Abstract
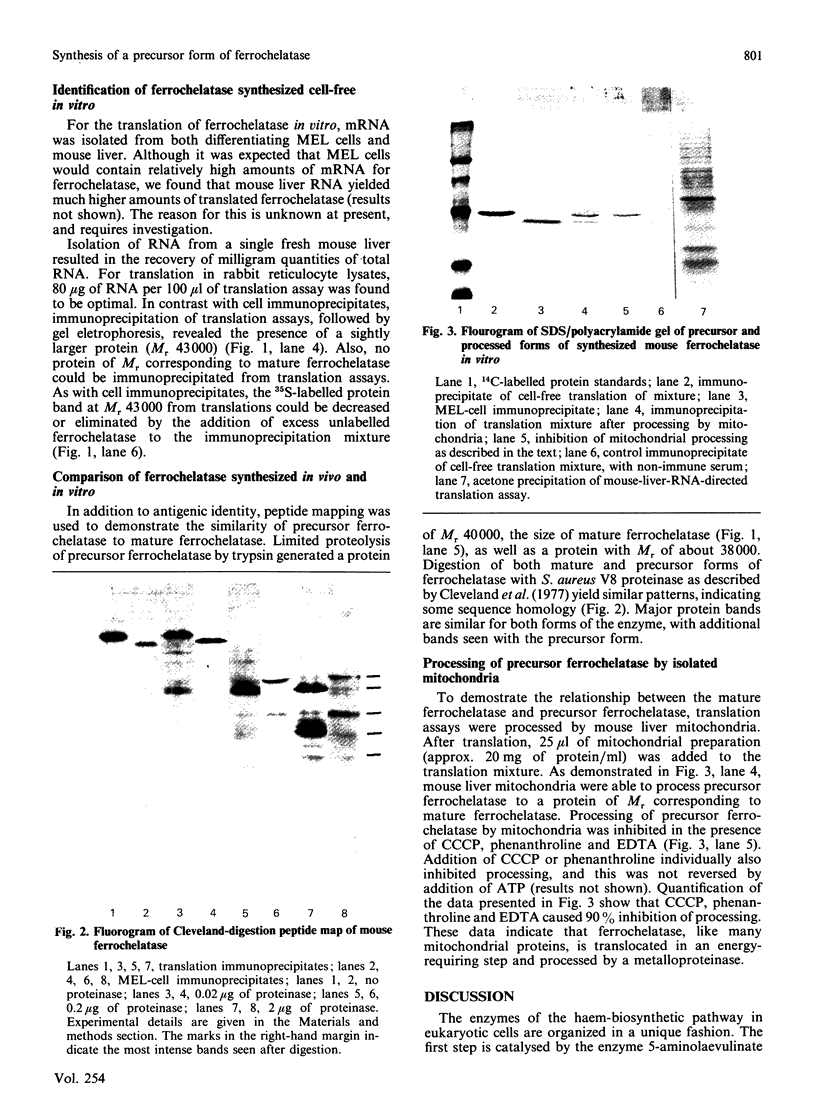
Ferrochelatase (protohaem ferro-lyase, EC 4.99.1.1), the terminal enzyme of the haem-biosynthetic pathway, is an integral membrane protein of the mitochondrial inner membrane. When murine erythroleukaemia cells are labelled in vivo with [35S]methionine, lysed, and the extract is immunoprecipitated with rabbit anti-(mouse ferrochelatase) antibody, a protein of Mr 40,000 is isolated. However, when isolated mouse RNA is translated in a cell-free reticulocyte extract, a protein of Mr 43,000 is isolated. Incubation of this Mr 43,000 protein with isolated mitochondria resulted in processing of the Mr 43,000 precursor to the Mr 40,000 mature-sized protein. Addition of carbonyl cyanide m-chlorophenylhydrazone and/or phenanthroline inhibits this processing. These data indicate that ferrochelatase, like most mitochondrial proteins, is synthesized in the cytoplasm as a larger precursor and is then translocated and processed to a mature-sized protein in an energy-required step.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomer J. R. Characterization of deficient heme synthase activity in protoporphyria with cultured skin fibroblasts. J Clin Invest. 1980 Feb;65(2):321–328. doi: 10.1172/JCI109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer J. R., Hill H. D., Morton K. O., Anderson-Burnham L. A., Straka J. G. The enzyme defect in bovine protoporphyria. Studies with purified ferrochelatase. J Biol Chem. 1987 Jan 15;262(2):667–671. [PubMed] [Google Scholar]
- Bloomer J. R., Morton K. O., Reuter R. J., Ruth G. R. Bovine protoporphyria: documentation of autosomal recessive inheritance and comparison with the human disease through measurement of heme synthase activity. Am J Hum Genet. 1982 Mar;34(2):322–330. [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J Biol Chem. 1983 Oct 10;258(19):11453–11459. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E., Harbin B. M. Purification and characterization of mammalian and chicken ferrochelatase. Methods Enzymol. 1986;123:401–408. doi: 10.1016/s0076-6879(86)23049-9. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O. Evidence that the coproporphyrinogen oxidase activity of rat liver is situated in the intermembrane space of mitochondria. Biochem J. 1978 May 15;172(2):345–347. doi: 10.1042/bj1720345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. A., Rich C. B., Fletcher S., Karr S. R., Przybyla A. Translation of chick aortic elastin messenger ribonucleic acid. Comparison to elastin synthesis in chick aorta organ culture. Biochemistry. 1980 Mar 4;19(5):857–864. doi: 10.1021/bi00546a005. [DOI] [PubMed] [Google Scholar]
- Guerra F. C. Rapid isolation techniques for mitochondria: technique for rat liver mitochondria. Methods Enzymol. 1974;31:299–305. doi: 10.1016/0076-6879(74)31031-2. [DOI] [PubMed] [Google Scholar]
- Hanson J. W., Dailey H. A. Purification and characterization of chicken erythrocyte ferrochelatase. Biochem J. 1984 Sep 15;222(3):695–700. doi: 10.1042/bj2220695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbin B. M., Dailey H. A. Orientation of ferrochelatase in bovine liver mitochondria. Biochemistry. 1985 Jan 15;24(2):366–370. doi: 10.1021/bi00323a019. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Watanabe N., Kikuchi G. Inhibition by hemin of in vitro translocation of chicken liver delta-aminolevulinate synthase into mitochondria. Biochem Biophys Res Commun. 1983 Sep 15;115(2):700–706. doi: 10.1016/s0006-291x(83)80201-0. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Poulson R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J Biol Chem. 1976 Jun 25;251(12):3730–3733. [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G., Borthwick I. A., Brooker J. D., May B. K., Elliott W. H. Purification of rat liver mitochondrial delta-aminolaevulinate synthase. Biochem Biophys Res Commun. 1982 Nov 30;109(2):305–312. doi: 10.1016/0006-291x(82)91721-1. [DOI] [PubMed] [Google Scholar]
- Taketani S., Tokunaga R. Rat liver ferrochelatase. Purification, properties, and stimulation by fatty acids. J Biol Chem. 1981 Dec 25;256(24):12748–12753. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K., Hayashi N., Kikuchi G. Translocation of delta-aminolevulinate synthase from the cytosol to the mitochondria and its regulation by hemin in the rat liver. J Biol Chem. 1980 Feb 25;255(4):1746–1751. [PubMed] [Google Scholar]