Abstract
As the geographic distributions of medically important ticks and tick-borne pathogens continue to expand in the United States, the burden of tick-borne diseases continues to increase along with a growing risk of coinfections. Coinfection with multiple tick-borne pathogens may amplify severity of disease and complicate diagnosis and treatment. By testing 13,400 Ixodes ticks from 17 US states spanning five geographical regions for etiological agents of Lyme disease (Borrelia burgdorferi sensu stricto [s.s.] and Borrelia mayonii), Borrelia miyamotoi disease (Borrelia miyamotoi), anaplasmosis (Anaplasma phagocytophilum), and babesiosis (Babesia microti) we show that B. burgdorferi s.s. was the most prevalent and widespread pathogen. Borrelia miyamotoi, A. phagocytophilum, and B. microti were widespread but less prevalent than B. burgdorferi s.s. Coinfections with B. burgdorferi s.s. and A. phagocytophilum or B. microti were most common in the Northeast and occurred at rates higher than expected based on rates of single infections in that region.
Keywords: Ticks, Ixodes, Borrelia, Anaplasma, Babesia, Surveillance, Coinfection
1. Introduction
Tick-borne diseases are becoming increasingly more common and geographically widespread in the United States (Rosenberg et al., 2018). This trend is explained, in part, by expanding ranges of medically important ticks and an accelerating rate of new tick-borne pathogen discovery (Eisen and Paddock, 2020). The majority of tick-borne disease cases are associated with the blacklegged tick, Ixodes scapularis, a tick that was restricted to focal regions of the U.S. and not even considered a medically important tick before the 1970s but is now ubiquitous in the eastern U.S. and recognized as a vector of seven human pathogens (Eisen and Eisen, 2018; Eisen et al., 2016). As the geographic range of this tick and its associated pathogens continue to expand, the human population at risk for exposure to I. scapularis-borne infections increases as does the risk of coinfections. Coinfection with multiple Ixodes-borne pathogens may increase severity of disease and complicate diagnosis and treatment (Belongia, 2002; Krause et al., 1996). Understanding the true rate of coinfections in humans is challenging as many epidemiological studies reporting human coinfection fail to distinguish concurrent and sequential infections (Chmielewska-Badora et al., 2012; Mitchell et al., 1996).
Humans can become coinfected from the bite of a single tick that is infected with and transmits multiple pathogens, or by simultaneous or successive bites from multiple ticks each transmitting a different pathogen. Assessing differences in prevalence of single and coinfections in host-seeking ticks across regions and life stages can aid in estimating acarological risk of infections or coinfections in humans. While several previous studies have reported prevalence of single or coinfections in Ixodes ticks at local scales (Adelson et al., 2004; Aliota et al., 2014; Hersh et al., 2014; Holden et al., 2003; Holman et al., 2004; Hutchinson et al., 2015; Johnson et al., 2017, 2018; Little and Molaei, 2020; Piesman et al., 1986; Prusinski et al., 2014; Schauber et al., 1998; Schulze et al., 2013, 2005; Schwartz et al., 1997; Varde et al., 1998; Xu et al., 2016), comparison across regions is often complicated by use of varying pathogen detection assays, differences in the suite of pathogens included, and the blood feeding status of the ticks tested. In this study we used a consistent pathogen detection assay (Graham et al., 2018) and we restricted testing to host-seeking nymphs and adults. We tested 13,400 I. scapularis and I. pacificus ticks collected from 2013 through 2019 from 17 US states spanning five geographical regions for etiological agents of Lyme disease (Borrelia burgdorferi sensu stricto [s.s.] and Borrelia mayonii), Borrelia miyamotoi disease (Borrelia miyamotoi), anaplasmosis (Anaplasma phagocytophilum), and babesiosis (Babesia microti). We 1) summarize single- and coinfection prevalence for these pathogens in ticks by species, life stage and geographic region, and 2) evaluate if coinfections occur more commonly than expected based on prevalence of single infections.
2. Methods
2.1. Collection sites
From 2013 through 2019, host-seeking I. scapularis or I. pacificus nymphs or adults were collected by dragging, flagging or CO2 trapping from a total of 261 counties in 17 states and Washington D.C. (Fig. 1). Sampling was conducted either as part of the Centers for Disease Control and Prevention’s (CDC) national tick and tick-borne pathogen surveillance program (CDC, 2018b; Eisen and Paddock, 2020) or as part of collaborative research projects with academic or public health partners. All ticks were submitted to CDC’s Division of Vector-Borne Diseases, Bacterial Diseases Branch for pathogen testing.
Fig. 1.
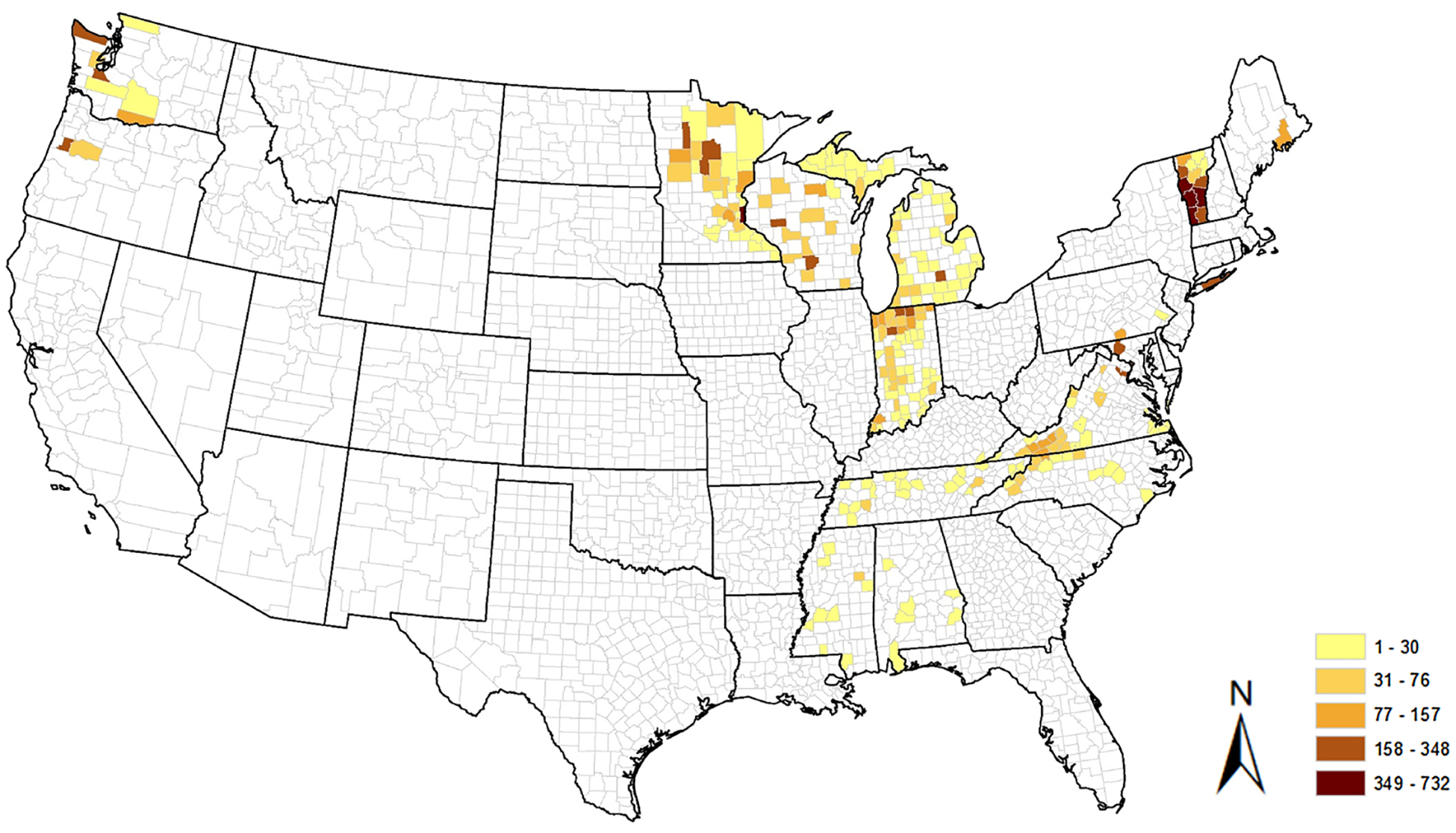
Number of Ixodes ticks tested for presence of pathogens per county.
