Abstract
Ipomoea batatas (L.) Lam is a dicotyledonous plant originally from tropical regions, with China and Spain acting as the main producers from outside and within the EU, respectively. The root, including only flesh, is the edible part, and the peel, leaves, stems, or shoots are considered by-products, which are generated due to being discarded in the field and during processing. Therefore, this study aimed to perform a comprehensive review of the nutritional value, phytochemical composition, and health-promoting activities of purple-fleshed sweet potato and its by-products, which lead to its potential applications in bakery products for the development of functional foods. The methodology is applied to the selected topic and is used to conduct the search, review abstracts and full texts, and discuss the results using different general databases. The studies suggested that purple-fleshed sweet potato parts are characterized by a high content of essential minerals and bioactive compounds, including anthocyanins belonging to the cyanidin or the peonidin type. The flesh and leaves are also high in phenolic compounds and carotenoids such as lutein and β-carotene. The high content of phenolic compounds and anthocyanins provides the purple-fleshed sweet potato with high antioxidant and anti-inflammatory power due to the modulation effect of the transcription factor Nrf2 and NF-kB translocation, which may lead to protection against hepatic and neurological disorders, among others. Furthermore, purple-fleshed sweet potato and its by-products can play a dual role in food applications due to its attractive color and wide range of biological activities which enhance its nutritional profile. As a result, it is essential to harness the potential of the purple-fleshed sweet potato and its by-products that are generated during its processing through an appropriate agro-industrial valorization system.
Keywords: sweet potato, Ipomoea batatas (L.) Lam., food application, antioxidants, anthocyanins
1. Introduction
Ipomoea batatas (L.) Lam. or sweet potato (SP) is a dicotyledonous plant and herbaceous perennial vine that is native to the neotropics [1,2,3]. The migration of the plant spread its growth to 114 countries worldwide [4]. It belongs to the series Ipomoea batatas, which is taxonomically placed in the genus Ipomoea; recent research has found that 14 wild species are related to the SP. These are the following: I. cordatotriloba Dennstedt, I. cynanchifolia Meisn, I. grandifolia O’Donell, I. lacunose L., I. leucantha Jacquin, I. littoralis Blume, I. ramosissima Choisy, I. splendor-sylvae House, I. tabascana McDonald and Austin, I. tenuissima Choisy, I. tiliacea (Willd.) Choisy in D. C, I. trifida (H. B. K.) G. Don, and I. triloba L. [5]. All these species belong to the family Convolvulaceae, although Ipomoea batatas is the only known cropped and major-economic-importance species in the family [6].
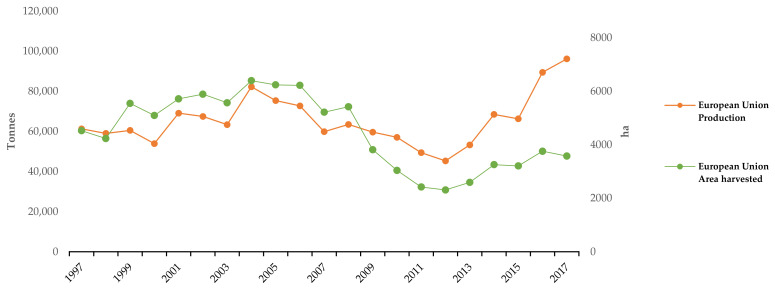
SP is the seventh most important crop worldwide [7]. According to FAOSTAT, the world production of SP was 86.4 million tons in 2022. The main destination of the total amount produced was the food supply (58%), followed by feed (32%) and waste (7%). Asia and Africa are the biggest SP producers, making up 61% and 34% of the world’s total production, respectively. The Chinese SP total production accounts for 54% of the total global production, while only 2% of the production belongs to industrialized countries, mainly in the USA and Japan [8]. In the European Union, SP production has increased since 2012, predicting an upward trend in future years (Figure 1). In this context, the latest data from the FAO database show Spain’s leadership as the largest SP producer in the EU, with 83 thousand tons being produced in 2022 [8]. In Spain, the crop is located, mainly, in Andalucía and Valencia, but also in Extremadura, Baleares, Aragón, and Murcia, raising its production to 79 thousand tons in 2021 [9]. Due to its neotropical origin, SP has been a traditional Mediterranean harvest crop. SP crops are grown as annual plants by vegetative propagation with a short growing period of 90 to 120 days [10]. The planting period requires a moderate temperature (21–26 °C), plenty of sunshine, sandy loam with clay subsoil, and a soil pH range between 5.5 and 6.5 [11]. However, SP is sensitive to salinity and alkalinity conditions [12,13].
Figure 1.
European Union production and harvested area of SP from 1997 to 2017 [8].
The SP cultivar has an elongated and tapered appearance, and this plant may produce 40–50 roots reaching an average length of 30 cm, with a weight between 100 and 1000 g, although it differs among commercial cultivars [14]. The edible part of the SP is the root, the most consumed worldwide and used as food or as a raw material for starch production [15]. However, SP leaves, young stems, and shoots are often discarded in the field or used as livestock feed [16]. In fact, these above-ground parts are also edible and consumed as a leafy or green vegetable in parts of Asia and Africa due to the richness in minerals, vitamins, proteins, pigments, and polyphenols [17,18]. The processing of SP generates a wide variety of bio-waste and by-products depending on whether they are a result of the agricultural phase or the industrial processing phase [19]. In the field, bio-waste is generated from the removal of leaves, young stems, and shoots and also from the root tubers that do not achieve size requirements or are damaged due to harvesting techniques [20]. During processing, by-products come from peels, trimming, chunks of tuber, and nutrient-rich wastewater (Figure 2) [21].
Figure 2.
(A) Cross-section of a mature PFSP roots; (B) Cultivar of PFSP; (C) Different parts of a PFSP plant.
SP color has led to a divide among commercial cultivars, resulting in two categories, which depend on (i) the color of the skin and (ii) the flesh color. SP skin colors include white, cream, yellow, orange, pink, red, and purple. SP flesh colors include white, cream, yellow, orange, and purple. The differences between these commercial cultivars only extend to the bioactive and non-nutrient compounds [22]. Due to the wide variety of SP commercial cultivars, this review is going to focus on the study of purple-fleshed sweet potato (PFSP). The high accumulation of acylated anthocyanins in the root leads to the common purple color of the skin and the flesh [23,24].
The therapeutic and medicinal benefits of PFSP have been known since its domestication 5000 years ago, playing a significant role in combating food shortage and malnutrition because of its nutritive values and biological activities [25]. The high content of bioactive compounds, such as phenolic acids, anthocyanins, vitamins, dietary fiber, or resistant starch, provides purple-fleshed sweet potato with beneficial effects against a variety of diseases. Numerous studies have found that PFSPs present an anti-inflammatory [26], antioxidant [27], antimicrobial [28], antidiabetic [29], antimutation [30], anti-tumor [31], and hypouricemic [32] effect, as well as hepatic [33], and neuroprotective action [34]. On the other hand, new alternatives use for PFSP bio-wastes have been proposed to avoid environmental problems [35]. Purple-fleshed sweet potato by-products in combination with ultrasound and microwave extraction techniques can be a valuable raw material to obtain the pure bioactive compounds, anthocyanins and dietary fiber, for the manufacture of value-added products such as functional foods [36,37,38].
Due to the growing interest in research on agricultural by-products and its beneficial effects for applications in food industry, especially in bakery products, this article aims to review the existing literature about the functional characteristics, health benefits, and food application of purple-fleshed sweet potato.
2. Materials and Methods
This comprehensive review followed four steps: selecting the topic, conducting the literature search, reviewing abstracts and full texts, and discussing the results. For this purpose, the Science Direct, Google Scholar, PubMed, Web of Science, Scopus, and Dialnet databases were searched to recognize the appropriate studies, according to the review’s aim. The final search was conducted in July 2024 and included English and Spanish-language-based international articles, including reviews and research, reports, and theses. The keyword “purple-fleshed sweet potato” was utilized combined with other terms such as antioxidant, anti-inflammatory, bakery products, food waste, anthocyanins, phenolic acids, minerals, peel, or hepatoprotective effects. After the full search, duplicates were removed, and the abstracts and their specific sections of the articles were read to ensure that they addressed the review inclusion criteria. The eligible criteria were studies that analyzed PFSP in at least one of the three dimensions focused on in this review (nutritional characteristics, health benefits, and food application). Therefore, the studies of interest focusing on sweet potato, or sweet potato varieties different from the purple one, were summarized and synthesized to integrate into the comprehensive review. Finally, no specific platforms were necessary to document the comprehensive search due to the nature of this review.
3. Nutritional Characteristics
3.1. Proximate Composition of Purple-Fleshed Sweet Potato
There is a large variability in nutrients between the different botanical parts of PFSP (flesh, leaves, stems, stalks, shoots, or peels). Genetic [39], agricultural practices [40], geographic [41], maturation stage [42], and environmental factors also contribute to this variation. According to the U.S. Department of Agriculture [43], PFSP provides 85 kcal per 100 g of FW of the edible portion and is considered a high-calorie food due to its high moisture content (ranging from 62.6 to 73.6%) [44].
Carbohydrates represent up to 72.10 g/100 g (Table 1) of the dry weight (DW) of PFSP. Free sugars of low molecular weight or reducing sugars constitute a small fraction of the total carbohydrates, reporting ranges between 1.01 and 5.94 g/100 g depending on the variety or the maturity stage, with maltose, sucrose, and glucose + fructose being the predominant reducing sugars with 11.98, 8.33, and 6.52% of FW, respectively [45,46]. However, a large part of these constitute starches reaching values of 56.7 g/100 g of DW [47,48]. Native starch is composed of amylose and amylopectin, and its starch digestion rate depends on the proportion of amylose/amylopectin generating different absorption rates dividing starch into three categories: (i) rapidly digestible starch (RDS), (ii) slowly digestible starch (SDS), and (iii) resistant starch (RS) [49]. The results shown in Table 1 fit with the values reported by another study that compares the physicochemical characterization of seven PFSP varieties establishing an amylose content that varied from 18.2 to 27.2%, and RDS, SDS, and RS contents from 40.66% to 53.50%, from 10.40% to 23.84%, and from 29.25% to 43.50%, respectively [50]. Wang et al. [51] revealed that starch degradation provided abundant substrates for anthocyanin biosynthesis in PFSP roots since the most abundant PFSP starch, phosphorylase (SP), and phosphoglucomutase (PGM) promoted the synthesis of precursors for anthocyanin metabolism explaining the high anthocyanins content of PFSP (Section 3.2.1).
Dietary fiber is defined as a group of non-digestible carbohydrates that can lower blood glucose and act as substrates in the intestinal tract reducing digestive tract disorders, among other functions [52,53]. PFSP roots present around 16% DW total dietary fiber, having an insoluble fiber content higher than the soluble fiber content (Table 1). However, the content of this nutrient varies in other parts of the PFSP considered as by-products, being higher in peels containing more than 60% dietary fiber, of which 77.6% is insoluble dietary fiber [54].
Purple-fleshed sweet potato root has a low protein content of 2.33 g/100 g DW (Table 1), with the finding that the crude protein extracts are rich in amino acids indicating that glutamic acid, aspartate, arginine, alanine, and leucine had the five highest amino acids contents, with 565.75 mg/kg, 479.74 mg/kg, 413.54 mg/kg, 371.87 mg/kg, and 336.67 mg/kg, respectively [55,56]. Despite the low protein content of the flesh, some studies have revealed a high protein content reached in the leaves ranging from 16.2 to 30.3 g/100 g DW across diverse cultivars [57,58]. In addition, some studies have indicated that polysaccharides from PFSP contain proportions of proteins and uric acids that could enhance their antioxidant activities [59].
Table 1.
Proximal composition of the edible part of PFSP (flesh).
| Nutrient | Value | Ref. | |
|---|---|---|---|
| Moisture | (g/100 g FW) | 63.51 ± 0.20 | [60] |
| Ash | (g/100 g DW) | 2.06 ± 0.01 | [61] |
| Proteins | (g/100 g DW) | 2.33 ± 0.04 | [62] |
| Crude Fat | (g/100 g DW) | 0.51 ± 0.04 | [63] |
| Total Dietary Fiber | (g/100 g DW) | 15.8 ± 0.50 | [64] |
| Insoluble Dietary Fiber | (g/100 g DW) | 8.4 ± 1.30 | [64] |
| Soluble Dietary Fiber | (g/100 g DW) | 7.4 ± 1.40 | [64] |
| Carbohydrates | (g/100 g DW) | 79.10 ± 0.03 | [65] |
| Starch | (g/100 g DW) | 56.7 ± 0.35 | [63] |
| RDS | (%) | 46.1 ± 0.20 | [66] |
| SDS | (%) | 10.4 ± 0.20 | [66] |
| RS | (%) | 43.5 ± 0.20 | [66] |
| Reducing Sugar | (g/100 g DW) | 3.09 ± 0.01 | [63] |
DW: dry weight; FW: fresh weight; RDS: rapidly digestible starch; SDS: slowly digestible starch; RS: resistant starch.
3.2. Bioactive Compounds
More than 100 different bioactive compounds have been identified from diverse PFSP parts, including flesh, skin, and leaves, such as flavonoids, non-flavonoids, carotenoids, or organic acids. However, a large variety of these bioactive compounds show values that vary sharply in different PFSP varieties from trace to elevated content due to factors such as the genotype, harvest, postharvest, or extraction procedures [67]. The following sections described the major bioactive compounds located in PFSP.
