Abstract
Background
Although bronchiectasis has been shown to be associated with cardiovascular disease, there is limited evidence of an association with subclinical atherosclerosis, especially carotid intima-media thickness (CIMT).
Methods
This prospective study compared CIMT among patients with and without bronchiectasis, and among bronchiectatic patients classified according to disease severity using the FACED score. The study was carried out at a major regional hospital and tertiary respiratory referral centre in Hong Kong.
Results
Total 155 Chinese patients with non-cystic fibrosis (CF) bronchiectasis and 512 controls were recruited. The mean CIMT was 0.58 ± 0.10 mm, 0.63 ± 0.11 mm and 0.66 ± 0.08 mm respectively among controls, patients with mild-to-moderate bronchiectasis and patients with severe bronchiectasis. There was no statistically significant difference in CIMT between patients with mild-to-moderate bronchiectasis and controls. Multivariate linear regression revealed that CIMT was significantly increased in patients with severe bronchiectasis relative to controls. The same phenomenon was observed among patients without a history of cardiovascular disease or cardiovascular risk factors.
Conclusions
CIMT was significantly increased in patients with severe bronchiectasis compared with controls without bronchiectasis, but not among patients with mild-to-moderate bronchiectasis, which suggested the subclinical atherosclerosis to be more prevalent among patients with severe bronchiectasis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04129-x.
Keywords: Bronchiectasis, Carotid initial thickness, Subclinical atherosclerosis, Cardiovascular disease
Introduction
Bronchiectasis is characterized by airway inflammation, abnormal mucus clearance and bacterial colonization with consequent progressive airway destruction and distortion. There is accumulating evidence that airway inflammation and immune dysregulation play a central role in the evolution of non-CF bronchiectasis [1].
An association of systemic inflammation with cardiovascular diseases has been demonstrated, while baseline C-reactive protein (CRP) level has been shown to predict the long-term risk of a first myocardial infarction, ischemic stroke, and peripheral artery disease [2–4]. Guidelines suggest measurement of high-sensitivity CRP in patients at intermediate risk of coronary heart disease (CHD) [5–7]. Other inflammatory markers such as interleukin-6, [8, 9] leukocyte enzyme myeloperoxidase, [7, 10–13] white blood cell count, erythrocyte sedimentation rate, IL-18, tumor necrosis factor alpha, transforming growth factor beta, soluble intercellular adhesion molecule-1, P-selectin, cathepsin S, and lipoprotein-associated phospholipase A2 have also been reported as markers of increased CHD risk [12, 14–20].
There is growing evidence of an association between bronchiectasis and cardiovascular diseases [21–26]. Bronchiectasis was associated with the development of cardiovascular disease in a population-based study conducted in the United Kingdom, as well as increased risks for coronary heart disease and stroke [22].
To assess underlying atherosclerotic burden and predict adverse cardiovascular events, various non-invasive functional and structural surrogate markers of vascular health can be measured. These include assessment of endothelial function by brachial-artery flow-mediated dilatation (FMD), and measurement of carotid intima-media thickness (CIMT) and arterial stiffness and ankle-brachial index (ABI). A case control study also showed that patients with bronchiectasis were at greater risk of endothelial dysfunction as measured by FMD but not CIMT [27]. Nonetheless this study comprised only 80 patients with bronchiectasis and 80 controls. The study also did not stratify patients with bronchiectasis according to disease severity.
A large-scale study to compare CIMT in patients with bronchiectasis and healthy subjects is warranted to assess the risks of subclinical atherosclerosis, taking into account disease severity. In view of this knowledge gap, we conducted this study with the objective being assessing burden of subclinical atherosclerosis as measured by CIMT among patients with bronchiectasis of different severity, as well as comparing CIMT between patients with bronchiectasis and healthy controls.
Materials and methods
A prospective study was conducted at the University Department of Medicine, Queen Mary Hospital (QMH). The Divisions of Respiratory Medicine and Neurology at Queen Mary Hospital are tertiary referral centers for the territory as well as major receiving units for patients with various respiratory diseases (including bronchiectasis) and neurological diseases in the Hong Kong West Cluster. Subjects above the age of 18 years old with a confirmed diagnosis of bronchiectasis based on high-resolution computed tomography (HRCT) scan were included. Those with co-existent systemic inflammatory diseases (Rheumatological diseases, inflammatory bowel diseases and other autoimmune diseases) and respiratory co-morbidities (asthma, chronic obstructive pulmonary disease and interstitial lung diseases) were excluded. HRCT and lung function tests were performed within 12 months prior to recruitment to the study. Severity of bronchiectasis was defined according to the FACED [forced expiratory volume in 1 s (FEV1), age, chronic colonization, extension, and dyspnea score] score, with mild, moderate and severe bronchiectasis defined as a FACED score of 0–2, 3–4, and 5–7 points respectively [28]. Subjects with bronchiectasis were recruited from 1st October to 31st December 2021 from the bronchiectasis clinic of QMH. Controls without bronchiectasis, as assessed by symptom questionnaires and checking of electronic patient records of the Hospital Authority of Hong Kong, were recruited between July 2018 and June 2020 from the community by open advertisements.
As gender and smoking status are the important factors that could contribute to differences in CIMT, while they are also significantly different in the two groups, they are chosen to be factors for propensity score matching. Control patients were individually matched by gender and smoking status using propensity score matching with a 1:2 matching ratio and caliper of 0.2. Covariates that were not well-matched were adjusted in multivariate analysis.
Vascular ultrasound examination of carotid intima-medial thickness (CIMT) was performed by a standard B-mode ultrasound examination with a 7.5 MHz linear array transducer and a high-resolution ultrasound system in accordance with the Mannheim Carotid Intima-Media Thickness and Plaque Consensus [29–31]. In patients with bronchiectasis, ultrasound examination was performed by a single experienced operator (Kwok Wang Chun) using General Electric LOGIQ e. In controls, scans were performed by one of four operators (Matthew SS Hsu, Kkts Pijarnvanit, Carman Nga-Man Cheung, Yick Hin Chow) using a Samsung Ultrasound RS80A. All subjects were examined in a supine position. Ultrasound scans of the right and left common carotid artery in three different projections (anterior, lateral, and posterior) were performed. CIMT was determined by measuring the distance between the lumen-intima and media-adventitia border at a 10 mm straight arterial segment near the bulb at the far wall of both common carotid arteries. CIMT was calculated using automated IMT software. CIMT was measured at each projection (anterior, lateral, and posterior) from each side giving six measurements for each patient and the mean value calculated.
