Abstract
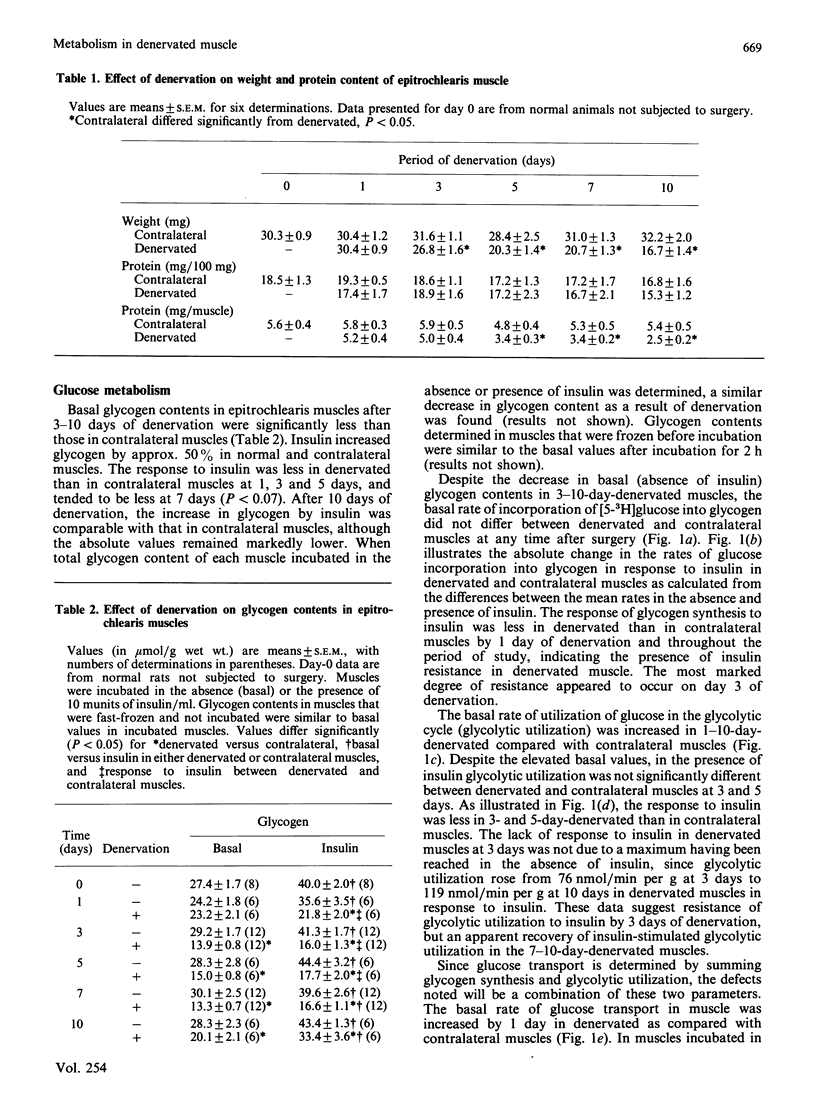
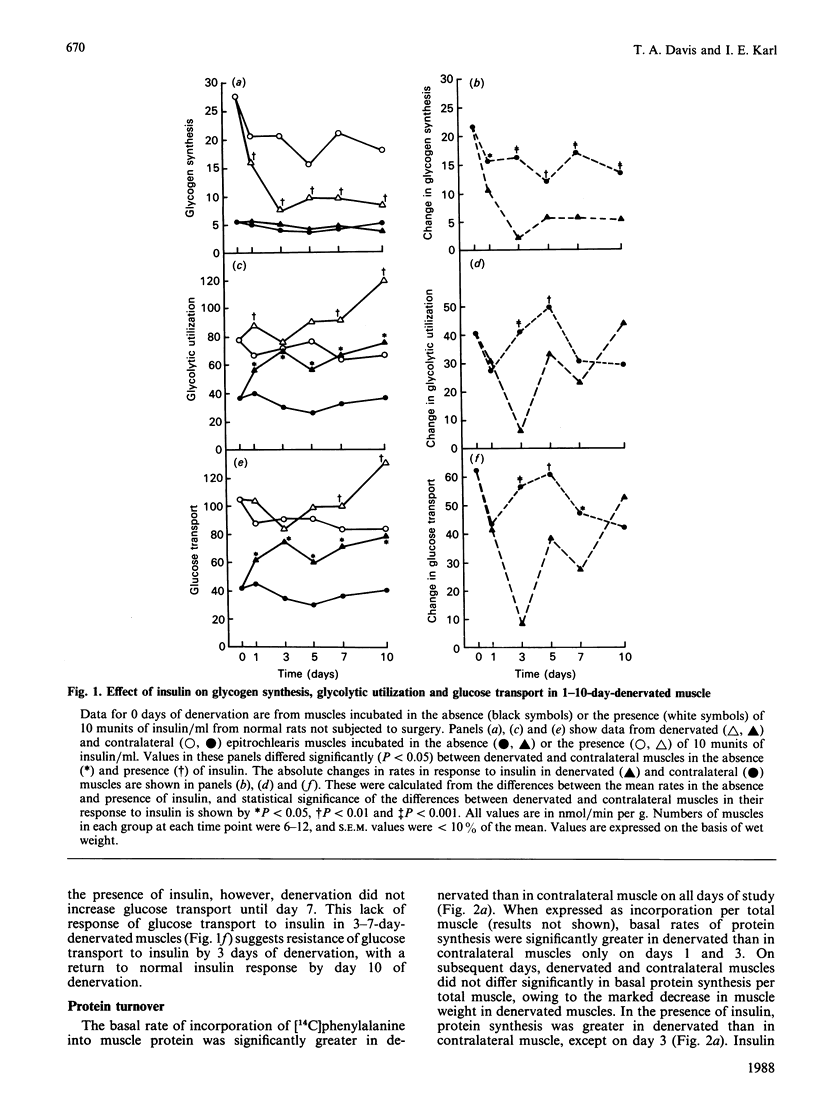
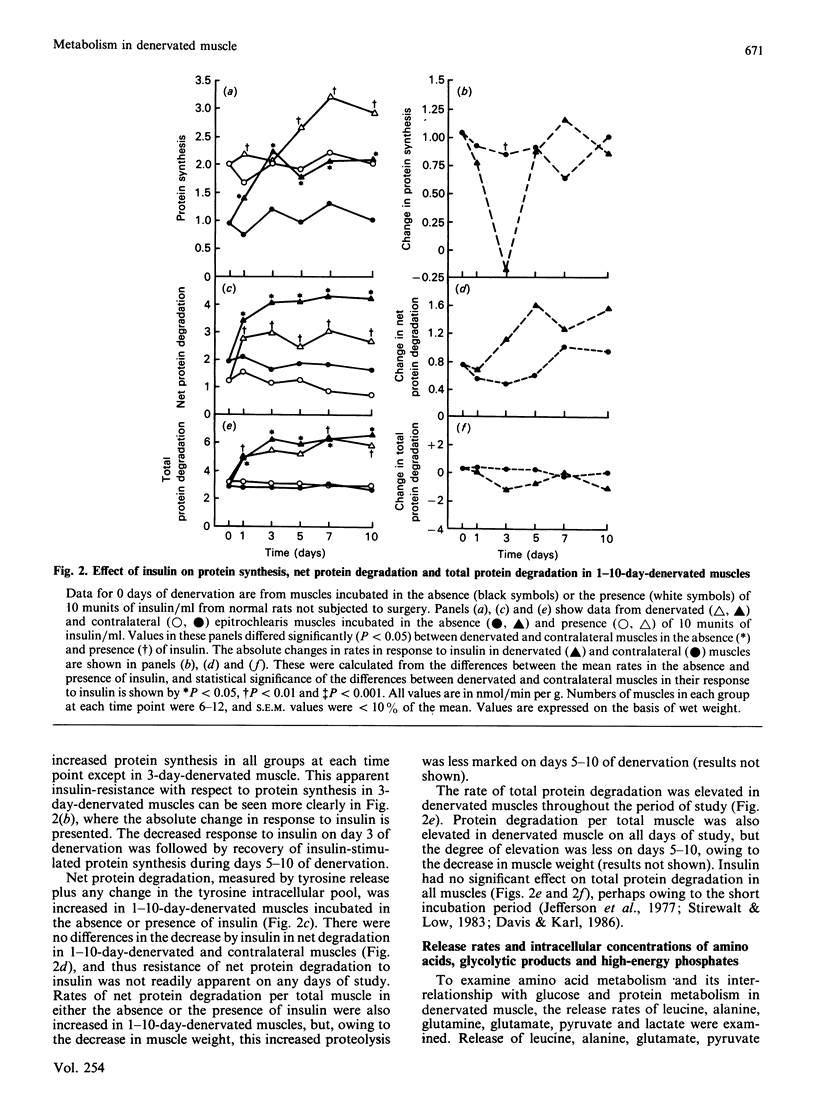
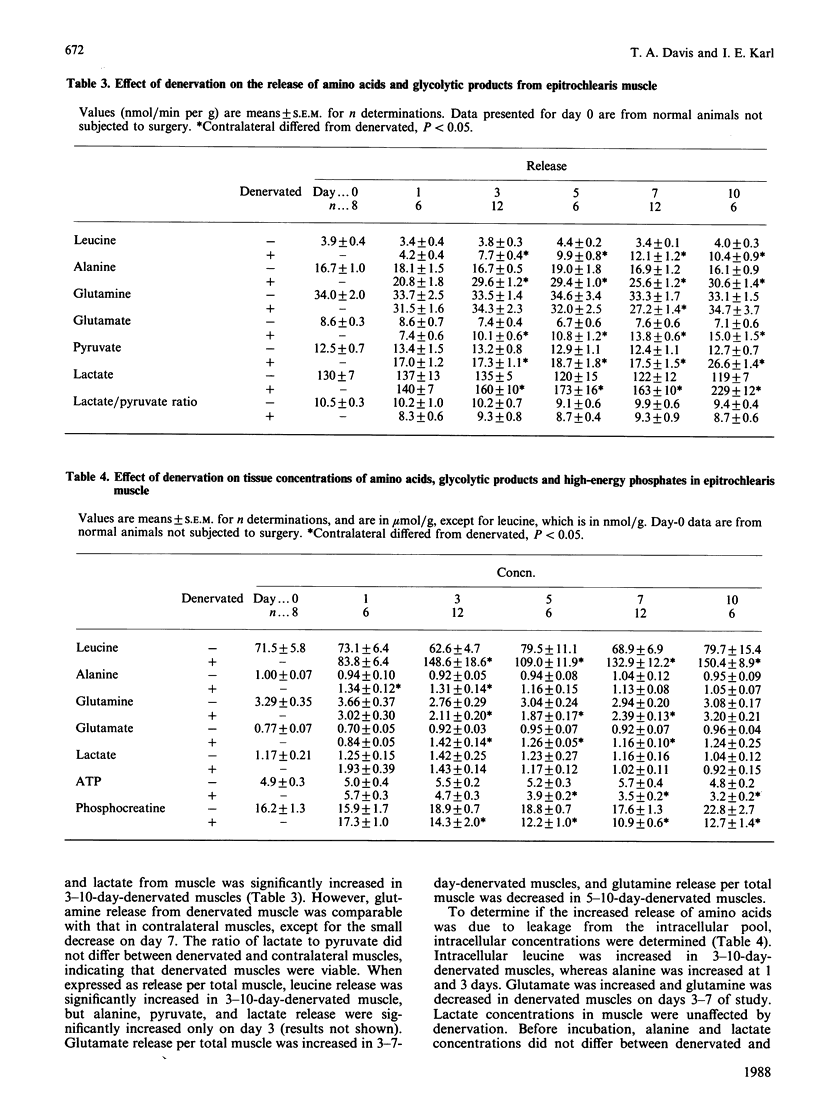
Denervated (1-10 days) rat epitrochlearis muscles were isolated, and basal and insulin-stimulated protein and glucose metabolism were studied. Although basal rates of glycolysis and glucose transport were increased in 1-10-day-denervated muscles, basal glycogen-synthesis rates were unaltered and glycogen concentrations were decreased. Basal rates of protein degradation and synthesis were increased in 1-10-day-denervated muscles. The increase in degradation was greater than that in synthesis, resulting in muscle atrophy. Increased rates of proteolysis and glycolysis were accompanied by elevated release rates of leucine, alanine, glutamate, pyruvate and lactate from 3-10-day-denervated muscles. ATP and phosphocreatine were decreased in 3-10-day-denervated muscles. Insulin resistance of glycogen synthesis occurred in 1-10-day denervated muscles. Insulin-stimulated glycolysis and glucose transport were inhibited by day 3 of denervation, and recovered by day 10. Inhibition of insulin-stimulated protein synthesis was observed only in 3-day-denervated muscles, whereas regulation by insulin of net proteolysis was unaffected in 1-10-day-denervated muscles. Thus the results demonstrate enhanced glycolysis, proteolysis and protein synthesis, and decreased energy stores, in denervated muscle. They further suggest a defect in insulin's action on protein synthesis in denervated muscles as well as on glucose metabolism. However, the lack of concurrent changes in all insulin-sensitive pathways and the absence of insulin-resistance for proteolysis suggest multiple and specific cellular defects in insulin's action in denervated muscle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V., Greenberg R. E., Goldberg A. L. Influence of calcium and other divalent cations on protein turnover in rat skeletal muscle. Am J Physiol. 1986 Jun;250(6 Pt 1):E702–E710. doi: 10.1152/ajpendo.1986.250.6.E702. