Abstract
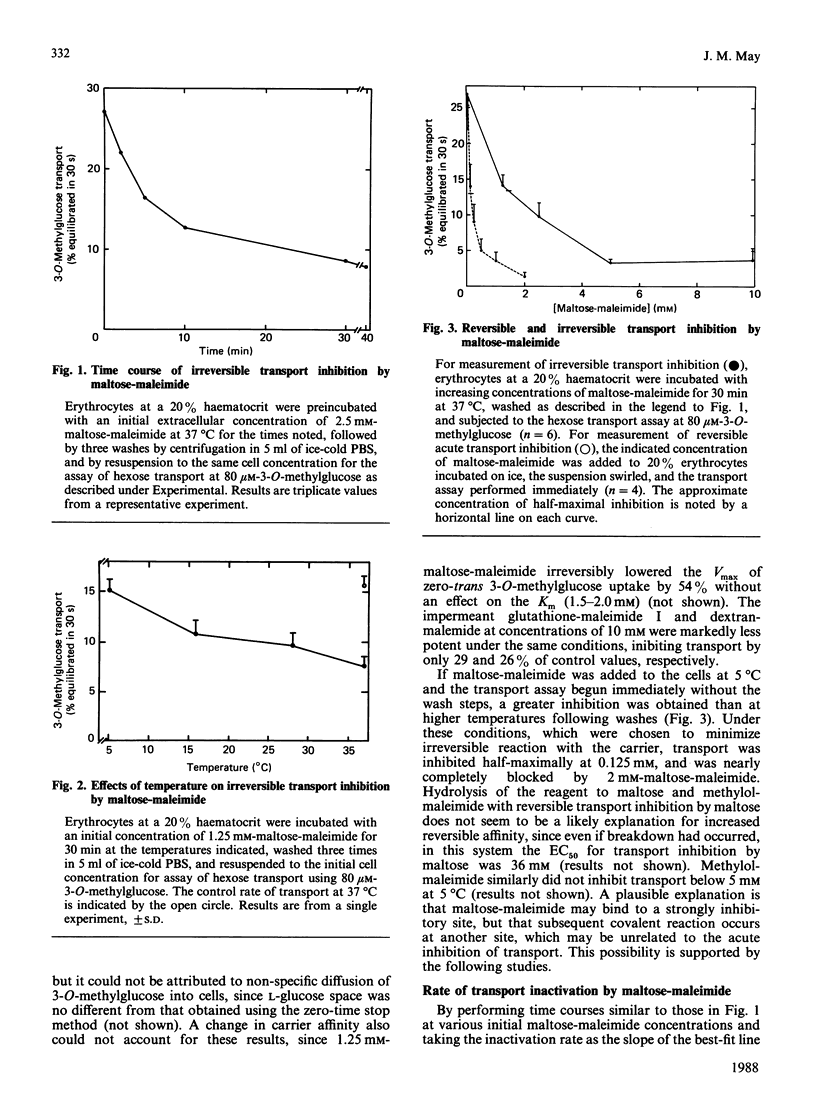
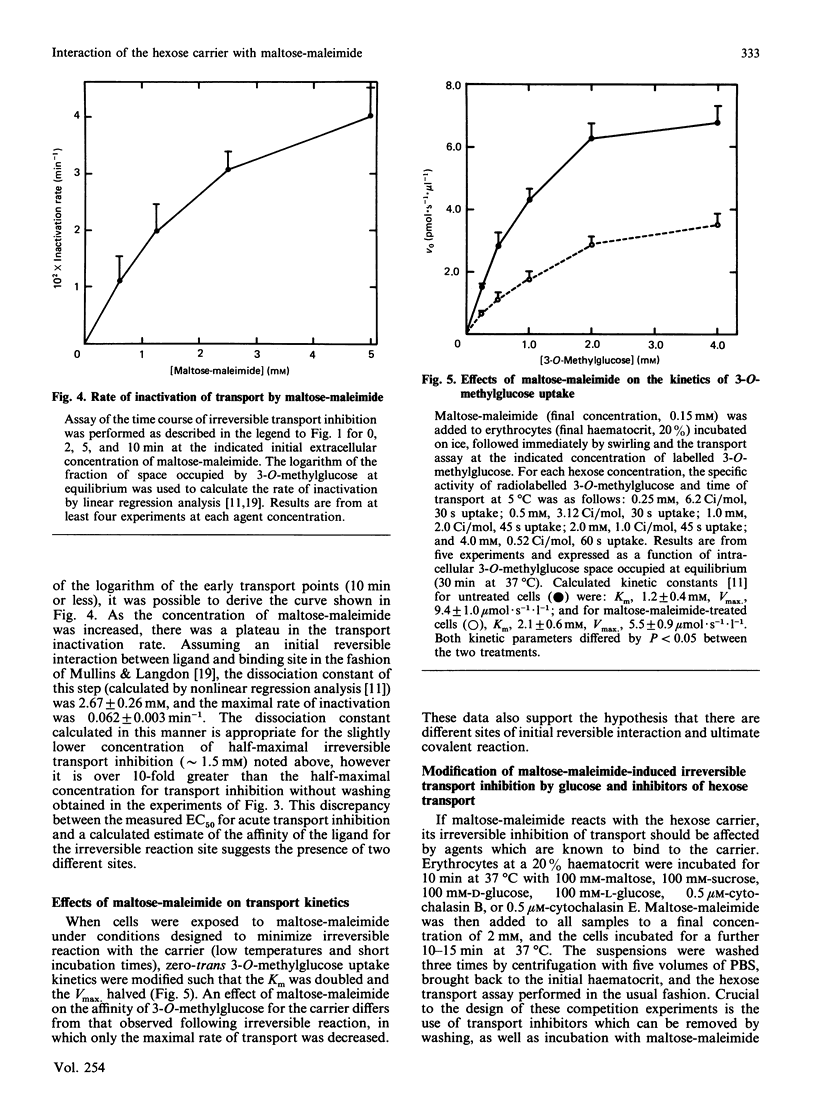
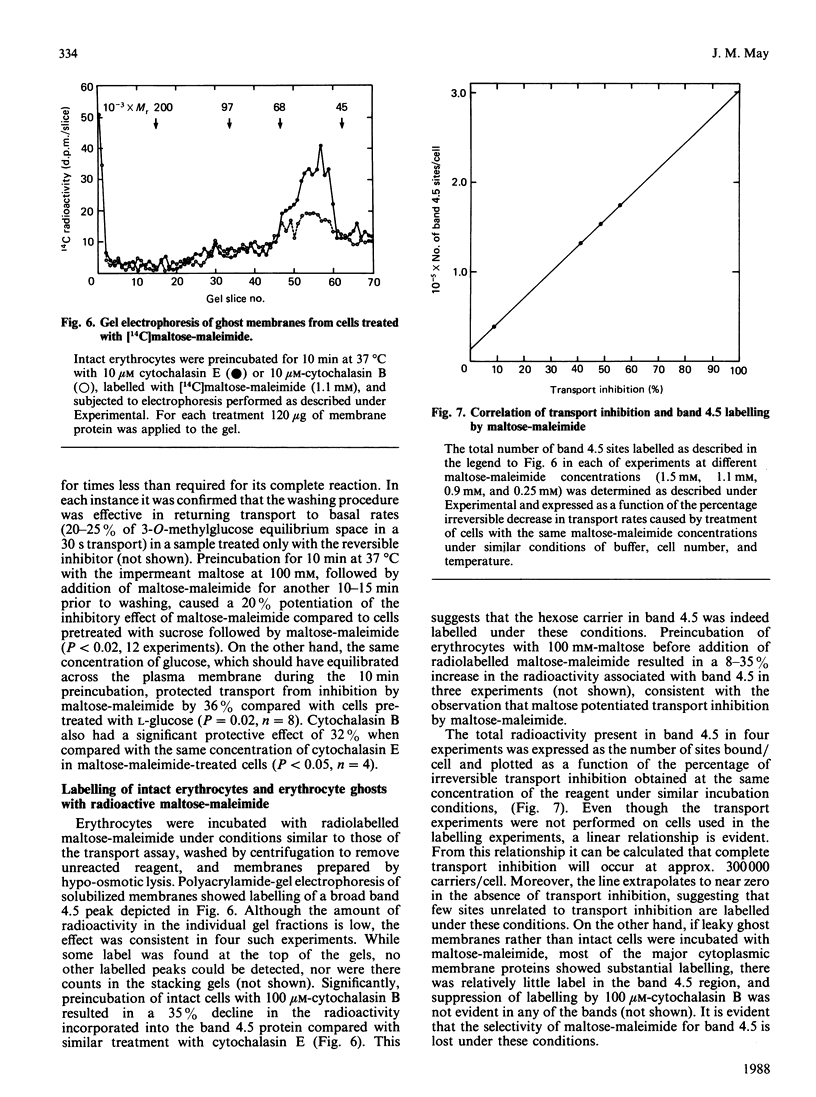
Maltose-maleimide was synthesized as a potential affinity label for the facilitative hexose carrier with selectivity for exofacial sulphydryl groups. This reagent, although probably a mixture of isomers, did not significantly penetrate the plasma membrane of human erythrocytes at concentrations below 5 mM at 37 degrees C. When allowed to react to completion, it irreversibly inhibited the uptake of 3-O-methylglucose, with a half-maximal response at about 1.5-2.0 mM-reagent. The rate of transport inactivation was a saturable function of the maltose-maleimide concentration. Studies of reaction kinetics and effects of known transport inhibitors demonstrated that irreversible reaction occurred on the exofacial outward-facing carrier, although not at a site involved in substrate binding. Reaction of intact erythrocytes with [14C]maltose-maleimide resulted in labelling of a broad band 4.5 protein of Mr (average) 45,000-66,000 in electrophoretic gels. This protein was very likely the hexose carrier, since its labelling was inhibited by cytochalasin B. Exofacial band 4.5 labelling was stoichiometric with respect to transport inhibition, yielding an estimated 300,000 carriers/cell. These results suggest that the exofacial sulphydryl which reacts with maltose-maleimide is distinct from the substrate binding site on the hexose carrier, but that it confers substantial labelling selectivity to impermeant maleimides. Additionally, the high efficiency of carrier labelling obtained with maltose-maleimide is useful in quantifying numbers of carriers in whole cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. E., Schachter D., Batt E. R., Flamm M. Sulfhydryl substituents of the human erythrocyte hexose transport mechanism. Am J Physiol. 1986 Jun;250(6 Pt 1):C853–C860. doi: 10.1152/ajpcell.1986.250.6.C853. [DOI] [PubMed] [Google Scholar]