3.2.1. Anthocyanins
Anthocyanins, responsible for the color of purple-fleshed sweet potato, represent one of the most important constituent groups of PFSP. These compounds are secondary metabolites and water-soluble pigments belonging to the phenolic group with fundamental functions in the plant [68]. However, PFSP anthocyanins are usually glycated and acylated, with 3-sophoroside-5-glucoside and mono- or di-acylation with phenolic acids such as caffeic, ferulic, vanillic, p-coumaric, or p-hydroxybenzoic acids being the common glycation and acylation forms, respectively [69]. Concretely, these anthocyanins belong to the cyanidin or the peonidin type acylated with caffeic, ferulic, and p-hydroxybenzoic acids [70]. The acylation form guarantees heat stability, improving their application in heat-treated products [71]. Therefore, the importance of these compounds resides mainly in their function as antioxidant agents, being considered one of the most potent antioxidants of PFSP leaves, flesh, and peel [72,73].
The most abundant anthocyanins in PFSP are cyanidin 3-sophoroside-5-glucoside, cyanidin 3-(6,6′-dicaffeoyl-sophoroside)-5-glucoside, cyanidin 3-(6,6′-caffeoylphydroxybenzoyl sophoroside)-5-glucoside, cyanidin 3-(6,6′-caffeoylferuloylsophoroside)-5-glucoside, peonidin 3-(6,6′-dicaffeoylsophoroside)-5-glueoside, and peonidin 3-(6,6′-caffeoylphydroxybenzoyl sophoroside)-5-glucoside, which have been identified in diverse flesh, leaves, and peels as the richest anthocyanins. Although these cyanidins and peonidin derivatives are found mainly in purple sweet potato flesh [74], higher levels of cyanidin 3-(6″-feruloyl sophoroside)-5-glucoside and caffeoylated (cyanidin 3-sophoroside-5-glucoside) have been reported in leaves and peel [75]. Furthermore, the anthocyanins distribution in leaves and peel is similar to that in flesh with the former mainly consisting of cyanidin derivatives regardless of the cultivar.
However, relevant differences are appreciated in the supporting data indicating that TA seems higher in leaves and peels. In contrast, Su et al. [76] reported that the total contents of anthocyanins in PFSP var P40 leaves were much lower than those in the roots, suggesting an exceedingly diverse phenotype of anthocyanin biosynthesis between leaves and roots. This fact was attributed some years ago by Mano et al. [77] to the presence of a one-member transcriptional factor, MYB, which induces all structural anthocyanin biosynthesis genes, and is predominantly expressed in the roots but not in stems, leaves, or flowers either in the roots of orange-, yellow-, or white-fleshed varieties.
Finally, the increasing attention to PFSP anthocyanins, due to their high contents and multiple biological activities, has led to new anthocyanins constantly being identified in flesh, leaves, and peels, yet absent from stems [78,79].
3.2.2. Phenolic Acids
A rich diversity of phenolic acids has been successfully quantified and identified in PFSP (Table 2). Around 30 phenolic acids and their derivatives have been reported, specifically hydroxybenzoic, chlorogenic, caffeic, ferulic, and p-coumaric acids are the primary phenolic acids in the flesh, leaves, and peels of purple sweet potato. Among the multiple functions they contribute, it has been shown how some phenolic compounds, such as ferulic and caffeic acids, increase anthocyanin stability via intermolecular co-pigmentation shielding the flavylium cation from nucleophilic attack by water and improving its functional structure [80,81].
Table 2.
Micronutrients and bioactive compounds of diverse botanical parts of PFSP.
| Composition | Flesh | Leaves | Peel | |
|---|---|---|---|---|
| Minerals | ||||
| Macroelements | ||||
| K | (mg/100 g DW) | 1200 1 | 1786.56 14 | 5572 11 |
| Ca | (mg/100 g DW) | 98.20 1 | 0.26 14 | 134.30 11 |
| Mg | (mg/100 g DW) | 120.40 1 | 0.16 14 | 49.7 11 |
| Na | (mg/100 g DW) | 55.00 1 | 0.31 14 | 38310 11 |
| Microelements | ||||
| Zn | (mg/100 g DW) | 1.11 1 | 1.99 14 | 7.5 11 |
| Se | (mg/100 g DW) | 0.23 10 | - | - |
| Fe | (mg/100 g DW) | 1.95 1 | 22.01 14 | 4.10 11 |
| Mn | (mg/100 g DW) | 2.08 1 | 3.90 14 | 15 11 |
| Cu | (mg/100 g DW) | 0.69 1 | 0.005 14 | 0.94 11 |
| Vitamins | ||||
| Vitamin B1 | (mg/100 g DW) | 1.89 1 | - | - |
| Vitamin B2 | (mg/100 g DW) | 0.83 1 | - | - |
| Vitamin B3 | (mg/100 g DW) | 2.56 1 | - | - |
| Vitamin C | (mg/100 g DW) | 63.40 1 | 0.30 14 | - |
| (mg/100 mL DW) | - | - | 0.74 16 | |
| Vitamin E | (mg/100 g DW) | 11.2 15 | - | - |
| No Flavonoids | ||||
| Phenolic Compounds | ||||
| m-Hydroxybenzoic acid | (mg/g DW) | 0.11 2 | - | - |
| P-hydroxybenzoic acid | (mg/100 g DW) | 11.34 22 | - | - |
| 4-Hydroxybenzoic acid | (μg/g DW) | 3.67 23 | - | - |
| Protocatechuic acid-3-glucoside | (mg/g DW) | 0.12 2 | - | - |
| Chlorogenic acid | (mg/g DW) | 0.34 2 | 24.90 24 | - |
| Neochlorogenic acid | (mg/100 g DW) | - | 158.40 24 | - |
| Cryptochlorogenic acid | (mg/g DW) | 0.07 2 | 2.06 24 | - |
| Isochlorogenic acid | (mg/g DW) | 0.20 2 | - | - |
| Caffeic acid | (mg/g DW) | 0.17 6 | - | 0.34 25 |
| (mg/Kg DW) | - | 629.45 17 | - | |
| Caffeoyl-hexoside | (mg/100 g DW) | - | - | 142 25 |
| 5-O-caffeoylquinic acid | (mg/100 g DW) | 47 | 93 | 245.30 25 |
| 3,4-di-O-caffeoylqunic acid | (mg/g DW) | - | 3.79 24 | 0.50 25 |
| 3,5-di-O-caffeoylqunic acid | (mg/g DW) | - | 4.16 24 | 6.07 25 |
| Dicaffeoyl quinic acid isomer 1 | (mg/g DW) | 4.76 2 | - | - |
| (mg/Kg DW) | - | 1051 17 | - | |
| Dicaffeoyl quinic acid isomer 2 | (mg/g DW) | 2.56 2 | - | - |
| (mg/Kg DW) | - | 191.32 17 | - | |
| p-Coumaric acid | (mg/g DW) | 0.72 6 | - | - |
| Trans-p-coumaric acid | (mg/100 g DW) | 5 04 22 | - | - |
| Coumaroyl-hexoside | (mg/100 g DW) | - | - | 60 25 |
| Ferulic acid | (mg/g DW) | 0.15 2 | - | - |
| Feruloyl glucose | (mg/g DW) | 8.09 2 | - | - |
| Feruloyl sucrose | (mg/g DW) | 9.52 2 | - | - |
| Feruloylquinic acid | (mg/100 g DW) | - | - | 22.10 25 |
| 3-Feruloyl quinic acid | (mg/g DW) | 10.62 2 | - | - |
| 4-Feruloyl quinic acid | (mg/g DW) | 11.77 2 | - | - |
| 1,5-Diferuloyl quinic acid | (mg/g DW) | 29.56 2 | - | - |
| 3-Feruloyl-4-caffeoyl quinic acid | (mg/g DW) | 11.77 2 | - | - |
| 1-Feruloyl-5-caffeoyl quinic acid | (mg/g DW) | 191.57 2 | - | - |
| Salicylic acid | (μg/g DW) | 197.88 23 | - | - |
| Protocatechuic acid | (μg/g DW) | 74.72 23 | - | - |
| Vanillic acid | (mg/100 g DW) | 1.98 22 | - | - |
| Flavonoids | ||||
| Anthocyanins | ||||
| Cyanidin 3-sophoroside-5-glucoside | (mg PN3GE/kg DW) | 312.10 7 | 3.80 7 | - |
| (mg/100 g DW) | - | - | 50.6 25 | |
| Cyanidin 3-(6″-caffeoyl sophoroside)-5-glucoside | (mg/100 g DW) | 58.00 19 | - | 3.6 25 |
| Cyanidin 3-dicaffeoyl sophoroside-5-glucoside | (mg/g DW) | 12.20 20 | - | - |
| Cyanidin 3-(6″-feruloyl sophoroside)-5-glucoside | (mg/100 g DW) | 95.00 19 | - | 178.4 25 |
| Cyanidin 3-caffeoyl-p-gydroxybenzoyl sophoroside-5-glucoside | (mg/g DW) | 14.80 20 | - | - |
| Cyanidin 3-caffeoyl-feruloyl sophoroside-5-glucoside | (mg/g DW) | 16.20 20 | - | - |
| Peonidin 3-sophoroside-5-glucoside | (mg PN3GE/kg DW) | 52.90 7 | 0.80 7 | - |
| (mg/100 g DW) | - | - | 120 25 | |
| p-hydroxybenzoylated (Cyanidin 3-sophoreside-5-glucoside) | (mg PN3GE/kg DW) | 604.60 7 | 2.70 7 | - |
| Caffeoylated (Cyanidin 3-sophoroside-5-glucoside) | (mg PN3GE/kg DW) | 180.10 7 | 3.30 7 | - |
| p-hydroxybenzoylated (Peonidin 3-sophoroside-5-glucoside) | (mg PN3GE/kg DW) | 132.80 7 | 1.00 7 | - |
| Caffeoylated (Peonidin 3-sopheroside-5-glucoside) | (mg PN3GE/kg DW) | 46.60 7 | 1.00 7 | - |
| Feruloylated (Cyanidin 3-sophoroside-5-glucoside) | (mg PN3GE/kg DW) | 297 7 | 1.10 7 | - |
| Cyanidin 3-(6,6′-dicaffeoyl-sophoroside)-5-glucoside | (mg PN3GE/kg DW) | 1481.40 7 | 11.60 7 | - |
| Cyanidin 3-(6,6′-caffeoylphydroxybenzoyl sophoroside)-5-glucoside | (mg PN3GE/kg DW) | 5667.90 7 | 9.80 7 | - |
| Cyanidin 3-(6,6′-caffeoylferuloylsophoroside)-5-glucoside | (mg PN3GE/kg DW) | 1877.30 7 | 1.90 7 | - |
| Peonidin 3-(6″-feruloyl sophoroside)-5-glucoside | (mg/100 g DW) | 29 19 | - | - |
| Peonidin 3-(6,6′-dicaffeoylsophoroside)-5-glueoside | (mg PN3GE/kg DW) | 381.60 7 | 2.30 7 | - |
| Peonidin 3-feruloyl-p-hydroxybenzoyl sophoroside-5-glucoside | (mg/g DW) | 5.81 20 | - | 1.12 25 |
| Peonidin 3-(6″, 6‴-diferuloyl sophoroside)-5-glucoside | (mg/g DW) | 2.43 20 | - | - |
| Peonidin 3-(6‴-caffeoyl sophoroside)-5-glucoside | (mg/g DW) | 2.29 20 | - | - |
| Peonidin 3-feruloyl sophoroside-5-glucoside | (mg/g DW) | 7.12 20 | - | - |
| Peonidin 3-dicaffeoyl sophoroside-5-glucoside | (mg/g DW) | 57.90 20 | - | - |
| Peonidin 3-caffeoyl sophoroside-5-glucoside | (mg/100 g DW) | 275 19 | - | - |
| Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside | (mg/100 g DW) | 116 19 | - | - |
| Peonidin 3-(6,6′-caffeoylphydroxybenzoyl sophoroside)-5-glucoside | (mg PN3GE/kg DW) | 1620.90 7 | 1.30 7 | - |
| Peonidin 3-(6,6′-caffeoylferuloylsophoroside)-5-glucoside | (mg PN3GE/kg DW) | 344.30 7 | 1.00 7 | - |
| Peonidin 3-caffeoyl-feruloyl sophoroside-5-glucoside | (mg/g DW) | 69.20 20 | - | - |
| Peonidin 3-caffeoyl-p-coumarylsophoroside-5-glucoside | (mg PN3GE/kg DW) | 59.30 7 | 1.10 7 | - |
| Peonidin 3-coumaryl-p-hydroxybenzoyl sophoroside-5-glucoside | (mg/g DW) | 1.81 20 | - | - |
| Cyanidin-based anthocyanin | (mg/kg DW) | 6964 8 | - | - |
| (μg/g FW) | - | 83.32 13 | - | |
| Peonidin-based anthocyanin | (mg/kg DW) | 2269 8 | - | - |
| (μg/g FW) | - | 44.01 13 | - | |
| Flavonols and Flavones | ||||
| Kaempferol | (μg/g DW) | 23.38 21 | - | - |
| (mg/100 g DW) | nd 9 | 60.9 9 | nd 9 | |
| Luteolin | (μg/g DW) | 15.17 21 | - | - |
| Myricetin | (μg/g DW) | 152.11 21 | - | - |
| (mg/100 g DW) | 42.1 9 | 36.7 9 | 20.6 9 | |
| Apigenin-6-C-glucoside-8-C-arabinoside | (mg/g DW) | 0.82 2 | - | - |
| Naringenin | (mg/g DW) | 1.12 2 | - | - |
| Naringenin-glucoside | (mg/g DW) | 0.24 2 | - | - |
| Isoquercitin | (mg/100 g DW) | 59.9 9 | 268.3 9 | 33.9 9 |
| Quercetin-3-galactoside | (mg/g DW) | 0.91 2 | 0.78 24 | - |
| (mg/Kg DW) | - | 455.13 17 | - | |
| Quercetin diglucoside | (mg/g DW) | 1.02 2 | - | - |
| Isorhamnetin-3-O-glucoside | (mg/g DW) | 0.94 2 | - | - |
| Isorhamnetin-3-glucoside 4-rhamnoside | (mg/g DW) | 2.12 2 | - | - |
| Epicatechin derivative | (mg/g DW) | 0.10 2 | - | - |
| 3′-O-Methylepicatechin derivatives | (mg/g DW) | 0.05 2 | - | - |
| 4′-Methyl-epigallocatechin derivatives | (mg/g DW) | 0.09 2 | - | - |
| 4′-Methyl-epigallocatechin derivatives | (mg/g DW) | 0.02 2 | - | - |
| Carotenoids | ||||
| Lutein | (mg/100 g DW) | - | 100.22 17 | - |
| (μg/g DW) | 0.28 21 | - | - | |
| Zeaxanthin | (mg/100 g DW) | - | 33.60 17 | - |
| (μg/g DW) | 0.11 21 | |||
| β-Cryptoxanthin | (mg/100 g DW) | 0.07 18 | - | - |
| α-Carotene | (μg/g DW) | nd 21 | - | - |
| (All E)-β-Carotene | (μg/g DW) | 1.53 21 | - | - |
| (9Z)-β-Carotene | (μg/g DW) | 0.02 21 | - | - |
| (13Z)-β-Carotene | (μg/g DW) | 0.28 21 | - | - |
| All-Trans-β-carotene | (mg/100 g DW) | 0.30 18 | 56.94 17 | - |
| Cis-β-carotene | (mg/100 g DW) | - | 7.11 17 | - |
| ABTS | (mg AAE/100 g DW) | 710 9 | 5300 9 | 880 9 |
| DPPH | (mg TE/100 mg DW) | 0.77 5 | - | 0.03 11 |
| (mg AAE/100 g DW) | 330 9 | 2920 9 | 410 9 | |
| FRAP | (mg TE/100 g DW) | 46 9 | 550 9 | 47 9 |
| TA | (mg GAE/100 mg DW) | 94.80 4 | - | - |
| (mg CGE/100 g DW) | 170 9 | 1010 9 | 230 9 | |
| TPC | (mg GAE/100 mg DW) | 167.40 3 | 3.68 7 | 289.01 12 |
