Abstract
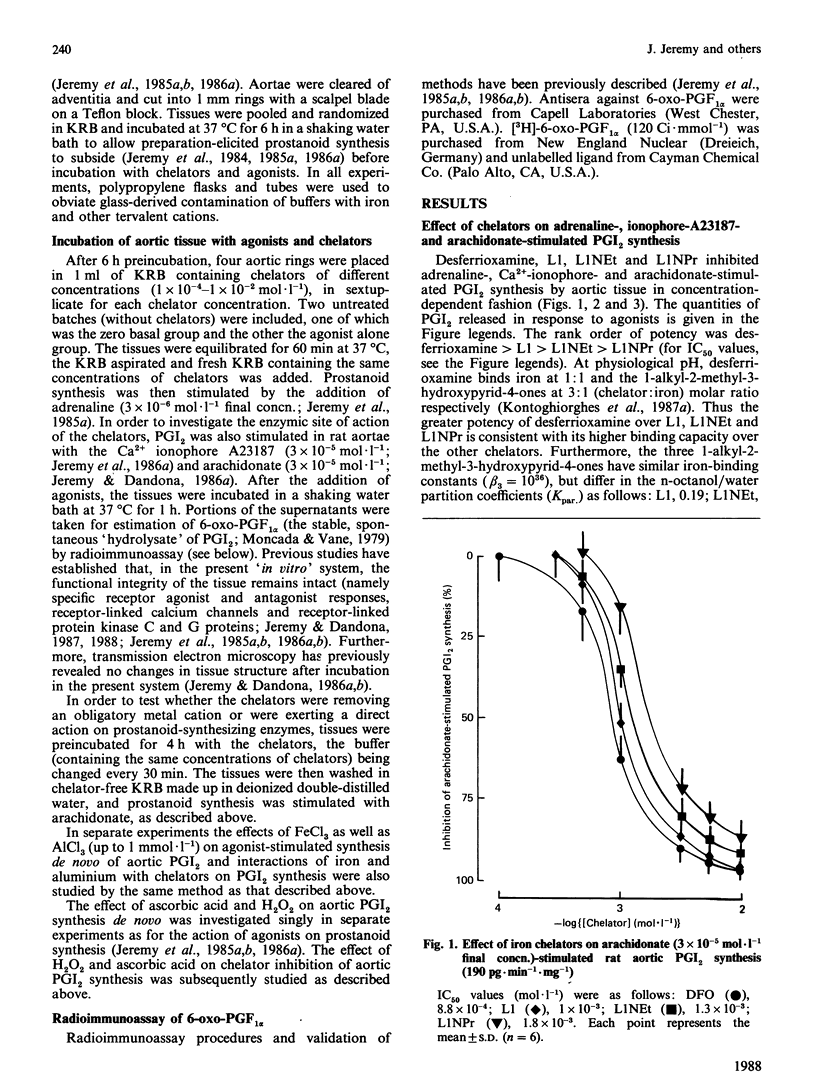
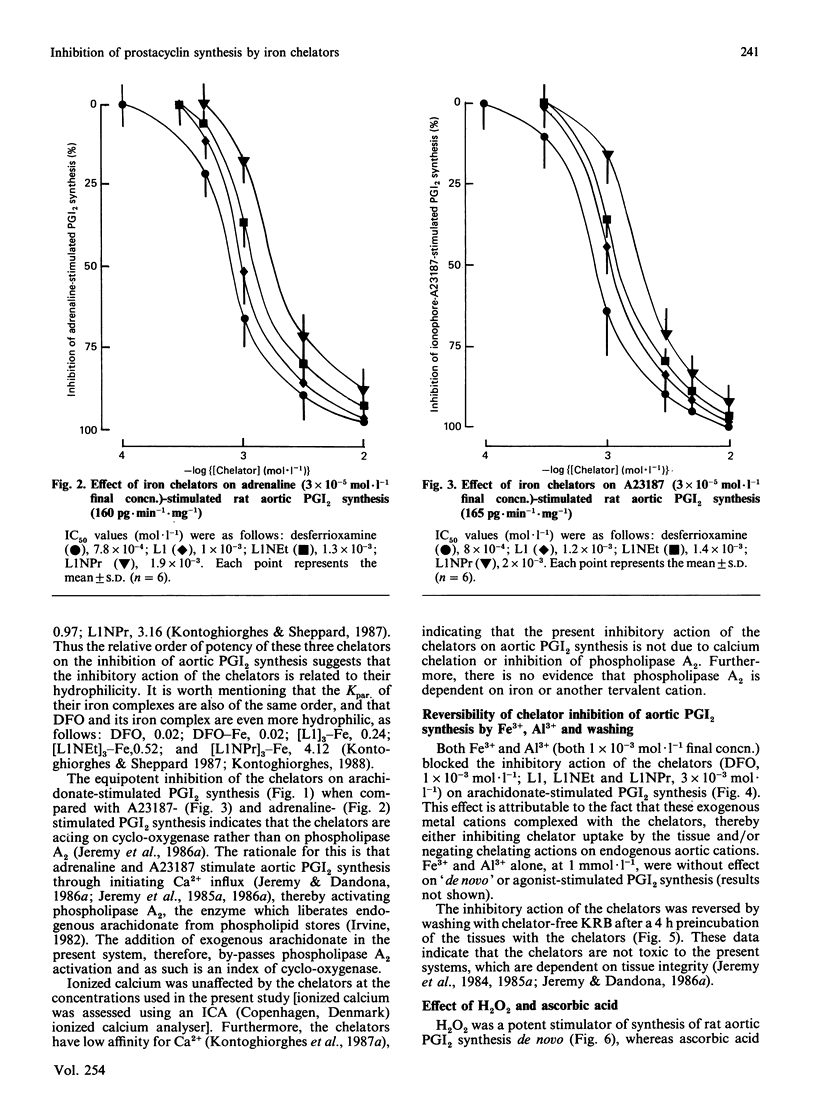
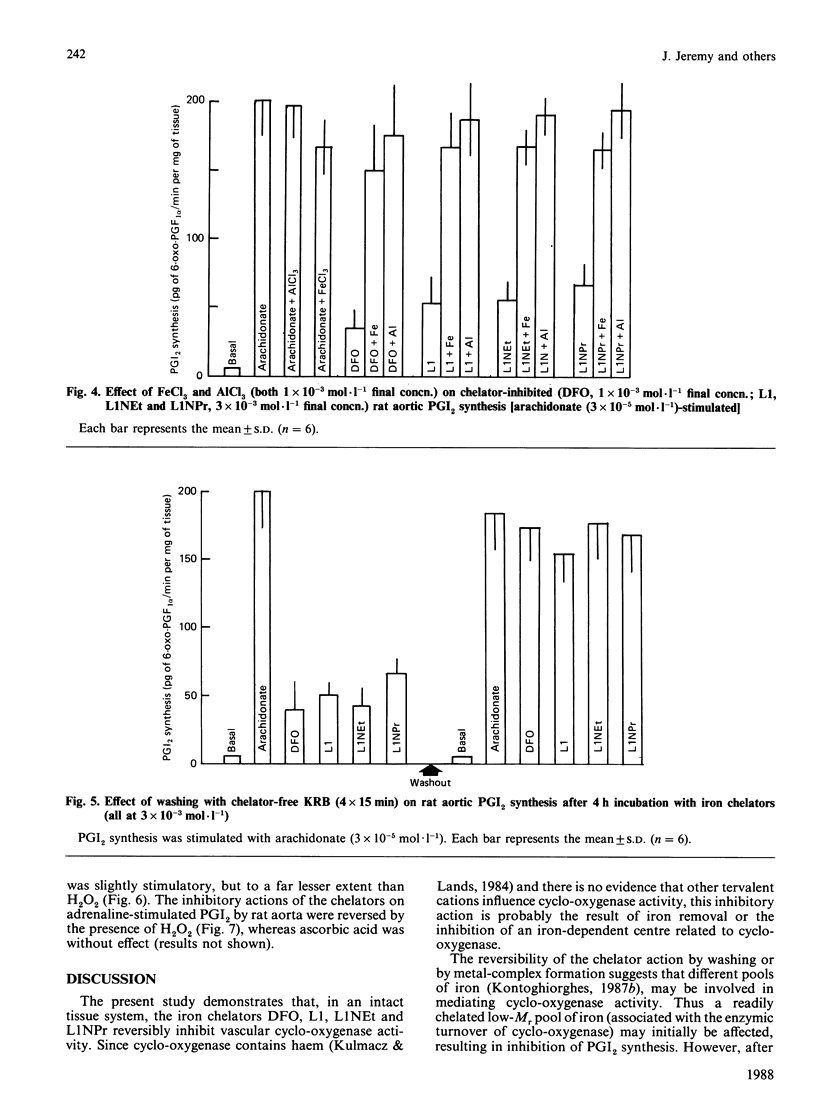
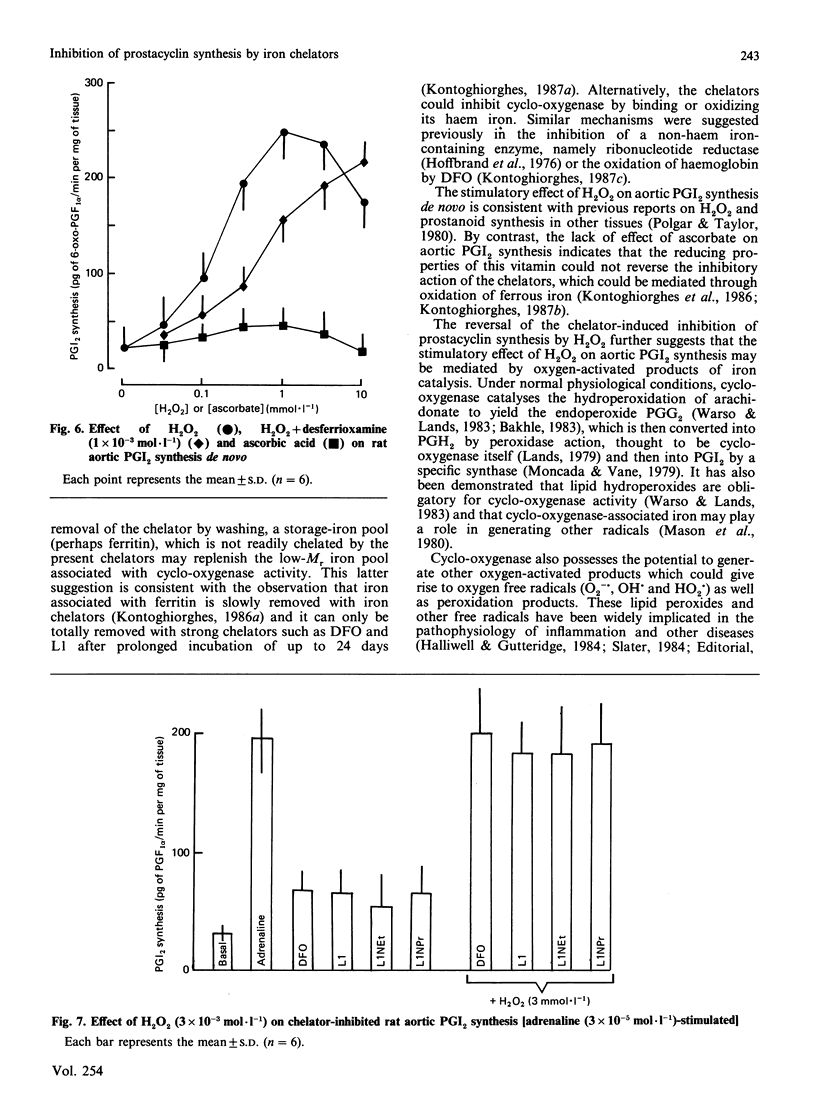
The iron chelators desferrioxamine (DFO), 1,2-dimethyl(L1)-, 1-ethyl-2-methyl(L1NEt)- and 1-propyl-2-methyl(L1NPr)-3-hydroxypyrid-4-ones inhibited rat aortic prostacyclin (PGI2) synthesis in vitro (rank order of potency: DFO greater than L1 greater than L1NEt greater than L1NPr) when stimulated with adrenaline, arachidonate and the Ca2+ ionophore A23187. The inhibitory action of the chelators was blocked by Fe3+ and Al3+ and reversed by washing and H2O2, but not by ascorbate. These data suggest that iron chelators inhibit prostanoid synthesis in intact tissue through the removal or binding of Fe3+ linked to cyclo-oxygenase. These iron chelators may be of therapeutic value in the treatment of inflammatory and other diseases via two mechanisms: (1) the inhibition of pro-inflammatory prostanoid synthesis and (2) the inhibition of toxic-free-radical generation by cyclo-oxygenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhle Y. S. Synthesis and catabolism of cyclo-oxygenase products. Br Med Bull. 1983 Jul;39(3):214–218. doi: 10.1093/oxfordjournals.bmb.a071821. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1973 Mar;70(3):899–903. doi: 10.1073/pnas.70.