Abstract
Purpose
To investigate the correlation between whole eye movement (WEM) parameters measured using Corvis ST and axial length (AL) to explore whether AL affects WEMs.
Methods
This single-center, cross-sectional study included data from healthy subjects and patients preparing for refractive surgery at the Qingdao Eye Hospital of Shandong First Medical University. Data were collected from July 2021 to April 2022. We first determined the correlations of WEMs at the time of corneal first applanation (A1_WEM), highest concavity (HC_WEM), and second applanation (A2_WEM), as well as the maximum value of WEM (WEM_Max) with AL. Subsequently, we established a series of regression models to analyze the relationships between different WEM values and AL.
Results
AL was negatively correlated with HC_WEM, A2_WEM, and WEM_Max (r = − 0.28, − 0.23, and − 0.22, respectively; P < 0.001). The correlation between AL and A1_WEM was not significant (P = 0.77). According to the adjusted regression models, AL was negatively associated with HC_WEM (Model 2: β = −7.39, P < 0.001) and WEM_Max (Model 4: β = −3.52, P = 0.02), while the associations of AL with A1_WEM (Model 1: P = 0.61) and A2_WEM (Model 3: P = 0.23) were not significant.
Conclusions
AL is an independent negative influencing factor for HC_WEM. WEM is a potentially useful parameter that reflects the biomechanical properties of the eye behind the cornea in myopia.
Keywords: Whole eye movement, Axial length, Corneal biomechanical behavior, Dynamic corneal response, Axial myopia
Introduction
In recent years, the advent of in vivo biomechanical evaluation devices, such as Corneal Visualization Scheimpflug Technology (Corvis ST), has significantly advanced our understanding of the corneal structure and its material properties. These technological advancements have facilitated progress in analyzing corneal biomechanical parameters, promoting the development of methods for refractive correction and corneal cross-linking. The implications of these advancements extend to evaluating the pathogenesis of glaucomatous optic nerve damage, thyroid-associated ophthalmopathy, and other ocular disorders [1–4]. However, our understanding of corneal biomechanics in myopic eyes remains limited, primarily because axial myopia is characterized by elongation of the posterior sclera [5, 6]. In vivo corneal biomechanical parameters predominantly reflect the cornea’s structural and material properties through the dynamic corneal response (DCR) [7]. Although previous studies have suggested a reduction in the corneal stiffness parameters of the DCR in myopic eyes, these changes do not adequately represent the alterations occurring in the posterior sclera, which is a key feature of axial myopia. The biomechanical characteristics of myopia, whether associated with the posterior sclera or the cornea, encompass both structural and material properties, making it essential to differentiate between them. Extensive in vivo evaluations of the corneal wall’s material properties in myopic eyes have been conducted [8–10]. Our previous studie [11, 12] demonstrated that the stress-strain index (SSI) proposed by Eliasy et al. is an appropriate parameter for reflecting corneal material properties in patients with axial myopia, as it excludes the influence of structural parameters such as central corneal thickness (CCT). However, this also means that SSI cannot reflect the effect of posterior scleral lengthening on the DCR.