2.2. Pathogen detection
To extract DNA, we homogenized individual ticks in lysis buffer using a Mini-Beadbeater-96 (BioSpec Products, Bartlesville, OK, USA) and then processed approximately 40 % of each tick lysate using the QIAcube HT system and the cador Pathogen 96 QIAcube HT kit (Qiagen, Valencia, CA, USA) as described previously (Graham et al., 2018; Johnson et al., 2017, 2018), or using the KingFisher Flex and the Mag-Max CORE Nucleic Acid Purification Kit (ThermoFisher Scientific, Waltham, MA). To prepare homogenates for processing on the KingFisher Flex, we followed the manufacturer’s “complex method” with modifications. Briefly, we mixed 200 μL homogenate with 450 μL lysis solution for 5 min at moderate speed, then we mixed 30 μL bead/proteinase K mix with the lysate for 2 min at vigorous speed. Finally, we added 350 μL binding solution and processed the samples using the MagMax_CORE_Flex_96W program (ThermoFisher Scientific).
All ticks were screened for B. burgdorferi s.s., B. mayonii, B. miyamotoi, A. phagocytophilum, and B. microti (Table 1) except for a minority of ticks that were not tested for B. mayonii because they were submitted before B. mayonii was integrated into the standard testing algorithm in 2017.
Table 1.
Prevalence of human pathogens by Ixodes species and life-stage by state and region, 2013 – 2019.
| Region State† | Species, life stage | Total no. positive ticks (% positive [95 % C.I.]) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Borrelia burgdorferi s.s. | Borrelia miyamotoi | Borrelia mayonii †† | Anaplasma phagocytophilum | Babesia microti | |||||||
| Northeast | |||||||||||
| ME | I. scapularis | No. ticks tested | No. ticks tested | No. ticks tested | No. ticks tested | No. ticks tested | |||||
| Nymph | 154 | 27 (17.53 [12.34–24.31]) | 154 | 1 (0.65 [0.03–3.59]) | – | – | 154 | 5 (3.25 [1.39–7.37]) | 154 | 6 (3.90 [1.80–8.24]) | |
| Adult | – | – | – | – | – | – | – | – | – | – | |
| NY | I. scapularis | ||||||||||
| Nymph | 299 | 50 (16.72 [12.92–21.37]) | 299 | 9 (3.01 [1.59–5.62]) | – | – | 299 | 27 (9.03 [6.28–12.82]) | 299 | 36 (12.04 [8.82–16.22]) | |
| Adult | – | – | – | – | – | – | – | – | – | – | |
| PA | I. scapularis | ||||||||||
| Nymph | 115 | 26 (22.61 [15.92–31.07]) | 115 | 0 (0.00 [0.00–3.23]) | 1 | 0 (0.00 [0.00–94.87]) | 115 | 3 (2.61 [0.89–7.39]) | 115 | 0 (0.00 [0.00–3.23]) | |
| Adult | – | – | – | – | – | – | – | – | – | – | |
| VT | I. scapularis | ||||||||||
| Nymph | 716 | 170 (23.74 [20.77–26.99]) | 716 | 5 (0.70 [0.30–1.62]) | 716 | 0 (0.00 [0.00–0.53]) | 716 | 39 (5.45 [4.01–7.36]) | 716 | 31 (4.33 [3.07–6.08]) | |
| Adult | 2152 | 1249 (58.04 [55.94–60.11]) | 2155 | 24 (1.11 [0.75–1.65]) | 2153 | 0 (0.00 [0.00–0.18]) | 2155 | 174 (8.07 [7.00–9.30]) | 2155 | 76 (3.53 [2.83–4.39]) | |
| Total | |||||||||||
| Nymph | 1284 | 273 (21.26 [19.11–23.58]) | 1284 | 15 (1.20 [0.71–1.92]) | 717 | 0 (0.00 [0.00–0.53]) | 1284 | 74 (5.76 [4.62–7.17]) | 1284 | 73 (5.69 [4.55–7.10]) | |
| Adult | 2152 | 1249 (58.04 [55.94–60.11]) | 2155 | 24 (1.11 [0.75–1.65]) | 2153 | 0 (0.00 [0.00–0.18]) | 2155 | 174 (8.07 [6.99–9.30]) | 2155 | 76 (3.53 [2.83–4.39]) | |
| Mid-Atlantic | |||||||||||
| DC | I. scapularis | ||||||||||
| Nymph | 253 | 62 (24.51 [19.61–30.16]) | 253 | 2 (0.79 [0.22–2.84]) | – | – | 253 | 1 (0.40 [0.02–2.20]) | 253 | 0 (0.00 [0.00–1.50]) | |
| Adult | – | – | – | – | – | – | – | – | – | – | |
| KY | I. scapularis | ||||||||||
| Nymph | 13 | 0 (0.00 [0.00–22.81]) | 13 | 0 (0.00 [0.00–22.81]) | 13 | 0 (0.00 [0.00–22.81]) | 13 | 0 (0.00 [0.00–22.81]) | 13 | 0 (0.00 [0.00–22.81]) | |
| Adult | – | – | – | – | – | – | – | – | – | – | |
| MD | I. scapularis | ||||||||||
| Nymph | 168 | 39 (23.21 [17.47–30.15]) | 168 | 4 (2.38 [0.93–5.96]) | – | – | 168 | 4 (2.38 [0.93–5.96]) | 168 | 0 (0.00 [0.00–2.24]) | |
| Adult | – | – | – | – | – | – | – | – | – | – | |
| NC | I. scapularis | ||||||||||
| Nymph | 378 | 46 (12.17 [9.25–15.85]) | 378 | 4 (1.06 [0.41–2.69]) | 378 | 0 (0.00 [0.00–1.01]) | 378 | 5 (1.32 [0.57–3.06]) | 378 | 0 (0.00 [0.00–1.01]) | |
| Adult | 89 | 38 (42.70 [32.93–53.06]) | 89 | 1 (1.12 [0.06–6.09]) | 89 | 0 (0.00 [0.00–4.14]) | 89 | 2 (2.25 [0.62–7.83]) | 89 | 0 (0.00 [0.00–4.14]) | |
| VA | I. scapularis | ||||||||||
| Nymph | 1276 | 205 (16.07 [14.15–18.18]) | 1277 | 17 (1.33 [0.83–2.12]) | 804 | 0 (0.00 [0.00–0.48]) | 1277 | 51 (3.99 [3.05–5.21]) | 1277 | 2 (0.16 [0.04–0.57]) | |
| Adult | 329 | 129 (39.21 [34.09–44.58]) | 329 | 9 (2.74 [1.45–5.12]) | 329 | 0 (0.00 [0.00–1.15]) | 329 | 10 (3.04 [1.66–5.50]) | 329 | 0 (0.00 [0.00–1.15]) | |
| Total | |||||||||||
| Nymph | 2088 | 352 (16.85 [15.31–18.52]) | 2089 | 27 (1.29 [0.89–1.87]) | 1195 | 0 (0.00 [0.00–0.32]) | 2089 | 61 (2.92 [2.28–3.73]) | 2089 | 2 (0.09 [0.02–0.35]) | |