DW: dry weight; FW: fresh weight; FRAP: ferric reducing ability of plasma; TA: total content of anthocyanins; TPC: total content of phenolic compounds; GAE: gallic acid equivalent; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic) acid; nd: not detected; PN3GE: peonidin 3-glucoside equivalent; AAE: ascorbic acid equivalent; TE: Trolox equivalent; CGE: cyandin-3-glucoside equivalent. 1 Mean values of PFSP cultivated in Wanju-Gun rural guidance center [65]; 2 Mean values of dry hydroalcoholic purple sweet potato [82]; 3 Mean values of the purple-fleshed sweet potato genotype “JNRX12” [83]; 4 Mean values of the purple-fleshed sweet potato genotypes “JNRX2” [83]; 5 Mean values of the purple-fleshed sweet potato genotypes “Trabuca” [83]; 6 Mean values of the purple-fleshed sweet potato genotype “JNRX1” [83]; 7 Mean values leaves or flesh of PFSP “P40” [74]; 8 Mean values of the purple-fleshed sweet potato variety “Gyebone108” [84]; 9 Mean values of the PFSP variety “Yuzi No. 7” [85]; 10 Mean values of the PFSP variety “Jishu No.2” [63]; 11 Mean value of a PFSP variety bought from the King’s market, Akure, Ondo State, Nigeria [86]; 12 Mean value of Ipomoea batatas Lam cv. Anggun 1 harvested in Malaysia [87]; 13 Mean value of the PFSP cultivar Fushu No. 317 [74]; 14 Mean value of PFSP from community gardens in Koya Koso Jayapura [88]; 15 Mean value of PFSP accession from a farmer’s field for planting material [89]; 16 Mean value of Ipomoea batatas Lam cv. Anggun 1 [90]; 17 Mean value of PFSP leaves of Purple-purple X VS-8 WAP, and Purple-purple X TMS-16 WAP samples [91]; 18 Mean value of the PFSP variety Yeonjami [92]; 19 Mean value of the PFSP variety P40 [93]; 20 Mean value of Ipomoea batatas L. cultivar Eshu No. 8 [33]; 21 Mean value of the PFSP cultivar Sinjami [94]; 22 Mean value of the PFSP cultivar Dphi potato 1, Wuxi, and Jiangsu [95]; 23 Mean value of the PFSP cv. Yeonjami [96]; 24 Mean value of a purple sweet potato cultivar [97]; 25 Mean value of the PFSP genotype Ea3-1 [75].
Chlorogenic and caffeic acid and their derivatives are the most widely distributed phenolic acids in the leaves and peels of purple sweet potato, while salicylic and ferulic acids are more frequently present in the flesh. As mentioned, Jang et al. [98] quantified that 3,5-dicaffeoylquinic acid was the most abundant in the leaves of all varieties analyzed. Another research study conducted by Ooi et al. [99] evaluated the phenolic content of the skin and flesh from a purple sweet potato variety obtaining a similar total phenolic content in flesh and skin with 52.80 ± 0.84 and 48.19 ± 1.29 mg GAE/g, respectively. In addition, purple sweet potato varieties contain a significantly higher total phenolic content as compared to yellow and orange varieties due to genotype variations that influence the accumulation and types of synthesized phenolic acids [100,101].
Finally, phenolic acids are not distributed uniformly through the plant, suggesting that their distribution and content depend on a set of factors such as the extraction method, solvent, genotype, plant part, and environment, with flesh color being a relevant factor affecting the total content of phenols in sweet potatoes that could explain the wide range of data supported [102,103,104].
3.2.3. Flavonols, Flavones, Carotenoids, and Other Bioactive Compounds
Non-anthocyanins flavonoids have also been identified in PFSP including flavonols, flavanes, and flavones [105]. A good source of flavonols and flavones is found in the flesh of the tuber where kaempferol, luteolin, and myricetin are the major constituents, while quercetin is mainly found in their leaves and peel. These compounds play significant biological regulatory functions such as their remarkable effect on protein regulation by reversibly combining with various proteins and enzymes in the body [106]. Furthermore, 18 organic acids from the roots of PFSP have been reported such as acetic, lactic, or pyruvic acids, although more studies are needed to quantify [107].
On the other hand, the considerable number of carotenoids located in the flesh and leaves of the tuber make it a valuable source of these compounds. Lutein, zeaxanthin, and carotene are the main carotenoid compounds identified. However, total carotenoid content varies depending on the extraction and drying method, as well as environmental factors where climate temperature influences the total carotenoid content in vegetables and fruit [108].
3.3. Minerals and Vitamins
PFSP and its by-products represent a rich source of essential minerals for the organism [58]. Compared to the mineral composition of other vegetables reported in the literature, purple-fleshed sweet potato presents a good source of Na, and mainly K, and Mg (Table 2) [109]. Deficiency of these minerals can lead to several metabolic disorders, such as DNA and RNA synthesis [110], neurological [111], and cardiovascular alterations [112]. The concentration of macro- and microelements varies depending on the botanical part of I. batatas (L.) Lam [113], although generally PFSP can cover part of the mineral requirements [86]. However, relevant differences could be attributed to genotype variation [41]. A significant mineral amount can be found in their flesh, leaves, and peel, with major values in most of the evaluated minerals in the peels compared to the flesh and leaves.
The purple-fleshed sweet potato has a high content of vitamins C and E (Table 2); although the higher contents are found in the flesh, some studies have found low quantities in peels and leaves. Furthermore, B group vitamins, such as B1, B2, and B3, have been detected in the flesh of the tuber in minor proportions. In addition, it has been shown that the content of vitamin C in PFSP is reduced after cooking methods such as boiling and frying with a loss of 72, and 61%, respectively [114]. Purple sweet potato has a high vitamin C content, reaching similar values to those of lemon, oranges, and grapefruits [115]. Vitamin C is involved in the biosynthesis of collagen, cholesterol metabolism, modulation of the iron pathway, and scavenging reactive oxygen and nitrogen species as part of its antioxidant mechanism [116].
4. Health Benefits
4.1. Antioxidant Activity
As described above, PFSP contains many bioactive compounds, such as caffeic, chlorogenic, and ferulic acid derivatives, naringenin, quercetin, and diverse anthocyanins. Apart from performing essential functions in the plant, these compounds, once ingested, can perform protective functions against the generation of ROS and oxidative damage, as well as playing a crucial role in the color stabilization of anthocyanins [117,118]. Even though ROS act as signaling molecules in physiological functions, the overproduction of ROS can damage lipids, membranes, proteins, or DNA, resulting in oxidative stress and damage [119,120]. Phenolic compounds provide purple-fleshed sweet potato with a high antioxidant capacity, showing significantly higher ABTS (2,2-azino-bis-3-(ethylbenzothiazoline-6-sulfonic acid)) and ORAC (oxygen radical absorbance capacity) activity, and up to 10 times higher DPPH (2,2-diphenyl-1-picrylhydrazyl) values than other sweet potato varieties such as white-fleshed, yellow-fleshed, or orange-fleshed sweet potato [121,122]. This high antioxidant capacity is mainly found in the peels, which contain the major levels of both total and individual phenolic compounds, although the rest of the PFSP by-products represent a good source of these phenolic compounds [75].
Several studies have observed the potential action of anthocyanins and polyphenols from PFSP against oxidative stress at in vitro levels in different types of cell lines. Esatbeyoglu et al. [123] reported that the application of three different polyphenol extracts from PFSP, mainly peonidin, and cyanidin derivatives, (1, 5, 10, 25, 50, and 75 µg/mL) in the Huh7 human cell line, resulting in a decrease in xanthine oxidase enzyme (XO), an enzyme involved in the generation of reactive oxygen species [124], and an increase in nuclear factor E2-related factor 2 transcription (Nrf2), a transcription factor that regulates the expression of genes involved in the oxidative stress response [125]. Ye et al. [126] observed similar results in the protective effects of PFSP anthocyanin on PC12 cells, obtaining a reduction in intracellular reactive oxygen species (ROS) generation and lipid peroxidation, in a dose-dependent manner. In addition, Insanu et al. [127] found that the higher content of phenolic acids and flavonoids had higher antioxidative activity, identifying that the highest antioxidative activity was in the leaves.
The antioxidant effect of PFSP has also been confirmed in in vivo experiments [128,129]. Zhang et al. [130] investigated the effects of HFD-induced rats treated with PFSP anthocyanin extracts daily for 6 weeks, reporting a level reduction in ROS and an inhibition of the receptor of advanced glycation end products (AGEs). It involved anti-obesity effects via attenuation of oxidative stress. Chang et al. [131] explored the effect of consumption of PFSP leaves on oxidative stress markers in healthy, nontrained, young male populations, revealing that consuming a high-polyphenol diet can modulate antioxidative status and decrease exercise-induced oxidative damage and pro-inflammatory cytokine secretion. In addition, Kano et al. [132] evaluated the antioxidative activity of anthocyanins from the PFSP cultivar Ayamurasaki in in vitro, and vivo trials, with rats and volunteers. The in vitro results suggested that the PFSP anthocyanin pigment showed higher radical-scavenging activity than ones from grape skin, elderberry, red cabbage, purple corn, and even ascorbic acid. Meanwhile, the in vivo results revealed that the urine of rats and humans that ingested PSFP increased their radical-scavenging activity.
However, a relevant point in in vivo experiments is the stability and antioxidant activity of these bioactive compounds after gastrointestinal digestion. Yang et al. [133] investigated the bioaccessibility and antioxidant activity of PFSP anthocyanins after intestinal digestion, obtaining a significant decline after digestion, although its stability depended on the type and number of acylated groups.
Several mechanisms explain the antioxidant action of the bioactive compounds present in PFSP. Oxidative stress is an imbalance between the production of ROS and their elimination by a protective mechanism [134]. Anthocyanins can induce the expression of antioxidants via the nuclear erythroid 2-related factor 2 (Nrf2) pathway and by reducing inflammation [135]. Polyphenols can reduce the catalytic activity of enzymes involved in ROS generation such as nitric oxide synthases (NOs), or XO [136,137]. However, the antioxidant capacity of these bioactive compounds can be attributed to their specific structural characteristics. Phenolic compounds and flavonoids react with ROS and thus terminate the chain reaction before cell viability is seriously affected [138]. For instance, chlorogenic acids possess one to two aromatic rings linked to hydroxyl groups, which lead to forming complexes with free radicals that are quickly broken down into further products that cannot generate any free radicals [139]. In fact, some studies have reported that antioxidant activity was positively correlated with the TPC of leaves and roots and the TA content of roots in sweet potato [140,141].
4.2. Hepatoprotective Action
The liver is an essential organ for a variety of physiological processes including macronutrients, alcohol, and drug metabolism, detoxification, endocrine control, cholesterol homeostasis, or immune defense [142]. Liver disease is the eleventh-leading cause of death annually and accounts for 4% of all deaths worldwide [143]. Dysfunction is associated with numerous liver diseases, such as alcohol-associated liver disease (AALD), non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), drug-induced liver injury (DILI), cholestasis, viral hepatitis, and hepatocellular carcinoma (HCC) [144]. Recently, it has been suggested that the antioxidant and anti-inflammatory properties of PFSP bioactive constituents, such as phenolic compounds, anthocyanins, or polysaccharides, could prevent liver diseases by a reduction in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzyme levels [145,146].
