Abstract
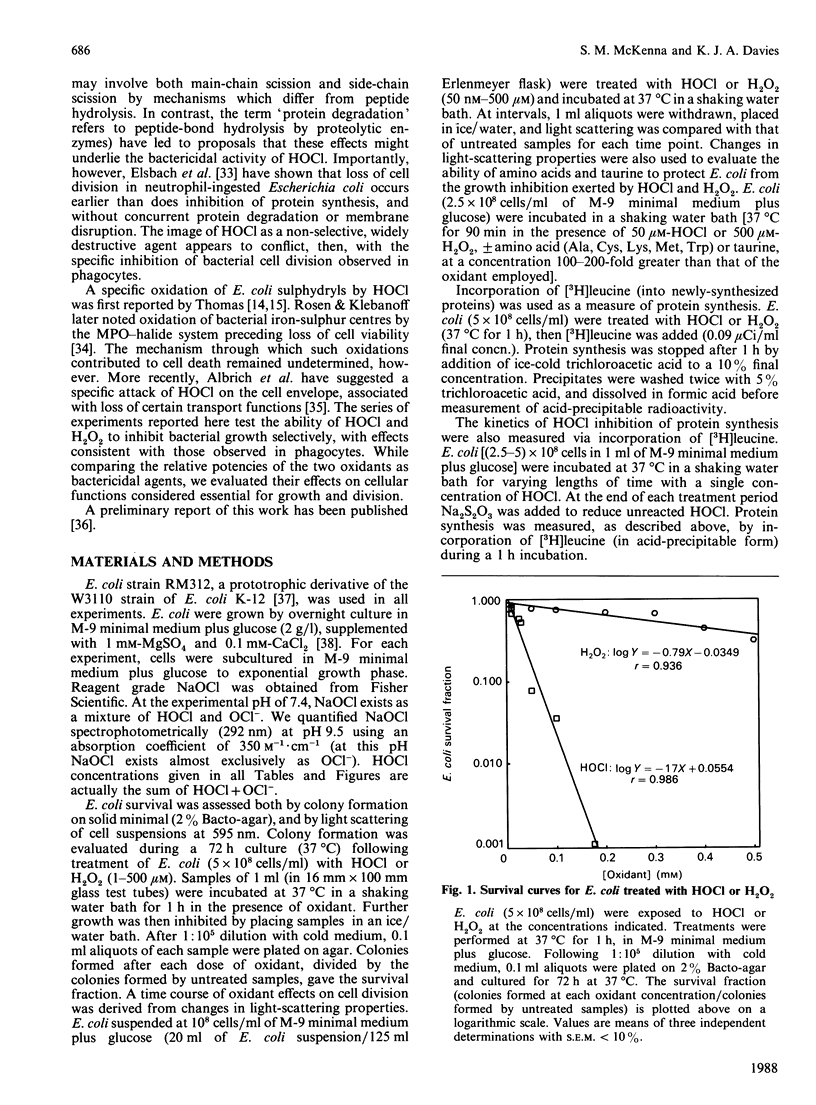
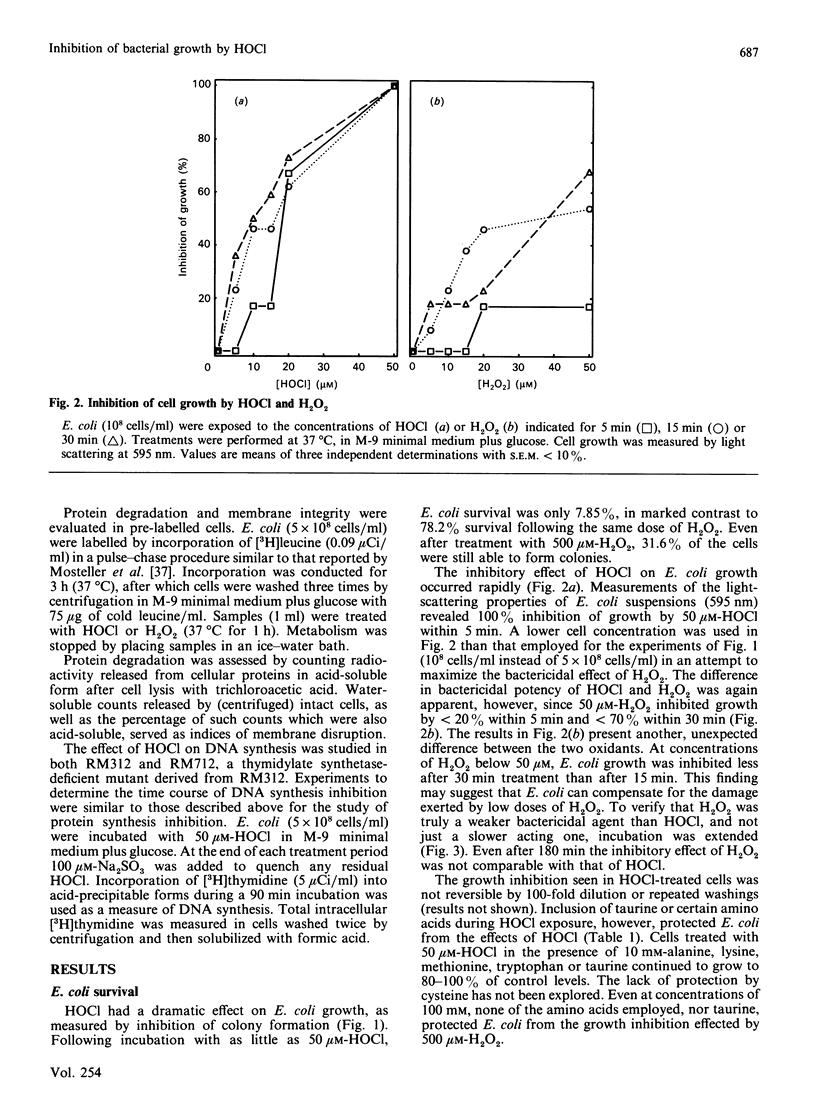
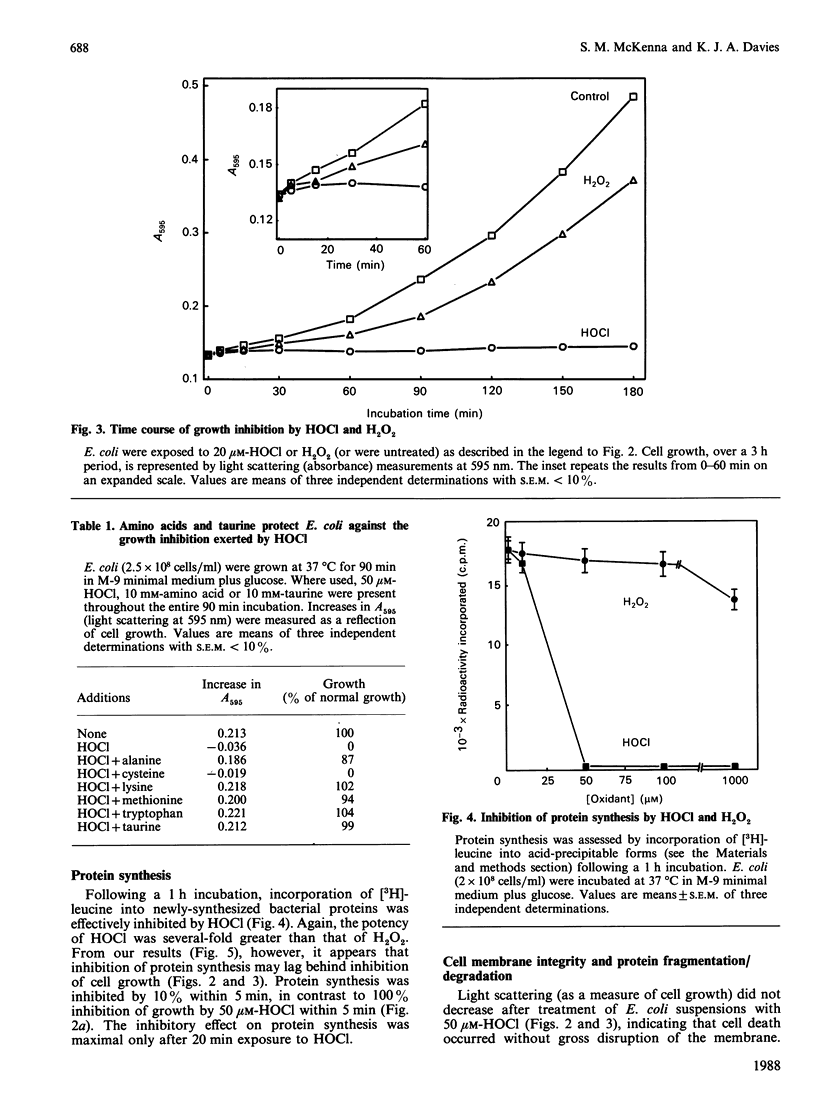
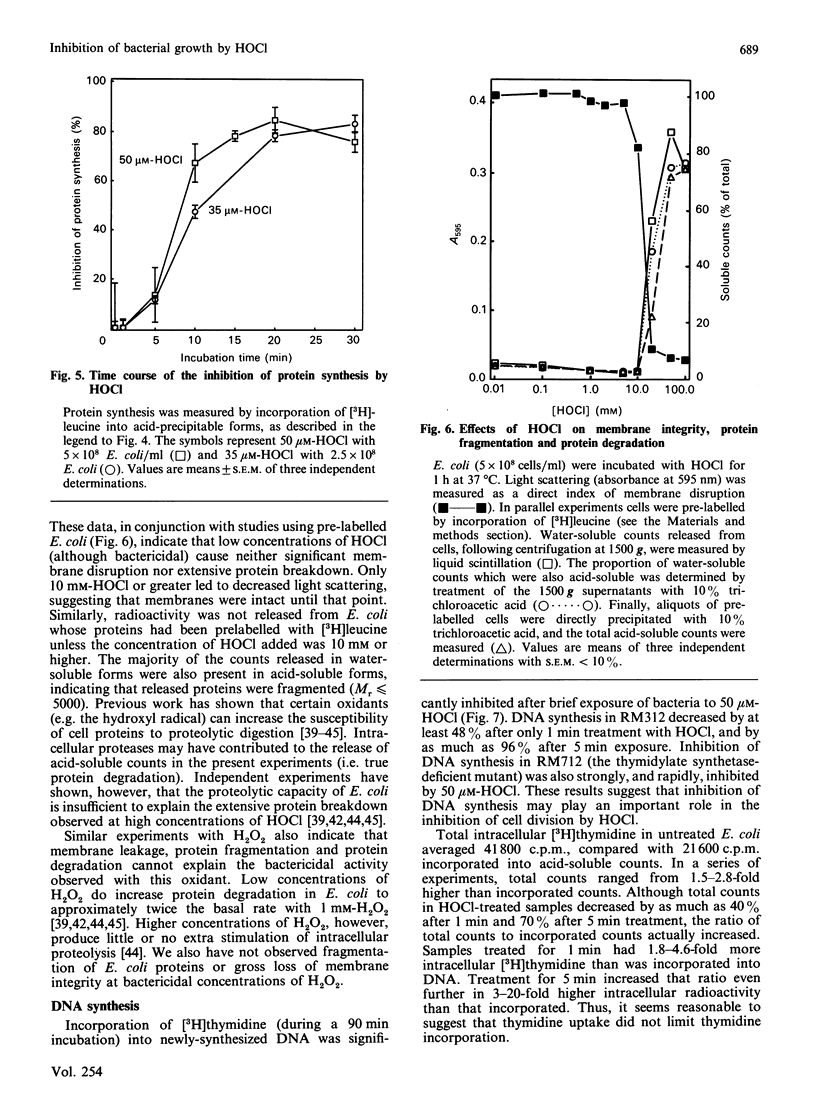
The 'respiratory burst' of phagocytes such as neutrophils generates superoxide which forms H2O2 by dismutation. H2O2 and Cl- ions serve as substrates for the enzyme myeloperoxidase to generate hypochlorous acid (HOCl). HOCl is thought to play an important role in bacterial killing, but its mechanism of action is not well characterized. Furthermore, although many studies in vitro have shown HOCl to be a damaging oxidant with little or no specificity (particularly at high concentrations), bacteria which have been ingested by phagocytes appear to experience a rapid and selective inhibition of cell division. Bacterial membrane disruption, protein degradation, and inhibition of protein synthesis, do not seem to occur in the early phases of phagocyte action. We have now found that low concentrations of HOCl exert a rapid and selective inhibition of bacterial growth and cell division, which can be blocked by taurine or amino acids. Only 20 microM-HOCl was required for 50% inhibition of bacterial growth (5 x 10(8) Escherichia coli/ml), and 50 microM-HOCl completely inhibited cell division (colony formation). These effects were apparent within 5 min of HOCl exposure, and were not reversed by extensive washings. DNA synthesis (incorporation of [3H]-thymidine) was significantly affected by even a 1 min exposure to 50 microM-HOCl, and decreased by as much as 96% after 5 min. In contrast, bacterial membrane disruption