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffbrand A. V., Ganeshaguru K., Hooton J. W., Tattersall M. H. Effect of iron deficiency and desferrioxamine on DNA synthesis in human cells. Br J Haematol. 1976 Aug;33(4):517–526. doi: 10.1111/j.1365-2141.1976.tb03570.x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremy J. Y., Dandona P. Fluoride stimulates in vitro vascular prostacyclin synthesis: interrelationship of G proteins and protein kinase C. Eur J Pharmacol. 1988 Feb 9;146(2-3):279–284. doi: 10.1016/0014-2999(88)90303-2. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Dandona P. Inhibition by hydrocortisone of prostacyclin synthesis by rat aorta and its reversal with RU486. Endocrinology. 1986 Aug;119(2):661–665. doi: 10.1210/endo-119-2-661. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Dandona P. RU486 antagonizes the inhibitory action of progesterone on prostacyclin and thromboxane A2 synthesis in cultured rat myometrial explants. Endocrinology. 1986 Aug;119(2):655–660. doi: 10.1210/endo-119-2-655. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Dandona P. The role of the diacylglycerol-protein kinase C system in mediating adrenoceptor-prostacyclin synthesis coupling in the rat aorta. Eur J Pharmacol. 1987 Apr 29;136(3):311–316. doi: 10.1016/0014-2999(87)90303-7. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Dandona P. Adrenergic modulation of vascular prostacyclin (PGI2) secretion. Eur J Pharmacol. 1985 Aug 7;114(1):33–40. doi: 10.1016/0014-2999(85)90517-5. