Gao YF. Artesunate promotes Th1 differentiation from CD4+ T cells to enhance cell apoptosis in ovarian cancer via miR-142. Braz J Med Biol Res. 2019;52:e7992. doi: 10.1590/1414-431x20197992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorgham K, Amoura Z, Parizot C, Arnaud L, Frances C, Pionneau C, Devilliers H, Pinto S, Zoorob R, Miyara M, et al. Ultraviolet light converts propranolol, a nonselective β-blocker and potential lupus-inducing drug, into a proinflammatory AhR ligand. Eur J Immunol. 2015;45:3174–3187. doi: 10.1002/eji.201445144. [DOI] [PubMed] [Google Scholar]
- 42.Guo NH, Fu X, Zi FM, Song Y, Wang S, Cheng J. The potential therapeutic benefit of resveratrol on Th17/Treg imbalance in immune thrombocytopenic purpura. Int Immunopharmacol. 2019;73:181–192. doi: 10.1016/j.intimp.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 43.Delmas D, Limagne E, Ghiringhelli F, Aires V. Immune Th17 lymphocytes play a critical role in the multiple beneficial properties of resveratrol. Food Chem Toxicol. 2020;137:111091. doi: 10.1016/j.fct.2019.111091. [DOI] [PubMed] [Google Scholar]
- 44.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 45.Choi JY, Ho JHE, Pasoto SG, Bunin V, Kim ST, Carrasco S, Borba EF, Gonçalves CR, Costa PR, Kallas EG, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: Association with disease activity. Arthritis Rheumatol. 2015;67:988–999. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sequeira J, Boily G, Bazinet S, Saliba S, He X, Jardine K, Kennedy C, Staines W, Rousseaux C, Mueller R, McBurney MW. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314:3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q, Yan C, Xin M, Han L, Zhang Y, Sun M. Sirtuin 1 (Sirt1) overexpression in BaF3 cells contributes to cell proliferation promotion, apoptosis resistance and pro-inflammatory cytokine production. Med Sci Monit. 2017;23:1477–1482. doi: 10.12659/MSM.900754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delmas D, Lin HY. Role of membrane dynamics processes and exogenous molecules in cellular resveratrol uptake: Consequences in bioavailability and activities. Mol Nutr Food Res. 2011;55:1142–1153. doi: 10.1002/mnfr.201100065. [DOI] [PubMed] [Google Scholar]
- 51.Ho Y, Li ZL, Shih YJ, Chen YR, Wang K, Whang-Peng J, Lin HY, Davis PJ. Integrin αvβ3 in the mediating effects of dihydrotestosterone and resveratrol on breast cancer cell proliferation. Int J Mol Sci. 2020;21:2906. doi: 10.3390/ijms21082906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Schwager J, Richard N, Widmer F, Raederstorff D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement Altern Med. 2017;17:309. doi: 10.1186/s12906-017-1823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svajger U, Obermajer N, Jeras M. Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology. 2010;129:525–535. doi: 10.1111/j.1365-2567.2009.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sener G, Tuğtepe H, Yüksel M, Cetinel S, Gedik N, Yeğen BC. Resveratrol improves ischemia/reperfusion-induced oxidative renal injury in rats. Arch Med Res. 2006;37:822–829. doi: 10.1016/j.arcmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Wang W, Sun L, Zhang P, Song J, Liu W. An anti-inflammatory cell-free collagen/resveratrol scaffold for repairing osteochondral defects in rabbits. Acta Biomater. 2014;10:4983–4995. doi: 10.1016/j.actbio.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 57.Orsu P, Murthy BVSN, Akula A. Cerebroprotective potential of resveratrol through anti-oxidant and anti-inflammatory mechanisms in rats. J Neural Transm (Vienna) 2013;120:1217–1223. doi: 10.1007/s00702-013-0982-4. [DOI] [PubMed] [Google Scholar]
- 58.Bo S, Ciccone G, Castiglione A, Gambino R, De Michieli F, Villois P, Durazzo M, Cavallo-Perin P, Cassader M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20:1323–1331. doi: 10.2174/0929867311320100009. [DOI] [PubMed] [Google Scholar]