- Abbott R. E., Schachter D. Impermeant maleimides. Oriented probes of erythrocyte membrane proteins. J Biol Chem. 1976 Nov 25;251(22):7176–7183. [PubMed] [Google Scholar]
- Basketter D. A., Widdas W. F. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol. 1978 May;278:389–401. doi: 10.1113/jphysiol.1978.sp012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt E. R., Abbott R. E., Schachter D. Impermeant maleimides. Identification of an exofacial component of the human erythrocyte hexose transport mechanism. J Biol Chem. 1976 Nov 25;251(22):7184–7190. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Jung C. Y., Rothstein A. The topology of the major band 4.5 protein component of the human erythrocyte membrane: characterization of reactive cysteine residues. Biochim Biophys Acta. 1985 Sep 25;819(1):83–92. doi: 10.1016/0005-2736(85)90198-1. [DOI] [PubMed] [Google Scholar]
- Deziel M., Pegg W., Mack E., Rothstein A., Klip A. Labelling of the human erythrocyte glucose transporter with 3H-labelled cytochalasin B occurs via protein photoactivation. Biochim Biophys Acta. 1984 May 30;772(3):403–406. doi: 10.1016/0005-2736(84)90157-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry. 1981 Sep 1;20(18):5108–5113. doi: 10.1021/bi00521a003. [DOI] [PubMed] [Google Scholar]
- Groman E. V., Schultz R. M., Engel L. L. Catalytic competence: a direct criterion for affinity labeling. Methods Enzymol. 1977;46:54–58. doi: 10.1016/s0076-6879(77)46010-5. [DOI] [PubMed] [Google Scholar]
- Hissin P. J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976 Jul;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Parkar B. A., Midgley P. J. Exofacial photoaffinity labelling of the human erythrocyte sugar transporter. Biochim Biophys Acta. 1986 Feb 13;855(1):115–126. doi: 10.1016/0005-2736(86)90195-1. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Rees W. D. Side-specific analogues for the rat adipocyte sugar transport system. Biochim Biophys Acta. 1982 Feb 8;685(1):78–86. doi: 10.1016/0005-2736(82)90037-2. [DOI] [PubMed] [Google Scholar]