Seventeen randomly selected controls underwent CIMT re-measurement, performed with General Electric LOGIQ e by the same operator who assessed bronchiectasis patients to identify any inter-machine variability.
Automated IMT function was used to measure CIMT in both bronchiectasis patients and control, which has been demonstrated to have high accuracy and reproducibility [32–34]. Automated IMT measurement allows automated contour detection of lumen-intima and media-adventitia vessel walls and calculation of IMT quality index. The advantage of having automated IMT measurement is that it avoided some of the problems from manual measurement of CIMT, including being user-dependent, measurement not fully standardized, subjective, time-consuming and prone to errors [35].
The primary outcome was the difference in CIMT between patients with bronchiectasis and controls, and among patients with bronchiectasis of different severity as determined by FACED score. The study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (UW19-624).
Statistical analysis
The demographic and clinical data are presented as actual frequency, mean ± standard deviation (SD) or median (interquartile range, IQR) as appropriate. Categorical variables were compared using χ2 test. For continuous variables, two-group comparisons were performed using unpaired t test or Mann-Whitney U test as appropriate. One-way ANOVA was employed to compare multi-group continuous variables. Multivariate linear regression analyses were performed to adjust for cofounders that included age, gender, smoking history, body mass index (BMI), cardiovascular risk factors and history of cardiovascular diseases. Statistical significance was determined at the level of p = 0.05. All statistical analyses were performed using R V.4.2.2 (R Foundation for Statistical Computing) statistical software.
Results
A total of 155 Chinese patients with non-CF bronchiectasis managed at QMH and 512 controls were included. The baseline characteristics of the patients with bronchiectasis and the controls are summarized in Table 1.
Table 1.
Baseline demographic and clinical characteristics of all subjects
| Controls (n = 512) |
Bronchiectasis patients (n = 155) |
P-value | |
|---|---|---|---|
| Age (years), mean ± SD | 54.5 ± 6.6 | 69.0 ± 11.4 | < 0.01* |
| Male (%) | 255 (45.5%) | 54 (34.8%) | < 0.01* |
| Smoking status (%) | 0.18 | ||
| Current smoker | 38 (7.4%) | 5 (3.2%) | |
| Former smoker | 71 (13.9%) | 21 (13.6%) | |
| Non-smoker | 403 (78.7%) | 129 (83.2%) | |
| Body mass index (kg/m2), mean ± SD | 24.0 ± 4.6 | 22.2 ± 3.9 | < 0.01* |
| CIMT (mm), mean ± SD | 0.58 ± 0.10 | 0.64 ± 0.11 | < 0.01* |
| Hypertension (%) | 91 (17.8%) | 47 (30.3%) | < 0.01* |
| Diabetes mellitus (%) | 19 (3.7%) | 17 (11.0%) | < 0.01* |
| Hyperlipidemia (%) | 66 (12.9%) | 28 (18.1%) | 0.11 |
| Ischemic heart disease (%) | 1 (0.2%) | 11 (7.1%) | < 0.01* |
| Ischemic stroke/Transient ischemic attack (%) | 0 (0%) | 3 (1.9%) | 0.01* |
| FEV1 (L), mean ± SD | - | 1.69 ± 0.66 | - |
| FEV1 (% predicted), mean ± SD | - | 86.3 ± 24.5 | - |
| FVC (L), mean ± SD | - | 2.50 ± 0.85 | - |
| FVC (% predicted), mean ± SD | - | 95.7 ± 20.6 | - |
| FEV1/FVC Ratio (%), mean ± SD | - | 68.2 ± 12.5 | - |
| Extent of involvement ≥ 3 lobes (%) | - | 60 (38.7%) | - |
| Pseudomonas aeruginosa colonization (%) | - | 49 (31.6%) | - |
| Colonization by other organisms (%) | 16 (10.3%) | - | |
| Exacerbation(s) in the past 1 year (%) | - | 33 (21.3%) | - |
| Number of exacerbation(s) in the past 1 year (%) | |||
| 1 | - | 17 (11.0%) | - |
| 2 | - | 7 (4.5%) | - |
| 3 | - | 4 (2.6%) | - |
| 4 | - | 3 (1.9%) | - |
| 5 | - | 2 (1.3%) | - |
| Hospitalized exacerbation(s) in the past 1 year (%) | - | 12 (7.7%) | - |
| Number of hospitalized exacerbation(s) in the past 1 year (%) | |||
| 1 | - | 10 (6.5%) | - |
| 2 | - | 2 (1.3%) | - |
| Baseline FACED score, Median (IQR) | - | 3 (2–4) | - |
| Baseline BSI score, mean ± SD | - | 5.5 ± 3.2 | - |
| Baseline blood neutrophil count (x cells/µL), mean ± SD | - | 4.10 ± 1.95 | - |
| Baseline blood lymphocyte (x cells/µL), mean ± SD | - | 1.89 ± 0.71 | - |
| Etiology of bronchiectasis | |||
| Post-tuberculosis | - | 32 (20.6%) | - |
| Non-tuberculosis mycobacteria infection | - | 14 (9.0%) | - |
| Post-irradiation | - | 3 (1.9%) | - |
| Diffuse pan-bronchiolitis | - | 1 (0.6%) | - |
| Post-haemopoietic stem cell transplantation | - | 3 (1.9%) | - |
| Primary ciliary dyskinesia | - | 3 (1.9%) | - |
| Idiopathic | - | 99 (63.9%) | - |
| Medication for bronchiectasis (%) | |||
| Macrolide | - | 25 (16.1%) | - |
| Inhaled corticosteroid | - | 57 (36.8%) | - |
| LABA | 35 (22.6%) | - | |
| Theophylline | - | 7 (4.5%) | - |
SD = standard deviation; CIMT = carotid intima-medial thickness; mL = milliliter; * = statistically significant; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity, BSI = Bronchiectasis severity index, LABA = Long-acting beta-agonist
Correlation of CIMT among controls using different ultrasound machines
Both the General Electric LOGIQ e and Samsung Ultrasound RS80A were used to measure CIMT in 17 controls. All scans were performed by the same investigator. Measurements of CIMT by both machines had a high Pearson Correlation Coefficient of 0.856 (95% confidence interval [CI] = 0.627–0.949, p < 0.001).