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Synthesis of acetylcholine receptor by denervated rat diaphragm muscle. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1368–1372. doi: 10.1073/pnas.72.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burant C. F., Lemmon S. K., Treutelaar M. K., Buse M. G. Insulin resistance of denervated rat muscle: a model for impaired receptor-function coupling. Am J Physiol. 1984 Nov;247(5 Pt 1):E657–E666. doi: 10.1152/ajpendo.1984.247.5.E657. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. In vitro and in vivo activation of the insulin receptor kinase in control and denervated skeletal muscle. J Biol Chem. 1986 Jul 5;261(19):8985–8993. [PubMed] [Google Scholar]
- Bylund-Fellenius A. C., Ojamaa K. M., Flaim K. E., Li J. B., Wassner S. J., Jefferson L. S. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol. 1984 Apr;246(4 Pt 1):E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- Davis T. A., Karl I. E. Response of muscle protein turnover to insulin after acute exercise and training. Biochem J. 1986 Dec 15;240(3):651–657. doi: 10.1042/bj2400651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. A., Karl I. E., Tegtmeyer E. D., Osborne D. F., Klahr S., Harter H. R. Muscle protein turnover: effects of exercise training and renal insufficiency. Am J Physiol. 1985 Mar;248(3 Pt 1):E337–E345. doi: 10.1152/ajpendo.1985.248.3.E337. [DOI] [PubMed] [Google Scholar]
- Davis T. A., Klahr S., Tegtmeyer E. D., Osborne D. F., Howard T. L., Karl I. E. Glucose metabolism in epitrochlearis muscle of acutely exercised and trained rats. Am J Physiol. 1986 Feb;250(2 Pt 1):E137–E143. doi: 10.1152/ajpendo.1986.250.2.E137. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- DuBois D. C., Max S. R. Effect of denervation and reinnervation on oxidation of [6-14C]glucose by rat skeletal muscle homogenates. J Neurochem. 1983 Mar;40(3):727–733. doi: 10.1111/j.1471-4159.1983.tb08039.x. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Kitabchi A. E. Insulin metabolism and degradation. Endocr Rev. 1981 Spring;2(2):210–233. doi: 10.1210/edrv-2-2-210. [DOI] [PubMed] [Google Scholar]