- Jacquez J. A. Modulation of glucose transport in human red blood cells by ATP. Biochim Biophys Acta. 1983 Jan 19;727(2):367–378. doi: 10.1016/0005-2736(83)90422-4. [DOI] [PubMed] [Google Scholar]
- Krupka R. M. Asymmetrical binding of phloretin to the glucose transport system of human erythrocytes. J Membr Biol. 1985;83(1-2):71–80. doi: 10.1007/BF01868739. [DOI] [PubMed] [Google Scholar]
- Krupka R. M., Devés R. Looking for probes of gated channels: studies of the inhibition of glucose and choline transport in erythrocytes. Biochem Cell Biol. 1986 Nov;64(11):1099–1107. doi: 10.1139/o86-145. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Gorga F. R., Orasky J. E., Jr, Zoccoli M. A. Monosaccharide transport system of the human erythrocyte. Identification of the cytochalasin B binding component. Biochemistry. 1977 Nov 1;16(22):4921–4926. doi: 10.1021/bi00641a028. [DOI] [PubMed] [Google Scholar]
- Lin S., Spudich J. A. Biochemical studies on the mode of action of cytochalasin B. Cytochalasin B binding to red cell membrane in relation to glucose transport. J Biol Chem. 1974 Sep 25;249(18):5778–5783. [PubMed] [Google Scholar]
- Lowe A. G., Walmsley A. R. The kinetics of glucose transport in human red blood cells. Biochim Biophys Acta. 1986 May 28;857(2):146–154. doi: 10.1016/0005-2736(86)90342-1. [DOI] [PubMed] [Google Scholar]
- May J. M. Labeling of human erythrocyte band 3 with maltosylisothiocyanate. Interaction with the anion transporter. J Biol Chem. 1987 Mar 5;262(7):3140–3145. [PubMed] [Google Scholar]
- May J. M. Photoaffinity labeling of glyceraldehyde-3-phosphate dehydrogenase by an aryl azide derivative of glucosamine in human erythrocytes. J Biol Chem. 1986 Feb 25;261(6):2542–2547. [PubMed] [Google Scholar]