Polysaccharides exert hepatoprotective effects by modulating different signaling pathways. They downregulate key molecule expressions in the TLR4-P2X7R/NLRP3 signaling pathway, which controls inflammatory responses. Furthermore, polysaccharides activate the PI3K/AKT signaling pathway to recover redox balance and inhibit the expression of NF-kβ to mitigate pro-inflammatory cytokine expression (Table 3). This dual function ameliorates oxidative stress and liver inflammation [147]. The study by Sun et al. [148] observed changes in liver weight and size, increased scavenging activity and reducing power, and increased levels of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione (GSH) after the consumption of PFSP polysaccharides for 31 days in mice, as well as a reduction in ALT and AST and the hepatic lipid peroxidation marker malondialdehyde (MDA). Another study observed the hepatoprotective effect of PFSP extracts in CCl4-induced oxidative hepatotoxicity fibrosis mice, reporting a reduction in serum levels of ALT, AST, and MDA, and increased SOD and GPx activity levels [149].
On the other hand, a relevant number of studies have found changes in the lipid profile after PFSP intake [150]. The reduction in triacylglycerides (TG) and total cholesterol (TC) decreases the lipid hepatic accumulation after the intake of PFSP leaves, suggesting that PFSP could exert a hypolipidemic action [151]. This effect could be attributed to the presence of chlorogenic acid, and its derivatives, a PFSP leaf constituent that regulates lipid metabolism by reducing preadipocyte differentiation and inhibiting fatty acids and total cholesterol synthesis [152,153,154]. Therefore, the hepatoprotective effect of PFSP extracts is achieved through multiples signaling pathways and mechanisms.
Table 3.
Health benefits of different PFSP parts.
| Type | Botanical Part | Experiment Design | Results | Ref. |
|---|---|---|---|---|
| Antioxidant activity | ||||
| Clinical trial | Leaves | Consumption of PSPL (200 g/day) by basketball players for 7 weeks | ↑ Plasma polyphenol concentration, vitamin E and C levels, and LDL lag time | [129] |
| ↓ 8-OHdG | ||||
| Clinical trial | Leaves | Healthy adults were treated with PSPL (200 g/day) for 7 weeks | ↑ LDL lag time, glutathione concentration, and urinary total phenol excretion | [128] |
| ↓ 8-OHdG | ||||
| In vivo | Flesh | Eight-week-old male Sprague Dawley strain rats were administered with 100, 200, and 400 mg of PFSP anthocyanin/kg b.w. once a day for 6 weeks | ↓ ROS and AGESs | [130] |
| In vitro | Leaves | Evaluation of the inhibitory effect of PFSP leaves on endothelial cell-mediated LDL oxidation | ↑ Free radical scavenging activity | [155] |
| ↑ Lag time for LDL oxidation | ||||
| In vitro | Stem, Leaves, and Flesh | Application of extracts from PFSP stems, leaves, and flesh to evaluate in vitro antioxidant activities by DPPH and CUPRAC assay | ↑ DPPH and CUPRAC activity | [127] |
| ↑ TPC and TFC level | ||||
| In vitro | Flesh | Huh7 cells were treated with three different PFSP-derived polyphenol extracts (1, 5, 10, 25, 50, and 75 μg/mL) for 24 h | ↓ α-amylase, α-glucosidase, and XO enzyme | [123] |
| ↑ Nrf2 factor and PON 1 transactivation | ||||
| In vitro | Flesh and Peel | Isolation and quantification of colorless caffeoyl compounds from PFSP to test their antioxidant abilities | ↑ Total antioxidant capacity | [156] |
| ↑ Reducing power and DPPH | ||||
| In vivo | Flesh and Peel | Six healthy volunteers were administered with a PFSP beverage, rich in anthocyanins (2.49 mg/mL), collecting blood and urine samples at fixed times (0, 0.5, 1, 2, 3, 4, 6, 8, and 24 h) after feeding | ↑ Urinary DPPH activity | [132] |
| In vitro | Flesh | PFSP species Guijingshu 09-7 was treated to four different cooking methods (raw, boiling, roasting, and steaming) | ↑ FRAP and DPPH | [157] |
| In vitro | Flesh | Utilization of PFSP anthocyanin (0, 0.1, 1, 10, 20, 40, 50, 80, 100, and 200 g/mL) on PC12 cells for 24 h | ↓ Aβ-induced cytotoxicity and Ca2+ concentration | [158] |
| ↓ Intracellular ROS generation and LPO | ||||
| Clinical trial | Leaves | PSPL (200 g/day for lunch and dinner) was consumed by 15 healthy male volunteer for 5 weeks | ↑ TPC and FRAP | [131] |
| ↓ TBARS, plasma PC, oxidative damage, and IL-6 | ||||
| In vitro | Flesh | PFSP anthocyanins were isolated from Ipomoea Batatas Poir Cv (5–20 µg/mL) and administered in PC12 cells for 24 h | ↓ Aβ-induced toxicity, ROS, and lipid peroxidation levels | [126] |
| ↓ Ca2+ intracellular concentration, and mitochondria dysfunction | ||||
| Hepatoprotective action | ||||
| In vivo | Leaves | Five-week-old male C57BL/6 mice received alcohol + PSPE (400 mg/kg bw for 7 days) | ↓ ALT and AST enzyme levels | [151] |
| ↓ Blood alcohol concentration and inflammatory cells | ||||
| ↓ TG and TC levels | ||||
| In vivo | Flesh | Application of PSPP-1 (200 and 400 mg/kg; once daily until Day 28) in control mice (without any liver injury) and concanavalin A-induced liver injury mice | ↓ ALT and AST enzyme levels | [147] |
| ↑ SOD and GSH levels | ||||
| ↓ MDA level | ||||
| ↓ TNF-α and IFN-γ levels | ||||
| In vivo | Flesh | Application of a novel polysaccharide (PSPP-A) extracted and isolated from PFSP in C57BL/6J male mice fed for 8 weeks with a high-fat diet blended with PSPP-A (100 mg/kg, 200 mg/kg and 400 mg/kg) | ↓ Body weight and liver index | [145] |
| ↓ ALT and AST content | ||||
| ↓ TG and TC levels | ||||
| In vivo | Flesh | Female ICR mice were treated with three kinds of polysaccharides obtained from PFSP (100, 200 and 400 mg/kg bw of each extract per day) for 31 days | ↓ Relative liver weight | [148] |
| ↓ ALT, AST, alkaline phosphatase and MDA levels | ||||
| ↑ SOD, CAT, and GSH-Px enzymes | ||||
| ↑ GSH and T-AOC levels | ||||
| In vivo | Flesh | Utilization of anthocyanins extract from PFSP (227.5, 455, and 910 mg/kg bw) in male mice after hepatic fibrosis induced by carbon tetrachloride for 3 weeks | ↓ Relative liver weight | [149] |
| ↓ ALT, AST, and MDA levels | ||||
| ↑ SOD and GPx activity levels | ||||
| In vitro | Flesh | HepG2 hepatocytes were treated with AF (0, 50, 100, and 200 μg/mL) | ↑ AMPK and ACC phosphorylation | [150] |
| ↓ TG and TC levels | ||||
| In vitro | Flesh | HepG2 cells were treated with raw, steamed, microwaving and roasted PFSP (100 μg/mL) for 24 h. | ↓ ROS, GPx, and GR | [159] |
| ↑ GSH levels | ||||
| ↑ HO-1, NQO1, and GCLC expression | ||||
| Anti-inflammatory effect | ||||
| In vitro | Leaves | HAECs were treated with 100 μg/mL PSPLE for 24 h | ↓ TNF-α-induced monocyte-endothelial cell adhesion | [160] |
| ↓ ERK1, and ERK2 expression | ||||
| ↓ VCAM-1, IL-8, and CD40 expression | ||||
| In vitro | Flesh | Effect of PFSP TNG 75 extracts ((1, 2, 3, 4, and 5 mg/mL) on RAW264.7 murine macrophage cells for 24 h | ↓ NO production | [161] |
| ↓ NF-kβ, IL-6, and TNF-α levels | ||||
| In vitro | Flesh | Application of two anthocyanins, FAC-PSP and p-BAC-PSP (25, 50, 100, and 200 μg/mL), on RAW264.7 macrophages | ↓ NO production level | [162] |
| ↓ NO production level | ||||
| In vivo | Flesh | (DSS)-induced colitis mice treated with 400 mg/kg of ASPP once per day for 30 days | ↓ TNF-α release level | [163] |
| ↓ IL-1β, IL-6, and TNF-α | ||||
| In vitro | Leaves | Monosodium urate-induced RAW264.7 cells were treated with different concentrations (20, 40, 60 μg/mL) of PSPLP for 24 h | ↑ SCFAs contents | [164] |
| ↓ IL-1β, IL-6, and TNF-α | ||||
| In vivo | Flesh | Male Wistar rats were given purple sweet potato extract (400 mg/kg/day for 9 days, once per day) | ↓ IL-1β, MDA, COMP, and MMP-3 levels | [165] |
| ↑ chondrocytes | ||||
| In vitro | Leaves | Differentiated 3T3-L1 cells treated with PSPLE (0, 1, 2, and 4 mg/mL) for 72 h | ↓ IL-6 and TNF-α expression | [166] |
| ↑ PARP and cellular apoptosis | ||||
| Hypoglycemic and antidiabetic effect | ||||
| In vivo | Leaves | Alloxan-induced diabetic male Wistar rats of 8–10 weeks old were treated with purple sweet potato leaves (50, 100, and 200 mg/kg bw) for 15 days | ↓ MDA and blood glucose levels | [167] |
| ↑ Pancreatic histopathological features | ||||
| In vitro | Flesh | Utilization of three anthocyanins (3-caffeoyl-phydroxybenzoyl-sophoroside-5-glucoside, peonidin 3-caffeoyl sophoroside-5-glucoside, and peonidin 3-(6″-caffeoyl-6‴-feruloyl sophoroside)-5-glucoside) from PFSP (0, 10, and 50 μg/mL; 3 h) on human HepG2 cells | ↓ Glucose production | [168] |
| In vivo | Flesh | Evaluating the effect of anthocyanin on fasting blood glucose levels in 6-week-old male C57BL/6 mice fed a 60% high-fat diet for 14 weeks | ↓ Glucose production | [168] |
| In vivo | Flesh | Application of diacylated AF-PSPs (25 and 50 mg/kg bw) into free (SPF)-grade male Kun-Ming strain mice induced by a high-fructose/high-fat diet for nine weeks | ↓ TG, TC, MDA, fasting blood glucose values, and blood glucose levels | [169] |
| ↑ T–SOD activity | ||||
| In vivo | Flesh | Male mice were fed with a high-fat diet and STZ to induce T2DM. The model mice were treated with 0, 227.5, 455, or 910 mg/kg bw of PSPA for ten days | ↓ Blood glucose levels | [170] |
| ↑ GSH-Px level | ||||
| In vitro | Leaves | Application of four crude extracts (IBH, IBM, IBB, and IBW; 0.1 mg/mL) in 3T3-L1 preadipocytes | ↑ PI3K, AKT, and Glut4 phosphorylation | [171] |
| ↑ Glucose uptake | ||||
| Clinical trial | Flesh and Peel | Double blinded pre-post test control group design in patients with T2DM (75 mL of PFSP that contained 11 g of anthocyanins—3 times per day 30 min after meal) for 4 weeks | ↓ MDA | [172] |
| ↓ Fasting plasma glucose and 2hpppg levels | ||||
| ↓ Glycated albumin level | ||||
| Neuroprotective effect | ||||
| In vivo | Flesh | Application of PFSP anthocyanin (700 mg/kg/day) in eight-week-old C57BL 6J mice for 20 weeks by oral gavage | ↑ Memory function, HFD-induced impairment mouse behavior, and IL-10 level | [173] |
| ↓ Body weight, fat content, hyperlipemia, and endotoxin level | ||||
| ↓ COX-2, TNF-α, IL-1β, IL-6, iNOS, ERK, JNK, and NF-kβ | ||||
| In vivo | Flesh | Utilization of purple sweet potato water extract (200 mg/kg bw*day) in d-galactose-induced male Wistar rats for 70 days | ↓ TNF-α, p53, and GFAP expression | [174] |
| ↑ BDNF levels and spatial working memory | ||||
| In vitro | Leaves | BV-2 microglia cells were treated with purple sweet potato leaf extract (10–200 µg/mL) for 24 h | ↓ NO, iNOS, COX-2, and TNF-α levels | [175] |
| In vivo | Flesh | 15-month-old D-galactose-induced male Kunming mice were treated with PFSP anthocyanins (500 mg/kg*day bw) for 8 weeks by oral gavage | ↓ Step-through latency, AGEs, Cu/Zn-SOD, and CAT activity | [176] |
| ↑ Spatial learning and memory ability | ||||
| ↓ JNK and cytochrome c levels | ||||
| In vivo | Flesh | Anthocyanins extracted from “Balinese” cultivar of PFSP administered to rat models of induced ischemic stroke | ↑ Bcl-2 expression | [177] |
| ↓ Cytochrome C, caspase-3 levels, and apoptosis rate | ||||
| In vivo | Flesh | 9-week-old male Kunming mice induced by D-galactose were administrated with PFSP anthocyanins (100 mg/kg*day) for 4 weeks via the oral route | ↓ GFAP, COX-2, NF-kβ, and iNOS expression | [178] |
| ↓ MDA content | ||||
| ↑ Cu/Zn-SOD and CAT activity | ||||
| Antimicrobial and prebiotic activity | ||||
| In vitro | Flesh | Application of five peonidin-based anthocyanins from PFSP (0, 0.5, 1, 1.5, 2, and 2.5 mg/mL) to test the growth of probiotics and harmful bacteria | ↑ Bifidobacterium bifidum, Bifidobacterium adolescentis, Bifidobacterium infantis, and Lactobacillus acidophilus | [179] |
| ↓ Staphylococcus aureus and Salmonella typhimurium | ||||
| In vivo | Flesh and peel | Three polysaccharides were extracted from PFSP and administered in female ICR mice (400 mg/kg bw) for 30 days by oral gavage | ↑ Bacteroidetes, Ruminococcaceae, Lachnospiraceae, Ruminococcus, and Oscillospir | [180] |
| ↓ Firmicutes, Proteobacteria, Alcaligenaceae, and Sutterella | ||||
| In vitro | Flesh and Peel | Utilization of PFSP anthocyanin to evaluate the modulatory effect on human intestinal microbiota using fecal samples from volunteers (1% w/v) | ↑ Bifidobacterium and Lactobacillus/Enterococcus spp. | [181] |
| ↓ Bacteroides-Prevotella and Clostridium histolyticum | ||||
| ↑ SCFA concentration | ||||