- Felber J. P., Thiébaud D., Maeder E., Jéquier E., Hendler R., DeFronzo R. A. Effect of somatostatin-induced insulinopenia on glucose oxidation in man. Diabetologia. 1983 Oct;25(4):325–330. doi: 10.1007/BF00253195. [DOI] [PubMed] [Google Scholar]
- Forsayeth J. R., Gould M. K. Inhibition of insulin-stimulated xylose uptake in denervated rat soleus muscle: a post-receptor effect. Diabetologia. 1982 Dec;23(6):511–516. doi: 10.1007/BF00254301. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J Biol Chem. 1976 Feb 10;251(3):826–835. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem. 1976 Feb 10;251(3):836–843. [PubMed] [Google Scholar]
- Goldberg A. L., Chang T. W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc. 1978 Jul;37(9):2301–2307. [PubMed] [Google Scholar]
- Goldspink D. F. The effects of denervation on protein turnover of rat skeletal muscle. Biochem J. 1976 Apr 15;156(1):71–80. doi: 10.1042/bj1560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Landau B. R. Contribution of the pentose cycle to glucose metabolism in muscle. Arch Biochem Biophys. 1965 Sep;111(3):569–575. doi: 10.1016/0003-9861(65)90236-5. [DOI] [PubMed] [Google Scholar]
- Harter H. R., Karl I. E., Klahr S., Kipnis D. M. Effects of reduced renal mass and dietary protein intake on amino acid release and glucose uptake by rat muscle in vitro. J Clin Invest. 1979 Aug;64(2):513–523. doi: 10.1172/JCI109489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan E. L., Dawson D. M., Romanul F. C. Enzymatic changes in denervated muscle. II. Biochemical studies. Arch Neurol. 1965 Sep;13(3):274–282. doi: 10.1001/archneur.1965.00470030054004. [DOI] [PubMed] [Google Scholar]
- Huijing F. A rapid enzymic method for glycogen estimation in very small tissue samples. Clin Chim Acta. 1970 Dec;30(3):567–572. doi: 10.1016/0009-8981(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Joffe M., Savage N., Isaacs H. Increased muscle calcium. A possible cause of mitochondrial dysfunction and cellular necrosis in denervated rat skeletal muscle. Biochem J. 1981 Jun 15;196(3):663–667. doi: 10.1042/bj1960663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kameyama T., Etlinger J. D. Calcium-dependent regulation of protein synthesis and degradation in muscle. Nature. 1979 May 24;279(5711):344–346. doi: 10.1038/279344a0. [DOI] [PubMed] [Google Scholar]