and extensive protein degradation/fragmentation (release of acid-soluble counts from [3H]leucine-labelled cells) were not observed at concentrations below 5 mM-HOCl. Protein synthesis (incorporation of [3H]leucine) was only inhibited by 10-30% following 5 min exposure to 50 microM-HOCl, although longer exposure produced more marked reductions (80% after 30 min). Neutrophils deficient in myeloperoxidase cannot convert H2O2 to HOCl, yet can kill bacteria. We have found that H2O2 is only 6% as effective as HOCl in inhibiting E. coli growth and cell division (0.34 mM-H2O2 required for 50% inhibition of colony formation), and taurine or amino acids do not block this effect. Our results are consistent with a rapid and selective inhibition of bacterial cell division by HOCl in phagocytes. H2O2 may substitute for HOCl in myeloperoxidase deficiency, but by a different mechanism and at a greater metabolic cost.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrich J. M., Gilbaugh J. H., 3rd, Callahan K. B., Hurst J. K. Effects of the putative neutrophil-generated toxin, hypochlorous acid, on membrane permeability and transport systems of Escherichia coli. J Clin Invest. 1986 Jul;78(1):177–184. doi: 10.1172/JCI112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrich J. M., Hurst J. K. Oxidative inactivation of Escherichia coli by hypochlorous acid. Rates and differentiation of respiratory from other reaction sites. FEBS Lett. 1982 Jul 19;144(1):157–161. doi: 10.1016/0014-5793(82)80591-7. [DOI] [PubMed] [Google Scholar]