| In vivo | Flesh | 7-week-old male Fischer 344 rats were treated with PFSP polyphenols (1% bw) for 27 days | ↑ Dorea, cecal mucin, and cecal IgA level | [182] |
| ↓ Oscillospira and Bacteroides, and indole production | ||||
| In vitro | Flesh | Assessment of PFSP polyphenols (0.16%) by colonic fermentation using pig colonic digest under anaerobic conditions at 37 °C for 48 h | ↑ Eubacterium spp., Lactobacillus spp., Bifidobacterium spp., Collinsella stercoris, and Bulleidia p1630cJ | [183] |
| ↓ Clostridium spp. and Acidaminococcus spp. | ||||
| Hypouricemic action | ||||
| In vivo | Flesh | SPF grade 8-week male Kun-Ming induced-hyperuricemia mice were treated with PFSP anthocyanins (25 mg/kg bw) and allopurinol (2.5 and 5 mg/kg bw) | ↓ Serum uric acid level | [184] |
| ↓ TNF-α, IL-1β, IL-6, and TGF-β1 expression | ||||
| In vivo | Flesh | Oral application of PFSP anthocyanins (100 mg/kg bw) in three-week-old potassium oxonate-induced hyperuricemia ICR male mice | ↓ Serum acid uric concentration | [185] |
| In vitro | Flesh and Peel | PFSP anthocyanins were evaluated for their inhibitory activity on commercial XO by spectrophotometrically measuring the formation of UA | ↑ Inhibition XO activity rate | [186] |
| In vivo | Flesh and Peel | Hyperuricemia mice were administered with PFSP anthocyanins (25 and 100 mg/kg bw) orally for 7 days | ↓ Uric acid level | [32] |
| ↓ 5′-NT and XO enzyme activity | ||||
| In vivo | Flesh | Utilization of an anthocyanin-rich purple sweet potato extract (75, 150, and 300 mg/kg bw, once daily) in potassium oxonate-induced hyperuricemia male Kun-Ming strain mice for 7 days | ↓ Serum uric acid level | [187] |
| ↓ BUN and Cr levels | ||||
| Antitumoral and antimutation activity | ||||
| In vitro | Flesh | NALM6 human B-ALL cells were treated with PFSP anthocyanins (0, 20, 40, and 60 μg/mL) for 24 h | ↓ NALM6 cell viability and S100A4 protein expression | [188] |
| ↑ NALM6 cells apoptosis and p38 | ||||
| In vitro | Flesh | Utilization of three polysaccharides, PSPP1-1, PSPP2-1, and PSPP3-1, isolated from PFSP (100, 200, 300, 400, 500 μg/mL for SGC7901; 200, 400, 600, 800, 1000 μg/mL for SW620) on SGC7901 and SW620 tumor cells | ↑ % Inhibition of tumor cells rate | [59] |
| In vitro | Leaves and Flesh |
Application of anthocyanins isolated from the PFSP cultivar Bhu Krishma and the leaves of accession S-1467 (100, 200, and 400 μg/mL) in human mammalian epithelial cells (MCF-10A) | ↑ MCF-7, HeLa, and HCT-116 cells’ apoptosis | [189] |
| In vitro | Flesh | PFSP glucan was extracted and tested (0, 15.625, 31.25, 62.5, 125, 250, 500, and 1000 μg/mL) on HepG2, LOVO, MCF-7, LO2, GES-1, MCF-10A, NCM460, SGC-7901, and HGC-27 cells for 72 h | ↑ % Inhibition in liver, colonic, and breast cells | [190] |
| In vitro | Flesh | Human colon cancer HT-29 cells were treated with PFSP polysaccharide (0, 10, 20, 40, 80, 160, and 320 μg/mL) for 24, 36, and 48 h | ↓ Tumor cell viability | [191] |
| In vivo | Flesh | Evaluation of PFSP anthocyanin (100, 500, or 1000 mg/kg bw) in SPF-grade ICR mice implanted with mice S180 anal sarcoma cells for 5 weeks by oral gavage | ↑ % Inhibition of tumor cells rate | [192] |
| In vivo | Flesh and Peel | C57BL/6J-APCMIN/+ mice were treated with purple sweet potato flesh and peel (10%) for 18 weeks | ↓ Adenoma number | [193] |
FAC-PSP: free anthocyanin compounds from purple sweet potato; p-BAC-PSP: protein-bound anthocyanin compounds from purple sweet potato; NO: nitric oxide; TNF-α: tumor necrosis factor-α; PSPP1-1: purple sweet potato polysaccharide composed of rhamnose, xylose, glucose, and galactose and their corresponding molar ratios of 17.54:1.00:2.67:1.10; PSPP2-1: purple sweet potato polysaccharide composed of rhamnose and galactose; PSPP3-1: purple sweet potato polysaccharide composed of rhamnose, xylose, glucose, and galactose, and their corresponding molar ratios of 3.51:1.92:1.44:1.00; DSS: dextran sulphate sodium; ASPP: alkali-soluble polysaccharide from purple flesh sweet potato; IL: interleukin; PSPLP: purple sweet potato leaf polyphenols; PSPP-1: purple sweet potato polysaccharide of glucose, galacturonic acid, galactose, arabinose, rhamnose, and glucuronic acid (molar ratio 320:20:19:10:8:2); ALT: alanine aminotransferase; AST: aspartate aminotransferase; IFN-γ: interferon-γ; T-SOD/SOD: total superoxide dismutase; GSH: glutathione; MDA: malondialdehyde; PSPP-A: purple sweet potato polysaccharide composed of L-rhamnose, D-arabinose, D-galactose, D-glucose, and D-glucuronic acid (molar ratios 1.89:8.45:1.95:1.13:1); TC: total cholesterol; TG: triglyceride; bw: body weight; ICR: Institute for Cancer Research; T-AOC: total antioxidant capacity; CAT: catalase; GSH-Px/GPx: glutathione peroxidase; AF: anthocyanin fraction from purple-fleshed sweet potato; AMPK: adenosine monophosphate-activated protein kinase; ACC: acetyl-coenzyme A carboxylase; ROS: reactive oxygen species; GR: glutathione reductase; HO-1: heme oxygenase-1; NQO1: NAD(P)H quinone oxidoreductase 1; GCLC: gamma glutamate-cysteine ligase; AF-PSPs: diacylated anthocyanins from purple sweet potato; AGESs: advanced glycation end products; COMP: cartilage oligomeric matrix protein; MMP-3: matrix metalloproteinase-3; LDL: low-density lipoprotein; CUPRAC: cupric reducing antioxidant capacity assay; TFC: total flavonoids content; IBH: n-hexane-fraction; IBM: 95% MeOH-fraction; IBB: n-BuOH-fraction; IBW: H2O-soluble fraction; PI3K: phosphoinositide 3 kinase; AKT: protein kinase B; Glut4: glucose transporter type 4; PSPLE: water-extracted purple sweet potato leaves authenticated by the National Plant Genetic Resources Centre of Taiwan Agricultural Research Institute with the account number Pin 375; PARP: cleaved caspase-3 and poly ADP-ribose polymerase; XO: xanthine oxidase enzyme; Nrf2: nuclear factor E2-related factor 2 transcription; PON 1: paraoxonase 1 enzyme; PFSP TNG 75: purple-fleshed sweet potato var. “Tainung 73”; NF-κβ: Nuclear factor kappa-light-chain-enhancer of activated B cells; HAECs: human aortic endothelial cells; PSPLE/PSPL: purple sweet potato leaf extract/purple sweet potato leaves; ERK/ERK1/ERK2; extracellular signal-regulated kinase; VCAM-1: vascular cell adhesion molecule 1; CD40: Cluster of differentiation 40; Bcl-2: B-cell lymphoma 2; GFAP: glial fibrillary acidic protein; iNOS: inducible nitric oxide synthase; COX-2: cyclooxygenase-2; Cu/Zn-SOD: copper/zinc superoxide dismutase; 2hpppg: 2 h post-prandial plasma glucose levels; 2DM: type 2 diabetes mellitus; Aβ: β-amyloid peptide; LPO: lipid peroxidation; HepG2: human hepatoma cell line; LOVO: human colonic carcinoma cell line; MCF-7: human breast carcinoma cell line; LO2: human normal hepatocyte GES-1: human normal stomach mucosa epithelial cell line; MCF-10A: human normal breast epithelial cell line; NCM460: human normal colon epithelial cell; SGC-7901 and HGC-27: human gastric carcinoma cell lines; TBARS: thiobarbituric acid-reactive substance; Plasma PC: protein carbonyl, a marker of protein oxidation; HFD: high-fat diet; JNK: c-Jun N-terminal kinase; p53: tumor protein P53; GFAP: glial fibrillary acidic protein; BDNF: brain-derived neurotrophic factor; TGF-β1: Transforming growth factor β; BUN: serum blood urea nitrogen; Cr: creatine; SCFA: short-chain fatty acid; IgA: immunoglobulin A; 8-OHdG: urinary 8-hydroxy-2-deoxyguanosine; 5′-NT: 5′-nucleotidase enzyme; STZ: streptozotocin; DPPH: 2,2-diphenyl-1-picrylhydrazyl, ↑ increase; ↓ decrease.
4.3. Anti-Inflammatory Effect
Numerous research studies have exhibited that oxidative stress is correlated with the development of inflammation [194]. The inflammatory process is often triggered by factors such as diet, alcohol, smoking, insufficient sleep, infections, antibiotics, or dysfunction [195]. During the inflammation process, inflammatory mediators, ROS, and cytokines play crucial roles [196]. Several studies conducted in vivo and in vitro have shown evidence about how certain bioactive compounds obtained from PFSP report anti-inflammatory properties [113,197].
Jiang et al. [162] measured the anti-inflammatory effect of two anthocyanins, FAC-PSP and p-BAC-PSP, obtained from the root of PFSP after an in vitro experiment evaluating the effect on inflammatory markers in LPS-induced RAW264.7 macrophages. Both anthocyanins showed significant anti-inflammatory activity by attenuating LPS production of NO, TNF-α, and ROS. Another study reported the potential benefits of a novel ASPP from PFSP on inflammation after an in vivo intervention in (DSS)-induced colitis mice. The alkali-soluble polysaccharide significantly inhibited the IL-1β, IL-6, and TNF-α cytokine levels in colitis tissue mice [163]. Sun et al. [164] investigated the effect of polyphenols from purple potato leaves on hyperuricemia, and their results showed that PSPLP significantly reduced the secretion of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in a dose-dependent manner [32].
Many of the beneficial properties of PFSP are attributed to the large number of bioactive compounds present in their flesh, peel, leaves, or stems. The mechanism involved in the anti-inflammatory effect appears to be similar for every bioactive compound. For instance, the anti-inflammatory property related to the higher anthocyanin content resulted in reduced protein expression of TNF-α, IL-1β, and IL-6 in colonic cells due to the prevention of NF-kB translocation reducing phosphorylation levels and the gene expression of pro-inflammatory cytokines and mediators [198,199,200]. However, the anti-inflammatory property related to the higher complex polysaccharides content is related to their ability to produce SCFAs modulating gut microbiota since human microbiota is not capable of digesting these polysaccharides, lowering the inflammation status [201,202]. While chlorogenic acid, the main polyphenol substance in the leaves of PFSP, has a proven capacity to inhibit the production of pro-inflammatory cytokines and chemokines because chlorogenic acid significantly attenuates the nuclear translocation, with the inhibition of the NF-kB signaling pathway being the mechanism responsible for the suppression of pro-inflammatory cytokines [203].
4.4. Hypoglycemic and Antidiabetic Effect
Diabetes mellitus (DM) is a heterogeneous metabolic disorder characterized by a chronic hyperglycemia status; a physiologically abnormal condition caused by disturbed insulin secretion, insulin effect, or both [204,205]. Approximately 95% of patients with DM present type 2 diabetes (T2DM) based on insulin resistance and β-cell dysfunction [206,207].
Many studies have evidenced the ability of PFSP to show beneficial effects due to its hypoglycemic action [208]. Solihah et al. [167] evaluated the effects of PFSP leaves in alloxan-induced diabetic rats, obtaining positive results with a reduction in blood glucose levels. The treatment with the leaf extract also had significantly lower MDA levels and better pancreatic histopathological features than untreated animals. A study in T2DM patients with the consumption of PFSP tubers treated with three daily oral doses of 75 mL for four weeks showed similar results, improving fasting and two hours post-prandial plasma glucose, as well as reducing the glycated albumin level [172].
The possible mechanisms responsible for the hypoglycemic effects of PFSP are multiple and unknown. However, it has been evidenced that polyphenols, anthocyanins, and protein-bound anthocyanins can increase AMP-activated protein kinase (AMPK) leading to an increased level of glucose transporter type 2 (GLUT2), glucokinase protein (GK), and insulin receptor α (INSR) [57]. Acetylated anthocyanins, mainly peonidin and cyanidin, revealed a low glucose production using an in vivo model with high-fructose/high-fat diet-induced mice for nine weeks, as well as reduced TG, TC, and MDA concentrations [169]. The cyanidin 3-caffeoyl-p-hydroxybenzoylsophoroside-5-glucoside showed glucose tolerance improvement and inhibition of hepatic gluconeogenesis in in vitro and in vivo experiments [168]. These facts are attributed to the capacity of anthocyanins to show affinity with insulin-regulated glucose transporter 4 (GLUT 4) and the competition with glucose in the small intestine of rats [209]. As a result, the hypoglycemic and antidiabetic effect of PFSP is determined by the diverse bioactive compounds with different target actions.