- Karl I. E., Garber A. J., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. III. Dietary and hormonal regulation. J Biol Chem. 1976 Feb 10;251(3):844–850. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKee E. E., Cheung J. Y., Rannels D. E., Morgan H. E. Measurement of the rate of protein synthesis and compartmentation of heart phenylalanine. J Biol Chem. 1978 Feb 25;253(4):1030–1040. [PubMed] [Google Scholar]
- Nesher R., Karl I. E., Kipnis D. M. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol. 1985 Sep;249(3 Pt 1):C226–C232. doi: 10.1152/ajpcell.1985.249.3.C226. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Waxman L., Goldberg A. L. The stimulation of protein degradation in muscle by Ca2+ is mediated by prostaglandin E2 and does not require the calcium-activated protease. J Biol Chem. 1982 Aug 10;257(15):8716–8723. [PubMed] [Google Scholar]
- Ryan M. P., Gifford J. D., Solomon S. S., Duckworth W. C. The calcium dependence of insulin degradation by rat skeletal muscle. Endocrinology. 1985 Oct;117(4):1693–1698. doi: 10.1210/endo-117-4-1693. [DOI] [PubMed] [Google Scholar]
- Shoji S. Effect of denervation on glucose uptake in rat soleus and extensor digitorum longus muscles. Muscle Nerve. 1986 Jan;9(1):69–72. doi: 10.1002/mus.880090111. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Kilberg M. S., Oxender D. L. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983 May 24;737(2):267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- Smith R. H., Palmer R. M., Reeds P. J. Protein synthesis in isolated rabbit forelimb muscles. The possible role of metabolites of arachidonic acid in the response to intermittent stretching. Biochem J. 1983 Jul 15;214(1):153–161. doi: 10.1042/bj2140153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Lawrence J. C., Jr Insulin action in denervated rat hemidiaphragms. Decreased hormonal stimulation of glycogen synthesis involves both glycogen synthase and glucose transport. J Biol Chem. 1984 Feb 25;259(4):2201–2207. [PubMed] [Google Scholar]