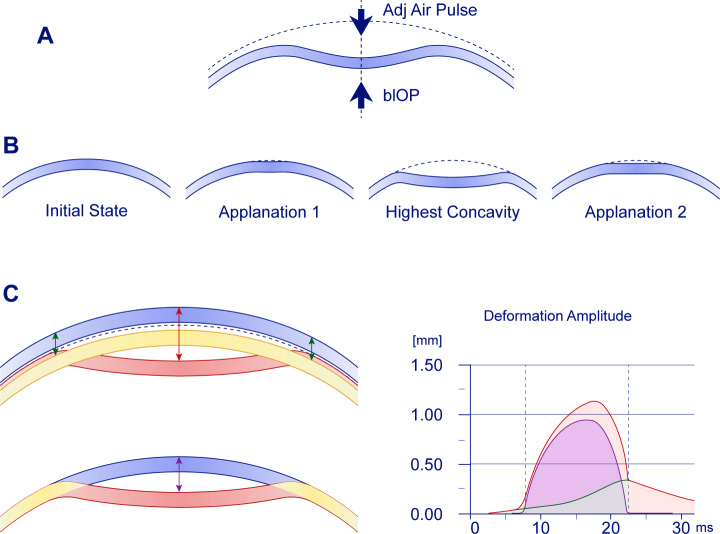
Few studies have investigated the biomechanical properties of the tissues behind the cornea through DCR parameters (DCRs), especially in myopia. Whole eye movement (WEM) is one of the DCRs measured by Corvis ST and is expected to provide information about the structural characteristics of the tissues behind the cornea. It has been reported that WEM can be used to quantify the biomechanical parameters of orbital soft tissue [4]. When the jet pulse impinges on the anterior surface of the eyeball, the whole eyeball, including the cornea, begins to move backward (Fig. 1). The total translational movement of the corneal vertex in the axial direction (deformation amplitude [DeformAmp]) minus the corneal deflection (deviation) gives the WEM value at that moment. The axial translation motion of the cornea induced by an air jet includes both WEM and deflection amplitude (DeflAmp). From the moment the jet impacts the anterior surface of the cornea, the amplitudes of WEM and DeflAmp increase simultaneously. The cornea then transitions through the inward-motion convex phase, also known as the first corneal applanation (A1), and the inward-motion concave phase. During this period, the WEM increases slowly and linearly. Subsequently, the corneal deflection associated with maximum resistance to further deviation peaks and enters the oscillating phase. Despite the presence of the highest concavity (HC) DeflAmp and DeformAmp (HC_DeflAmp and HC_DeformAmp) during the oscillating period, the WEM does not peak but instead transitions from linearly increasing motion to more rapid, non-linear increase. As the airflow pulse reaches its maximum and begins to decrease, the cornea starts to move forward, entering the outward-motion concave phase. However, during this period, the WEM continues to increase backward even as air pressure decreases. After the second corneal applanation (A2), the cornea returns to its initial position through the convex phase of outward movement. The maximum value of WEM (WEM_Max) appears around A2 [13].
Fig. 1.
Corneal deformation during the Corvis ST measurement. The whole eye movement (WEM) was measured at a distance of 4 mm from the cornea center. (A) The cornea deforms and moves backward when the jet pulse hits the front surface of the eye. (B) From left to right: Initial state; first corneal applanation (A1); highest concavity (HC); second corneal applanation (A2). (C) Relationships among DeformAmp, DeflAmp, and WEM. Upper left of C: The blue cornea represents the initial moment, the red cornea represents the moment of HC_DeflAmp, and the yellow cornea represents the moment of WEM_Max. Since WEM is still progressing when the cornea is at its maximum deflection, HC_DeflAmp occurs before WEM_Max. Red arrow: DeformAmp; green arrow: WEM; purple arrow: DeflAmp. The red, green, and purple curves in the right panel represent DeflAmp, WEM, and DeflAmp, respectively, with WEM occurring after the complete recovery of the corneal flexure. The right side of C is a schematic of these data
Specifically, WEM reflects the overall movement of the cornea because it is calculated based on the corneal position before deformation. Therefore, we hypothesize that WEM is influenced by the structural and material properties behind the cornea, including the sclera, vitreous body, orbital soft tissue, and other ocular components. If this hypothesis is correct, the process of vitreous cavity compression should be related to the vitreous cavity volume or axial length (AL). It may then be possible to obtain partial information about the AL by isolating the relevant parameters of the vitreous cavity compression process using a finite element model (FEM). The hypothesis of this study is that WEM is a potentially useful parameter for reflecting the biomechanical properties of the eye behind the cornea in myopia. The aim of this study is to verify the correlation between WEM and AL to determine whether axial myopia affects WEM.
Methods
Clinical data
This cross-sectional comparative study was conducted at the Eye Institute of Shandong First Medical University in Qingdao, China. It adhered to the principles outlined in the Declaration of Helsinki and received approval from the Ethics Committee of the Qingdao Eye Hospital of Shandong First Medical University.