Interrater variability of CIMT among controls
Ultrasound measurement of CIMT in controls was performed by four operators. The interrater variability was determined by repeat ultrasound CIMT measurement in 11 controls by different investigators. The intraclass correlation coefficient was 0.977.
Whole cohort with 155 patients with bronchiectasis and 512 controls
CIMT in patients with bronchiectasis and controls (whole cohort, n = 667)
Patients with bronchiectasis had significantly increased CIMT compared with controls (0.64 ± 0.11 mm vs. 0.58 ± 0.10 mm respectively, p < 0.001). The association was statistically significant after adjusting for age, gender, BMI, smoking status, any cardiovascular risk factors and any history of cardiovascular diseases (p = 0.020) (Supplementary Table S1).
CIMT in patients with bronchiectasis and controls without a history of cardiovascular disease or cardiovascular risk factors (Bronchiectasis, n = 92; control, n = 384)
The mean CIMT, as compared by unpaired t test, among patients with bronchiectasis and controls was 0.61 ± 0.10 mm and 0.57 ± 0.10 mm respectively (p < 0.001). The association was statistically significant after adjusting for age, gender, BMI, smoking status, any cardiovascular risk factors, and any history of cardiovascular disease in multivariate linear regression (p = 0.007) (Supplementary Table S1).
CIMT in patients with bronchiectasis of different severity and controls (Mild-to-moderate bronchiectasis,n = 126; Severe bronchiectasis,n = 29; control,n = 512)
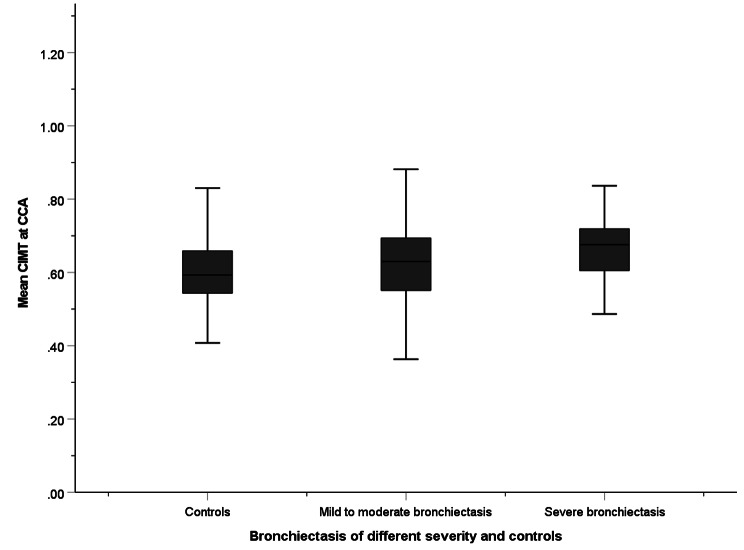
The CIMT, compared by one-way ANOVA, was 0.58 ± 0.10 mm, 0.63 ± 0.11 mm and 0.66 ± 0.08 mm among controls, patients with mild-to-moderate bronchiectasis and patients with severe bronchiectasis respectively (Fig. 1). The association was statistically significant after adjusting for age, gender, BMI, smoking status, any cardiovascular risk factors, and any history of cardiovascular disease in multivariate linear regression (p = 0.021) (Supplementary Table S1).
Fig. 1.
Mean CIMT among all patients with mild-to-moderate bronchiectasis, severe bronchiectasis, and controls in the whole cohort. CIMT are compared by one-way ANOVA. CIMT: Carotid intima-media thickness
CIMT in patients with bronchiectasis of different severity and controls with no history of cardiovascular disease or cardiovascular risk factors (Mild-to-moderate bronchiectasis,n = 76; Severe bronchiectasis,n = 16; control,n = 384)
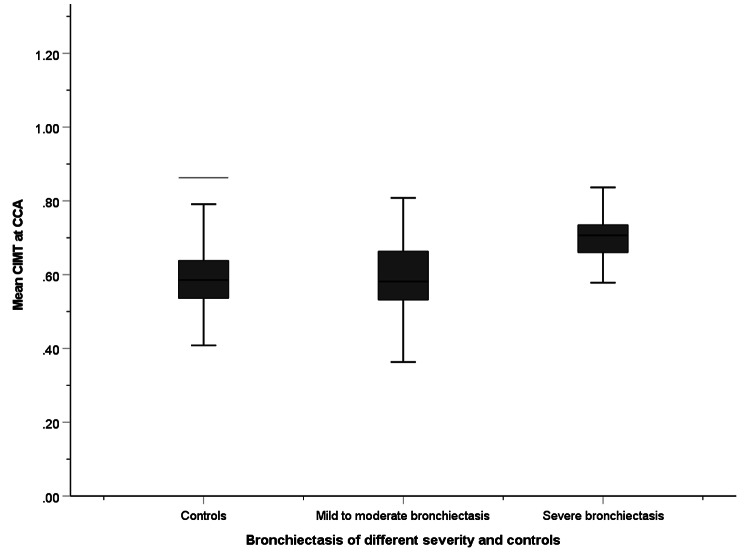
The CIMT, compared by one-way ANOVA, was 0.57 ± 0.10 mm, 0.60 ± 0.10 mm and 0.69 ± 0.06 mm among controls, patients with mild-to-moderate bronchiectasis and patients with severe bronchiectasis (Fig. 2). The association was statistically significant after adjusting for age, gender, BMI, smoking status, any cardiovascular risk factor, and any history of cardiovascular disease in multivariate linear regression (p < 0.001) (Supplementary Table S1).
