Abstract
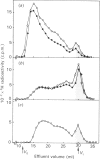
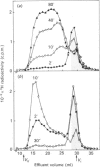
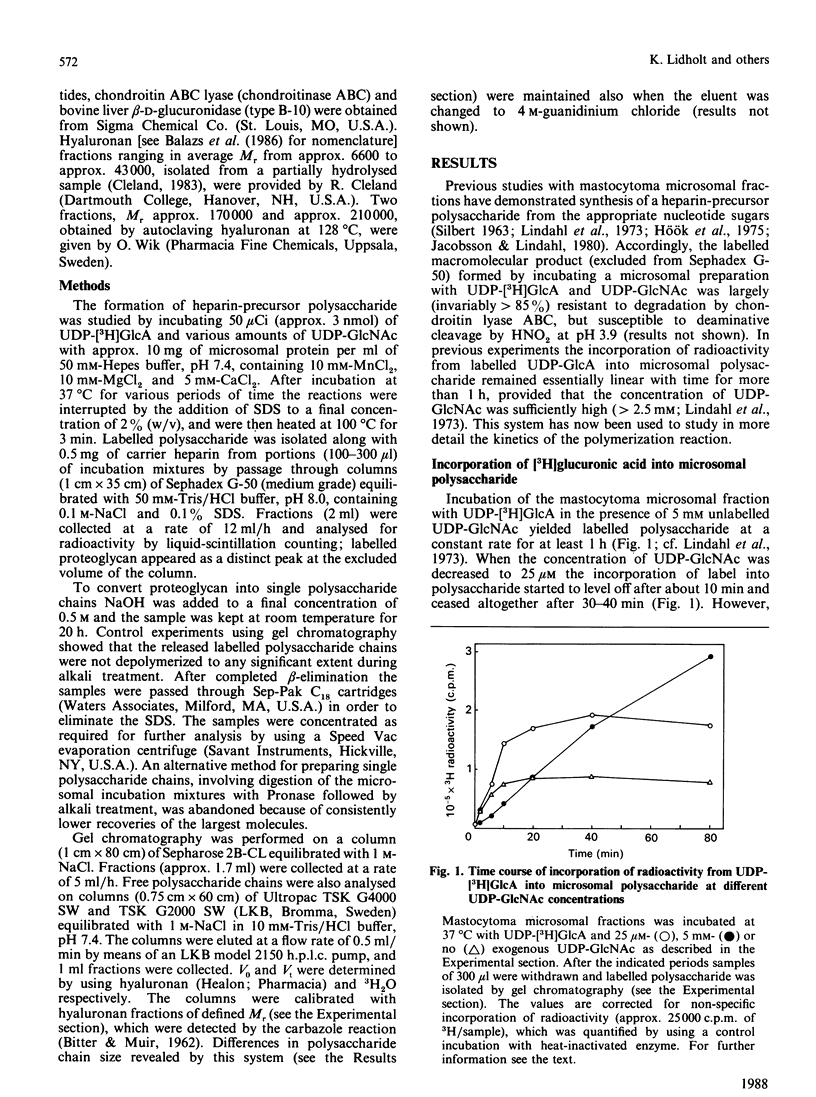
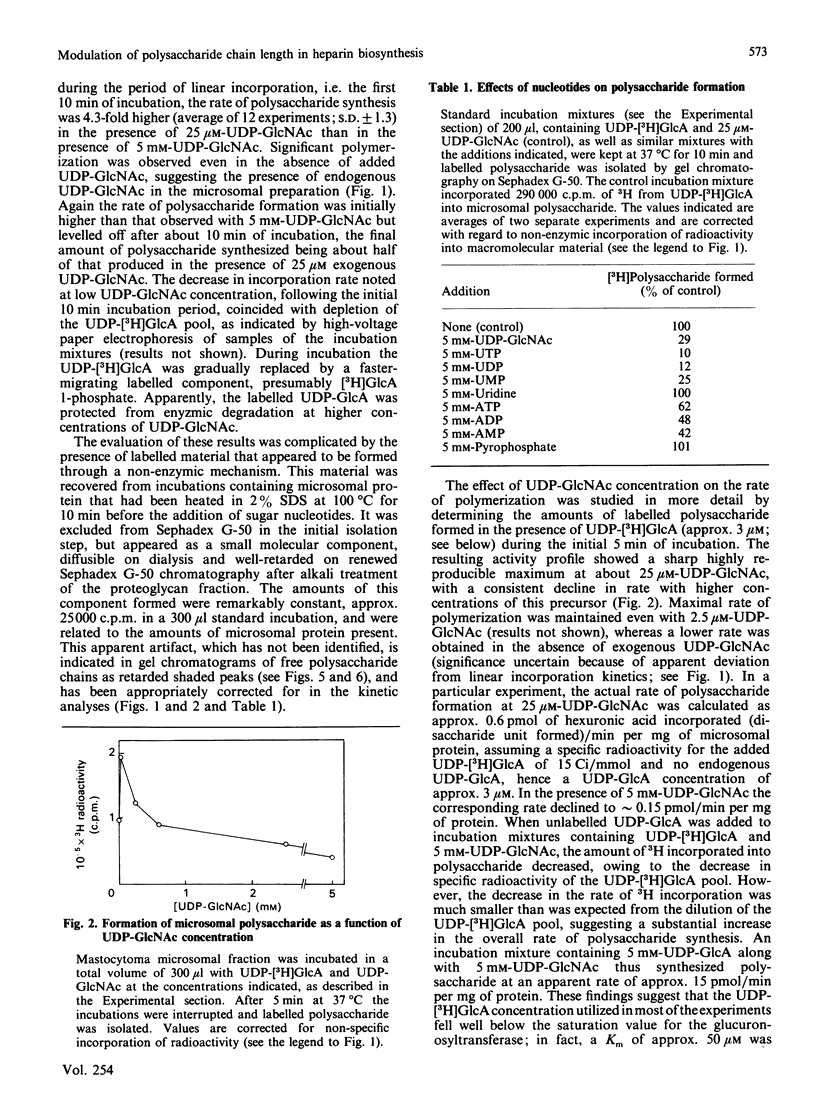
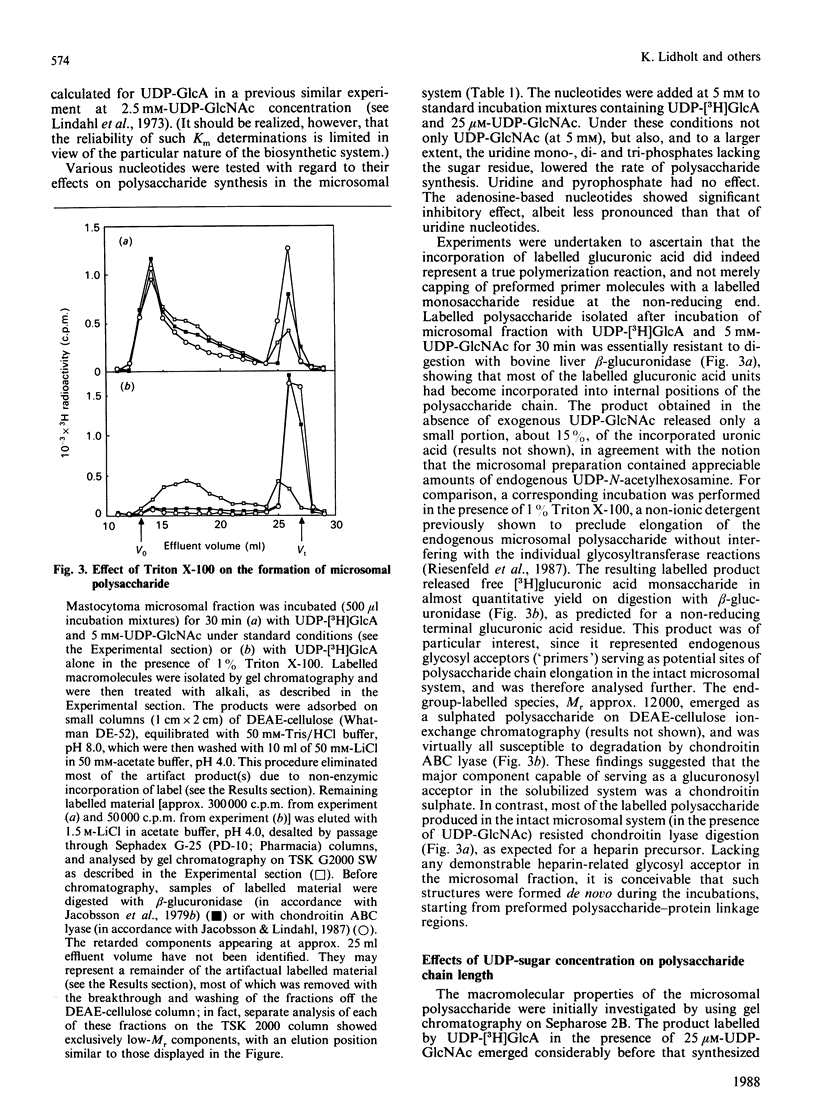
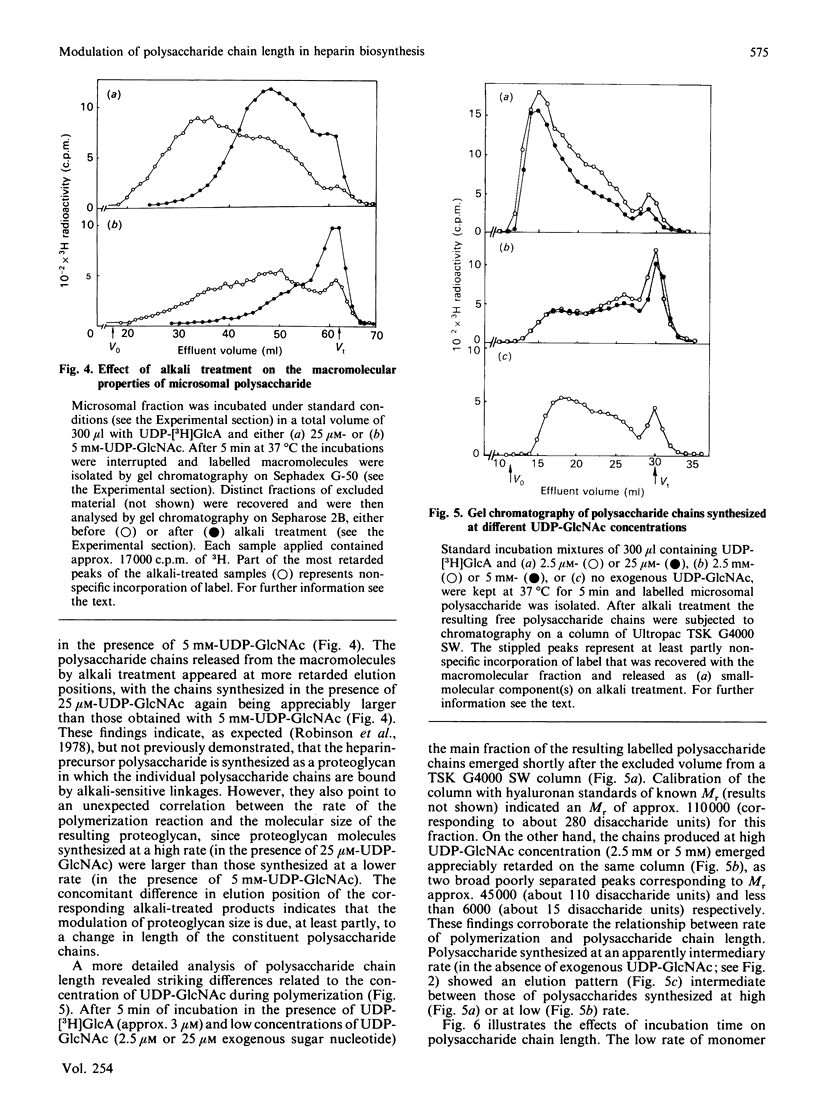
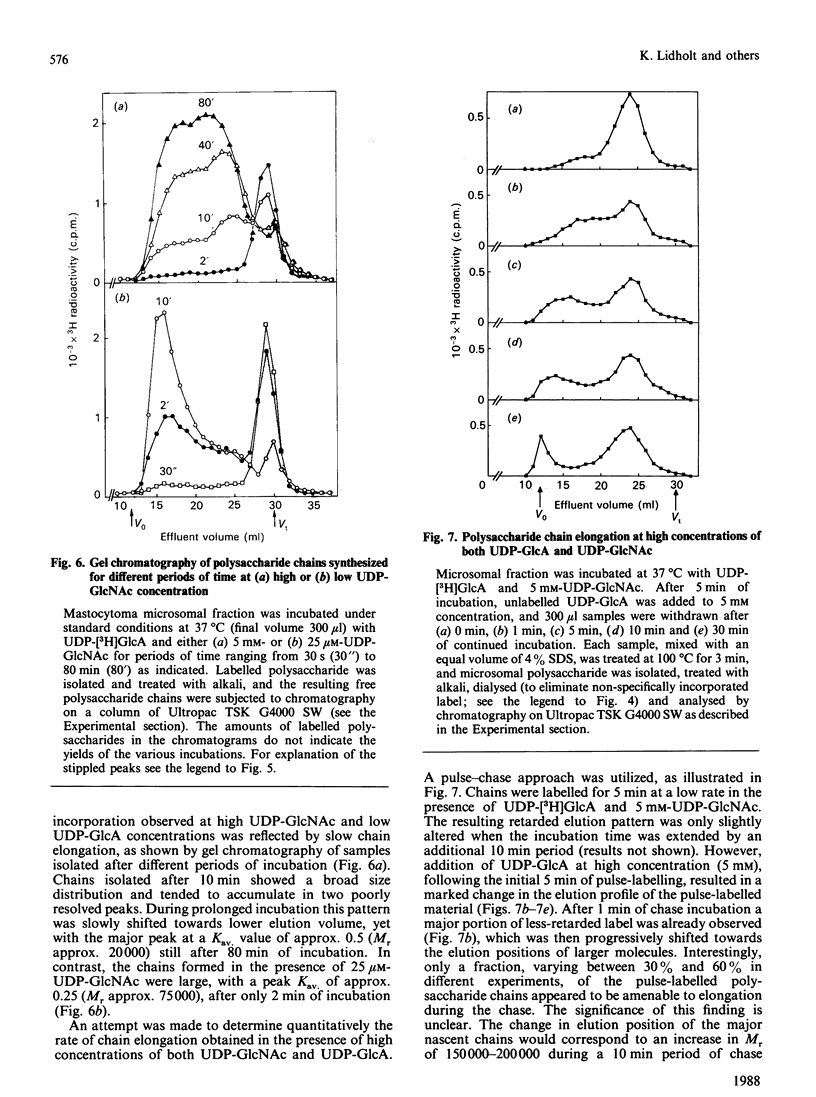
The formation of heparin-precursor polysaccharide (N-acetylheparosan) was studied with a mouse mastocytoma microsomal fraction. Incubation of this fraction with UDP-[3H]GlcA and UDP-GlcNAc yielded labelled macromolecules that could be depolymerized, apparently to single polysaccharide chains, by alkali treatment, and thus were assumed to be proteoglycans. Label from UDP-[3H]GlcA (approx. 3 microM) is transiently incorporated into microsomal polysaccharide even in the absence of added UDP-GlcNAc, probably owing to the presence of endogenous sugar nucleotide. When the concentration of exogenous UDP-GlcNAc was increased to 25 microM the rate of incorporation of 3H increased and proteoglycans carrying polysaccharide chains with an Mr of approx. 110,000 were produced. Increasing the UDP-GlcNAc concentration to 5 mM led to an approx. 