- 59.Balkrishna A, Sinha S, Kumar A, Arya V, Gautam AK, Valis M, Kuca K, Kumar D, Amarowicz R. Sepsis-mediated renal dysfunction: Pathophysiology, biomarkers and role of phytoconstituents in its management. Biomed Pharmacother. 2023;165:115183. doi: 10.1016/j.biopha.2023.115183. [DOI] [PubMed] [Google Scholar]
- 60.Liu FC, Tsai HI, Yu HP. Organ-protective effects of red wine extract, resveratrol, in oxidative stress-mediated reperfusion injury. Oxid Med Cell Longev. 2015;2015:568634. doi: 10.1155/2015/568634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Souza Andrade MM, Leal VNC, Fernandes IG, Gozzi-Silva SC, Beserra DR, Oliveira EA, Teixeira FME, Yendo TM, Sousa MDGT, Teodoro WR, et al. Resveratrol downmodulates neutrophil extracellular trap (NET) generation by neutrophils in patients with severe COVID-19. Antioxidants (Basel) 2022;11:1690. doi: 10.3390/antiox11091690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rieder SA, Nagarkatti P, Nagarkatti M. Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. Br J Pharmacol. 2012;167:1244–1258. doi: 10.1111/j.1476-5381.2012.02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Q, Huyan T, Ye LJ, Li J, Shi JL, Huang QS. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. J Agric Food Chem. 2014;62:10928–10935. doi: 10.1021/jf502950u. [DOI] [PubMed] [Google Scholar]
- 64.Wang ZL, Luo XF, Li MT, Xu D, Zhou S, Chen HZ, Gao N, Chen Z, Zhang LL, Zeng XF. Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PLoS One. 2014;9:e114792. doi: 10.1371/journal.pone.0114792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao Y, Zhu J, Qin S, Zhou Z, Zeng Q, Long R, Mao Z, Dong X, Zhao R, Zhang R, et al. Resveratrol induces autophagy impeding BAFF-stimulated B-cell proliferation and survival by inhibiting the Akt/mTOR pathway. Biochem Pharmacol. 2022;202:115139. doi: 10.1016/j.bcp.2022.115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wållberg M, Cooke A. Immune mechanisms in type 1 diabetes. Trends Immunol. 2013;34:583–591. doi: 10.1016/j.it.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Battaglia M. Neutrophils and type 1 autoimmune diabetes. Curr Opin Hematol. 2014;21:8–15. doi: 10.1097/MOH.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 68.Kaur G, Padiya R, Adela R, Putcha UK, Reddy GS, Reddy BR, Kumar KP, Chakravarty S, Banerjee SK. Garlic and resveratrol attenuate diabetic complications, loss of β-cells, pancreatic and hepatic oxidative stress in streptozotocin-induced diabetic rats. Front Pharmacol. 2016;7:360. doi: 10.3389/fphar.2016.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee SM, Yang H, Tartar DM, Gao B, Luo X, Ye SQ, Zaghouani H, Fang D. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia. 2011;54:1136–1146. doi: 10.1007/s00125-011-2064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yonamine CY, Pinheiro-Machado E, Michalani ML, Freitas HS, Okamoto MM, Corrêa-Giannella ML, Machado UF. Resveratrol improves glycemic control in insulin-treated diabetic rats: Participation of the hepatic territory. Nutr Metab (Lond) 2016;13:44. doi: 10.1186/s12986-016-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boirivant M, Cossu A. Inflammatory bowel disease. Oral Dis. 2012;18:1–15. doi: 10.1111/j.1601-0825.2011.01811.x. [DOI] [PubMed] [Google Scholar]