Fig. 2.
Mean CIMT among patients with mild-to-moderate bronchiectasis, severe bronchiectasis, and controls with no cardiovascular risk factors or history of cardiovascular diseases in the whole cohort. CIMT are compared by one-way ANOVA. CIMT: Carotid intima-media thickness
Matched cohort (n = 465)
There were 155 subjects with bronchiectasis and 310 controls in the propensity score matched cohort (Table 2).
Table 2.
Baseline demographic and clinical characteristics of subjects in matched cohort
| - | Controls (n = 310) |
Bronchiectasis patients (n = 155) |
P-value |
|---|---|---|---|
| Age (years), mean ± SD | 55.0 ± 6.6 | 69.0 ± 11.4 | < 0.01* |
| Male (%) | 108 (34.8%) | 54 (34.8%) | 1.00 |
| Smoking status (%) | 0.275 | ||
| Current smoker | 20 (6.5%) | 5 (3.2%) | |
| Former smoker | 34 (11.0%) | 21 (13.6%) | |
| Non-smoker | 256 (82.6%) | 129 (83.2%) | |
| Body mass index (kg/m2), mean ± SD | 23.7 ± 5.2 | 22.2 ± 3.9 | < 0.01* |
| CIMT (mm), mean ± SD | 0.58 ± 0.10 | 0.64 ± 0.11 | < 0.01* |
| Hypertension (%) | 50 (16.1%) | 47 (30.3%) | < 0.01* |
| Diabetes mellitus (%) | 7 (2.3%) | 17 (11.0%) | < 0.01* |
| Hyperlipidemia (%) | 35 (11.3%) | 28 (18.1%) | 0.06 |
| Ischemic heart disease (%) | 0 (0%) | 11 (7.1%) | < 0.01* |
| Ischemic stroke/Transient ischemic attack (%) | 0 (0%) | 3 (1.9%) | 0.01* |
| FEV1 (L), mean ± SD | - | 1.69 ± 0.66 | - |
| FEV1 (% predicted), mean ± SD | - | 86.3 ± 24.5 | - |
| FVC (L), mean ± SD | - | 2.50 ± 0.85 | - |
| FVC (% predicted), mean ± SD | - | 95.7 ± 20.6 | - |
| FEV1/FVC Ratio (%), mean ± SD | - | 68.2 ± 12.5 | - |
| Extent of involvement ≥ 3 lobes (%) | - | 60 (38.7%) | - |
| Pseudomonas aeruginosa colonization (%) | - | 49 (31.6%) | - |
| Colonization by other organisms (%) | 16 (10.3%) | - | |
| Exacerbation(s) in the past 1 year (%) | - | 33 (21.3%) | - |
| Number of exacerbation(s) in the past 1 year (%) | |||
| 1 | - | 17 (11.0%) | - |
| 2 | - | 7 (4.5%) | - |
| 3 | - | 4 (2.6%) | - |
| 4 | - | 3 (1.9%) | - |
| 5 | - | 2 (1.3%) | - |
| Hospitalized exacerbation(s) in the past 1 year (%) | - | 12 (7.7%) | - |
| Number of hospitalized exacerbation(s) in the past 1 year (%) | |||
| 1 | - | 10 (6.5%) | - |
| 2 | - | 2 (1.3%) | - |
| Baseline FACED score, Median (IQR) | - | 3 (2–4) | - |
| Baseline BSI score, mean ± SD | - | 5.5 ± 3.2 | - |
| Severity of bronchiectasis by FACED score | |||
| Severe | - | 29 (18.7%) | - |
| Moderate | - | 60 (38.7%) | - |
| Mild | - | 66 (42.6%) | - |
| Baseline blood neutrophil count (x cells/µL), mean ± SD | - | 4.10 ± 1.95 | - |
| Baseline blood lymphocyte (x cells/µL), mean ± SD | - | 1.89 ± 0.71 | - |
| Etiology of bronchiectasis | |||
| Post-tuberculosis | - | 32 (20.6%) | - |
| Non-tuberculosis mycobacteria infection | - | 14 (9.0%) | - |
| Post-irradiation | - | 3 (1.9%) | - |
| Diffuse pan-bronchiolitis | - | 1 (0.6%) | - |
| Post-haemopoietic stem cell transplantation | - | 3 (1.9%) | - |
| Primary ciliary dyskinesia | - | 3 (1.9%) | - |
| Idiopathic | - | 99 (63.9%) | - |
| Medication for bronchiectasis (%) | |||
| Macrolide | - | 25 (16.1%) | - |
| Inhaled corticosteroid | - | 57 (36.8%) | - |
| LABA | 35 (22.6%) | - | |
| Theophylline | - | 7 (4.5%) | - |
SD = standard deviation; CIMT = carotid intima-medial thickness; mL = milliliter; * = statistically significant; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity
CIMT in patients with bronchiectasis and controls
Patients with bronchiectasis had significantly increased CIMT compared with controls (0.64 ± 0.11 mm and 0.58 ± 0.10 mm respectively, p < 0.001), as compared by unpaired t-test. The association was statistically significant after adjusting for age, gender, BMI, smoking status, any cardiovascular risk factor, and any history of cardiovascular disease in multivariate linear regression (p = 0.020) (Supplementary Table S2).
CIMT in patients with bronchiectasis and controls with no history of cardiovascular disease or cardiovascular risk factors (Bronchiectasis, n = 92; control,n = 235)
The mean CIMT, compared by unpaired t-test, among patients with bronchiectasis and controls was 0.61 ± 0.10 mm and 0.57 ± 0.09 mm respectively (p < 0.001). The association was statistically significant after adjusting for age, gender, BMI, smoking status, any cardiovascular risk factor and any history of cardiovascular disease in multivariate linear regression (p = 0.017) (Supplementary Table S2).
CIMT in patients with bronchiectasis of different severity and controls (Mild-to-moderate bronchiectasis,n = 126; Severe bronchiectasis,n = 29; control,n = 310)
The mean CIMT, as compared by one-way ANOVA, was 0.58 ± 0.10 mm, 0.63 ± 0.11 mm and 0.66 ± 0.08 mm among controls, patients with mild-to-moderate and severe bronchiectasis respectively. The association was statistically significant after adjusting for age, gender, BMI, smoking status, any cardiovascular risk factor and any history of cardiovascular disease in multivariate linear regression (p = 0.004) (Supplementary Table S2).