Data were collected from July 2021 to April 2022 from sources including the Picture Archiving and Communication System and the Hospital Information System at Qingdao Eye Hospital of Shandong First Medical University. The study included samples from healthy subjects undergoing physical examinations and patients preparing for refractive surgery. Subjects were excluded if they: (i) had astigmatism of ≥ 3 diopters (D) in power, (ii) had ocular hypertension (intraocular pressure > 21 mmHg) or glaucoma, (iii) used contact lenses, (iv) had a history or suspicion of corneal diseases such as keratoconus, or (v) had a history of eye surgery. Medical records were included if they met the following criteria: (i) had a complete medical history and (ii) had ophthalmic examinations conducted on the same day, including comprehensive optometry results after mydriasis, AL measurements (OA 2000, Tomey, Japan), anterior corneal surface radius of curvature (CR, calculated as the mean of Kflat and Ksteep) in a 3 mm diameter range (OA 2000, Tomey, Japan), and the corneal biomechanical parameter stress-strain index (SSI) (Corvis ST, Oculus, Wetzlar, Germany). Only measurements with an “OK” quality score were included in the analysis. Data from the right eye of each individual were analyzed due to the strong correlation of corneal parameters between the right and left eyes. Based on the measured AL of the right eye, subjects were divided into two groups: AL < 26 mm and AL ≥ 26 mm.
Statistical analysis
Statistical analyses and data visualization were performed using R software (version 4.3.1). The significance level was set at P < 0.05. Continuous variables are presented as mean (standard deviation [SD]), while categorical variables are presented as sample size (percentage). Pearson’s correlation analysis was conducted to examine the relationship between AL and WEM at A1 (A1_WEM), HC (HC_WEM), A2 (A2_WEM), and WEM_Max. A matrix of plots was created to analyze the correlations among these variables. To explore potential associations between AL and the WEM parameters (A1_WEM, HC_WEM, A2_WEM, and WEM_Max), a series of linear regression models were established. All models used AL as the dependent variable. Models 1, 2, 3, and 4 used A1_WEM, HC_WEM, A2_WEM, and WEM_Max as the independent variables, respectively. Each model was adjusted for sex, age, CR, CCT, biomechanically corrected intraocular pressure (bIOP), SSI, and time-histories of each moment. Several key assumptions of linear regression, including normality of residuals, homogeneity of variance, linearity, and independence, were checked, and our models did not violate these key assumptions.
Results
Sample characteristics
Baseline characteristics
Table 1 displays the demographic information and clinical variables of the subjects included in this study. Overall, 258 participants were included in the present study.
Table 1.
Characteristics of the subjects included in the study
| Characteristic | AL < 26 mm1 | AL ≥ 26 mm1 | Overall1 |
|---|---|---|---|
| Age, years | 20 (8) | 24.0 (6.7) | 21.98 (7.65) |
| Sex | |||
| Female | 73 (55%) | 68 (54%) | 141 (55%) |
| Male | 60 (45%) | 57 (46%) | 117 (45%) |
| AL, mm | 24.88 (0.85) | 27.07 (0.87) | 25.94 (1.39) |
| CR, mm | 7.71 (0.24) | 7.85 (0.22) | 7.78 (0.24) |
| SER, diopter (D) | -4.20 (2.23) | -7.66 (1.93) | −5.88 (2.72) |
| CCT, mm | 543 (35) | 547 (32) | 544.61 (33.64) |
| bIOP, mmHg | 17.47 (2.58) | 17.70 (2.44) | 17.78 (2.99) |
| SSI | 0.85 (0.15) | 0.79 (0.14) | 0.82 (0.15) |
| A1 | |||
| Time, ms | 7.76 (0.39) | 7.81 (0.35) | 7.78 (0.37) |
| Velocity, m/s | 0.150 (0.023) | 0.147 (0.023) | 0.15 (0.02) |
| DeflAmp, mm | 0.093 (0.008) | 0.092 (0.005) | 0.09 (0.007) |
| DeformAmp, mm | 0.142 (0.010) | 0.141 (0.009) | 0.14 (0.009) |
| HC | |||
| Time, ms | 17.40 (0.61) | 17.45 (0.55) | 17.43 (0.58) |
| DeflAmp, mm | 0.86 (0.13) | 0.89 (0.12) | 0.88 (0.12) |
| DeformAmp, mm | 1.03 (0.12) | 1.04 (0.11) | 1.04 (0.12) |
| A2 | |||
| Time, ms | 22.12 (0.49) | 22.00 (0.40) | 22.06 (0.45) |
| Velocity, m/s | -0.25 (0.05) | -0.26 (0.04) | −0.26 (0.04) |
| DeflAmp, mm | 0.102 (0.011) | 0.100 (0.010) | 0.10 (0.01) |
| DeformAmp, mm | 0.36 (0.05) | 0.33 (0.05) | 0.34 (0.05) |
| WEM Max, mm | 0.26 (0.05) | 0.23 (0.05) | 0.25 (0.05) |
| WEM Max time, ms | 22.07 (0.66) | 21.91 (0.75) | 21.99 (0.71) |
1 n (%); mean (SD). AL, axial length; CR, corneal curvature; SER, spherical equivalent error; CCT, central corneal thickness; bIOP, biomechanically corrected intraocular pressure; SSI, stress‒strain index; A1, first applanation; HC, highest concavity; A2, second applanation; DeflAmp, deflection amplitude; DeformAmp, deformation amplitude; WEM, whole eye movement; WEM_Max, maximum value of WEM.