- Mullins R. E., Langdon R. G. Maltosyl isothiocyanate: an affinity label for the glucose transporter of the human erythrocyte membrane. 1. Inhibition of glucose transport. Biochemistry. 1980 Mar 18;19(6):1199–1205. doi: 10.1021/bi00547a025. [DOI] [PubMed] [Google Scholar]
- Mullins R. E., Langdon R. G. Maltosyl isothiocyanate: an affinity label for the glucose transporter of the human erythrocyte membrane. 2. Identification of the transporter. Biochemistry. 1980 Mar 18;19(6):1205–1212. doi: 10.1021/bi00547a026. [DOI] [PubMed] [Google Scholar]
- Roberts S. J., Tanner M. J., Denton R. M. Properties of N-maleoylmethionine sulphone, a novel impermeant maleimide, and its use in the selective labelling of the erythrocyte glucose-transport system. Biochem J. 1982 Jul 1;205(1):139–145. doi: 10.1042/bj2050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. F. Characterization of cytochalasin B photoincorporation into human erythrocyte D-glucose transporter and F-actin. Biochemistry. 1983 May 24;22(11):2750–2756. doi: 10.1021/bi00280a024. [DOI] [PubMed] [Google Scholar]
- Taverna R. D., Langdon R. G. Reversible association of cytochalasin B with the human erythrocyte membrane. Inhibition of glucose transport and the stoichiometry of cytochalasin binding. Biochim Biophys Acta. 1973 Oct 11;323(2):207–219. doi: 10.1016/0005-2736(73)90145-4. [DOI] [PubMed] [Google Scholar]
- VANSTEVENINCK J., WEED R. I., ROTHSTEIN A. LOCALIZATION OF ERYTHROCYTE MEMBRANE SULFHYDRYL GROUPS ESSENTIAL FOR GLUCOSE TRANSPORT. J Gen Physiol. 1965 Mar;48:617–632. doi: 10.1085/jgp.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. F., Falke J. J., Chan S. I. A proton NMR study of the mechanism of the erythrocyte glucose transporter. Proc Natl Acad Sci U S A. 1986 May;83(10):3277–3281. doi: 10.1073/pnas.83.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]