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Dandona P. Prostanoid synthesis by the rat urinary bladder: evidence for stimulation through muscarine receptor-linked calcium channels. Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):463–467. doi: 10.1007/BF00569387. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Dandona P. The effect of nifedipine, nimodipine and nisoldipine on agonist- and trauma-stimulated vascular prostacyclin synthesis in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):70–73. doi: 10.1007/BF00633200. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Dandona P. Thromboxane A2 analogue (U-46619) stimulates vascular PGI2 synthesis. Eur J Pharmacol. 1985 Jan 2;107(2):259–262. doi: 10.1016/0014-2999(85)90066-4. [DOI] [PubMed] [Google Scholar]
- Jeremy J. Y., Mikhailidis D. P., Dandona P. Vascular trauma and prostacyclin release. Microcirc Endothelium Lymphatics. 1984 Oct;1(5):629–644. [PubMed] [Google Scholar]
- Kontoghiorghes G. J., Aldouri M. A., Sheppard L., Hoffbrand A. V. 1,2-Dimethyl-3-hydroxypyrid-4-one, an orally active chelator for treatment of iron overload. Lancet. 1987 Jun 6;1(8545):1294–1295. doi: 10.1016/s0140-6736(87)90545-9. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J. Iron mobilization from ferritin using alpha-oxohydroxy heteroaromatic chelators. Biochem J. 1986 Jan 1;233(1):299–302. doi: 10.1042/bj2330299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoghiorghes G. J., Jackson M. J., Lunec J. In vitro screening of iron chelators using models of free radical damage. Free Radic Res Commun. 