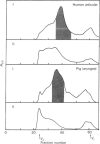
Abstract
Low molecular mass proteoglycans (PG) were isolated from human articular cartilage and from pig laryngeal cartilage, which contained protein cores of similar size (Mr 40-44 kDa). However, the PG from human articular cartilage contained dermatan sulphate (DS) chains (50% chondroitinase AC resistant), whereas chains from pig laryngeal PG were longer and contained only chondroitin sulphate (CS). Disaccharide analysis after chondroitinase ABC digestion showed that the human DS-PG contained more 6-sulphated residues (34%) than the pig CS-PG (6%) and both contained fewer 6-sulphated residues than the corresponding high Mr aggregating CS-PGs from these tissues (86% and 20% from human and pig respectively). Cross-reaction of both proteoglycans with antibodies to bovine bone and skin DS-PG-II and human fibroblasts DS-PG suggested that the isolated proteoglycans were the humans DS-PG-II and pigs CS-PG-II homologues of the cloned and sequenced bovine proteoglycan. Polyclonal antibodies raised against the pig CS-PG-II were shown to cross-react with human DS-PG-II. SDS/polyacrylamide-gel analysis and immunoblotting of pig and human cartilage extracts showed that some free core protein was present in the tissues in addition to the intact proteoglycan. The antibodies were used in a competitive radioimmunoassay to determine the content of this low Mr proteoglycan in human cartilage extracts. Analysis of samples from 5-80 year-old humans showed highest content (approximately 4 mg/g wet wt.) in those from 15-25 year-olds and lower content (approximately 1 mg/g wet wt.) in older tissue (greater than 55 years). These changes in content may be related to the deposition and maintenance of the collagen fibre network with which this class of small proteoglycan has been shown to interact.
Full text
PDF

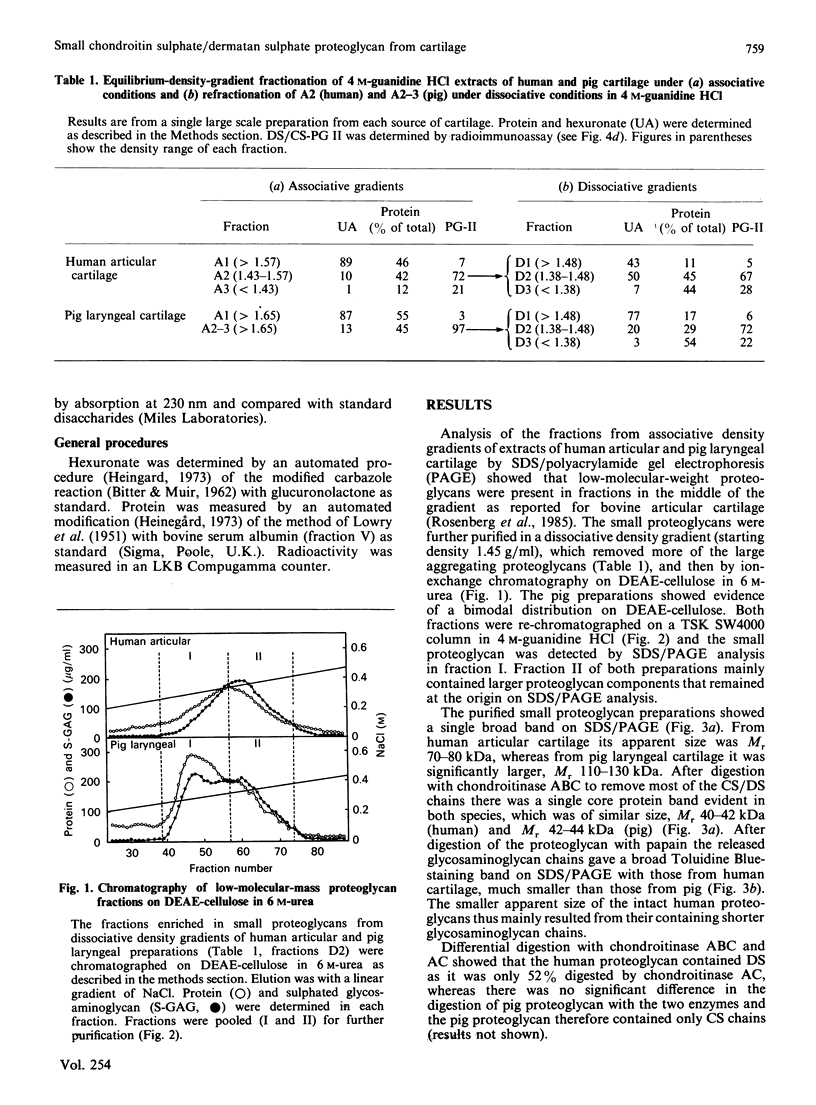

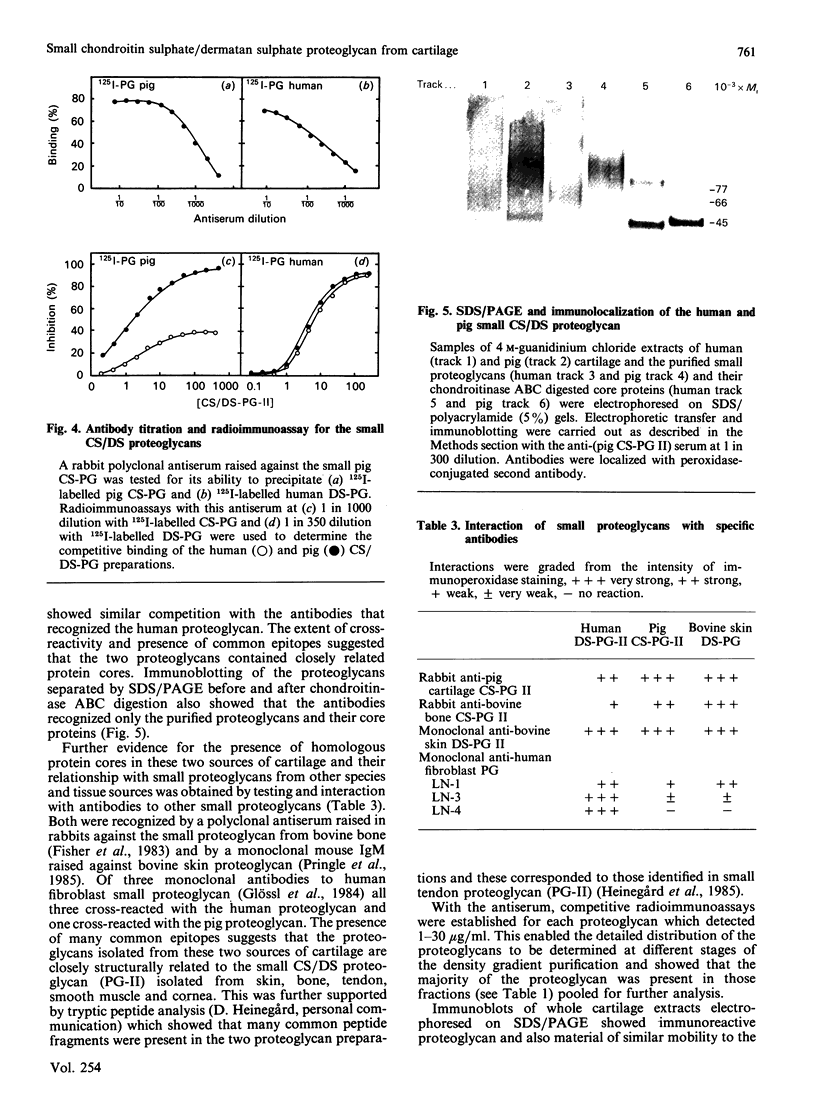
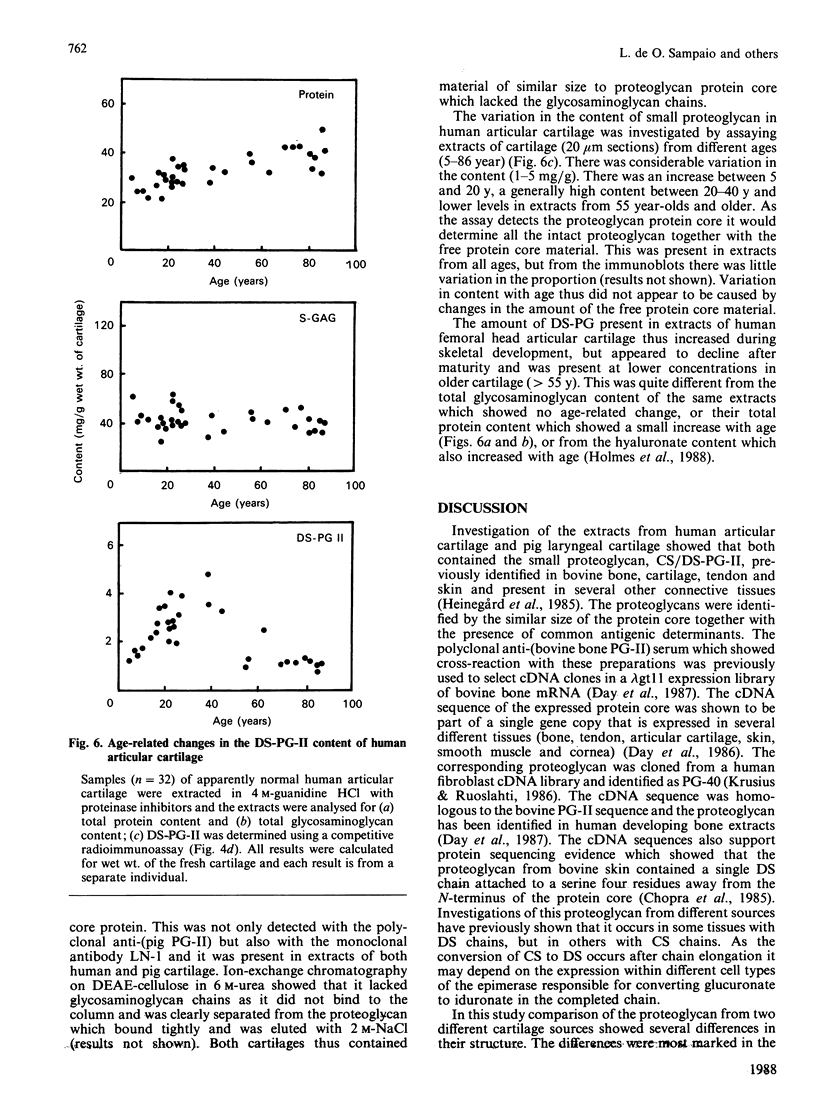