CIMT in patients with bronchiectasis of different severity and controls with no history of cardiovascular disease or cardiovascular risk factors (Mild-to-moderate bronchiectasis,n = 76; Severe bronchiectasis,n = 16; control,n = 235)
The mean CIMT was 0.56 ± 0.09 mm, 0.60 ± 0.10 mm and 0.69 ± 0.06 mm respectively among controls, patients with mild-to-moderate and patients with severe bronchiectasis. The association was statistically significant after adjusting for age, gender, BMI, smoking status, any cardiovascular risk factor, and any history of cardiovascular disease (p < 0.001) (Supplementary Table S2).
Subgroup analysis – age > 60
There were 126 subjects with bronchiectasis and 102 controls above the age of 60 years in the propensity score matched cohort. Age is reported to be associated with CIMT [36], with age 60 years a commonly used cut-off to define age group in CIMT studies [37–40]. The results in this subgroup show consistent results as in the primary analysis. The findings of subgroup analysis were summarized in Supplementary Table S3 and S4.
Discussion
To the best of our knowledge, this is the first report of a significant association of severe bronchiectasis with CIMT, but not mild-to-moderate bronchiectasis. The findings were consistent among patients with or without cardiovascular risk factors or cardiovascular disease. Among patients with severe bronchiectasis, defined by a FACED score 5 or above, CIMT was increased compared with controls.
There is growing evidence of an association of adverse cardiovascular outcomes with bronchiectasis. A possible link between the two is chronic inflammation, a hallmark of bronchiectasis. Nonetheless no previous study has suggested that CIMT is increased in patients with bronchiectasis. A small-scale case control study identified only a difference in FMD, not CIMT. This may have been due to the small sample size of only 80 patients with bronchiectasis and 80 controls. Our study has a larger sample size so overcomes the potential problem of the previous case control study that lacked statistical power to detect CIMT differences. In addition, previous study did not take account of disease severity. In our study, bronchiectasis as a whole group (a group with varying severity) may not account for the differences in CIMT. Nonetheless based on analysis according to disease severity, we determined that severe bronchiectasis, not mild-to-moderate, was associated with increased CIMT. We postulate that there is an interplay of various factors that underlie the association of bronchiectasis and CIMT. This finding is consistent with the proposal that CIMT, as a marker of subclinical atherosclerosis, is related to the degree of inflammation. The more inflamed the airway is, the thicker the CIMT. This finding is supported by studies in other inflammatory diseases. In rheumatoid arthritis, features of more severe disease, including extra-articular manifestations, erosions, high inflammatory parameters, and long disease duration, were associated with greater CIMT [41]. A similar phenomenon has been observed in systemic lupus erythematosus and psoriasis [42, 43]. This may explain why CIMT is increased in patients with severe bronchiectasis but not mild-to-moderate cases. We postulate that as an inflammatory disease mainly affecting the respiratory tract, the severity of bronchiectasis is closely related to the degree of systemic inflammation and hence CIMT. For patients with mild-to-moderate bronchiectasis, the degree of systemic inflammation is low and CIMT is not increased.
CIMT was also being assessed in other respiratory diseases before. In chronic obstructive pulmonary disease (COPD), 32% of patients with mild COPD and 36% with moderate to severe COPD had increased CIMT, compared with 23% in patients without COPD [44]. CIMT was also found to be positively correlated with exacerbation rate in past year and negatively correlated with FEV1 among patients with COPD, which suggested that CIMT is related to COPD severity [45]. CIMT was also reported to be increased among patients with asthma, [46] which may have differences in different phenotypes. In Atherosclerosis Risk in Communities (ARIC) study, CIMT was increased among women with adult-onset asthma but not childhood-onset asthma [47]. This could be related to the severity of asthma as adult-onset asthma which is well reported to be associated with higher morbidity and mortality [48, 49]. In cystic fibrosis, CIMT was increased among patients who are pancreatic insufficient but not those who are pancreatic sufficient [50].
In patients with severe bronchiectasis, as defined by FACED score, they are more likely to have Pseudomonas colonization, worse lung function, and more symptomatic as measured by mMRC dyspnoea scale. They are also older with more lobes of the lungs being involved. By having Pseudomonas colonization, this will lead to chronic low-grade airway inflammation. The chronic inflammation can contribute to an increase in CIMT as in other inflammatory diseases. Worse lung function by FEV1, higher mMRC dyspnoea scale and more extensive disease could reflect the airway damage from chronic airway inflammation, which is linked to the increase in CIMT. The chronic inflammatory state in these patients with severe bronchiectasis could eventually lead to subclinical atherosclerosis with an increase in CIMT. The constellation of all these factors, as reflected in FACED score, ultimately translate into increase in CIMT, mediated through heightened inflammatory state in these patients.
Our findings provide a pathophysiological basis for the observed association of bronchiectasis with cardiovascular diseases. Although systemic inflammatory diseases have been reported to be associated with cardiovascular diseases, the evidence for bronchiectasis is weaker, especially from a pathogenic aspect. The only evidence for subclinical atherosclerosis derived from a small-scale study of FMD [27] and brachial-ankle pulse wave velocity (baPWV), a measure of arterial stiffness [51]. Our study is the first to show evidence of an association of bronchiectasis with increased CIMT in a severity-dependent manner. Together with the previous reports on FMD and baPWV, [27, 51] these findings provide a stronger pathogenic basis to support the reported association of bronchiectasis with cardiovascular diseases. In previous literature, bronchiectasis has been shown to be associated the adverse cardiovascular outcomes. But as a heterogenous disease, the exact pathophysiological mechanism is not established. This could be attributed by smoking, advanced age and co-existing diseases. Our study provides data to suggest that the association of bronchiectasis and cardiovascular diseases could be contributed by bronchiectasis itself, if severe enough. As bronchiectasis is associated with cardiovascular diseases, we believe that using CIMT as a non-invasive tool can help to identify at risk population, especially patients with severe bronchiectasis and underlying cardiovascular risk factors. The findings from our study also call for the screening and monitoring of cardiovascular health in patients with bronchiectasis, especially if it is a severe one. CIMT is one of the options while other clinical parameters such as blood pressure, glucose and lipid level shall also be assessed. These patients may need better control of bronchiectasis and cardiovascular risk factors, for example with smoking cessation, lowering of lipid level and achieve optimal blood pressure control, to prevent future cardiovascular events. Although bronchiectasis cannot be reversed, yet, it can be controlled with various medications like macrolide and inhaled antibiotics, which may help to prevent bronchiectasis exacerbation and probable subsequent cardiovascular events after bronchiectasis exacerbation.