| Adult | 418 | 167 (39.95 [35.37–44.72]) | 418 | 10 (2.39 [1.30–4.35]) | 418 | 0 (0.00 [0.00–0.91]) | 418 | 12 (2.87 [1.65–4.95]) | 418 | 0 (0.00 [0.00–0.91]) | |
| Midwest | |||||||||||
| IN | I. scapularis | ||||||||||
| Nymph | 721 | 107 (14.84 [12.43–17.62]) | 721 | 10 (1.39 [0.76–2.53]) | 721 | 0 (0.00 [0.00–0.53]) | 721 | 6 (0.83 [0.38–1.80]) | 721 | 0 (0.00 [0.00–0.53]) | |
| Adult | 1686 | 612 (36.30 [34.04–38.62]) | 1686 | 21 (1.25 [0.82–1.90]) | 1686 | 0 (0.00 [0.00–0.23]) | 1686 | 41 (2.43 [1.80–3.28]) | 1686 | 0 (0.00 [0.00–0.23]) | |
| MI | I. scapularis | ||||||||||
| Nymph | 287 | 16 (5.57 [3.46–8.86]) | 287 | 1 (0.35 [0.02–1.95]) | 287 | 0 (0.00 [0.00–1.32]) | 287 | 6 (2.09 [0.96–4.49]) | 287 | 0 (0.00 [0.00–1.32]) | |
| Adult | 535 | 113 (21.12 [17.87–24.78]) | 535 | 4 (0.75 [0.29–1.91]) | 535 | 0 (0.00 [0.00–0.71]) | 536 | 31 (5.78 [4.10–8.09]) | 536 | 0 (0.00 [0.00–0.71]) | |
| MN | I. scapularis | ||||||||||
| Nymph | 2004 | 464 (23.15 [21.36–25.05]) | 2004 | 19 (0.95 [0.61–1.48]) | 2004 | 12 (0.60 [0.34–1.04]) | 2004 | 109 (5.44 [4.53–6.52]) | 2004 | 85 (4.24 [3.44–5.21]) | |
| Adult | 148 | 48 (32.43 [25.42–40.34]) | 148 | 4 (2.70 [1.06–6.74]) | 148 | 0 (0.00 [0.00–2.53]) | 148 | 7 (4.73 [2.31–9.44]) | 148 | 0 (0.00 [0.00–2.53]) | |
| WI | I. scapularis | ||||||||||
| Nymph | 929 | 930 | 930 | 930 | 930 | ||||||
| 122 (13.13 [11.11–15.46]) | 16 (1.72 [1.06–2.78]) | 3 (0.32 [0.11–0.94]) | 38 (4.09 [2.99–5.56]) | 12 (1.29 [0.74–2.24]) | |||||||
| Adult | 69 | 35 (50.72 [39.21–62.17]) | 69 | 4 (5.80 [2.28–13.98]) | 69 | 0 (0.00 [0.00–5.27]) | 69 | 7 (10.14 [5.00–19.49]) | 69 | 7 (10.14 [5.00–19.49]) | |
| Total | |||||||||||
| Nymph | 3941 | 709 (17.99 [16.82–19.22]) | 3942 | 46 (1.17 [0.88–1.55]) | 3942 | 15 (0.38 [0.23–0.62]) | 3942 | 159 (4.03 [3.46–4.69]) | 3942 | 97 (2.46 [2.02–2.99]) | |
| Adult | 2438 | 808 (33.14 [31.30I 35.04]) | 2438 | 33 (1.35 [0.97–1.89]) | 2438 | 0 (0.00 [0.00–0.16]) | 2439 | 86 (3.53 [2.86–4.33]) | 2439 | 7 (0.29 [0.14I 0.59]) | |
| Southeast | |||||||||||
| AL | I. scapularis | n | n | n | n | n | |||||
| Nymph | 3 | 0 (0.00 [0.00–56.15]) | 3 | 0 (0.00 [0.00–56.15]) | 3 | 0 (0.00 [0.00–56.15]) | 3 | 0 (0.00 [0.00–56.15]) | 3 | 0 (0.00 [0.00–56.15]) | |
| Adult | 22 | 0 (0.00 [0.00–14.87]) | 22 | 0 (0.00 [0.00–14.87]) | 22 | 0 (0.00 [0.00–14.87]) | 22 | 0 (0.00 [0.00–14.87]) | 22 | 0 (0.00 [0.00–14.87]) | |
| MS | I. scapularis | ||||||||||
| Nymph | – | – | – | – | – | – | – | – | – | – | |
| Adult | 70 | 0 (0.00 [0.00–5.20]) | 70 | 0 (0.00 [0.00–5.20]) | 70 | 0 (0.00 [0.00–5.20]) | 70 | 0 (0.00 [0.00–5.20]) | 70 | 0 (0.00 [0.00–5.20]) | |
| TN | I. scapularis | ||||||||||
| Nymph | 3 | 0 (0.00 [0.00–56.15]) | 3 | 0 (0.00 [0.00–56.15]) | 3 | 0 (0.00 [0.00–56.15]) | 3 | 0 (0.00 [0.00–56.15]) | 3 | 0 (0.00 [0.00–56.15]) | |
| Adult | 211 | 5 (2.37 [1.02–5.43]) | 211 | 2 (0.95 [0.26–3.39]) | 211 | 0 (0.00 [0.00–1.79]) | 211 | 0 (0.00 [0.00–1.79]) | 211 | 0 (0.00 [0.00–1.79]) | |
| Total | |||||||||||
| Nymph | 6 | 0 (0.00 [0.00–0.39]) | 6 | 0 (0.00 [0.00–0.39]) | 6 | 0 (0.00 [0.00–0.39]) | 6 | 0 (0.00 [0.00–0.39]) | 6 | 0 (0.00 [0.00–0.39]) | |
| Adult | 303 | 5 (1.65 [0.71–3.80]) | 303 | 2 (0.66 [0.18–2.37]) | 303 | 0 (0.00 [0.00–1.25]) | 303 | 0 (0.00 [0.00–1.25]) | 303 | 0 (0.00 [0.00–1.25]) | |
| Northwest | |||||||||||
| OR | I. pacificus | ||||||||||
| Nymph | – | – | – | – | – | – | – | – | – | – | |
| Adult | 243 | 0 (0.00 [0.00–1.56]) | 243 | 2 (0.82 [0.23–2.95]) | 243 | 0 (0.00 [0.00–1.56]) | 243 | 0 (0.00 [0.00–1.56]) | 243 | 0 (0.00 [0.00–1.56]) | |
| WA | I. pacificus | ||||||||||
| Nymph | 20 | 1 (5.00 [0.26–23.61]) | 20 | 0 (0.00 [0.00–16.11]) | 15 | 0 (0.00 [0.00–20.39]) | 20 | 0 (0.00 [0.00–16.11]) | 20 | 0 (0.00 [0.00–16.11]) | |
| Adult | 501 | 17 (3.39 [2.13–5.37]) | 501 | 11 (2.20 [1.23–3.89]) | 387 | 0 (0.00 [0.00 – 0.98]) | 501 | 8 (1.60 [0.81–3.12]) | 501 | 0 (0.00 [0.00 – 0.76]) | |
| Total | |||||||||||
| Nymph | 20 | 1 (5.00 [0.89–23.61]) | 20 | 0 (0.00 [0.00–16.11]) | 15 | 0 (0.00 [0.00–20.39]) | 20 | 0 (0.00 [0.00–16.11]) | 20 | 0 (0.00 [0.00–16.11]) | |
| Adult | 744 | 17 (2.28 [1.43–3.63]) | 744 | 13 (1.75 [1.03–2.97]) | 630 | 0 (0.00 [0.00 – 0.61]) | 744 | 8 (1.08 [0.55–2.11]) | 744 | 0 (0.00 [0.00–0.51]) | |
| Total | Ixodes spp. | ||||||||||
| Nymph | 7336 | 1335 (18.20 [17.33–19.10]) | 7341 | 88 (1.20 [0.97–1.47]) | 5872 | 15 (0.26 [0.15–0.42]) | 7341 | 294 (4.00 [3.58–4.45]) | 7341 | 172 (2.34 [2.02–2.71]) | |
| Adult | 5963 | 2246 (37.67 [36.44–38.90]) | 5988 | 82 (1.37 [1.10–1.70]) | 5850 | 0 (0.00 [0.00–0.07]) | 6059 | 280 (4.62 [4.12–5.18]) | 6059 | 83 (1.37 [1.11–1.69]) | |
ME: Maine; NY: New York; PA: Pennsylvania; VT: Vermont; DC: Washington, D.C.; KY: Kentucky; MD: Maryland; NC: North Carolina; VA: Virginia; IN: Indiana; MI: Michigan; MN: Minnesota; WI: Wisconsin; AL: Alabama; MS: Mississippi; TN: Tennessee; OR: Oregon; WA: Washington.