- Albrich J. M., McCarthy C. A., Hurst J. K. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Cech P., Papathanassiou A., Boreux G., Roth P., Miescher P. A. Hereditary myeloperoxidase deficiency. Blood. 1979 Mar;53(3):403–411. [PubMed] [Google Scholar]
- Clark R. A. Modulation of the inflammatory response by the neutrophil myeloperoxidase system. Adv Exp Med Biol. 1982;141:207–216. doi: 10.1007/978-1-4684-8088-7_22. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Szot S. The myeloperoxidase-hydrogen peroxide-halide system as effector of neutrophil-mediated tumor cell cytotoxicity. J Immunol. 1981 Apr;126(4):1295–1301. [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem. 1987 Jun 15;262(17):8220–8226. [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J Biol Chem. 1987 Jun 15;262(17):8227–8234. [PubMed] [Google Scholar]
- Davies K. J., Lin S. W. Degradation of oxidatively denatured proteins in Escherichia coli. Free Radic Biol Med. 1988;5(4):215–223. doi: 10.1016/0891-5849(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Lin S. W. Oxidatively denatured proteins are degraded by an ATP-independent proteolytic pathway in Escherichia coli. Free Radic Biol Med. 1988;5(4):225–236. doi: 10.1016/0891-5849(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Lin S. W., Pacifici R. E. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem. 1987 Jul 15;262(20):9914–9920. [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- Elsbach P., Pettis P., Beckerdite S., Franson R. Effects of phagocytosis by rabbit granulocytes on macromolecular synthesis and degradation in different species of bacteria. J Bacteriol. 