In bronchiectasis, one of the goals of pharmacotherapy is immunomodulation, such as by macrolide and inhaled corticosteroid (ICS). Whether these treatments can have effect on CIMT and subsequent cardiovascular events worth further research, Macrolide has been shown to reduce the frequency of exacerbations and improve quality of life in bronchiectasis [52]. It acts by suppressing bacterial infection and reducing airway inflammation. ICS works in selected group of patients stratified by the blood eosinophil count [53]. ICS exerts the effect through altering macrophage gene expression, decreasing interferon (IFN)-γ expression and upregulating chemokine production [54]. The clinical benefits of these treatments in bronchiectasis are mainly on the respiratory outcomes such as bronchiectasis exacerbation. It is interesting to know if they can offer cardiovascular protective effect through dampening down the degree of airway inflammation and later reduction in CIMT, which is a marker of subclinical atherosclerosis.
There are some limitations to our study. First, it involved only a single centre with all the patients included being Chinese. This could have potential implication of generalizability of the findings in different ethnic groups, in which the etiology of bronchiectasis could be different. Also, as a single centre study, the sample size is relatively small, especially for patients with severe bronchiectasis. Nonetheless as a tertiary medical centre, the respiratory unit receives referrals from all other health care facilities across the territory. Patients diagnosed with bronchiectasis are managed in a designated bronchiectasis clinic at our centre. And the association of CIMT and severity of bronchiectasis can be demonstrated in this study with statistical significance. A large-scale multi-centre study involving different ethnic group will be worthwhile to conduct to assess if the same phenomenon is observed across different ethnic groups. Second, CIMT was measured using different ultrasound machines for patients with bronchiectasis and controls. Although there may be inter-machine variability for measurement of CIMT, we validated the findings in 17 controls and demonstrated a good correlation for measurements obtained by the two ultrasound machines. In propensity score matching, ideally, all factors that are significantly different should be matched. However, given a relatively small sample size, especially in the severe bronchiectasis group. Matching all the factors that are significantly different such as age and co-morbidities would lead to loss of some of the patients with severe bronchiectasis which cannot be matched. As such, we matched only the gender and smoking status, while adjusting the other factors that are significantly different but not matched are included in multi-variate analysis as confounder. Another limitation is that lung function and blood test results available. They were identified by symptom questionnaire and electronic health record. This may potentially include some patients with minute lung function abnormality or any systemic disorder. Yet, they have been screened for any underlying respiratory diseases by questionnaire and their electronic health record. As they are free of respiratory symptoms without physician diagnosed respiratory diseases, the possibility of erroneously recruiting healthy controls with major respiratory or systemic diseases that affect the CIMT is very low. Also, in the bronchiectasis group, the mean baseline FEV1 was 86.3 ± 24.5%, with 104 patients with baseline FEV1 > 70%. The baseline neutrophil and lymphocyte count in the bronchiectasis group is also relatively normal. The impact from lung function and abnormal blood count on the result is considered to be minute, if there is any.
Conclusions
CIMT was significantly increased in patients with severe bronchiectasis compared with controls without bronchiectasis, but not among patients with mild-to-moderate bronchiectasis, which suggested the subclinical atherosclerosis to be more prevalent among patients with severe bronchiectasis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 2: Appendix 1 Questionnaire for symptom screening in healthy control
Acknowledgements
Nil.
Abbreviations
- AMI ankle
Brachial index
- baPWV brachial
Ankle pulse wave velocity
- BMI
Body mass index
- CHD
Coronary heart disease
- CIMT
Carotid intima-media thickness
- CRP
C-reactive protein
- FMD
Flow-mediated dilatation
- HRCT
High-resolution computed tomography
- IQR
Interquartile range
- SD
Standard deviation
Author contributions
Dr. Wang Chun Kwok and Gary Kui Kai Lau were involved with study concept and design, analysis and interpretation of data, acquisition of data, drafting of manuscript, and approval of the final version of the manuscript. Dr. Kay Cheong Teo, Chung Ki Tsui and Sze Him Isaac Leung, Matthew SS Hsu, Kkts Pijarnvanit, Mr. Yick Hin Chow and Ms. Carman Nga-Man Cheung were involved with critical revision of the manuscript for important intellectual content and approval of the final version. Dr. James Chung Man Ho was involved with the study concept and design, drafting of manuscript, critical revision of the manuscript for important intellectual content, study supervision, and approval of the final version. All the authors had full access to the data, contributed to the study, approved the final version of the publication and took all the responsibility of its accuracy and integrity.
Funding
The study was supported by a Hong Kong College of Physicians Young Investigator Research Grant.
Data availability
Dataset supporting the conclusion of this article is included within this article and no additional data will be provided. Research data is not shared.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (UW 19–624).
Informed consents were obtained.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Clinical Trial Details
Not Applicable. We do not have a Clinical Trial Number as it is not a clinical trial. We only have IRB approval number.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wang Chun Kwok and Kui Kai Lau contributed equally to this work.