Testing for B. mayonii was not initiated until 2017, thus samples tested prior to 2017 were not tested for B. mayonii.
First, using probe-based real-time PCR reactions, we screened all samples using a series of paired multiplex assays to detect multiple targets from each pathogen: genes encoding P44 outer membrane surface proteins (p44) and major surface protein 4 (msp4) for A. phagocytophilum; genes encoding secreted antigen 1 (sa1) and 18S rRNA (18S) for B. microti; a flagellin filament cap gene (fliD) for B. burgdorferi s.s. and B. mayonii; and a genomic Borrelia target (gB31) present in B. burgdorferi s.s. and B. miyamotoi (Hojgaard et al., 2014) or a 16S rDNA (16S) a pan-Borrelia target for Borrelia spp. (Graham et al., 2018). Reaction conditions were as described previously (Graham et al., 2018; Hojgaard et al., 2014). The multiplex assays also incorporated an I. scapularis actin target that was previously shown to verify DNA integrity in both I. scapularis and I. pacificus (Graham et al., 2018, 2016).
We screened all Borrelia-positive ticks for B. miyamotoi using a pair of B. miyamotoi specific targets for adenylosuccinate lyase (purB) and glycerophosphodiesterase (glpQ) genes as described previously (Graham et al., 2016). Among the small minority of ticks tested before 2017, we identified B. burgdorferi s.s.-positive samples by amplifying and sequencing B. burgdorferi s.l. ClpA protease subunit A (clpA) and/or Dipeptidyl amino-amino-peptidase (pepX) targets from all B. burgdorferi s.l.-positive I. pacificus and from a representative sample of B. burgdorferi s.l.-positive I. scapularis as described previously (Johnson et al., 2017). To detect and differentiate B. burgdorferi s.s. and B. mayonii in all Borrelia-positive samples tested after 2017, we used a pair of TaqMan real-time PCR duplex assays targeting the oligopeptide permease periplasmic A2 gene (oppA2) as described previously (Graham et al., 2018). All PCR reactions were performed using a C1000 Touch thermal cycler with a CFX96 real time system (Bio-Rad, Hercules, CA, USA). We analyzed the samples using the CFX Manager 3.1 software (Bio-Rad) with the quantitation cycle (Cq) determination set to regression.
2.3. Statistical analysis
We calculated the infection prevalence and associated 95 % confidence intervals for all pathogens, and all possible combinations of pathogens for each state and each geographic region. The 95 % confidence intervals were calculated using the Wilson-score method for binomial probabilities. Having computed confidence intervals for single parameters, we use these to compare prevalence among regions, realizing that this increases our Type II error.
Permutation tests were used to determine whether an observed co-infection prevalence was different than the expected coinfection prevalence based on single infections. If coinfections occur independently, then coinfection prevalence equals the product of the marginal infection prevalences. Approximate null distributions of coinfection prevalences (assumes independence of infections) were constructed by permuting testing results for one of the pathogens ten thousand times to determine the prevalence of coinfection. The observed coinfection prevalence was then compared to the 2.5th and 97.5th quantiles of the null distribution to assess whether the observed coinfection prevalence fell within this boundary. Observed coinfection prevalences that fell outside of this boundary were assumed to occur either more or less than expected than if infections occur independently. All analyses were conducted in R (Team, 2013).
3. Results
Of the 13,400 Ixodes ticks tested from 17 U.S. states and the District of Columbia, 6,059 (45.21 %) were adults and 7,341 (54.78 %) were nymphs (Fig. 1). Host seeking nymphs were rarely submitted from the southeastern U.S., where adults were the predominant life stage submitted for testing. In general, with the exception of B. burgdorferi s.s. in the Northwest, infection prevalence was higher in adults compared with nymphs (Table 1).
Borrelia burgdorferi s.s. was the most prevalent and geographically widespread pathogen, detected in each of the states from which ticks were submitted except for Kentucky, Alabama, Mississippi, and Oregon; however, sample sizes were relatively low from most of these states. Among all ticks tested, 18.20 % (17.33–19.10 %) of nymphs and 37.67 % (36.44–38.90 %) of adults were infected with B. burgdorferi s.s. (Table 1). Infection prevalence in nymphs (21.26 % [19.11–23.58 %]) and adults (58.04 % [55.94–60.11 %]) was highest in the Northeast compared with all other regions. Prevalence of B. burgdorferi s.s. was similar between the Mid-Atlantic (16.85 % [15.31–18.52 %]; 39.95 % [35.37–44.72 %], in nymphs and adults, respectively) and Midwest (17.99 % [16.82–19.22%]; 33.14 % [31.30–35.04 %]). Nymphal infection prevalence was significantly lower in the Southeast (0.00 % [0.00–0.39 %]) compared with all other regions, whereas prevalence of infection in adults was similar between the Southeast (1.65 % [0.71–3.80 %]) and Northwest (2.28 % [1.43–3.63 %]) and lower in both these regions compared with all others.
Borrelia miyamotoi, A. phagocytophilum, and B. microti were widespread but less prevalent than B. burgdorferi s.s. (Table 1). Borrelia miyamotoi was detected in ticks collected from each region, with nymphal infection prevalence similar among the Northeast (1.20 % [0.71–1.92 %), Midwest (1.17 % [0.88–1.55 %]) and Mid-Atlantic (1.29 % [0.89–1.87 %]), which trended higher than nymphal infection prevalence in the Northwest and the Southeast where infections were not detected in tested nymphs; prevalence of infection in adult ticks was similar among regions with an overall average of 1.37 % (1.10–1.70 %) infected. Anaplasma phagocytophilum was detected in ticks from each region except the Southeast, with highest prevalence of infection recorded in the Northeast (5.76 % [4.62–7.17 %] and 8.07 % [6.99–9.30 %] in nymphs and adults, respectively). In the Northeast, B. microti was detected at similar prevalence (5.69 % [4.55–7.10 %] and 3.53 % [2.83–4.39 %] in nymphs and adults, respectively) to A. phagocytophilum. Babesia microti was less commonly detected in the Midwest (2.46 % [2.02–2.99 %] in nymphs; 0.29 % [0.14–0.59 %] in adults) compared with the Northeast and within the Midwest, B. microti was less prevalent in ticks compared with A. phagocytophilum (4.03 % [3.46–4.69 %] in nymphs and 3.53 % [2.86–4.33 %] in adults). Babesia microti was detected in only a single state (Virginia) in the Mid-Atlantic region and overall prevalence for that region was low (0.09 % [0.02–0.35 %] in nymphs and 0.00 % [0.00–0.91 % in adults); no B. microti infections were detected in the Southeast or Northwest. Borrelia mayonii was detected only in nymphal ticks from Wisconsin (0.32 % [0.11–0.94 %]) and Minnesota (0.60 % [0.34–1.04 %]) and occurred at very low prevalence (<1 %) (Table 1).
