Abstract
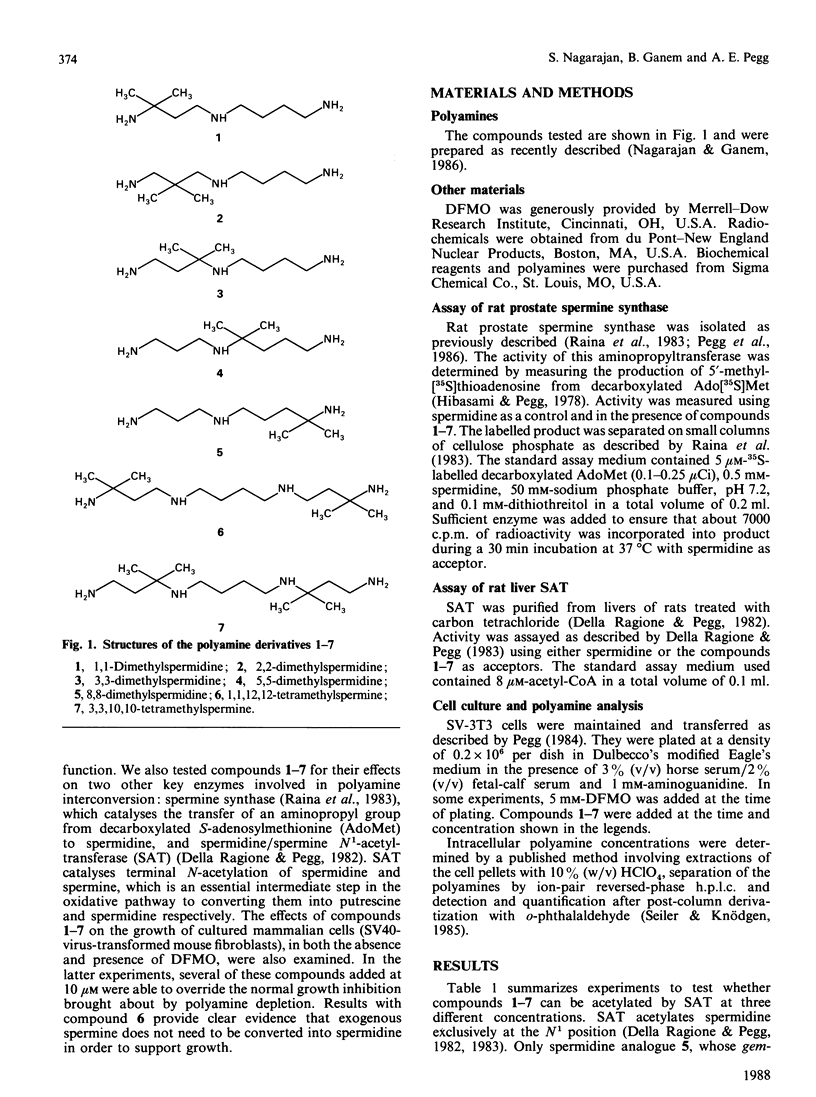
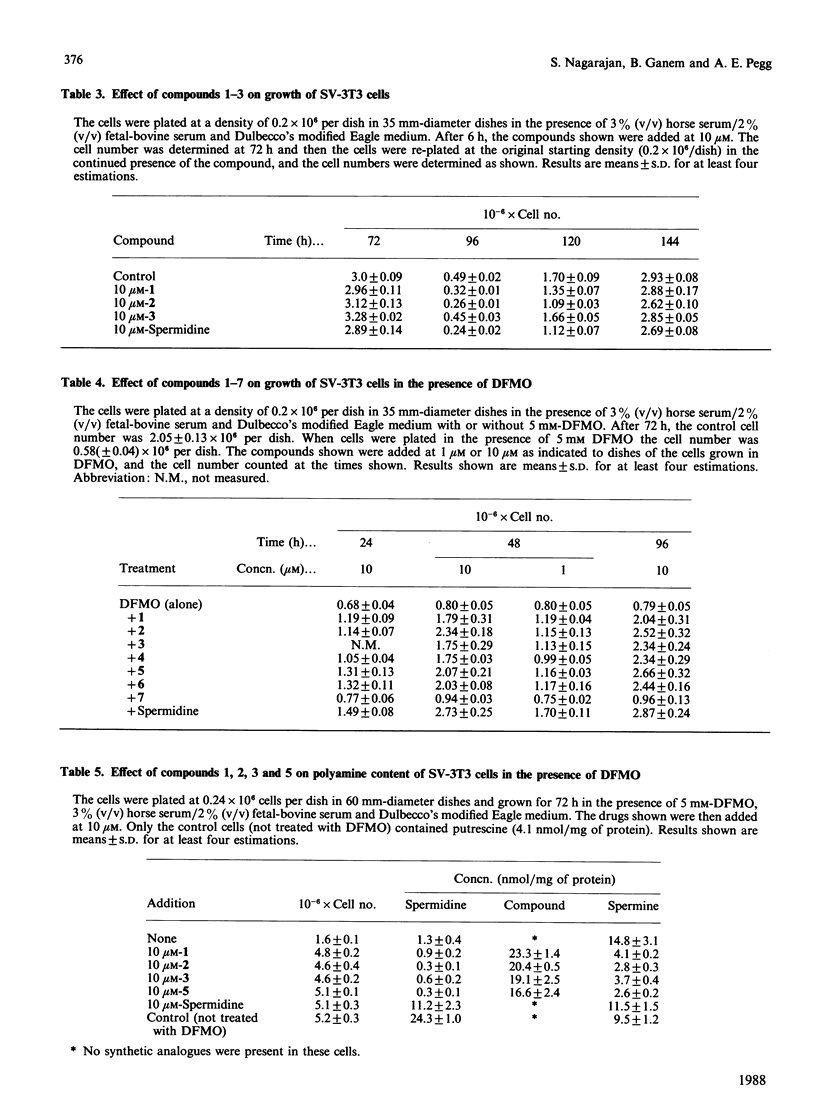
A number of synthetic polyamine derivatives that included five achiral gem-dimethylspermidines and two analogous tetramethylated spermines were tested for their abilities to serve as substrates for enzymes metabolizing polyamines and for their capacities to substitute for the natural polyamines in cell growth. It was found that none of the compounds were effective substrates for spermine synthase, and only one, namely 8,8-dimethylspermidine, was a substrate for spermidine/spermine N1-acetyltransferase. However, all of the spermidine derivatives and 1,1,12,12-tetramethylspermine were able to support the growth of SV-3T3 cells in which endogenous polyamine synthesis was prevented by the addition of alpha-difluoromethylornithine. These results suggest that either spermidine or spermine can support cell growth without the need for metabolic interconversion. In contrast with the result with 1,1,12,12-tetramethylspermine, 3,3,10,10-tetramethylspermine did not restore growth of polyamine-depleted SV-3T3 cells. Comparison of the properties of these derivatives may prove valuable in understanding the physiological role of polyamines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship J., Marchant P. E. Metabolism of N1-acetylspermidine and N8-acetylspermidine in rats. Proc Soc Exp Biol Med. 1984 Oct;177(1):180–187. doi: 10.3181/00379727-177-41930. [DOI] [PubMed] [Google Scholar]
- Canellakis Z. N., Theoharides T. C. Stimulation of ornithine decarboxylase synthesis and its control by polyamines in regenerating rat liver and cultured rat hepatoma cells. J Biol Chem. 1976 Jul 25;251(14):4436–4441. [PubMed] [Google Scholar]
- Casero R. A., Jr, Bergeron R. J., Porter C. W. Treatment with alpha-difluoromethylornithine plus a spermidine analog leads to spermine depletion and growth inhibition in cultured L1210 leukemia cells. J Cell Physiol. 1984 Dec;121(3):476–482. doi: 10.1002/jcp.1041210305. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Della Ragione F., Erwin B. G., Pegg A. E. Studies of the acetyl-CoA-binding site of rat liver spermidine/spermine N1-acetyltransferase. Biochem J. 1983 Sep 1;213(3):707–712. doi: 10.1042/bj2130707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Ragione F., Pegg A. E. Studies of the specificity and kinetics of rat liver spermidine/spermine N1-acetyltransferase. Biochem J. 1983 Sep 1;213(3):701–706. doi: 10.1042/bj2130701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin B. G., Persson L., Pegg A. E. Differential inhibition of histone and polyamine acetylases by multisubstrate analogues. Biochemistry. 1984 Aug 28;23(18):4250–4255. doi: 10.1021/bi00313a036. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Pegg A. E. Rapid and convenient method for the assay of aminopropyltransferases. Biochem J. 1978 Mar 1;169(3):709–712. doi: 10.1042/bj1690709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., Pösö H., Scalabrino G., Jänne J. Regulation of ornithine decarboxylase by diamines in regenerating rat liver. FEBS Lett. 1977 Feb 1;73(2):229–234. doi: 10.1016/0014-5793(77)80987-3. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Control of ornithine decarboxylase activity in stimulated human lymphocytes by putrescine and spermidine. Biochem J. 1973 Apr;132(4):791–796. doi: 10.1042/bj1320791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P. S., Danzin C., Kolb M., Gerhart F., Bey P., Sjoerdsma A. Marked and prolonged inhibition of mammalian ornithine decarboxylase in vivo by esters of (E)-2-(fluoromethyl)dehydroornithine. Biochem Pharmacol. 1986 Jan 15;35(2):159–165. doi: 10.1016/0006-2952(86)90509-5. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Hornsperger J. M., Böhlen P. Two distinct mechanisms for ornithine decarboxylase regulation by polyamines in rat hepatoma cells. J Cell Physiol. 1979 May;99(2):183–190. doi: 10.1002/jcp.1040990204. [DOI] [PubMed] [Google Scholar]
- McGovern K. A., Clark R. S., Pegg A. E. Effect of 1,3,6-triaminohexane and 1,4,7-triaminoheptane on growth and polyamine metabolism in SV-3T3 cells treated with 2-difluoromethylornithine. J Cell Physiol. 1986 May;127(2):311–316. doi: 10.1002/jcp.1041270219. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Pajula R. L., Raina A., Eloranta T. Polyamine synthesis in mammalian tissues. Isolation and characterization of spermine synthase from bovine brain. Eur J Biochem. 