WEM, DeflAmp and deformAmp
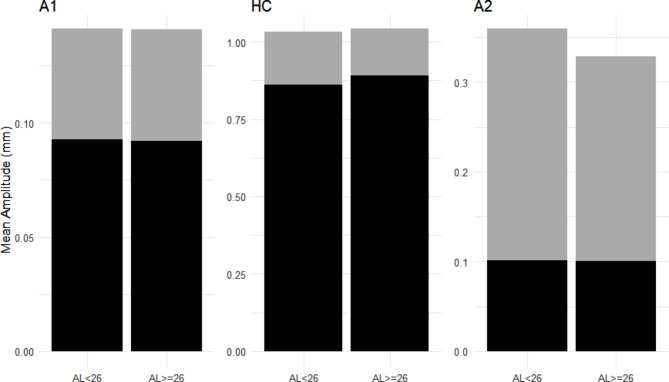
WEM was measured as the difference between DeformAmp and DeflAmp. Figure 2 illustrates the relationships among these three variables.
Fig. 2.
Whole eye movement (WEM), deflection, and deformation. The panel shows the amplitudes of the DeflAmp and WEM in subjects with axial length (AL) < 26 mm and AL ≥ 26 mm. The black area represents DeflAmp, and the gray area represents WEM. The sum of these two regions represents the DeformAmp region. DeflAmp, deflection amplitude; DeformAmp, deformation amplitude
Correlation between AL and whole eye movement
Correlation between AL and WEMs
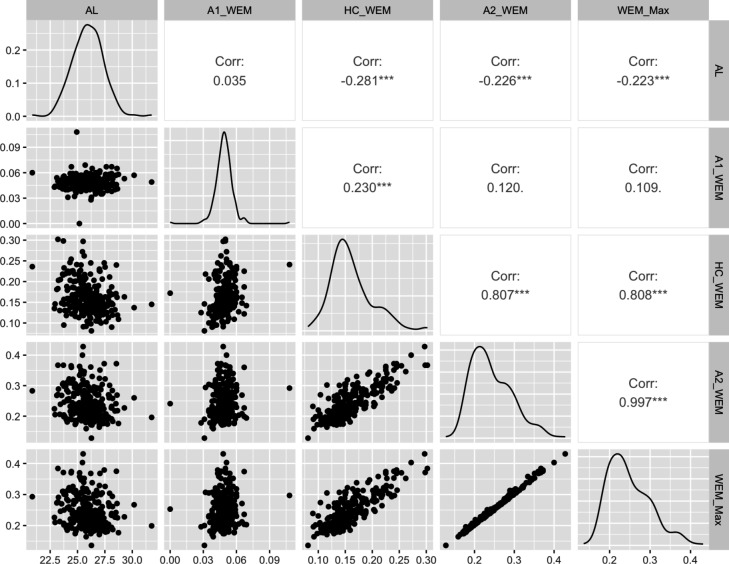
As shown in Fig. 3, Pearson’s correlation analyses indicated that AL was negatively correlated with HC_WEM, A2_WEM, and WEM_Max (r = − 0.28, − 0.23, and − 0.22, respectively; P < 0.001). The correlation between AL and A1_WEM was not significant (P = 0.77).
Fig. 3.
Matrix of plots showing the correlations between axial length (AL) and whole eye movement (WEM) at each time point. A1_WEM, whole eye movement at A1; HC_WEM, whole eye movement at HC; A2_WEM, whole eye movement at A2; WEM_Max, maximum value of whole eye movement. *** P < 0.001
Regression models between axial myopic parameters and whole eye movement
Several multiple linear regression models were established and are summarized in Table 2. According to the adjusted models, AL was negatively associated with HC_WEM (Model 2: β = −7.39, P < 0.001) and WEM_Max (Model 4: β = −3.52, P = 0.02). However, the associations of AL with A1_WEM (Model 1: P = 0.61) and A2_WEM (Model 3: P = 0.23) were not significant.