Coinfections were more common in the Northeast compared with other regions (Table 2). Looking only at the three most common pathogens (B. burgdorferi s.s., A. phagocytophilum and B. microti), coinfections were most commonly detected in the Northeast where B. burgdorferi s.s. and either A. phagoctyphilum or B. microti were reported in roughly 3% of nymphs; approximately 1% of nymphs were coinfected with A. phagocytophilum and B. microti. Compared with the Northeast, coinfection rates were substantially lower in the Midwest and Mid-Atlantic and no coinfections were detected in ticks tested from the Southeast or Northwest (Table 2).
Table 2.
Prevalence of Borrelia burgdorferi s.s., Anaplasma phagocytophilum, and Babesia microti coinfections by Ixodes species and life-stage at the state-level, 2013 – 2019.
| Region State† | Tick species and life stage | No. ticks tested | Total no. ticks co-infected (% [95 % C.I.]) | ||
|---|---|---|---|---|---|
| Borrelia burgdorferi s.s. and Anaplasma phagocytophilum | Borrelia burgdorferi s.s. and Babesia microti | Anaplasma phagocytophilum and Babesia microti | |||
| Northeast | |||||
| ME | I. scapularis | ||||
| Nymph | 154 | 4 (2.6 [1.01–6.49]) | 3 (1.95 [0.66–5.57]) | 0 (0.00 [0.00–2.43]) | |
| Adult | – | ||||
| NY | I. scapularis | ||||
| Nymph | 299 | 9 (3.01 [1.59–5.62]) | 16 (5.35 [3.32–8.51]) | 5 (1.67 [0.72–3.85]) | |
| Adult | – | ||||
| PA | I. scapularis | ||||
| Nymph | 115 | 1 (0.87 [0.04–4.76]) | 0 (0.00 [0.00–3.23]) | 0 (0.00 [0.00–3.23]) | |
| Adult | – | ||||
| VT | I. scapularis | ||||
| Nymph | 716 | 26 (3.63 [2.49–5.27]) | 23 (3.21 [2.15–4.77]) | 11 (1.54 [0.86–2.73]) | |
| Adult | 2155 | 132 (6.13 [5.19–.22]) | 66 (3.06 [2.41–3.88]) | 14 (0.65 [0.39–1.09]) | |
| Total | |||||
| Nymph | 1284 | 40 (3.12 [2.30–4.21]) | 42 (3.27 [2.42–4.39]) | 16 (1.25 [0.77–2.01]) | |
| Adult | 2155 | 132 (6.13 [5.19–7.22]) | 66 (3.06 [2.41–3.88]) | 14 (0.65 [0.39–1.09]) | |
| Mid-Atlantic | |||||
| DC | I. scapularis | ||||
| Nymph | 253 | 0 (0.00 [0.00–1.50]) | 0 (0.00 [0.00–1.50]) | 0 (0.00 [0.00–1.50]) | |
| Adult | – | ||||
| KY | I. scapularis | ||||
| Nymph | 13 | 0 (0.00 [0.00–22.81]) | 0 (0.00 [0.00–22.81]) | 0 (0.00 [0.00–22.81]) | |
| Adult | – | ||||
| MD | I. scapularis | ||||
| Nymph | 168 | 0 (0.00 [0.00–2.24]) | 0 (0.00 [0.00–2.24]) | 0 (0.00 [0.00–2.24]) | |
| Adult | – | ||||
| NC | I. scapularis | ||||
| Nymph | 378 | 0 (0.00 [0.00–1.01]) | 0 (0.00 [0.00–1.01]) | 0 (0.00 [0.00–1.01]) | |
| Adult | 89 | 1 (1.12 (0.06–6.09]) | 0 (0.00 [0.00–4.14]) | 0 (0.00 [0.00–4.14]) | |
| VA | I. scapularis | ||||
| Nymph | 1277 | 4 (0.31 [0.12–0.80]) | 2 (0.16 [0.04–0.57]) | 0 (0.00 [0.00–0.30]) | |
| Adult | 329 | 2 (0.61 [0.17–2.19]) | 0 (0.00 [0.00–1.15]) | 0 (0.00 [0.00–1.15]) | |
| Total | |||||
| Nymph | 2089 | 4 (0.19 [0.07–0.49]) | 2 (0.10 [0.03–0.35]) | 0 (0.00 [0.00–0.18]) | |
| Adult | 418 | 3 (0.72 [0.24–2.09]) | 0 (0.00 [0.00–0.91]) | 0 (0.00 [0.00–0.91]) | |
| Midwest | |||||
| IN | I. scapularis | ||||
| Nymph | 721 | 0 (0.00 [0.00–0.53]) | 0 (0.00 [0.00–0.53]) | 0 (0.00 [0.00–0.53]) | |
| Adult | 1686 | 19 (1.13 [0.72–1.75]) | 0 (0.00 [0.00–0.23]) | 0 (0.00 [0.00–0.23]) | |
| MI | I. scapularis | ||||
| Nymph | 287 | 1 (0.35 [0.02–1.95]) | 0 (0.00 [0.00–1.32]) | 0 (0.00 [0.00–1.32]) | |
| Adult | 536 | 15 (2.80 [1.70–4.57]) | 0 (0.00 [0.00–0.71]) | 0 (0.00 [0.00–0.71]) | |
| MN | I. scapularis | ||||
| Nymph | 2006 | 63 (3.14 [2.46–4.00]) | 60 (2.99 [2.33–3.83]) | 25 (1.25 [0.85–1.83]) | |
| Adult | 148 | 3 (2.03 [0.69–5.79]) | 0 (0.00 [0.00–2.53]) | 0 (0.00 [0.00–2.53]) | |
| WI | I. scapularis | ||||
| Nymph | 930 | 9 (0.97 [0.51–1.83]) | 8 (0.86 [0.44–1.69]) | 1 (0.11 [0.01–0.61]) | |
| Adult | 69 | 4 (5.80 [2.28–13.98]) | 6 (8.70 [4.05–17.70]) | 1 (1.45 [0.07–7.76]) | |
| Total | |||||
| Nymph | 3944 | 73 (1.85 [1.47–2.32]) | 68 (1.72 [1.36–2.18]) | 26 (0.66 [0.45–0.96]) | |
| Adult | 2439 | 41 (1.68 [1.24–2.27]) | 6 (0.25 [0.11–0.54]) | 1 (0.04 [0.01–0.23]) | |
| Southeast | |||||
| AL | I. scapularis | ||||
| Nymph | 3 | 0 (0.00 [0.00–56.15]) | 0 (0.00 [0.00–56.15]) | 0 (0.00 [0.00–56.15]) | |
| Adult | 22 | 0 (0.00 [0.00–14.87]) | 0 (0.00 [0.00–14.87]) | 0 (0.00 [0.00–14.87]) | |
| MS | I. scapularis | ||||
| Nymph | – | ||||
| Adult | 70 | 0 (0.00 [0.00–5.20]) | 0 (0.00 [0.00–5.20]) | 0 (0.00 [0.00–5.20]) | |
| TN | I. scapularis | ||||
| Nymph | 3 | 0 (0.00 [0.00–56.15]) | 0 (0.00 [0.00–56.15]) | 0 (0.00 [0.00–56.15]) | |
| Adult | 211 | 0 (0.00 [0.00–1.79]) | 0 (0.00 [0.00–1.79]) | 0 (0.00 [0.00–1.79]) | |
| Total | |||||
| Nymph | 6 | 0 (0.00 [0.00–39.03]) | 0 (0.00 [0.00–39.03]) | 0 (0.00 [0.00–39.03]) | |
| Adult | 303 | 0 (0.00 [0.00–1.25]) | 0 (0.00 [0.00–1.25]) | 0 (0.00 [0.00–1.25]) | |
| Northwest | |||||
| OR | I. pacificus | ||||
| Nymph | – | ||||
| Adult | 243 | 0 (0.00 [0.00–1.56]) | 0 (0.00 [0.00–1.56]) | 0 (0.00 [0.00–1.56]) | |
| WA | I. pacificus | ||||
| Nymph | 20 | 0 (0.00 [0.00–16.11]) | 0 (0.00 [0.00–16.11]) | 0 (0.00 [0.00–16.11]) | |
| Adult | 501 | 0 (0.00 [0.00–0.76]) | 0 (0.00 [0.00–0.76]) | 0 (0.00 [0.00–0.76]) | |
| Total | |||||
| Nymph | 20 | 0 (0.00 [0.00–16.11]) | 0 (0.00 [0.00–16.11]) | 0 (0.00 [0.00–16.11]) | |
| Adult | 744 | 0 (0.00 [0.00–0.51]) | 0 (0.00 [0.00–0.51]) | 0 (0.00 [0.00–0.51]) | |
| Total | Ixodes spp. | 7343 | 117 (1.59 [1.33–1.91]) | 112 (1.53 [1.27–1.83]) | 42 (0.57 [0.42–0.77]) |
| Nymph | 7343 | 117 (1.59 [1.33–1.91]) | 112 (1.53 [1.27–1.83]) | 42 (0.57 [0.42–0.77]) | |
| Adult | 6059 | 176 (2.90 [2.51–3.36]) | 72 (1.19 [0.94–1.49]) | 15 (0.25 [0.15I 0.41]) | |
ME: Maine; NY: New York; PA: Pennsylvania; VT: Vermont; DC: Washington, D.C.; KY: Kentucky; MD: Maryland; NC: North Carolina; VA: Virginia; IN: Indiana; MI: Michigan; MN: Minnesota; WI: Wisconsin; AL: Alabama; MS: Mississippi; TN: Tennessee; OR: Oregon; WA: Washington.