4.5. Neuroprotective Effect
Neurodegenerative diseases (NDs) are a heterogeneous group of complex diseases characterized by neuronal loss and progressive degeneration of different areas of the nervous system, making aging the most important risk factor in the development of NDs [210,211]. The elderly have increased in the last years, accounting for 15% of the global population, and this will double over the next two decades [212,213]. Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) are the three most prevalent age-related neurodegenerative conditions in the elderly [214]. Although the action mechanism of these diseases has not been elucidated, a common feature is that neuronal damage is caused by the abnormal aggregation and deposition of proteins which alter specific molecular mechanisms leading to cell toxicity and degeneration [215].
In recent years, research has focused on the role of some PFSP bioactive compounds in the reduction in neuroinflammation [174,176,178]. Neuroinflammation is associated with NDs, with microglia and astrocytes being key regulators of inflammatory responses in the central nervous system (CNS). In response to neuronal damage, inflammatory mediators secreted by pro-inflammatory microglia, such as IL-1β or TNF-α, may activate pro-inflammatory astrocytes and induce an inflammatory response [216]. A recent study tested the effects of I. batatas L. purple-fleshed variety on neuroinflammation-induced male mice with an HFD [173], observing that the anthocyanins present in the tuber can significantly reduce inflammatory markers, such as IL-1β and IL-6, COX-2, TNF-α, body weight, fat content, hyperlipemia, and endotoxin level, as well as causing an improvement in memory function. Kang et al. [175] evaluated the neuroprotective effect of PFSP leaves in LPS-induced BV-2 microglial cells, reporting scavenging DPPH radicals in a dose-dependent manner, and also observing a reduction in the release of NO and the concentration of inflammatory mediators such as iNOS, COX-2, and TNF-α. In this way, the anthocyanins mechanism in neurodegenerative diseases has been studied and explained as the combination of four effects: (i) their radical scavenging ability to eliminate ROS and nitrogen reactive species (NRS) and their promotion of antioxidant enzymes, (ii) the inhibition of inflammatory pathways in the CNS, (iii) their cytoprotective and anti-apoptotic effects on neurons; and (iv) the promotion of cholinergic neurotransmission [216,217,218,219].
4.6. Antimicrobial and Prebiotic Action
Prebiotics have recently been redefined as a substrate that is selectively utilized by a host microorganism conferring a health benefit [220]. Some previous research studies have highlighted that bioactive compounds from PFSP can exert their anti-inflammatory effect by regulating the intestinal microbiota composition [221]. Concretely, two mechanisms are involved in this effect: (i) the reduction in intestinal pathogens, such as Clostridium spp. or Staphylococcus spp., which produce components and metabolites that can initiate the inflammatory response with the corresponding release of pro-inflammatory cytokines, and (ii) the improvement in the healthy gut microbiota profile promoting the proliferation of short-chain fatty acids (SCFAs) involved in the suppression of inflammatory pathways and strengthening of the intestinal barrier [222,223,224,225].
According to the above-mentioned, an in vitro fermentation was carried out using fecal samples from healthy volunteers to test the modulatory effect of purple-fleshed sweet potato anthocyanins on human intestinal microbiota, showing a significant increase in the proliferation of Bifidobacterium and Lactobacillus/Enterococcus spp. and the SCFAs level, as well as an inhibition of the growth of Clostridium histolyticum [181]. Another study revealed similar results using a pig colonic digest where the polyphenolic content of PFSP was responsible for the drastic reduction in putrefactive products, especially p-cresol, increasing the population of beneficial bacteria and decreasing the pathogenic bacteria [183]. This effect has been also confirmed in an in vivo experiment where cyclophosphamide (CTX)-treated mice were administered with three different polysaccharides from PFSP, obtaining an enhancement in the production of acetic, propionic, and butyric acid, as well as decreasing the ratio of Firmicutes/Bacteroidetes [180].
4.7. Hypouricemic Effect
Hyperuricemia status is caused by the impaired renal excretion of uric acid (UA) which reflects an extracellular fluid supersaturation for UA [226]. Uric acid is the product of the purine metabolism and excreted by the kidneys [227]. The enzymatic degradation of the purine pathway in humans transforms the oxidized hypoxanthine to xanthine, and then to uric acid by enzymes [228]. Hence, the regulative action on the key enzymes in the pathway of uric acid synthesis, 5′-nucleotidase (5′-NT), adenylate deaminase (ADA), and xanthine oxidase (XO), would be essential in their treatment [229,230].
In this line, some in vivo researchers have studied the hypouricemic effect of PFSP anthocyanins on hyperuricemic mice (Figure 3) [184,185]. Yang et al. [32] reported a significantly decreased level of UA, as well as 5′-NT and XO enzyme activity. The mechanism involved in this action was explained as the insertion of the acyl group of anthocyanins into the hydrophobic region of XO occupying the catalytic center to avoid the entrance of substrate due to the interaction with certain amino acid residues [186,231]. Similar results were reported by Zhang et al. [187] who evaluated the hypouricemic effect of PFSP anthocyanins in hyperuricemia mice showing a reduction in the serum UA level and a regulation of blood urea nitrogen (BUN) and (Cr) levels.
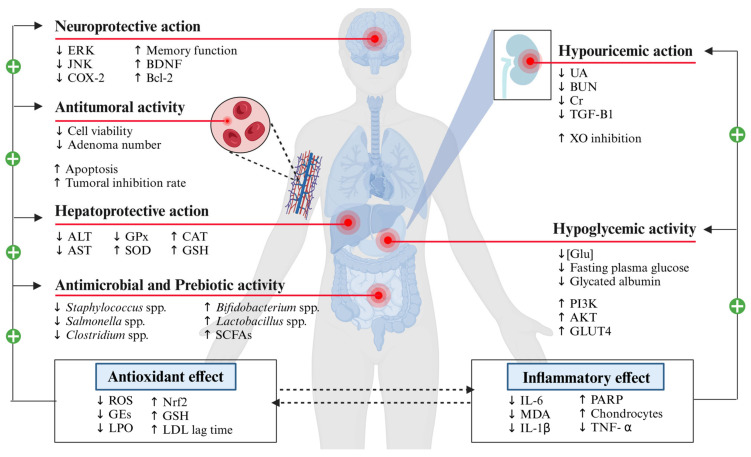
Figure 3.
Beneficial effects of Ipomoea batatas (L.) Lam. ↑ increase; ↓ decrease.
4.8. Antimutation and Antitumoral Effect
Cancer is a disease of uncontrolled proliferation by transformed cells subject to evolution by natural selection [232]. The most common cancers are breast, prostate, lung, colon, and bladder cancer which is expected to rise to 29.9 million new cancer cases by 2040 [233]. Several studies conducted in vitro and in vivo reported the antiproliferative and apoptotic effect of components of PFSP and its by-products through the enhancement of their anti-inflammatory and antioxidant properties [234]. Wu et al. [59] isolated three polysaccharides from PFSP and tested its in vitro antitumoral activities confirming that the three of them possessed an inhibitory effect against SGC7901 and SW620 cells in a dose-dependent manner. This effect is related to the structure and spatial conformation of polysaccharides since those with a lower molecular weight and higher sulphate content exhibited stronger antiproliferative activities [235,236]. Another in vitro research project studied the effect of fresh root tubers of the PFSP variety Bhu Krishna and the leaves accession S-1467 on multiple human cancer cell lines, reporting that the cyanidin-rich leaf extracts showed a superior action against colon and cervical cancer cell lines compared to the root [189]. The explanation for this effect is found in the B ring structure of cyanidin which has two hydroxyl groups instead of one in peonidin, predominantly in the leaves [237].
In addition, the antitumoral and antimutation roles of the principal bioactive compound of PFSP, anthocyanins, have been also tested. Guo et al. [188] evaluated the effects of PFSP anthocyanins on acute lymphoblastic leukemia cells (ALL) confirming their antileukemic action through the induction of the p38/c-Myc/CDK1-Cyclin B axis, essential in the progression of the G2 phase to the M phase and arresting tumoral DNA synthesis in cells [238,239]. Similar results were found in the administration of PFSP anthocyanins to SPF-grade ICR mice for one month, where the extract inhibited sarcoma S180 cell growth, and the sarcomas were significantly fewer and smaller than in the control mice, achieving an inhibition rate of 69.03% [192].
4.9. Other Biological Activities
Over the last few years, more biological activities have been tested, such as cardioprotective, anti-obesity, hypolipidemic, and immunomodulatory effects, as well as lung protection to elucidate the extent to which PFSP bioactive compounds can benefit a healthy status [240,241].
Potential cardioprotective effects of PFSP anthocyanins against low-density lipoprotein (LDL) oxidation in vitro, and atherosclerotic lesions in apolipoprotein E-deficient mice in vivo, reported a potential LDL oxidation protection in vitro and a significantly lower atherosclerotic plaque area, plasma soluble vascular cell adhesion molecule-1 (sVCAM-1), and thiobarbituric acid-reactive substances level than the control mice, suggesting a possible suppression of the development of atherosclerotic lesions [242]. Ju et al. [243] tested the anti-obesity action on high-fat diet-induced obese mice, achieving a reduced body weight and fat accumulation and an improvement in the lipid profile, as well as modulation of the energy intake, reducing the metabolic risk. The immunomodulatory effect of PFSP extracts in immune-deficient induced mice was investigated for 12 weeks, showing the inhibition of lymphadenopathy and the suppression of T- and B-cell proliferation and T helper 1/T helper 2 cytokine imbalance, as well as an increase in SOD and GPx enzyme levels, suggesting an amelioration of immune dysfunction [244]. Dong et al. [245] studied the therapeutic effect of PFSP anthocyanins on Klebsiella pneumoniae-infected mice to test the lung protective action. The results showed dampened lung injury, inflammatory responses, and bacterial systemic dissemination in vivo, as well as eliminating pyroptosis and restricting NLRP3 inflammasome activation in alveolar macrophages. According to the data, PFSP bioactive compounds may ameliorate and exert the protective actions mentioned above by modulating antioxidant and anti-inflammatory defense systems [246].
5. Food Application
Due to its remarkable health benefits, the use of purple-fleshed sweet potato may be a key opportunity for the food industry in the development of functional food to improve its nutraceutical and functional properties and as a strategy to reduce food waste and improve nutrition [247]. Several studies have considered the incorporation of the I. batatas L. purple variety in different food products, especially in bakery products [248,249,250,251,252]. However, none of them have used another PFSP by-product different from flesh and peels. The valorization of PFSP by-products in the form of extracts has become a promising possibility due to the high energetic value of the flesh, as well as the antioxidant properties of the leaves and peels because of their high content of phenolic compounds and anthocyanins (Table 4) [253].
Nowadays, bakery products formulated from refined wheat flour (WF) are dietary basics being consumed in the world despite their being nutritionally poor [254]. Enrichment, fortification, and replacement of some ingredients from staple foods are essential mechanisms to improve the nutrient intakes of the population, in both developed and undeveloped countries, when the staple food is being consumed daily [255]. Worldwide food markets supply a wide spectrum of bakery products, such as various types of bread, biscuits, muffins, cookies, pretzels, or pastries, with protein- and fiber-rich, gluten-free, or sugar-reduced new trends leading a diversification and innovation of the demanded products [256,257,258].
Bread, cookies, muffins, and biscuits are the bakery products studied for the introduction of purple-fleshed sweet potato. Its high anthocyanin and starch content and high-water holding capacity make purple-fleshed sweet potato a great option to incorporate into bread [259]. Several studies have used purple sweet potato flesh and peel in the form of flour to replace wheat, resulting in textural characteristic improvements, and bread with a higher firming rate related to a greater starch–gluten interaction [260,261]. Kweman et al. [262] investigated the physicochemical characteristics and glycemic index of bread made from purple sweet potato flesh flour, starch, and fiber obtained from the solid waste of PFSP starch processing and mixtures in different ratio proportions. The results revealed a higher dietary fiber content than bread made only from wheat flour, indicating its ability to reduce the blood glucose level due to its low glycemic index. Similar results were shown by Zhu et al. [263], who incorporated whole PFSP flour in different mixture ratios with wheat flour, resulting in a 10% replacement PFSP–WF with enhanced functional properties (polyphenols content and in vitro antioxidant activities) and sensory acceptance. However, part of the polyphenols were lost during the steaming stage, suggesting that microencapsulation would be a great strategy to avoid their degradation [264].
Incorporation in pastry products, such as cookies, has also been described [265]. Liu et at. [266] evaluated the quality characteristics and antioxidative activities of cookies using several proportions of PFSP flour, reporting that a concentration between 10 and 20% of PFSP powder was optimum to increase TPC and TA content and antioxidant effects with the best sensory evaluation without altering the sensorial cookie characteristics. Accordingly, another study revealed that PFSP can be incorporated into cookies at up to 20% without affecting the cookie quality and contributing to the deterrence of lipid oxidation after storage at 65 °C for 80 days [267]. Furthermore, Muhammad et al. [268] tested the addition of PSP mashed flesh into crackers, reporting that the PFSP trials were higher in fiber, carbohydrates, protein, anthocyanins, vitamins, and minerals, such as calcium.