- 72.Singh UP, Singh NP, Busbee B, Guan H, Singh B, Price RL, Taub DD, Mishra MK, Nagarkatti M, Nagarkatti PS. Alternative medicines as emerging therapies for inflammatory bowel diseases. Int Rev Immunol. 2012;31:66–84. doi: 10.3109/08830185.2011.642909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. 2017;2017:4535194. doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sánchez-Fidalgo S, Cárdeno A, Villegas I, Talero E, de la Lastra CA. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol. 2010;633:78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 75.Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: A randomized, double-blind, placebo-controlled pilot study. Arch Med Res. 2015;46:280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Hänsel A, Günther C, Ingwersen J, Starke J, Schmitz M, Bachmann M, Meurer M, Rieber EP, Schäkel K. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J Allergy Clin Immunol. 2011;127:787–794.e1-e9. doi: 10.1016/j.jaci.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 77.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: Toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141–150. doi: 10.1016/j.jaad.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 79.Kjær TN, Thorsen K, Jessen N, Stenderup K, Pedersen SB. Resveratrol ameliorates imiquimod-induced psoriasis-like skin inflammation in mice. PLoS One. 2015;10:e0126599. doi: 10.1371/journal.pone.0126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Navegantes KC, de Souza Gomes R, Pereira PAT, Czaikoski PG, Azevedo CHM, Monteiro MC. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J Transl Med. 2017;15:36. doi: 10.1186/s12967-017-1141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanaka T, Hishitani Y, Ogata A. Monoclonal antibodies in rheumatoid arthritis: comparative effectiveness of tocilizumab with tumor necrosis factor inhibitors. Biologics. 2014;8:141–153. doi: 10.2147/BTT.S37509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brzustewicz E, Bryl E. The role of cytokines in the pathogenesis of rheumatoid arthritis-Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine. 2015;76:527–536. doi: 10.1016/j.cyto.2015.08.260. [DOI] [PubMed] [Google Scholar]
- 83.Engler A, Tange C, Frank-Bertoncelj M, Gay RE, Gay S, Ospelt C. Regulation and function of SIRT1 in rheumatoid arthritis synovial fibroblasts. J Mol Med (Berl) 2016;94:173–182. doi: 10.1007/s00109-015-1332-9. [DOI] [PubMed] [Google Scholar]
- 84.Ma C, Wang Y, Dong L, Li M, Cai W. Anti-inflammatory effect of resveratrol through the suppression of NF-κB and JAK/STAT signaling pathways. Acta Biochim Biophys Sin (Shanghai) 2015;47:207–213. doi: 10.1093/abbs/gmu135. [DOI] [PubMed] [Google Scholar]
- 85.Lee SJ, Thien Quach CH, Jung KH, Paik JY, Lee JH, Park JW, Lee KH. Oxidized low-density lipoprotein stimulates macrophage 18F-FDG uptake via hypoxia-inducible factor-1α activation through Nox2-dependent reactive oxygen species generation. J Nucl Med. 2014;55:1699–1705. doi: 10.2967/jnumed.114.139428. [DOI] [PubMed] [Google Scholar]
- 86.Tsai MH, Hsu LF, Lee CW, Chiang YC, Lee MH, How JM, Wu CM, Huang CL, Lee IT. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int J Biochem Cell Biol. 2017;88:113–123. doi: 10.1016/j.biocel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 87.Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- 88.Wahba MGF, Messiha BAS, Abo-Saif AA. Protective effects of fenofibrate and resveratrol in an aggressive model of rheumatoid arthritis in rats. Pharm Biol. 2016;54:1705–1715. doi: 10.3109/13880209.2015.1125931. [DOI] [PubMed] [Google Scholar]
- 89.Avendaño-Vázquez SE, Dhir A, Bembich S, Buratti E, Proudfoot N, Baralle FE. Autoregulation of TDP-43 mRNA levels involves interplay between transcription, splicing, and alternative polyA site selection. Genes Dev. 2012;26:1679–1684. doi: 10.1101/gad.194829.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malaspina A, Puentes F, Amor S. Disease origin and progression in amyotrophic lateral sclerosis: An immunology perspective. Int Immunol. 2015;27:117–129. doi: 10.1093/intimm/dxu099. [DOI] [PubMed] [Google Scholar]