1973 Aug;115(2):490–497. doi: 10.1128/jb.115.2.490-497.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Kitahara M., Eyre H. J., Simonian Y., Atkin C. L., Hasstedt S. J. Hereditary myeloperoxidase deficiency. Blood. 1981 May;57(5):888–893. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- Knox W. E., Stumpf P. K., Green D. E., Auerbach V. H. The Inhibition of Sulfhydryl Enzymes as the Basis of the Bactericidal Action of Chlorine. J Bacteriol. 1948 Apr;55(4):451–458. [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller R. D., Goldstein R. V., Nishimoto K. R. Metabolism of individual proteins in exponentially growing Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2524–2532. [PubMed] [Google Scholar]
- Nauseef W. M., Metcalf J. A., Root R. K. Role of myeloperoxidase in the respiratory burst of human neutrophils. Blood. 1983 Mar;61(3):483–492. [PubMed] [Google Scholar]
- Parry M. F., Root R. K., Metcalf J. A., Delaney K. K., Kaplow L. S., Richar W. J. Myeloperoxidase deficiency: prevalence and clinical significance. Ann Intern Med. 1981 Sep;95(3):293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- Passo S. A., Weiss S. J. Oxidative mechanisms utilized by human neutrophils to destroy Escherichia coli. Blood. 1984 Jun;63(6):1361–1368. [PubMed] [Google Scholar]
- Quastel J. H., Wooldridge W. R. Experiments on Bacteria in relation to the Mechanism of Enzyme Action. Biochem J. 1927;21(5):1224–1251. doi: 10.1042/bj0211224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Oxidation of microbial iron-sulfur centers by the myeloperoxidase-H2O2-halide antimicrobial system. Infect Immun. 