Table 2.
Summary of linear regression models
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| (Intercept) | −6.23 | −2.20 | 34.76*** | 0.46 |
| [− 20.07, 7.61] | [− 7.90, 3.50] | [20.22, 49.29] | [− 5.79, 6.71] | |
| Male sex | −0.02 | 0.05 | 0.00 | 0.04 |
| [− 0.29, 0.25] | [− 0.22, 0.31] | [− 0.26, 0.26] | [− 0.23, 0.31] | |
| Age | 0.07*** | 0.06*** | 0.07*** | 0.07*** |
| [0.05, 0.08] | [0.05, 0.08] | [0.05 0.08] | [0.05, 0.08] | |
| CR | 3.18*** | 2.96*** | 2.98*** | 3.09*** |
| [2.62, 3.74] | [2.40, 3.52] | [2.45, 3.52] | [2.53, 3.65] | |
| CCT | 0.00 | 0.00 | 0.00 | 0.00 |
| [− 0.00, 0.00] | [− 0.00, 0.00] | [− 0.00, 0.01] | [− 0.00, 0.01] | |
| bIOP | −0.07 | 0.09*** | −0.10* | 0.08* |
| [− 0.35, 0.22] | [0.04, 0.14] | [− 0.18, − 0.01] | [0.02, 0.14] | |
| SSI | −3.73*** | −3.11*** | −4.01*** | −3.17*** |
| [− 4.67, − 2.78] | [− 4.11, − 2.11] | [− 5.10, − 2.97] | [− 4.19, − 2.15] | |
| Time-history# | 1.34 | 0.33* | −1.36*** | 0.09 |
| [− 0.95, 3.63] | [0.06, 0.59] | [− 1.94, − 0.78] | [− 0.13, 0.31] | |
| WEM# | −4.68 | −7.39*** | −1.62 | −3.52* |
| [− 22.87, 13.52] | [− 11.53, − 3.25] | [− 4.29, 1.05] | [− 6.46, − 0.59] | |
| N | 258 | 258 | 258 | 258 |
*** P < 0.001; ** P < 0.01; * P < 0.05.
# Models 1–4 included time history, A1_WEM, HC_WEM, A2_WEM, and WEM_Max as the independent variables. N represents the sample size in each model. The 95% confidence intervals are used to describe the uncertainties of coefficients. All coefficients are reported using unstandardized coefficients. *** P < 0.001; ** P < 0.01; * P < 0.05. CR, corneal curvature; CCT, central corneal thickness; bIOP, biomechanically corrected intraocular pressure; SSI, stress‒strain index; WEM, whole eye movement.
Discussion
Studies aimed at understanding the relationship between corneal biomechanical behavior and corneal material properties have made significant progress. However, determining the relationship between corneal biomechanical behavior and the material properties of the sclera or orbital soft tissue remains challenging. One major difficulty is the inability to observe the dynamic response of the fundus structure through the pupil. WEM reflects corneal displacement excluding corneal deflection and may be influenced by the dynamic response of the sclera, thus providing information on ocular AL. In this study, we analyzed the relationship between WEM and AL at different time points during the air puff test. First, we confirmed a negative correlation between AL and HC_WEM as well as A2_WEM. Second, among a series of regression models predicting AL using DCRs, HC_WEM was identified as the parameter most strongly correlated with AL after adjusting for corneal morphology and material properties. This finding is significant for studying corneal biomechanical behavior in axial myopia, as it suggests that characteristics of posterior scleral stiffness might be inferred through DCRs.