1986;2(1-2):115–124. doi: 10.3109/10715768609088062. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J. New orally active iron chelators. Lancet. 1985 Apr 6;1(8432):817–817. doi: 10.1016/s0140-6736(85)91472-2. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J. Orally active alpha-ketohydroxypyridine iron chelators: effects on iron and other metal mobilisations. Acta Haematol. 1987;78(2-3):212–216. doi: 10.1159/000205877. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J. Orally active alpha-ketohydroxypyridine iron chelators: studies in mice. Mol Pharmacol. 1986 Dec;30(6):670–673. [PubMed] [Google Scholar]
- Kontoghiorghes G. J., Sheppard L., Chambers S. New synthetic approach and iron chelating studies of 1-alkyl-2-methyl-3-hydroxypyrid-4-ones. Arzneimittelforschung. 1987 Oct;37(10):1099–1102. [PubMed] [Google Scholar]
- Kulmacz R. J., Lands W. E. Prostaglandin H synthase. Stoichiometry of heme cofactor. J Biol Chem. 1984 May 25;259(10):6358–6363. [PubMed] [Google Scholar]
- Lands W. E. The biosynthesis and metabolism of prostaglandins. Annu Rev Physiol. 1979;41:633–652. doi: 10.1146/annurev.ph.41.030179.003221. [DOI] [PubMed] [Google Scholar]