Coinfections with B. burgdorferi s.s. and either A. phagocytophilum or B. microti were observed more frequently than expected based on prevalence of single infections in the Northeast and Midwest, but this trend was not consistent in the Mid-Atlantic or Northwest where coinfections occurred at rates expected or lower than expected based on prevalence of single infections (Fig. 2).
Fig. 2.
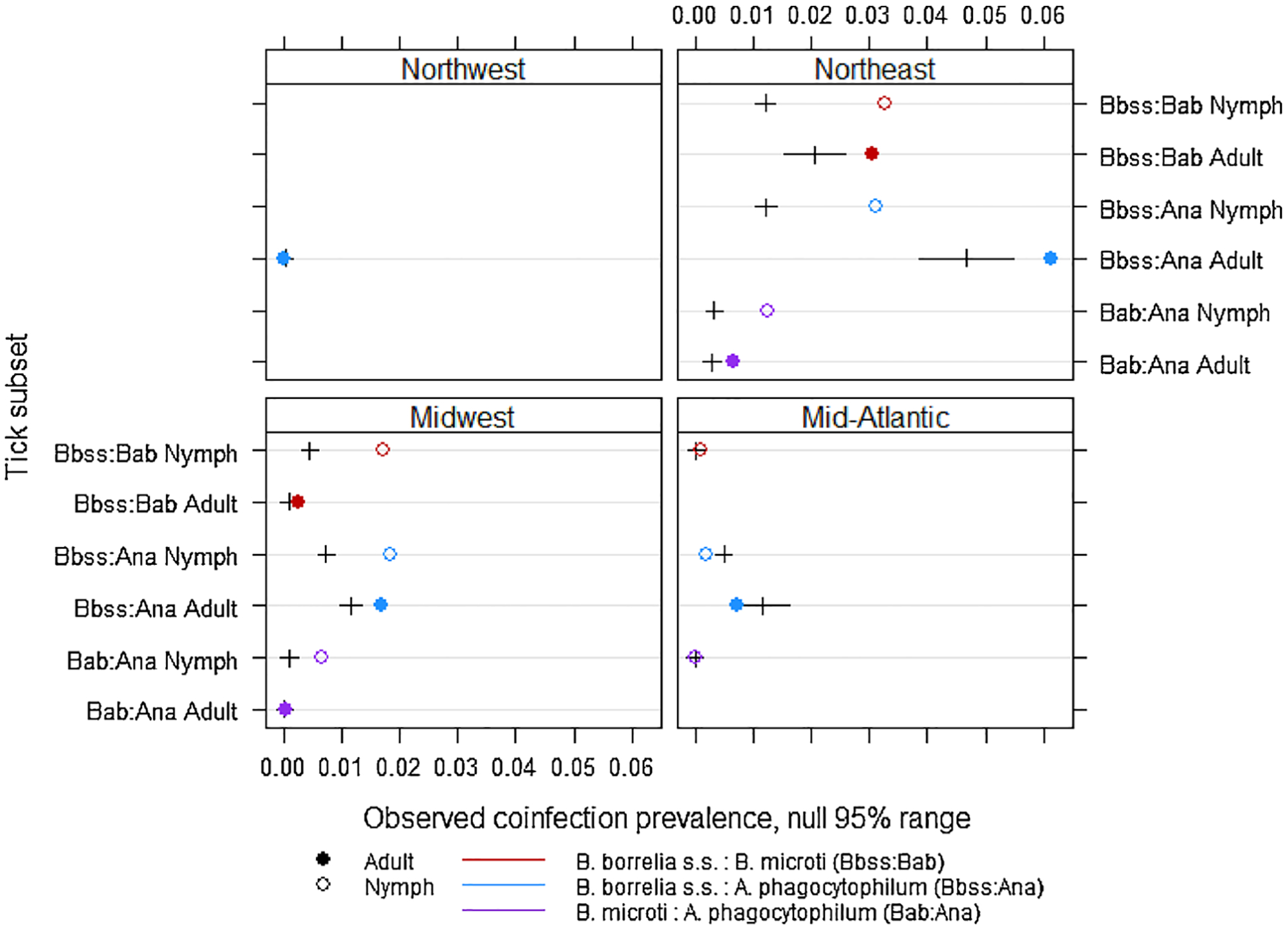
Observed coinfection prevalence and the null 95 % range estimated with permutation tests by tick life stage and geographical region. Ticks sampled from the Southeastern region did not have enough coinfections to be included in the permutation analysis.
4. Discussion
Surveillance of host-seeking ticks and pathogens in these ticks provide data that are complementary to human disease surveillance, which typically report human disease cases based on state or county of residence, rather than location of exposure. Such reporting of human cases may be misconstrued to give the false impression that risk of exposure to tick-borne infections is more geographically widespread than is real. Because of their limited mobility, testing host-seeking ticks provides spatially precise estimates of local infection presence and prevalence (Eisen and Paddock, 2020). Improved understanding of where in the United States ticks are biting people and which pathogens they carry can aid in resolving where exposure to tick-borne disease agents occurs. Such information is useful for targeting the delivery of prevention strategies to communities at risk for Ixodes-associated diseases. Moreover, tick surveillance data can provide estimates of human risk of exposure to tick-borne pathogens that cause diseases that are not nationally notifiable and for which information on the distribution of human disease cases therefore is limited (e.g., B. miyamotoi disease) (Eisen and Paddock, 2020).