- 91.Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao W, Varghese M, Yemul S, Pan Y, Cheng A, Marano P, Hassan S, Vempati P, Chen F, Qian X, Pasinetti GM. Peroxisome proliferator activator receptor gamma coactivator-1alpha (PGC-1α) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2011;6:51. doi: 10.1186/1750-1326-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McFarlane IG. Pathogenesis of autoimmune hepatitis. Biomed Pharmacother. 1999;53:255–263. doi: 10.1016/S0753-3322(99)80096-1. [DOI] [PubMed] [Google Scholar]
- 94.Ichiki Y, Aoki CA, Bowlus CL, Shimoda S, Ishibashi H, Gershwin ME. T cell immunity in autoimmune hepatitis. Autoimmun Rev. 2005;4:315–321. doi: 10.1016/j.autrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Gianchecchi E, Fierabracci A. Insights on the effects of resveratrol and some of its derivatives in cancer and autoimmunity: A molecule with a dual activity. Antioxidants (Basel) 2020;9:91. doi: 10.3390/antiox9020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang TH, Chen CC, Liu HM, Lee TY, Shieh SH. Resveratrol pretreatment attenuates concanavalin a-induced hepatitis through reverse of aberration in the immune response and regenerative capacity in aged mice. Sci Rep. 2017;7:2705. doi: 10.1038/s41598-017-02881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Biswas S, Bieber K, Manz RA. IL-10 revisited in systemic lupus erythematosus. Front Immunol. 2022;13:970906. doi: 10.3389/fimmu.2022.970906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Facciotti F, Larghi P, Bosotti R, Vasco C, Gagliani N, Cordiglieri C, Mazzara S, Ranzani V, Rottoli E, Curti S, et al. Evidence for a pathogenic role of extrafollicular, IL-10-producing CCR6+B helper T cells in systemic lupus erythematosus. Proc Natl Acad Sci USA. 2020;117:7305–7316. doi: 10.1073/pnas.1917834117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caielli S, Veiga DT, Balasubramanian P, Athale S, Domic B, Murat E, Banchereau R, Xu Z, Chandra M, Chung CH, et al. A CD4+ T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat Med. 2019;25:75–81. doi: 10.1038/s41591-018-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klonowska-Szymczyk A, Kulczycka-Siennicka L, Robak T, Smolewski P, Cebula-Obrzut B, Robak E. The impact of agonists and antagonists of TLR3 and TLR9 on concentrations of IL-6, IL10 and sIL-2R in culture supernatants of peripheral blood mononuclear cells derived from patients with systemic lupus erythematosus. Postepy Hig Med Dosw (Online) 2017;71:867–875. doi: 10.5604/01.3001.0010.5266. [DOI] [PubMed] [Google Scholar]
- 101.Voloshyna I, Teboul I, Littlefield MJ, Siegart NM, Turi GK, Fazzari MJ, Carsons SE, DeLeon J, Reiss AB. Resveratrol counters systemic lupus erythematosus-associated atherogenicity by normalizing cholesterol efflux. Exp Biol Med (Maywood) 2016;241:1611–1619. doi: 10.1177/1535370216647181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tian J, Huang T, Chen J, Wang J, Chang S, Xu H, Zhou X, Yang J, Xue Y, Zhang T, et al. SIRT1 slows the progression of lupus nephritis by regulating the NLRP3 inflammasome through ROS/TRPM2/Ca2+ channel. Clin Exp Med. 2023;23:3465–3478. doi: 10.1007/s10238-023-01093-2. [DOI] [PubMed] [Google Scholar]
- 104.Pannu N, Bhatnagar A. Combinatorial therapeutic effect of resveratrol and piperine on murine model of systemic lupus erythematosus. Inflammopharmacology. 2020;28:401–424. doi: 10.1007/s10787-019-00662-w. [DOI] [PubMed] [Google Scholar]
- 105.Pannu N, Bhatnagar A. Prophylactic effect of resveratrol and piperine on pristane-induced murine model of lupus-like disease. Inflammopharmacology. 2020;28:719–735. doi: 10.1007/s10787-019-00662-w. [DOI] [PubMed] [Google Scholar]
- 106.Feng X, Li H, Rumbin AA, Wang X, La Cava A, Brechtelsbauer K, Castellani LW, Witztum JL, Lusis AJ, Tsao BP. ApoE-/-Fas-/-C57BL/6 mice: A novel murine model simultaneously exhibits lupus nephritis, atherosclerosis, and osteopenia. J Lipid Res. 2007;48:794–805. doi: 10.1194/jlr.M600512-JLR200. [DOI] [PubMed] [Google Scholar]