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ridgway G. D., Ali S. Y. Delayed aggregation of proteoglycans in adult human articular cartilage. Biosci Rep. 1984 Oct;4(10):827–833. doi: 10.1007/BF01138164. [DOI] [PubMed] [Google Scholar]
- Byers S., Hopkins T. J., Kuettner K. E., Kimura J. H. The effect of zwitterionic detergents on the extraction and functional properties of cartilage proteoglycans. J Biol Chem. 1987 Jul 5;262(19):9166–9174. [PubMed] [Google Scholar]
- Caterson B., Baker J. R., Levitt D., Paslay J. W. Radioimmunoassay of the link proteins associated with bovine nasal cartilage proteoglycan. J Biol Chem. 1979 Oct 10;254(19):9369–9372. [PubMed] [Google Scholar]
- Day A. A., McQuillan C. I., Termine J. D., Young M. R. Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. Biochem J. 1987 Dec 15;248(3):801–805. doi: 10.1042/bj2480801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A. A., Ramis C. I., Fisher L. W., Gehron-Robey P., Termine J. D., Young M. F. Characterization of bone PG II cDNA and its relationship to PG II mRNA from other connective tissues. Nucleic Acids Res. 1986 Dec 22;14(24):9861–9876. doi: 10.1093/nar/14.24.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Dejter S. W., Jr, Whitson S. W., Yanagishita M., Kimura J. H., Hascall V. C., Kleinman H. K., Hassell J. R., Nilsson B. Proteoglycans of developing bone. J Biol Chem. 1983 May 25;258(10):6588–6594. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Biosynthesis of proteoglycans in cartilage slices. Fractionation by gel chromatography and equilibrium density-gradient centrifugation. Biochem J. 1972 Feb;126(4):791–803. doi: 10.1042/bj1260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L., Hayward J., Jr, Reynolds C. C. Treatment of bovine nasal cartilage proteoglycan with chondroitinases from Flavobacterium heparinum and Proteus vulgaris. J Biol Chem. 1972 Jul 25;247(14):4521–4528. [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Paulsson M., Inerot S., Carlström C. A novel low-molecular weight chondroitin sulphate proteoglycan isolated from cartilage. Biochem J. 1981 Aug 