Finally, PFSP extracts have also been used to prepare healthier biscuits and muffins [269,270]. Its use in the form of peel extract has also been investigated as an effective way of reducing PFSP by-products from the processing industry and improving functional and nutraceutical properties [271]. The findings suggested that the incorporation of 2% PSP peel powder was optimum for increasing dietary fiber, TPC, TA, and ABTS content, sensory acceptance, and texture preference, since higher concentrations of dry extract would disturb the development of the gluten matrix because of higher amounts of solids and a decreasing amount of gluten–protein content [272]. Nevertheless, limited research studies on PFSP by-product utilization have been performed. According to the outcomes in Table 4, there is a need to investigate the suitable addition form, as well as the optimal percentage that guarantees the health benefits without altering consumer preferences and new encapsulation techniques, which protect the bioactive compound, preventing their degradation and enhancing their stability and bioavailability [192,273,274,275].
Table 4.
Application of PFSP in bakery products.
| Food Product | Formulation | Outcomes | Ref. |
|---|---|---|---|
| Bread | Bread made from PSP flesh flour, starch, and fiber from a mixture with a ratio of 75:5:20 | Higher dietary fiber content and anthocyanins | [262] |
| Lower blood glucose response and glycemic index | |||
| Bread | Mixture of whole PFSP (roots and peels) transformed as a freeze-dried flour (5, 10, 20, 30, 40, and 50%), and wheat flour to incorporate into a traditional Chinese steamed bread | Higher TPC and antioxidant activities in the reformulated bread | [263] |
| A lower glycemic response | |||
| Improvement in the overall sensory acceptance at 5–10% levels | |||
| Cookies and Muffin | Replacement of wheat flour by the flesh of purple sweet potato in dried form (25, 30, 35, 40, and 45%) | Richness in amino acids and minerals | [270] |
| Higher global acceptability | |||
| Bread | Mixture of whole PFSP (flesh and peels) transformed as a freeze-dried flour in a proportion of 0, 25, 50, 75, and 100% with wheat flour into a traditional Chinese steamed bread | Decreased viscosity in PFSP breads | [260] |
| Increased TPC and antioxidant activity | |||
| Facilitated pasting wheat flour, decreasing the dough strength | |||
| Biscuit | Fresh, flour (using hot air-drying), and paste (by steaming, cooled, and mashed) PFSP was incorporated at 30% with wheat flour in biscuits | Enhancement of the purple color | [276] |
| Increased TPC, and TA content and antioxidant activity in a trial formulation | |||
| Cookies | Combination of PSP flesh flour (30.93–44.19%) with corn starch in cookies | Improvement in anthocyanin levels | [265] |
| Enhancement of the sensory attributes | |||
| Bread | 4% substitution of the original wheat flour content with PFSP flour in bread | Higher firming rate and greater starch–gluten interaction | [261] |
| Cookies | Substitution of 0, 10, 20, and 30% of PFSP powder | Highest TPC and TA contents and DPPH activity at 30% of addition | [266] |
| Increased sensory evaluation in cookies with 10, and 20% | |||
| Cracker | Addition of PSP mashed flesh (53%) to crackers | Higher content in fiber, vitamins, and minerals, such as Ca2+ | [268] |
| Elevated TA content | |||
| Biscuit | PFSP peel powder was incorporated, at 2, 5, and 10% in biscuits | Dietary fiber increased significantly | [271] |
| Enhancement of the TPC, total flavonoids content, and ABTS radical activity | |||
| Acceptable sensory evaluation at 2% biscuits | |||
| Biscuit | PSP flesh flour, fiber, and starch was added in different mixture proportions in biscuits | Biscuits produced from 75% flour and 25% fiber of PSP flour had a good physical and chemical quality | [269] |
PSP: purple sweet potato; PFSP: purple-fleshed sweet potato; TPC: total content of phenolic compounds; TA: total anthocyanin; DPPH: 2,2-diphenyl1-1-picrylhydrazyl; ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic) acid.
6. Conclusions and Future Trends
According to the Food and Agricultural Organization of the United Nations (FAO), food waste contributes to economic losses as well as environmental impact due to the depletion of natural resources such as inputs, water, land, or energy, and market policies result in discarding fit products for human consumption because of aesthetic criteria or due to market prices not being sufficient to cover costs. On the other hand, consumers are concerned about the importance of a healthy diet, generating a change in their food consumption preferences, and they are demanding natural and higher quality products. Additionally, the growing need for a vegetable-based diet where the consumption of animal-based foods is limited for reasons of sustainability, health, and animal welfare has led to the development of new trends in the food industry. Thus, these new trends are focusing more on the revalorization of agricultural by-products, prompting the industry to search for new ingredients that boost efficiency and sustainability. Purple-fleshed sweet potato and its by-products generated during processing have gained increasing attention from companies and consumers due to their richness in bioactive compounds, especially anthocyanins. The flesh, peel, or leaves can be processed into value-added products, which are particularly useful in bakery products with higher added value, such as replacing or substituting traditionally used wheat flour with purple-fleshed sweet potato flour to enhance the nutritional profile. Also, its high leaf protein content could be a good option to enrich pastry products. Hence, the incorporation of PFSP and its by-products could play a dual purpose: (i) integrating the circular economy concept and (ii) providing effects on hepatic, neurological, and glycemic disorders, among others. However, despite the widely recognized health benefit of purple-fleshed sweet potato through in vitro and in vivo studies, the existing literature on the application of its by-products in food is limited. More research is needed to effectively mask the color provided by PFSP, as well as new encapsulation techniques which could provide a promising approach to protect the bioactive compound, preventing its degradation after cooking and enhancing its stability and bioavailability. These aspects are crucial for optimally incorporating PFSP extracts in food products.
In conclusion, Ipomoea batatas (L.) Lam is an ideal food in terms of its contents of key bioactive compounds, which are essential for maintaining beneficial health activities that could be utilized for value-added purposes such as food fortification or food additives. For this reason, it is fundamental to harness the significant potential of the purple-fleshed sweet potato and the by-products generated during its processing through an appropriate agro-industrial valorization system.
Author Contributions
Conceptualization, M.d.l.Á.R. and G.N.; methodology, M.d.l.Á.R.; validation, P.A., J.Q., M.d.l.Á.R., R.P. and G.N.; investigation, M.d.l.Á.R.; writing—original draft preparation, M.d.l.Á.R.; writing—review and editing, P.A., J.Q., M.d.l.Á.R., R.P. and G.N.; visualization, G.N.; supervision, G.N.; project administration, G.N.; funding acquisition, G.N. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the Research Project PID2021-123628OB-C44 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”. The author Rosell María de los Ángeles is funded by the FPU Grant of the University of Murcia (UM, Spain).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mwanga R.O.M., Andrade M.I., Carey E.E., Low J.W., Craig Yencho G., Grüneberg W.J. Genetic Improvement of Tropical Crops. Springer; Cham, Switzerland: 2017. Sweetpotato (Ipomoea batatas L.) [Google Scholar]
- 2.Drapal M., Rossel G., Heider B., Fraser P.D. Metabolic Diversity in Sweet Potato (Ipomoea batatas, Lam.) Leaves and Storage Roots. Hortic. Res. 2019;6:2. doi: 10.1038/s41438-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan D. Sweet Potato: Production, Nutritional Properties and Diseases. Nova Science Publishers; New York, NY, USA: 2016. [Google Scholar]
- 4.Prohens J., Alvarez J.B., Khoury C.K. Distributions, Ex Situ Conservation Priorities, and Genetic Resource Potential of Crop Wild Relatives of Sweetpotato [Ipomoea batatas (L.) Lam., I. Series Batatas] Front. Plant Sci. 2015;6:251. doi: 10.3389/fpls.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang T., Ye S., Liao W., Wu M., He J., Mateus N., Oliveira H. The Botanical Profile, Phytochemistry, Biological Activities and Protected-Delivery Systems for Purple Sweet Potato (Ipomoea batatas (L.) Lam.): An up-to-Date Review. Food Res. Int. 2022;161:111811. doi: 10.1016/j.foodres.2022.111811. [DOI] [PubMed] [Google Scholar]
- 6.Hall M.R., Phatak S.C. Genetic Improvement of Vegetable Crops. Elsevier; Amsterdam, The Ntherlands: 1993. Sweet Potato: Ipomoea batatas (L.) Lam; pp. 693–708. [DOI] [Google Scholar]
- 7.Kays S.J. Sweetpotato Production Worldwide: Assessment, Trends and the Future. Acta Hortic. 2005;670:19–25. doi: 10.17660/ActaHortic.2005.670.2. [DOI] [Google Scholar]
- 8.Food and Agriculture Organization of the United Nations FAOSTAT. [(accessed on 20 July 2024)]. Available online: https://www.fao.org/faostat/en/#data/QCL.
- 9.Ministerio de Agricultura Pesca y Alimentación MAPA. [(accessed on 1 May 2024)]; Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2022/default.aspx?parte=3&capitulo=07&grupo=3.
- 10.Mohanraj R., Sivasankar S. Sweet Potato (Ipomoea batatas [L.] Lam)—A Valuable Medicinal Food: A Review. J Med. Food. 2014;17:733–741. doi: 10.1089/jmf.2013.2818. [DOI] [PubMed] [Google Scholar]
- 11.Nedunchezhiyan M., Gangadharan B., Jata S.K. Sweet Potato Agronomy. Fruit Veg. Cereal Sci. Biotechnol. 2012;6:1–10. [Google Scholar]
- 12.Doganay K.H., Kurunc A., Dincer C. Salinity Stress Effects on the Growth Yield and Quality Performance of Two Sweet Potato Varieties. ACS Agric. Sci. Technol. 2023;3:508–516. doi: 10.1021/acsagscitech.3c00069. [DOI] [Google Scholar]
- 13.Pushpalatha R., Gangadharan B. Climate Resilience, Yield and Geographical Suitability of Sweet Potato under the Changing Climate: A Review. Nat. Resour. Forum. 2024;48:106–119. doi: 10.1111/1477-8947.12309. [DOI] [Google Scholar]
- 14.Bovell-Benjamin A.C. Sweet Potato: A Review of Its Past, Present, and Future Role in Human Nutrition. Adv. Food Nutr. Res. 2007;52:1–59. doi: 10.1016/S1043-4526(06)52001-7. [DOI] [PubMed] [Google Scholar]
- 15.Tan W., Guo X., Wang Z., Zhang R., Tang C., Jiang B., Jia R., Deng Y., Yang S., Chen J. Metabolic Profiles and Morphological Characteristics of Leaf Tips among Different Sweet Potato (Ipomoea batatas Lam.) Varieties. J. Integr. Agric. 2024;23:494–510. doi: 10.1016/j.jia.2023.04.029. [DOI] [Google Scholar]
- 16.Cui L., Liu C.-Q., Li D.-J., Song J.-F. Effect of Processing on Taste Quality and Health-Relevant Functionality of Sweet Potato Tips. Agric. Sci. China. 2011;10:456–462. doi: 10.1016/S1671-2927(11)60025-4. [DOI] [Google Scholar]
- 17.Richardson M.L., Arlotta C.G. Growing Sweet Potatoes [Ipomoea batatas (L.) Lam.)] for Their Greens and the Impact on Storage Roots. J. Hortic. Sci. 2023;18:2. doi: 10.24154/jhs.v18i2.1932. [DOI] [Google Scholar]
- 18.Hamda, Ahmad M.Q., Saleem A., Yan H., Li Q. Biofortified Sweet Potato—An Ideal Source of Mitigating Hidden Hunger. Biofortification Grain Veg. Crops Mol. Breed. Approaches. 2024;12:239–253. doi: 10.1016/B978-0-323-91735-3.00013-3. [DOI] [Google Scholar]
- 19.Akoetey W., Britain M.M., Morawicki R.O. Potential Use of Byproducts from Cultivation and Processing of Sweet Potatoes. Ciência Rural. 2017;47:e20160610. doi: 10.1590/0103-8478cr20160610. [DOI] [Google Scholar]
- 20.Woolfe A.J. Sweet Potato: An Untapped Food Resource. Volume 30. Cambridge University Press; Cambridge, UK: 1992. [Google Scholar]
- 21.Nieto G., Bañón S., Garrido M. Administration of distillate thyme leaves into the diet of Segureña ewes: Effect on lamb meat quality. Animal. 2012;6:2048–2056. doi: 10.1017/S1751731112001012. [DOI] [PubMed] [Google Scholar]
- 22.Alam M.K. A Comprehensive Review of Sweet Potato (Ipomoea batatas [L.] Lam): Revisiting the Associated Health Benefits. Trends Food Sci. Technol. 2021;115:512–529. doi: 10.1016/j.tifs.2021.07.001. [DOI] [Google Scholar]