1979 Nov;101(2):619–626. doi: 10.1111/j.1432-1033.1979.tb19756.x. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Coward J. K., Talekar R. R., Secrist J. A., 3rd Effects of certain 5'-substituted adenosines on polyamine synthesis: selective inhibitors of spermine synthase. Biochemistry. 1986 Jul 15;25(14):4091–4097. doi: 10.1021/bi00362a016. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Inhibition of aminopropyltransferases. Methods Enzymol. 1983;94:294–297. doi: 10.1016/s0076-6879(83)94051-x. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Tang K. C., Coward J. K. Effects of S-adenosyl-1,8-diamino-3-thiooctane on polyamine metabolism. Biochemistry. 1982 Sep 28;21(20):5082–5089. doi: 10.1021/bi00263a036. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. The role of polyamine depletion and accumulation of decarboxylated S-adenosylmethionine in the inhibition of growth of SV-3T3 cells treated with alpha-difluoromethylornithine. Biochem J. 1984 Nov 15;224(1):29–38. doi: 10.1042/bj2240029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P., Hölttä E., Jänne O. A. Mutant strain of Chinese hamster ovary cells with no detectable ornithine decarboxylase activity. Mol Cell Biol. 1985 Jun;5(6):1385–1390. doi: 10.1128/mcb.5.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., Berger F. G., Pegg A. E., Ganis B., Bergeron R. J. Regulation of ornithine decarboxylase activity by spermidine and the spermidine analogue N1N8-bis(ethyl)spermidine. Biochem J. 1987 Mar 1;242(2):433–440. doi: 10.1042/bj2420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., Bergeron R. J. Spermidine requirement for cell proliferation in eukaryotic cells: structural specificity and quantitation. Science. 1983 Mar 4;219(4588):1083–1085. doi: 10.1126/science.6823570. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Cavanaugh P. F., Jr, Stolowich N., Ganis B., Kelly E., Bergeron R. J. Biological properties of N4- and N1,N8-spermidine derivatives in cultured L1210 leukemia cells. Cancer Res. 1985 May;45(5):2050–2057. [PubMed] [Google Scholar]