The existing studies have primarily focused on WEM_Max. In this study, the average WEM_Max was 0.25 ± 0.05 mm, which aligns closely with values reported in previous research [13–15]. However, Hwang et al. found that the average maximum value of WEM in 44 ophthalmologically normal subjects was 0.314 ± 0.083 mm [4]. This discrepancy may be attributed to differences in the age range of participants [16]. Additionally, our study found that WEM was negatively correlated with AL, consistent with previous reports [17]. While there is limited data on the value ranges of WEM at A1, HC, and A2, our results showed that WEM generally increased from A1 to A2. This increase was more pronounced in the AL < 26 mm group compared to the AL ≥ 26 mm group, although there was no significant difference in WEM at A1 between the two groups. The lack of significance in the correlation between A1_WEM and AL may be due to the fact that DCRs during the A1 and pre-A1 periods are less influenced by IOP or scleral biomechanical properties. Significant aqueous humor discharge and IOP elevation occur primarily during the concave phase of inward motion, which could explain this lack of significance [18]. At HC and A2, WEM showed the strongest correlation with AL, with longer ALs associated with smaller WEM values. Despite A2_WEM being the highest at A2, it was not as significant in predicting AL as HC_WEM in the regression model. This could be due to the influence of viscous damping properties of the cornea at A2. Previous studies using an ocular response analyzer (ORA) reported lower viscous damping in eyes with longer ALs [19–21]. We hypothesize that WEM may also be affected by the viscous damping properties of the eye wall at A2, which might obscure the relationship between A2_WEM and AL. Additionally, the decrease in IOP after the oscillation period and the reduction in A2_WEM due to vitreous cavity compression may also contribute to this effect.
In the present study, we investigated the relationship between WEMs and AL by analyzing the medical records of patients preparing for refractive surgery, confirming that AL affects WEMs in addition to influencing orbital soft tissue. However, there are some limitations to the current study. First, the underlying mechanism of the relationship between WEMs and AL remains undemonstrated. Second, while multivariate models were used to identify potential confounding effects of other factors, influences such as orbital volume and orbital pressure were not considered in these models. Utilizing FEM could be a feasible approach to analyzing the impact of these factors. This study suggests that it might be possible to isolate parameters related to the vitreous cavity compression process to gain insights into vitreous cavity volume or AL by analyzing the interactions of air pressure, bIOP, orbital pressure, ocular wall stiffness, and the biomechanical strength of orbital soft tissue through DCRs.
Acknowledgements
The authors thank Jie Zhang from Hangzhou Shansier Medical Technologies Ltd. for the statistical advice.
Author contributions
Y.Y. and Z.C. wrote the main manuscript text; L.C. analyzed the data; H.C. and Q.R. designed the study; All authors reviewed the manuscript.
Funding
There was no funding for this study.
Data availability
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Ethics Committee of Qingdao Eye Hospital of Shandong First Medical University. Informed consent was obtained from all subjects or their legal guardian(s). This study was performed in accordance with the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ye Yang and Zhe Chu contributed equally to this work and share first authorship.
Contributor Information
Hao Cheng, Email: chrischenghao@gzhmu.edu.cn.
Qi Ren, Email: renqi@sdfmu.edu.cn.
References
- 1.Kling S, Hafezi F. Corneal biomechanics - a review. Ophthalmic Physiol Opt. 2017;37(3):240–52. 10.1111/opo.12345 [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Cao H, Chen W, Bao F, Elsheikh A. Editorial: how can corneal biomechanics help with clinical applications? Front Bioeng Biotechnol. 2023;11:1186938. 10.3389/fbioe.2023.1186938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen SR, Fleming GP, Jain SG, Roberts CJ. A review of corneal biomechanics and scleral stiffness in topical prostaglandin Analog Therapy for Glaucoma. Curr Eye Res. 2023;48(2):172–81. 10.1080/02713683.2022.2099903 [DOI] [PubMed] [Google Scholar]