1985 Mar;47(3):613–618. doi: 10.1128/iai.47.3.613-618.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R. J., Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Oxidative peptide cleavage and decarboxylation by the MPO-H2O2-Cl- antimicrobial system. Infect Immun. 1974 Feb;9(2):255–260. doi: 10.1128/iai.9.2.255-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka A., LoBuglio A. F., Weiss S. J. A potential role for hypochlorous acid in granulocyte-mediated tumor cell cytotoxicity. Blood. 1980 Feb;55(2):347–350. [PubMed] [Google Scholar]
- Stendahl O., Coble B. I., Dahlgren C., Hed J., Molin L. Myeloperoxidase modulates the phagocytic activity of polymorphonuclear neutrophil leukocytes. Studies with cells from a myeloperoxidase-deficient patient. J Clin Invest. 1984 Feb;73(2):366–373. doi: 10.1172/JCI111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979 Feb;23(2):522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase-hydrogen peroxide-chloride antimicrobial system: effect of exogenous amines on antibacterial action against Escherichia coli. Infect Immun. 1979 Jul;25(1):110–116. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Peppin G., Ortiz X., Ragsdale C., Test S. T. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985 Feb 15;227(4688):747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Slivka A. Monocyte and granulocyte-mediated tumor cell destruction. A role for the hydrogen peroxide-myeloperoxidase-chloride system. J Clin Invest. 1982 Feb;69(2):255–262. doi: 10.1172/JCI110447. [DOI] [PMC free article] [PubMed] [Google Scholar]