Among the thousands of Ixodes ticks we tested for five human pathogens, B. burgdorferi s.s. was overwhelmingly the most common and was detected in each of the five geographical regions with an overall prevalence of 18 % in nymphs and 38 % in adults. By contrast, B. mayonii, which also causes Lyme disease, was the most geographically restricted and the least commonly detected pathogen, found only in the Midwest and in less than 1 % of ticks from two states in that region. Regional trends in the prevalence of B. burgdorferi s.s. infection in ticks are consistent with epidemiological trends showing greatest risk of Lyme disease concentrated in the Northeast, Mid-Atlantic and upper Midwest where host-seeking infected nymphs are more commonly encountered than in other regions of the United States (CDC, 2018a; Diuk-Wasser et al., 2012). Notably, prevalence of B. burgdorferi s.s. is relatively lower in areas where ticks feed commonly on lizards that are refractory to infection (e.g, the Southeast and West compared with the Northeast, Mid-Atlantic and upper Midwest); extensive feeding of I. pacificus nymphs on lizards that are capable of clearing B. burgdorferi s.s. from feeding ticks also contributes to explaining the observed lower prevalence of infection in adults compared with nymphs in the west (Lane and Quistad, 1998; Eisen et al., 2004a,b). Although vector ticks are widely distributed throughout the eastern and Pacific Coast states (Eisen et al., 2016), we report a more limited distribution of Lyme disease spiro-chetes, consistent with previous studies showing that B. burgorferi s.s. is rare in host-seeking I. scapularis nymphs from the southeast (Diuk--Wasser et al., 2012; Stromdahl and Hickling, 2012). Owing to their small size, which allows them to go undetected while feeding long enough for transmission to occur, nymphs are believed to contribute more than adults to the burden of Lyme disease (Eisen, 2018). However, in the southeastern U.S. where nymphs rarely ascend vegetation, adults might more commonly make contact with humans and cause human infections (Hickling et al., 2018; Stromdahl and Hickling, 2012). Among the small numbers of nymphs submitted from the southeast, we failed to detect B. burgdorferi s.s. in any; infections were detected in adult ticks, but at significantly lower prevalence than in other eastern regions. Limited contact between humans and infected nymphs, coupled with low prevalence of B. burgdorferi s.s. infection in adult ticks which are more likely than nymphs to be detected and removed prior to transmission occurring, contributes to explain why Lyme disease infections are less common in the Southeast compared with the Northeast, Mid-Atlantic and Midwest.
Similarly, human anaplasmosis and babesiosis cases are reported most commonly from the Northeast where prevalence of infection in the ticks was higher than for other regions (CDC, 2018a). Although consistent with reported disease trends, acarological risk of exposure to A. phagocytophilum might be over-estimated in our study because the pathogen detection assay employed does not distinguish the rodent-associated A. phagocytophilum variant (A. phagocytophilum-ha), which causes human infection, from the deer-associated variant (A. phagocytophilum-variant 1), which does not cause human disease (Graham et al., 2018). Borrelia miyamotoi disease is not a nationally notifiable condition, but consistent with other studies, our data suggest potential risk for exposure to infected ticks is geographically widespread, but the likelihood of encountering an infected tick is generally low (Wagemakers et al., 2015).
Incidence of coinfections in humans is not monitored through national surveillance systems. Our data suggest that risk of coinfections with Ixodes-borne pathogens is greatest in the Northeast where prevalence of the three most common pathogens (B. burgdorferi s.s., A. phagocytophilum, and B. microti) was highest and the prevalence of coinfections in ticks was higher than expected based on frequency of single infections. We report prevalence of coinfections similar to studies reviewed recently that showed coinfection prevalence in I. scapularis ranging from 1 to 28%, but commonly with fewer than 5–10 % of ticks coinfected (Eisen and Eisen, 2018). Previous studies suggested that B. burgdorferi s.s. and Ba. microti co-occur in I. scapularis more frequently than expected based on frequencies of individual infections, and this was explained by a shared reservoir host and because B. burgdorferi s.s. infection may facilitate Ba. microti transmission (Diuk-Wasser et al., 2016; Eisen and Eisen, 2018). Here we showed higher than expected rates of coinfection in the Northeast and in nymphs from the Midwest, but coinfection prevalence was observed at rates expected or lower than expected in other regions (Fig. 2). This might be explained by differences in host communities among regions, or attributable to the relatively low rates of B. microti outside the Northeast and Midwest where the pathogen has more recently established.
Although our data, derived using a common testing algorithm, provide insights into acarological risk of exposure to five Ixodes-associated pathogens and the findings are generally consistent with epidemiological trends, sampling was not conducted systematically. Thus, we caution against extrapolating results across regions to states that were not included in this assessment. Notably, several states that historically reported a high incidence of Lyme disease in the eastern U.S. (e.g., Pennsylvania, New Jersey, Rhode Island, Connecticut, Massachusetts and most counties in New York) and California in the western U.S. where incidence of Ixodes pacificus-associated diseases is generally higher than other western states included here (CDC, 2018a), were not represented in our study. Moreover, several southern states that typically report low incidence of Ixodes-associated diseases and low prevalence of infection in ticks, were not included (Diuk-Wasser et al., 2012; Stromdahl and Hickling, 2012). The reason for this is, in part, because recent tick surveillance efforts for which CDC provided testing support were differentially targeted to “leading edge” states or those neighboring states reporting high incidence of Lyme disease (Schwartz et al., 2017). Although tick surveillance was conducted in some high incidence states, several conduct their own tick testing and therefore pathogen data from these states were not included in our testing database. In addition, prevalence of infection in ticks described at the state level should not be assumed to be consistently observed among localities within the state. Indeed, previous studies have noted significant variability in infection prevalence among sampling sites (Johnson et al., 2017; Prusinski et al., 2014).
The data presented here report coarse trends in acarological risk of exposure to five Ixodes-borne infections across the U.S. Owing to lack of sufficient data, we did not explicitly present variability in infection prevalence among sampling sites within states or among years, which can be considerable. Nonetheless, we described regional trends that might be explained by multiple influences including, but not limited to: spatial variability in host abundance and composition, host-seeking phenology of ticks, and length of time pathogens have been established in a region (Lane et al., 1991; LoGiudice et al., 2003; Gatewood et al., 2009; Stromdahl et al., 2014). Continuing national tick surveillance efforts should provide improved information by providing estimates of the distribution and abundance of host-seeking ticks and presence and prevalence of human pathogens within ticks with greater coverage than presented here. Documentation of the expanding distribution of ticks and tick-borne pathogens serves as an important reminder of the urgent need to improve strategies to prevent human-tick encounters and ultimately reduce the burden of tick-borne diseases in the U.S.
Acknowledgments
We thank our public health partners who collected and submitted ticks for testing. This project was supported in part by an appointment to the Science Education and Workforce Development Programs at Oak Ridge National Laboratory, administered by ORISE through the U.S. Department of Energy Oak Ridge Institute for Science and Education.
Footnotes
Disclaimers
The findings and conclusions of this study are by the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
CRediT authorship contribution statement
Aine Lehane: Data curation, Methodology, Visualization, Formal analysis, Writing - original draft. Sarah E. Maes: Data curation, Methodology, Writing - original draft. Christine B. Graham: Data curation, Methodology, Writing - original draft. Emma Jones: Formal analysis, Methodology, Visualization, Writing - original draft. Mark Delorey: Formal analysis, Methodology. Rebecca J. Eisen: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing.
References
- Adelson ME, Rao RV, Tilton RC, Cabets K, Eskow E, Fein L, Occi JL, Mordechai E, 2004. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in Northern New Jersey. J. Clin. Microbiol 42, 2799–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Dupuis AP 2nd, Wilczek MP, Peters RJ, Ostfeld RS, Kramer LD, 2014. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis 14, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belongia EA, 2002. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2, 265–273. [DOI] [PubMed] [Google Scholar]
- CDC, 2018a. National notifiable diseases surveillance system, 2018 annual tables of infectious disease data. In: C.D.o.H.I.a (Ed.), Surveillance. Atlanta, GA. [Google Scholar]
- CDC, 2018b. Surveillance for Ixodes scapularis and Pathogens Found in this Tick Species in the United States (Accessed 13 September 2020). https://www.cdc.gov/ticks/surveillance/index.html.