1;197(2):355–366. doi: 10.1042/bj1970355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. W., Bayliss M. T., Muir H. Hyaluronic acid in human articular cartilage. Age-related changes in content and size. Biochem J. 1988 Mar 1;250(2):435–441. doi: 10.1042/bj2500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Pringle G. A., Dodd C. M., Osborn J. W., Pearson C. H., Mosmann T. R. Production and characterization of monoclonal antibodies to bovine skin proteodermatan sulfate. Coll Relat Res. 1985 Jan;5(1):23–39. doi: 10.1016/s0174-173x(85)80045-5. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Hardingham T. Cartilage proteoglycan binding region and link protein. Radioimmunoassays and the detection of masked determinants in aggregates. Biochem J. 1983 Aug 1;213(2):371–378. doi: 10.1042/bj2130371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985 Jan;5(1):71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- Torchia D. A., Hasson M. A., Hascall V. C. Investigation of molecular motion of proteoglycans in cartilage by 13C magnetic resonance. J Biol Chem. 1977 Jun 10;252(11):3617–3625. [PubMed] [Google Scholar]
- Vogel K. G., Fisher L. W. Comparisons of antibody reactivity and enzyme sensitivity between small proteoglycans from bovine tendon, bone, and cartilage. J Biol Chem. 1986 Aug 25;261(24):11334–11340. [PubMed] [Google Scholar]
- Vogel K. G., Heinegård D. Characterization of proteoglycans from adult bovine tendon. J Biol Chem. 1985 Aug 5;260(16):9298–9306. [PubMed] [Google Scholar]
- Vogel K. G., Koob T. J., Fisher L. W. Characterization and interactions of a fragment of the core protein of the small proteoglycan (PGII) from bovine tendon. Biochem Biophys Res Commun. 1987 Oct 29;148(2):658–663. doi: 10.1016/0006-291x(87)90927-2. [DOI] [PubMed] [Google Scholar]
- Zebrower M. E., Kieras F. J., Brown W. T. Analysis by high-performance liquid chromatography of hyaluronic acid and chondroitin sulfates. Anal Biochem. 1986 Aug 15;157(1):93–99. doi: 10.1016/0003-2697(86)90201-0. [DOI] [PubMed] [Google Scholar]