4-fold decrease in the rate of 3H incorporation and a decrease in the Mr of the resulting polysaccharide chains to approx. 6000 (predominant component). When both UDP-GlcA and UDP-GlcNAc were present at high concentrations (5 mM) the rate of polymerization and the polysaccharide chain size were again increased. The results suggest that the inhibition of polymerization observed at grossly different concentrations of the two sugar nucleotides, UDP-GlcA and UDP-GlcNAc, may be due either to interference with the transport of one of these precursors across the Golgi membrane or to competitive inhibition of one of the glycosyltransferases. The maximal rate of chain elongation obtained, under the conditions employed, was about 40 disaccharide units/min. The final length of the polysaccharide chains was directly related to the rate of the polymerization reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Balazs E. A., Laurent T. C., Jeanloz R. W. Nomenclature of hyaluronic acid. Biochem J. 1986 May 1;235(3):903–903. doi: 10.1042/bj2350903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström G., Hök M., Lindahl U., Feingold D. S., Malmström A., Rodén L., Jacobsson I. Biosynthesis of heparin. Assay and properties of the microsomal uronosyl C-5 epimerase. J Biol Chem. 1979 Apr 25;254(8):2975–2982. [PubMed] [Google Scholar]
- Capasso J. M., Hirschberg C. B. Effect of nucleotides on translocation of sugar nucleotides and adenosine 3'-phosphate 5'-phosphosulfate into Golgi apparatus vesicles. Biochim Biophys Acta. 1984 Oct 17;777(1):133–139. doi: 10.1016/0005-2736(84)90505-4. [DOI] [PubMed] [Google Scholar]
- Capasso J. M., Hirschberg C. B. Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7051–7055. doi: 10.1073/pnas.81.22.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. L. Viscometric study of the proteoglycan--hyaluronate (2:1) "dimer": minimum hyaluronate chain length. Biopolymers. 1983 Dec;22(12):2501–2506. doi: 10.1002/bip.360221203. [DOI] [PubMed] [Google Scholar]
- FURTH J., HAGEN P., HIRSCH E. I. Transplantable mastocytoma in the mouse containing histamine, heparin, 5-hydroxytryptamine. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):824–828. doi: 10.3181/00379727-95-23375. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Hallén A., Bäckström G. Biosynthesis of heparin. Studies on the microsomal sulfation process. J Biol Chem. 1975 Aug 10;250(15):6065–6071. [PubMed] [Google Scholar]
- Jacobsson I., Hök M., Pettersson I., Lindahl U., Larm O., Wirén E., von Figura K. Identification of N-sulphated disaccharide units in heparin-like polysaccharides. Biochem J. 1979 Apr 1;179(1):77–87. doi: 10.1042/bj1790077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U. Biosynthesis of heparin. Concerted action of late polymer-modification reactions. J Biol Chem. 1980 Jun 10;255(11):5094–5100. [PubMed] [Google Scholar]
- Jacobsson K. G., Lindahl U. Degradation of heparin proteoglycan in cultured mouse mastocytoma cells. Biochem J. 1987 Sep 1;246(2):409–415. doi: 10.1042/bj2460409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson K. G., Riesenfeld J., Lindahl U. Biosynthesis of heparin. Effects of n-butyrate on cultured mast cells. J Biol Chem. 1985 Oct 5;260(22):12154–12159. [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Mitchell D., Hardingham T. The control of chondroitin sulphate biosynthesis and its influence on the structure of cartilage proteoglycans. Biochem J. 1982 Feb 15;202(2):387–395. doi: 10.1042/bj2020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwayhid N., Glaser J. H., Johnson J. C., Conrad H. E., Hauser S. C., Hirschberg C. B. Xylosylation and glucuronosylation reactions in rat liver Golgi apparatus and endoplasmic reticulum. J Biol Chem. 1986 Oct 5;261(28):12936–12941. [PubMed] [Google Scholar]
- Otsu K., Inoue H., Tsuzuki Y., Yonekura H., Nakanishi Y., Suzuki S. A distinct terminal structure in newly synthesized chondroitin sulphate chains. Biochem J. 1985 Apr 1;227(1):37–48. doi: 10.1042/bj2270037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- SILBERT J. E. INCORPORATION OF 14C AND 3H FROM NUCLEOTIDE SUGARS INTO A POLYSACCHARIDE IN THE PRESENCE OF A CELL-FREE PREPARATION FROM MOUSE MAST CELL TUMORS. J Biol Chem. 1963 Nov;238:3542–3546. [PubMed] [Google Scholar]
- Telser A., Robinson H. C., Dorfman A. The biosynthesis of chondroitin sulfate. Arch Biochem Biophys. 1966 Sep 26;116(1):458–465. doi: 10.1016/0003-9861(66)90053-1. [DOI] [PubMed] [Google Scholar]