References
- 1.Boyton RJ, Altmann DM. Bronchiectasis: current concepts in Pathogenesis, Immunology, and Microbiology. Annu Rev Pathol. 2016;11:523–54. 10.1146/annurev-pathol-012615-044344 [DOI] [PubMed] [Google Scholar]
- 2.Koenig W, Sund M, Frohlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring trends and determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99(2):237–42. 10.1161/01.CIR.99.2.237 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–3. 10.1161/01.CIR.98.8.731 [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97(20):2007–11. 10.1161/01.CIR.97.20.2007 [DOI] [PubMed] [Google Scholar]
- 5.Force USPST, Curry SJ, Krist AH, et al. Risk Assessment for Cardiovascular Disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272–80. 10.1001/jama.2018.8359 [DOI] [PubMed] [Google Scholar]
- 6.Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Can J Cardiol. 2009;25(10):567–79. 10.1016/S0828-282X(09)70715-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. 10.1161/01.CIR.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- 8.Collaboration IRGCERF, Sarwar N, Butterworth AS, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379(9822):1205–13. 10.1016/S0140-6736(11)61931-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Interleukin-6 Receptor Mendelian Randomisation, Analysis C, Swerdlow DI, Holmes MV, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214–24. 10.1016/S0140-6736(12)60110-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349(17):1595–604. 10.1056/NEJMoa035003 [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286(17):2136–42. 10.1001/jama.286.17.2136 [DOI] [PubMed] [Google Scholar]
- 12.Zheng L, Nukuna B, Brennan ML, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114(4):529–41. 10.1172/JCI200421109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karakas M, Koenig W, Zierer A, et al. Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: results from the MONICA/KORA Augsburg study. J Intern Med. 2012;271(1):43–50. 10.1111/j.1365-2796.2011.02397.x [DOI] [PubMed] [Google Scholar]
- 14.Blankenberg S, Rupprecht HJ, Bickel C, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104(12):1336–42. 10.1161/hc3701.095949 [DOI] [PubMed] [Google Scholar]
- 15.Blankenberg S, Tiret L, Bickel C, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106(1):24–30. 10.1161/01.CIR.0000020546.30940.92 [DOI] [PubMed] [Google Scholar]
- 16.Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111(5):570–5. 10.1161/01.CIR.0000154553.12214.CD [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103(4):491–5. 10.1161/01.CIR.103.4.491 [DOI] [PubMed] [Google Scholar]
- 18.Roldan V, Marin F, Lip GY, Blann AD. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost. 2003;90(6):1007–20. 10.1160/TH02-09-0083 [DOI] [PubMed] [Google Scholar]
- 19.Tiret L, Godefroy T, Lubos E, et al. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation. 2005;112(5):643–50. 10.1161/CIRCULATIONAHA.104.519702 [DOI] [PubMed] [Google Scholar]
- 20.Valgimigli M, Ceconi C, Malagutti P, et al. Tumor necrosis factor-alpha receptor 1 is a major predictor of mortality and new-onset heart failure in patients with acute myocardial infarction: the cytokine-activation and long-term prognosis in myocardial infarction (C-ALPHA) study. Circulation. 2005;111(7):863–70. 10.1161/01.CIR.0000155614.35441.69 [DOI] [PubMed] [Google Scholar]
- 21.Saleh AD, Kwok B, Brown JS, Hurst JR. Correlates and assessment of excess cardiovascular risk in bronchiectasis. Eur Respir J 2017;50(5). [DOI] [PubMed]
- 22.Navaratnam V, Millett ER, Hurst JR, et al. Bronchiectasis and the risk of cardiovascular disease: a population-based study. Thorax. 2017;72(2):161–6. 10.1136/thoraxjnl-2015-208188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez R, Feced L, Alcaraz-Serrano V, et al. Cardiovascular events during and after bronchiectasis exacerbations and long-term mortality. Chest. 2022;161(3):629–36. 10.1016/j.chest.2021.10.013 [DOI] [PubMed] [Google Scholar]
- 24.Huang JT, Kuzmanova E, Dicker AJ, et al. Serum desmosine is Associated with Long-Term all-cause and Cardiovascular Mortality in Bronchiectasis. Am J Respir Crit Care Med. 2020;202(6):897–9. 10.1164/rccm.202002-0434LE [DOI] [PubMed] [Google Scholar]
- 25.Navaratnam V, Root AA, Douglas I, Smeeth L, Hubbard RB, Quint JK. Cardiovascular outcomes after a respiratory tract infection among adults with non-cystic fibrosis bronchiectasis: a General Population-based study. Ann Am Thorac Soc. 2018;15(3):315–21. 10.1513/AnnalsATS.201706-488OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi H, Kim SH, Han K, et al. Association between exercise and risk of cardiovascular diseases in patients with non-cystic fibrosis bronchiectasis. Respir Res. 2022;23(1):288. 10.1186/s12931-022-02202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao YH, Liu SX, Cui JJ, et al. Subclinical atherosclerosis in adults with steady-state bronchiectasis: a case-control study. Respir Med. 2018;134:110–6. 10.1016/j.rmed.2017.11.024 [DOI] [PubMed] [Google Scholar]
- 28.Costa JC, Machado JN, Ferreira C, Gama J, Rodrigues C. The Bronchiectasis Severity Index and FACED score for assessment of the severity of bronchiectasis. Pulmonology. 2018. [DOI] [PubMed]
- 29.Lau KK, Chan YH, Wong YK, et al. Garlic intake is an independent predictor of endothelial function in patients with ischemic stroke. J Nutr Health Aging. 2013;17(7):600–4. 10.1007/s12603-013-0043-6 [DOI] [PubMed] [Google Scholar]
- 30.Lau KK, Chan YH, Yiu KH, et al. Burden of carotid atherosclerosis in patients with stroke: relationships with circulating endothelial progenitor cells and hypertension. J Hum Hypertens. 2007;21(6):445–51. 10.1038/sj.jhh.1002178 [DOI] [PubMed] [Google Scholar]
- 31.Touboul PJ, Hennerici MG, Meairs S et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290–296. [DOI] [PMC free article] [PubMed]