- 107.Kasselman LJ, Renna HA, Voloshyna I, Pinkhasov A, Gomolin IH, Teboul I, De Leon J, Carsons SE, Reiss AB. Cognitive changes mediated by adenosine receptor blockade in a resveratrol-treated atherosclerosis-prone lupus mouse model. J Tradit Complement Med. 2022;12:447–454. doi: 10.1016/j.jtcme.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li C, Wang Z, Lei H, Zhang D. Recent progress in nanotechnology-based drug carriers for resveratrol delivery. Drug Deliv. 2023;30:2174206. doi: 10.1080/10717544.2023.2174206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 110.Sergides C, Chirilă M, Silvestro L, Pitta D, Pittas A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp Ther Med. 2016;11:164–170. doi: 10.3892/etm.2015.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Summerlin N, Soo E, Thakur S, Qu Z, Jambhrunkar S, Popat A. Resveratrol nanoformulations: Challenges and opportunities. Int J Pharm. 2015;479:282–290. doi: 10.1016/j.ijpharm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 112.Zupančič Š, Lavrič Z, Kristl J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur J Pharm Biopharm. 2015;93:196–204. doi: 10.1016/j.ejpb.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 113.Novelle MG, Wahl D, Diéguez C, Bernier M, de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH, Nasrallah GK, Pintus G. Potential adverse effects of resveratrol: A literature review. Int J Mol Sci. 2020;21:2084. doi: 10.3390/ijms21062084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hebbar V, Shen G, Hu R, Kim BR, Chen C, Korytko PJ, Crowell JA, Levine BS, Kong AN. Toxicogenomics of resveratrol in rat liver. Life Sci. 2005;76:2299–2314. doi: 10.1016/j.lfs.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 116.Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- 117.Liu S, Zhao M, Zhou Y, Wang C, Yuan Y, Li L, Bresette W, Chen Y, Cheng J, Lu Y, Liu J. Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: A potential risk to individuals with impaired kidney function. Phytomedicine. 2019;57:223–235. doi: 10.1016/j.phymed.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 118.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: Diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 119.Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases-safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 2011;4:1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N, Pedersen SB, Jørgensen JO. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mankowski RT, You L, Buford TW, Leeuwenburgh C, Manini TM, Schneider S, Qiu P, Anton SD. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults-A pilot study. Exp Gerontol. 2020;131:110821. doi: 10.1016/j.exger.2019.110821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramírez-Garza SL, Laveriano-Santos EP, Marhuenda-Muñoz M, Storniolo CE, Tresserra-Rimbau A, Vallverdú-Queralt A, Lamuela-Raventós RM. Health effects of resveratrol: Results from human intervention trials. Nutrients. 2018;10:1892. doi: 10.3390/nu10121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang T, He Q, Liu Y, Chen Z, Hu H. Efficacy and safety of resveratrol supplements on blood lipid and blood glucose control in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2021;2021:5644171. doi: 10.1155/2021/5644171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goh KP, Lee HY, Lau DP, Supaat W, Chan YH, Koh AFY. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int J Sport Nutr Exerc Metab. 2014;24:2–13. doi: 10.1123/ijsnem.2013-0045. [DOI] [PubMed] [Google Scholar]
- 125.Sattarinezhad A, Roozbeh J, Shirazi Yeganeh B, Omrani GR, Shams M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019;45:53–59. doi: 10.1016/j.diabet.2018.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.