- 4.Hwang HS, Kim EC, Kim MS, Yang SW. A novel method for quantifying the biomechanical parameters of orbital soft tissue using a corneal dynamic scheimpflug analyser: a retrospective study. BMC Ophthalmol. 2019;19(1):53. 10.1186/s12886-019-1064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flitcroft DI, He M, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, Rahi J, Resnikoff S, Vitale S, Yannuzzi L. IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20–30. 10.1167/iovs.18-25957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohno-Matsui K, Wu PC, Yamashiro K, Vutipongsatorn K, Fang Y, Cheung CMG, Lai TYY, Ikuno Y, Cohen SY, Gaudric A, et al. IMI Pathologic Myopia. Invest Ophthalmol Vis Sci. 2021;62(5):5. 10.1167/iovs.62.5.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kling S, Marcos S. Contributing factors to corneal deformation in air puff measurements. Invest Ophthalmol Vis Sci. 2013;54(7):5078–85. 10.1167/iovs.13-12509 [DOI] [PubMed] [Google Scholar]
- 8.Liu G, Rong H, Zhang P, Xue Y, Du B, Wang B, Hu J, Chen Z, Wei R. The effect of axial length elongation on corneal biomechanical property. Front Bioeng Biotechnol. 2021;9:777239. 10.3389/fbioe.2021.777239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li DL, Liu MX, Yin ZJ, Li YZ, Ma R, Zheng YJ, Qin Y, Liang G, Pan CW. Refractive associations with corneal biomechanical properties among young adults: a population-based Corvis ST study. Graefes Arch Clin Exp Ophthalmol 2023. [DOI] [PubMed]
- 10.Chen L, Huang Y, Zhang X, Shi Y, Gao Z, Sun B, Shen Y, Sun L, Cao Y, Zhang Q, et al. Corneal Biomechanical properties demonstrate anisotropy and correlate with axial length in myopic eyes. Investig Ophthalmol Vis Sci. 2023;64(10):27–27. 10.1167/iovs.64.10.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu Z, Ren Q, Chen M, Cheng L, Cheng H, Cui W, Bi W, Wu J. The relationship between axial length/corneal radius of curvature ratio and stress-strain index in myopic eyeballs: using Corvis ST tonometry. Front Bioeng Biotechnol. 2022;10:939129. 10.3389/fbioe.2022.939129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu Z, Ren Q, Su W, Cui W, Wu J. Effect of central corneal curvature on corneal material stiffness parameter acquired by dynamic corneal responses. Front Bioeng Biotechnol 2023, 11. [DOI] [PMC free article] [PubMed]
- 13.Roberts yJ, Mahmoud AM, Mendoza KAV Jr. RA: Interpreting dynamic corneal response parameters of the Corvis ST. In: Corneal biomechanics: from theory to practice edn. Edited by Roberts CJ, Liu J. Amsterdam: Kugler Publications; 2016.
- 14.Vellara HR, Ali NQ, Gokul A, Turuwhenua J, Patel DV, McGhee CNJ. Quantitative Analysis of Corneal Energy Dissipation and Corneal and Orbital Deformation in response to an air-pulse in healthy eyes. Investig Ophthalmol Vis Sci. 2015;56(11):6941–7. 10.1167/iovs.15-17396 [DOI] [PubMed] [Google Scholar]
- 15.Vellara HR, Hart R, Gokul A, McGhee CNJ, Patel DV. In vivo ocular biomechanical compliance in thyroid eye disease. Br J Ophthalmol. 2017;101(8):1076–9. 10.1136/bjophthalmol-2016-309532 [DOI] [PubMed] [Google Scholar]
- 16.Vinciguerra R, Elsheikh A, Roberts CJ, Ambrosio R Jr., Kang DS, Lopes BT, Morenghi E, Azzolini C, Vinciguerra P. Influence of Pachymetry and intraocular pressure on dynamic corneal response parameters in healthy patients. J Refract Surg. 2016;32(8):550–61. 10.3928/1081597X-20160524-01 [DOI] [PubMed] [Google Scholar]
- 17.Miki A, Maeda N, Ikuno Y, Asai T, Hara C, Nishida K. Factors Associated with corneal deformation responses measured with a dynamic Scheimpflug Analyzer. Invest Ophthalmol Vis Sci. 2017;58(1):538–44. 10.1167/iovs.16-21045 [DOI] [PubMed] [Google Scholar]
- 18.Roberts CJ, Liu J, Ebook Central Academic C. Corneal biomechanics: from theory to practice. Amsterdam: Kugler; 2016. [Google Scholar]
- 19.Jiang Z, Shen M, Mao G, Chen D, Wang J, Qu J, Lu F. Association between corneal biomechanical properties and myopia in Chinese subjects. Eye (Lond). 2011;25(8):1083–9. 10.1038/eye.2011.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang PY, Chang SW, Wang JY. Assessment of corneal biomechanical properties and intraocular pressure with the Ocular Response Analyzer in childhood myopia. Br J Ophthalmol. 2010;94(7):877–81. 10.1136/bjo.2009.158568 [DOI] [PubMed] [Google Scholar]
- 21.Wong YZ, Lam AK. The roles of cornea and axial length in corneal hysteresis among emmetropes and high myopes: a pilot study. Curr Eye Res. 2015;40(3):282–9. 10.3109/02713683.2014.922193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.