- Smith R. L., Lawrence J. C., Jr Insulin action in denervated skeletal muscle. Evidence that the reduced stimulation of glycogen synthesis does not involve decreased insulin binding. J Biol Chem. 1985 Jan 10;260(1):273–278. [PubMed] [Google Scholar]
- Smith R. L., Roach P. J., Lawrence J. C., Jr Insulin resistance in denervated skeletal muscle. Inability of insulin to stimulate dephosphorylation of glycogen synthase in denervated rat epitrochlearis muscles. J Biol Chem. 1988 Jan 15;263(2):658–665. [PubMed] [Google Scholar]
- Stauffacher W., Renold A. E. Effect of insulin in vivo on diaphragm and adipose tissue of obese mice. Am J Physiol. 1969 Jan;216(1):98–105. doi: 10.1152/ajplegacy.1969.216.1.98. [DOI] [PubMed] [Google Scholar]
- Stirewalt W. S., Low R. B. Effects of insulin in vitro on protein turnover in rat epitrochlearis muscle. Biochem J. 1983 Feb 15;210(2):323–330. doi: 10.1042/bj2100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turinsky J. Dynamics of insulin resistance in denervated slow and fast muscles in vivo. Am J Physiol. 1987 Mar;252(3 Pt 2):R531–R537. doi: 10.1152/ajpregu.1987.252.3.R531. [DOI] [PubMed] [Google Scholar]
- Wagner K. R., Max S. R. Neurotrophic regulation of glucose 6-phosphate dehydrogenase in rat skeletal muscle. Brain Res. 1979 Jul 20;170(3):572–576. doi: 10.1016/0006-8993(79)90978-8. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Gould M. K. Insulin-stimulated sugar transport and 125I-insulin binding by rat soleus muscle: permissive effect of ATP. Biochem Biophys Res Commun. 1977 Jul 11;77(1):203–210. doi: 10.1016/s0006-291x(77)80183-6. [DOI] [PubMed] [Google Scholar]
- Zorzano A., Balon T. W., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle alpha-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol. 1985 May;248(5 Pt 1):E546–E552. doi: 10.1152/ajpendo.1985.248.5.E546. [DOI] [PubMed] [Google Scholar]