- Chmielewska-Badora J, Moniuszko A, Zukiewicz-Sobczak W, Zwolinski J, Piatek J, Pancewicz S, 2012. Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp. and Babesia microti. Ann. Agric. Environ. Med 19, 271–274. [PubMed] [Google Scholar]
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc’h G, Melton F, Hickling GJ, Tsao JI, Bunikis J, Barbour AG, Kitron U, Piesman J, Fish D, 2012. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am. J. Trop. Med. Hyg 86, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vannier E, Krause PJ, 2016. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 32, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, 2018. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick. Dis 9, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, 2018. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 34, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Paddock CD, 2020. Tick and tick-borne pathogen surveillance as a public health tool in the United States. J. Med. Entomol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Mun J, Eisen L, Lane RS, 2004a. Life stage-related differences in density of questing ticks and infection with Borrelia burgdorferi sensu lato within a single cohort of Ixodes pacificus (Acari: Ixodidae). J. Med. Entomol 41, 768–773. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Lane RS, 2004b. Habitat-related variation in infestation of lizards and rodents with Ixodes ticks in dense woodlands in Mendocino County, California. Exp. Appl. Acarol 33, 215–233. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB, 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol 53, 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood AG, Liebman KA, Vourc’h G, Bunikis J, Hamer SA, Cortinas R, Melton F, Cislo P, Kitron U, Tsao J, Barbour AG, Fish D, Diuk-Wasser MA, 2009. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl. Environ. Microbiol 75, 2476–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ, 2016. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick. Dis 7, 1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CB, Maes SE, Hojgaard A, Fleshman AC, Sheldon SW, Eisen RJ, 2018. A molecular algorithm to detect and differentiate human pathogens infecting Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick. Dis 9, 390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh MH, Ostfeld RS, McHenry DJ, Tibbetts M, Brunner JL, Killilea ME, LoGiudice K, Schmidt KA, Keesing F, 2014. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS One 9, e99348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling GJ, Kelly JR, Auckland LD, Hamer SA, 2018. Increasing prevalence of Borrelia burgdorferi sensu stricto-infected blacklegged ticks in Tennessee Valley, Tennessee, USA. Emerg. Infect. Dis 24, 1713–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J, 2014. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick. Dis 5, 349–351. [DOI] [PubMed] [Google Scholar]
- Holden K, Boothby JT, Anand S, Massung RF, 2003. Detection of Borrelia burgdorferi, Ehrlichia chaffeensis, and Anaplasma phagocytophilum in ticks (Acari: Ixodidae) from a coastal region of California. J. Med. Entomol 40, 534–539. [DOI] [PubMed] [Google Scholar]
- Holman MS, Caporale DA, Goldberg J, Lacombe E, Lubelczyk C, Rand PW, Smith RP, 2004. Anaplasma phagocytophilum, Babesia microti, and Borrelia burgdorferi in Ixodes scapularis, southern coastal Maine. Emerg. Infect. Dis 10, 744–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson ML, Strohecker MD, Simmons TW, Kyle AD, Helwig MW, 2015. Prevalence rates of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in host-seeking Ixodes scapularis (Acari: Ixodidae) from Pennsylvania. J. Med. Entomol 52, 693–698. [DOI] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Boegler KA, Cherry CC, Maes SE, Pilgard MA, Hojgaard A, Buttke DE, Eisen RJ, 2017. Prevalence and diversity of tick-borne pathogens in nymphal Ixodes scapularis (Acari: Ixodidae) in eastern National Parks. J. Med. Entomol 54, 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Maes SE, Hojgaard A, Fleshman A, Boegler KA, Delory MJ, Slater KS, Karpathy SE, Bjork JK, Neitzel DF, Schiffman EK, Eisen RJ, 2018. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick. Dis 9, 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Telford SR 3rd, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH, 1996. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 275, 1657–1660. [PubMed] [Google Scholar]
- Lane RS, Quistad GB, 1998. Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J. Parasitol 84, 29–34. [PubMed] [Google Scholar]
- Lane RS, Piesman J, Burgdorfer W, 1991. Lyme borreliosis: relation of its causitive agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol 36, 587–609. [DOI] [PubMed] [Google Scholar]
- Little EAH, Molaei G, 2020. Passive tick surveillance: Exploring spatiotemporal associations of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Babesia microti (Piroplasmida: Babesiidae), and Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae) infection in Ixodes scapularis (Acari: Ixodidae). Vector Borne Zoonotic Dis. 20, 177–186. [DOI] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F, 2003. The ecology of infectious disease: effects of host diversit and community composition on Lyme disease risk. Proc. Nat. Acad. Sci. U.S.A 100, 567–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PD, Reed KD, Hofkes JM, 1996. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J. Clin. Microbiol 34, 724–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Mather TN, Telford SR 3rd, Spielman A, 1986. Concurrent Borrelia burgdorferi and Babesia microti infection in nymphal Ixodes dammini. J. Clin. Microbiol 24, 446–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, Backenson PB, 2014. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J. Med. Entomol 51, 226–236. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, Hooks H, Partridge SK, Visser SN, Beard CB, Petersen LR, 2018. Vital Signs: Trends in reported vectorborne disease cases - United States and territories, 2004–2016. Morb. Mortal. Wkly. Rep 67, 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber EM, Gertz SJ, Maple WT, Ostfeld RS, 1998. Coinfection of blacklegged ticks (Acari: Ixodidae) in Dutchess County, New York, with the agents of Lyme disease and human granulocytic ehrlichiosis. J. Med. Entomol 35, 901–903. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Schulze CJ, Mixson T, Papero M, 2005. Relative encounter frequencies and prevalence of selected Borrelia, Ehrlichia, and Anaplasma infections in Amblyomma americanum and Ixodes scapularis (Acari: Ixodidae) ticks from central New Jersey. J. Med. Entomol 42, 450–456. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Healy SP, Roegner VE, 2013. Detection of Babesia microti and Borrelia burgdorferi in host-seeking Ixodes scapularis (Acari: Ixodidae) in Monmouth County, New Jersey. J. Med. Entomol 50, 379–383. [DOI] [PubMed] [Google Scholar]
- Schwartz I, Fish D, Daniels TJ, 1997. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N. Engl. J. Med 337, 49–50. [DOI] [PubMed] [Google Scholar]
- Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ, 2017. Surveillance for lyme disease - United States, 2008–2015. Surveill. Summ 66, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromdahl EY, Hickling GJ, 2012. Beyond Lyme: aetiology of tick-borne human diseases with emphasis on the south-eastern United States. Zoonoses Public Health (59 Suppl 2), 48–64. [DOI] [PubMed] [Google Scholar]
- Stromdahl E, Hamer S, Jenkins S, Sloan L, Williamson P, Foster E, Nadolny R, Elkins C, Vince M, Pritt B, 2014. Comparison of phenology and pathogen prevalence, including infection with Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the midwestern and northeastern United States over a 15 year period (1997–2012). Parasit. Vectors 7, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC, 2013. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Varde S, Beckley J, Schwartz I, 1998. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County. Emerg. Infect. Dis 4, 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagemakers A, Staarink PJ, Sprong H, Hovius JW, 2015. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 31, 260–269. [DOI] [PubMed] [Google Scholar]
- Xu G, Mather TN, Hollingsworth CS, Rich SM, 2016. Passive surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts . Vector Borne Zoonotic Dis 16, 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]