- Lewis G. P. Immunoregulatory activity of metabolites of arachidonic acid and their role in inflammation. Br Med Bull. 1983 Jul;39(3):243–248. doi: 10.1093/oxfordjournals.bmb.a071827. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Kalyanaraman B., Tainer B. E., Eling T. E. A carbon-centered free radical intermediate in the prostaglandin synthetase oxidation of arachidonic acid. Spin trapping and oxygen uptake studies. J Biol Chem. 1980 Jun 10;255(11):5019–5022. [PubMed] [Google Scholar]
- Muirden K. D. Ferritin in synovial cells in patients with rheumatoid arthritis. Ann Rheum Dis. 1966 Sep;25(5):387–401. doi: 10.1136/ard.25.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugteren D. H., Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta. 1973 Dec 20;326(3):448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- Polgar P., Taylor L. Stimulation of prostaglandin synthesis by ascorbic acid via hydrogen peroxide formation. Prostaglandins. 1980 May;19(5):693–700. doi: 10.1016/0090-6980(80)90168-9. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B. ON THE INCORPORATION OF OXYGEN IN THE CONVERSION OF 8, 11, 14-EICOSATRIENOIC ACID TO PROSTAGLANDIN E1. J Am Chem Soc. 1965 Jul 5;87:3011–3013. doi: 10.1021/ja01091a043. [DOI] [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warso M. A., Lands W. E. Lipid peroxidation in relation to prostacyclin and thromboxane physiology and pathophysiology. Br Med Bull. 1983 Jul;39(3):277–280. doi: 10.1093/oxfordjournals.bmb.a071833. [DOI] [PubMed] [Google Scholar]