- Porter C. W., Ganis B., Vinson T., Marton L. J., Kramer D. L., Bergeron R. J. Comparison and characterization of growth inhibition in L1210 cells by alpha-difluoromethylornithine, an inhibitor of ornithine decarboxylase, and N1,N8-bis(ethyl)spermidine, an apparent regulator of the enzyme. Cancer Res. 1986 Dec;46(12 Pt 1):6279–6285. [PubMed] [Google Scholar]
- Porter C. W., McManis J., Casero R. A., Bergeron R. J. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987 Jun 1;47(11):2821–2825. [PubMed] [Google Scholar]
- Ragione F. D., Pegg A. E. Purification and characterization of spermidine/spermine N1-acetyltransferase from rat liver. Biochemistry. 1982 Nov 23;21(24):6152–6158. doi: 10.1021/bi00267a020. [DOI] [PubMed] [Google Scholar]
- Raina A., Pajula R. L., Eloranta T. Purification of spermidine aminopropyltransferase (spermine synthase) from bovine brain. Methods Enzymol. 1983;94:276–279. doi: 10.1016/s0076-6879(83)94048-x. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Sarhan S., Dezeure F., Seiler N. Putrescine derivatives as substrates of spermidine synthase. Int J Biochem. 1987;19(11):1037–1047. doi: 10.1016/0020-711x(87)90304-1. [DOI] [PubMed] [Google Scholar]
- Sarhan S., Knodgen B., Gerhart F., Seiler N. Chain-fluorinated polyamines as tumor markers--I. In vivo transformation of 2,2-difluoroputrescine into 6,6-difluorospermidine and 6,6-difluorospermine. Int J Biochem. 1987;19(9):843–852. doi: 10.1016/0020-711x(87)90244-8. [DOI] [PubMed] [Google Scholar]
- Seiler N. Functions of polyamine acetylation. Can J Physiol Pharmacol. 1987 Oct;65(10):2024–2035. doi: 10.1139/y87-317. [DOI] [PubMed] [Google Scholar]
- Shinki T., Kadofuku T., Sato T., Suda T. Spermidine N1-acetyltransferase has a larger role than ornithine decarboxylase in 1 alpha,25-dihydroxyvitamin D3-induced putrescine synthesis. J Biol Chem. 1986 Sep 5;261(25):11712–11716. [PubMed] [Google Scholar]
- Shinki T., Takahashi N., Kadofuku T., Sato T., Suda T. Induction of spermidine N1-acetyltransferase by 1 alpha,25-dihydroxyvitamin D3 as an early common event in the target tissues of vitamin D. J Biol Chem. 1985 Feb 25;260(4):2185–2190. [PubMed] [Google Scholar]
- Steglich C., Scheffler I. E. An ornithine decarboxylase-deficient mutant of Chinese hamster ovary cells. J Biol Chem. 1982 Apr 25;257(8):4603–4609. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]