- 32.Molinari F, Meiburger KM, Saba L, et al. Ultrasound IMT measurement on a multi-ethnic and multi-institutional database: our review and experience using four fully automated and one semi-automated methods. Comput Methods Programs Biomed. 2012;108(3):946–60. 10.1016/j.cmpb.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 33.Molinari F, Meiburger KM, Saba L et al. Automated carotid IMT measurement and its validation in low contrast ultrasound database of 885 patient Indian population epidemiological study: results of AtheroEdge® software. Multi-modality Atherosclerosis Imaging Diagnosis 2014:209–19. [PMC free article] [PubMed]
- 34.Saba L, Montisci R, Famiglietti L, et al. Automated analysis of intima-media thickness: analysis and performance of CARES 3.0. J Ultrasound Med. 2013;32(7):1127–35. 10.7863/ultra.32.7.1127 [DOI] [PubMed] [Google Scholar]
- 35.Naqvi T. Ultrasound vascular screening for cardiovascular risk assessment. Why, when and how? Minerva Cardioangiol. 2006;54(1):53–67. [PubMed] [Google Scholar]
- 36.van den Munckhof ICL, Jones H, Hopman MTE, et al. Relation between age and carotid artery intima-medial thickness: a systematic review. Clin Cardiol. 2018;41(5):698–704. 10.1002/clc.22934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuswardhani RT, Wiradharma KG, Kandarini Y, Widiana GR, Martadiani ED. Factors associated with carotid intima-media thickness in patients on maintenance hemodialysis. Int J Gen Med. 2019;12:1–6. 10.2147/IJGM.S178276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma M, Wang L, Zhong X, et al. Age and gender differences between carotid intima-media thickness and serum uric acid. Am J Cardiol. 2022;172:137–43. 10.1016/j.amjcard.2022.02.023 [DOI] [PubMed] [Google Scholar]
- 39.Chang CC, Chang ML, Huang CH, Chou PC, Ong ET, Chin CH. Carotid intima-media thickness and plaque occurrence in predicting stable angiographic coronary artery disease. Clin Interv Aging. 2013;8:1283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loboz-Rudnicka M, Jaroch J, Bociaga Z, et al. Impact of cardiovascular risk factors on carotid intima-media thickness: sex differences. Clin Interv Aging. 2016;11:721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Targonska-Stepniak B, Drelich-Zbroja A, Majdan M. The relationship between carotid intima-media thickness and the activity of rheumatoid arthritis. J Clin Rheumatol. 2011;17(5):249–55. 10.1097/RHU.0b013e3182290dbf [DOI] [PubMed] [Google Scholar]
- 42.Medeiros PBS, Salomao RG, Teixeira SR, et al. Disease activity index is associated with subclinical atherosclerosis in childhood-onset systemic lupus erythematosus. Pediatr Rheumatol Online J. 2021;19(1):35. 10.1186/s12969-021-00513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eder L, Jayakar J, Shanmugarajah S, et al. The burden of carotid artery plaques is higher in patients with psoriatic arthritis compared with those with psoriasis alone. Ann Rheum Dis. 2013;72(5):715–20. 10.1136/annrheumdis-2012-201497 [DOI] [PubMed] [Google Scholar]
- 44.Van Gestel YR, Flu W-J, van Kuijk J-P, et al. Association of COPD with carotid wall intima-media thickness in vascular surgery patients. Respir Med. 2010;104(5):712–6. 10.1016/j.rmed.2009.10.027 [DOI] [PubMed] [Google Scholar]
- 45.Gulbas G, Turan O, Sarioglu N, et al. Carotid intima-media thickness in chronic obstructive pulmonary disease and survival: a multicenter prospective study. Clin Respir J. 2019;13(6):391–9. 10.1111/crj.13024 [DOI] [PubMed] [Google Scholar]
- 46.Yılmaz M, Bozkurt Yılmaz HE, Şen N, Altın C, Tekin A, Müderrisoğlu H. Investigation of the relationship between asthma and subclinical atherosclerosis by carotid/femoral intima media and epicardial fat thickness measurement. J Asthma. 2018;55(1):50–6. 10.1080/02770903.2017.1313272 [DOI] [PubMed] [Google Scholar]
- 47.Onufrak S, Abramson J, Vaccarino V. Adult-onset asthma is associated with increased carotid atherosclerosis among women in the atherosclerosis risk in communities (ARIC) study. Atherosclerosis. 2007;195(1):129–37. 10.1016/j.atherosclerosis.2006.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai C-L, Delclos GL, Huang JS, Hanania NA, Camargo CA Jr. Age-related differences in asthma outcomes in the United States, 1988–2006. Ann Allergy Asthma Immunol. 2013;110(4):240–6. e241. 10.1016/j.anai.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 49.Enright PL, Kronmal RA, Higgins MW, Schenker MB, Haponik EF. Prevalence and correlates of respiratory symptoms and disease in the elderly. Chest. 1994;106(3):827–34. 10.1378/chest.106.3.827 [DOI] [PubMed] [Google Scholar]
- 50.Nowak JK, Wykrętowicz A, Mądry E, et al. Preclinical atherosclerosis in cystic fibrosis: two distinct presentations are related to pancreatic status. J Cyst Fibros. 2022;21(1):26–33. 10.1016/j.jcf.2021.06.010 [DOI] [PubMed] [Google Scholar]
- 51.Gao YH, Cui JJ, Wang LY, et al. Arterial stiffness in adults with steady-state bronchiectasis: association with clinical indices and disease severity. Respir Res. 2018;19(1):86. 10.1186/s12931-018-0790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly C, Chalmers JD, Crossingham I et al. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst Reviews 2018(3). [DOI] [PMC free article] [PubMed]
- 53.Kwok WC, Tam TCC, Lam DCL, Ip MSM, Ho JCM. Blood eosinophil percentage as a predictor of response to inhaled corticosteroid in bronchiectasis. Clin Respir J 2023. [DOI] [PMC free article] [PubMed]
- 54.Martínez-García MÁ, Oscullo G, García-Ortega A, Matera MG, Rogliani P, Cazzola M. Inhaled corticosteroids in adults with non-cystic fibrosis bronchiectasis: from bench to Bedside. A narrative review. Drugs. 2022;82(14):1453–68. 10.1007/s40265-022-01785-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 2: Appendix 1 Questionnaire for symptom screening in healthy control
Data Availability Statement
Dataset supporting the conclusion of this article is included within this article and no additional data will be provided. Research data is not shared.