- 23.Terahara N., Konczak I., Ono H., Yoshimoto M., Yamakawa O. Characterization of Acylated Anthocyanins in Callus Induced from Storage Root of Purple-Fleshed Sweet Potato, Ipomoea batatas L. J. Biomed. Biotechnol. 2004;2004:279–286. doi: 10.1155/S1110724304406056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philpott M., Gould K.S., Lim C., Ferguson L.R. In Situ and In Vitro Antioxidant Activity of Sweetpotato Anthocyanins. J. Agric. Food Chem. 2004;52:1511–1513. doi: 10.1021/jf034593j. [DOI] [PubMed] [Google Scholar]
- 25.Sanoussi A.F., Dansi A., Ahissou H., Adebowale A., Sanni L.O., Orobiyi A., Dansi M., Azokpota P., Sanni A. Possibilities of Sweet Potato [Ipomoea batatas (L.) Lam] Value Chain Upgrading as Revealed by Physico-Chemical Composition of Ten Elites Landraces of Benin. Afr. J. Biotechnol. 2016;15:481–489. doi: 10.5897/AJB2015.15107. [DOI] [Google Scholar]
- 26.Teow C.C., Den Truong V., McFeeters R.F., Thompson R.L., Pecota K.V., Yencho G.C. Antioxidant Activities, Phenolic and β-Carotene Contents of Sweet Potato Genotypes with Varying Flesh Colours. Food Chem. 2007;103:829–838. doi: 10.1016/j.foodchem.2006.09.033. [DOI] [Google Scholar]
- 27.Li A., Xiao R., He S., An X., He Y., Wang C., Yin S., Wang B., Shi X., He J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules. 2019;24:3816. doi: 10.3390/molecules24213816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang G.J., Sheu M.J., Chang Y.S., Lu T.L., Chang H.Y., Huang S.S., Lin Y.H. Isolation and Characterisation of Invertase Inhibitor from Sweet Potato Storage Roots. J. Sci. Food Agric. 2008;88:2615–2621. doi: 10.1002/jsfa.3380. [DOI] [Google Scholar]
- 29.Zhao J.G., Yan Q.Q., Lu L.Z., Zhang Y.Q. In Vivo Antioxidant, Hypoglycemic, and Anti-Tumor Activities of Anthocyanin Extracts from Purple Sweet Potato. Nutr. Res. Pract. 2013;7:359–365. doi: 10.4162/nrp.2013.7.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimoto M., Okuno S., Yamaguchi M., Yamakawa O. Antimutagenicity of Deacylated Anthocyanins in Purple-Fleshed Sweetpotato. Biosci. Biotechnol. Biochem. 2001;65:1652–1655. doi: 10.1271/bbb.65.1652. [DOI] [PubMed] [Google Scholar]
- 31.Wu Q., Qu H., Jia J., Kuang C., Wen Y., Yan H., Gui Z. Characterization, Antioxidant and Antitumor Activities of Polysaccharides from Purple Sweet Potato. Carbohydr. Polym. 2015;132:31–40. doi: 10.1016/j.carbpol.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y., Zhang Z.-C., Zhou Q., Yan J.-X., Zhang J.-L., Su G.-H. Hypouricemic Effect in Hyperuricemic Mice and Xanthine Oxidase Inhibitory Mechanism of Dietary Anthocyanins from Purple Sweet Potato (Ipomoea batatas L.) J. Funct. Foods. 2020;73:104151. doi: 10.1016/j.jff.2020.104151. [DOI] [Google Scholar]
- 33.Wang L., Zhao Y., Zhou Q., Luo C.L., Deng A.P., Zhang Z.C., Zhang J.L. Characterization and Hepatoprotective Activity of Anthocyanins from Purple Sweet Potato (Ipomoea batatas L. Cultivar Eshu No. 8) J. Food Drug Anal. 2017;25:607–618. doi: 10.1016/j.jfda.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuang J., Lu J., Wang X., Wang X., Hu W., Hong F., Zhao X.-X., Zheng Y.-L. Purple Sweet Potato Color Protects against High-Fat Diet-Induced Cognitive Deficits through AMPK-Mediated Autophagy in Mouse Hippocampus. J. Nutr. Biochem. 2019;65:35–45. doi: 10.1016/J.JNUTBIO.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 35.De Castro Vendrame L.P., de Castro e Melo R.A., da Silva G.O., Vargas P.F., Leonel M. Sweet Potato (Ipomoea batatas L. Lam.) Cultivation and Potentialities. Var. Landraces Cult. Pract. Tradit. Uses. 2023;2:245–259. doi: 10.1016/B978-0-323-90057-7.00007-3. [DOI] [Google Scholar]
- 36.Guo Z., Zhao B., Li H., Miao S., Zheng B. Optimization of Ultrasound-Microwave Synergistic Extraction of Prebiotic Oligosaccharides from Sweet Potatoes (Ipomoea batatas L.) Innov. Food Sci. Emerg. Technol. 2019;54:51–63. doi: 10.1016/j.ifset.2019.03.009. [DOI] [Google Scholar]
- 37.Rodríguez-Mena A., Ochoa-Martínez L.A., González-Herrera S.M., Rutiaga-Quiñones O.M., González-Laredo R.F., Olmedilla-Alonso B., Vega-Maturino S. Coloring Potential of Anthocyanins from Purple Sweet Potato Paste: Ultrasound-Assisted Extraction, Enzymatic Activity, Color and Its Application in Ice Pops. Food Chem. Adv. 2023;3:100358. doi: 10.1016/j.focha.2023.100358. [DOI] [Google Scholar]
- 38.Liu W., Yang C., Zhou C., Wen Z., Dong X. An Improved Microwave-Assisted Extraction of Anthocyanins from Purple Sweet Potato in Favor of Subsequent Comprehensive Utilization of Pomace. Food Bioprod. Process. 2019;115:1–9. doi: 10.1016/j.fbp.2019.02.003. [DOI] [Google Scholar]
- 39.Zhang L., Zhao L., Bian X., Guo K., Zhou L., Wei C. Characterization and Comparative Study of Starches from Seven Purple Sweet Potatoes. Food Hydrocoll. 2018;80:168–176. doi: 10.1016/j.foodhyd.2018.02.006. [DOI] [Google Scholar]
- 40.Gajanayake B., Raja Reddy K., Shankle M.W., Arancibia R.A., Villordon A.O., Arancibia R.A., Shankle M.W. Quantifying Storage Root Initiation, Growth, and Developmental Responses of Sweetpotato to Early Season Temperature. Agron. J. 2014;106:1795–1804. doi: 10.2134/agronj14.0067. [DOI] [Google Scholar]
- 41.Hossain M.M., Rahim M.A., Moutosi H.N., Das L. Evaluation of the Growth, Storage Root Yield, Proximate Composition, and Mineral Content of Colored Sweet Potato Genotypes. J. Agric. Food Res. 2022;8:100289. doi: 10.1016/j.jafr.2022.100289. [DOI] [Google Scholar]
- 42.Ivane N.M.A., Wang W., Ma Q., Wang J., Sun J. Harnessing the Health Benefits of Purple and Yellow-Fleshed Sweet Potatoes: Phytochemical Composition, Stabilization Methods, and Industrial Utilization—A Review. Food Chem. X. 2024;23:101462. doi: 10.1016/j.fochx.2024.101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Department of Agriculture . [Historical Record]: Purple Sweet Potatoes. US Department of Agriculture; Washington, DC, USA: 2021. [Google Scholar]
- 44.Martínez-Zamora L., Penalver R., Ros G., Nieto G. Substitution of synthetic nitrates and antioxidants by spices, fruits and vegetables in clean label Spanishchorizo. Food Res. Int. 2021;139:109835. doi: 10.1016/j.foodres.2020.109835. [DOI] [PubMed] [Google Scholar]
- 45.Fan H., Liang D., Fu F., Xu M., Li Z., Suo B., Ai Z. Processing Suitability of Different Varieties of Sweet Potatoes Cooked with Different Methods. J. Food Process. Preserv. 2022;46:e16944. doi: 10.1111/jfpp.16944. [DOI] [Google Scholar]
- 46.Lebot V. Rapid Quantitative Determination of Maltose and Total Sugars in Sweet Potato (Ipomoea batatas L. [Lam.]) Varieties Using HPTLC. J. Food Sci. Technol. 2017;54:718–726. doi: 10.1007/s13197-017-2510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H., Yang Q., Ferdinand U., Gong X., Qu Y., Gao W., Ivanistau A., Feng B., Liu M. Isolation and Characterization of Starch from Light Yellow, Orange, and Purple Sweet Potatoes. Int. J. Biol. Macromol. 2020;160:660–668. doi: 10.1016/j.ijbiomac.2020.05.259. [DOI] [PubMed] [Google Scholar]
- 48.Guo K., Liu T., Xu A., Zhang L., Bian X., Wei C. Structural and Functional Properties of Starches from Root Tubers of White, Yellow, and Purple Sweet Potatoes. Food Hydrocoll. 2019;89:829–836. doi: 10.1016/j.foodhyd.2018.11.058. [DOI] [Google Scholar]
- 49.Sun H., Fan J., Tian Z., Ma L., Meng Y., Yang Z., Zeng X., Liu X., Kang L., Nan X. Effects of Treatment Methods on the Formation of Resistant Starch in Purple Sweet Potato. Food Chem. 2022;367:130580. doi: 10.1016/j.foodchem.2021.130580. [DOI] [PubMed] [Google Scholar]
- 50.Yong H., Wang X., Sun J., Fang Y., Liu J., Jin C. Comparison of the Structural Characterization and Physicochemical Properties of Starches from Seven Purple Sweet Potato Varieties Cultivated in China. Int. J. Biol. Macromol. 2018;120:1632–1638. doi: 10.1016/j.ijbiomac.2018.09.182. [DOI] [PubMed] [Google Scholar]
- 51.Wang S., Pan D., Lv X., Song X., Qiu Z., Huang C., Huang R., Chen W. Proteomic Approach Reveals That Starch Degradation Contributes to Anthocyanin Accumulation in Tuberous Root of Purple Sweet Potato. J. Proteom. 2016;143:298–305. doi: 10.1016/j.jprot.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Stribling P., Ibrahim F. Dietary Fibre Definition Revisited—The Case of Low Molecular Weight Carbohydrates. Clin. Nutr. ESPEN. 2023;55:340–356. doi: 10.1016/j.clnesp.2023.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Han X., Ma Y., Ding S., Fang J., Liu G. Regulation of Dietary Fiber on Intestinal Microorganisms and Its Effects on Animal Health. Anim. Nutr. 2023;14:340–356. doi: 10.1016/j.aninu.2023.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu S., Sun H., Mu T., Li Q., Richel A. Preparation of Cellulose Nanocrystals from Purple Sweet Potato Peels by Ultrasound-Assisted Maleic Acid Hydrolysis. Food Chem. 2023;403:134496. doi: 10.1016/j.foodchem.2022.134496. [DOI] [PubMed] [Google Scholar]
- 55.Kurnianingsih N., Ratnawati R., Nazwar T.A., Ali M., Fatchiyah F. A Comparative Study on Nutritional Value of Purple Sweet Potatoes from West Java and Central Java, Indonesia. J. Phys. Conf. Ser. 2020;1665:012011. doi: 10.1088/1742-6596/1665/1/012011. [DOI] [Google Scholar]
- 56.Kurnianingsih N., Ratnawati R., Fatchiyah F., Barlianto W., Ali M.M., Safitri A., Suyanto E. The Difference of Amino Acid Profiling from Two Morphological Purple Sweet Potatoes from Kawi Mountain Cultivars, East Java, Indonesia. J. Phys. Conf. Ser. 2019;1374:012017. doi: 10.1088/1742-6596/1374/1/012017. [DOI] [Google Scholar]
- 57.Jiang T., Shuai X., Li J., Yang N., Deng L., Li S., He Y., Guo H., Li Y., He J. Protein-Bound Anthocyanin Compounds of Purple Sweet Potato Ameliorate Hyperglycemia by Regulating Hepatic Glucose Metabolism in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. J. Agric. Food Chem. 2020;68:1596–1608. doi: 10.1021/acs.jafc.9b06916. [DOI] [PubMed] [Google Scholar]
- 58.Tang C.C., Ameen A., Fang B.P., Liao M.H., Chen J.Y., Huang L.F., Zou H.D., Wang Z.Y. Nutritional Composition and Health Benefits of Leaf-Vegetable Sweet Potato in South China. J. Food Compos. Anal. 2021;96:103714. doi: 10.1016/j.jfca.2020.103714. [DOI] [Google Scholar]
- 59.Bañón S., Díaz P., Nieto G., Castillo M., Álvarez D. Modelling the Yield and Texture of Comminuted Pork Products Using Color and Temperature. Effect of Fat/Lean Ratio and Starch. Meat Sci. 2008;80:649–655. doi: 10.1016/j.meatsci.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Curayag Q.A.L., Dizon E.I., Hurtada W.A. Antioxidant Activity, Chemical and Nutritional Properties of Raw and Processed Purple-Fleshed Sweet Potato (Ipomoea batatas Lam.) Cogent. Food Agric. 2019;5:1662930. doi: 10.1080/23311932.2019.1662930. [DOI] [Google Scholar]
- 61.Ji H., Zhang H., Li H., Li Y. Analysis on the Nutrition Composition and Antioxidant Activity of Different Types of Sweet Potato Cultivars. Food Nutr. Sci. 2015;6:161. doi: 10.4236/fns.2015.61017. [DOI] [Google Scholar]
- 62.Munekata P.E.S., Pateiro M., Domínguez R., Nieto G., Kumar M., Dhama K., Lorenzo J.M. Bioactive compounds from fruits as preservatives. Foods. 2023;12:343. doi: 10.3390/foods12020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinanoglou J., Xu M., Li J., Yin J., Wu M., Zhou W., Yang X., Zhang R., He J. Color and Nutritional Analysis of Ten Different Purple Sweet Potato Varieties Cultivated in China via Principal Component Analysis and Cluster Analysis. Foods. 2024;13:904. doi: 10.3390/foods13060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim S., Xu J., Kim J., Chen T.Y., Su X., Standard J., Carey E., Griffin J., Herndon B., Katz B., et al. Role of Anthocyanin-Enriched Purple-Fleshed Sweet Potato P40 in Colorectal Cancer Prevention. Mol. Nutr. Food Res. 2013;57:1908–1917. doi: 10.1002/mnfr.201300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S.Y., Ryu C.H. Studies on the Nutritional Components of Purple Sweet Potato (Ipomoea batatas) Korean J. Food Sci. Technol. 1995;27:819–825. [Google Scholar]
- 66.Martínez L., Ros G., Nieto G. Fe, Zn and Se bioavailability in chicken meat emulsions enriched with minerals, hydroxytyrosol and extra virgin olive oil as measured by Caco-2 cell model. Nutrients. 2018;10:969. doi: 10.3390/nu10080969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grace M.H., Yousef G.G., Gustafson S.J., Truong V.D., Yencho G.C., Lila M.A. Phytochemical Changes in Phenolics, Anthocyanins, Ascorbic Acid, and Carotenoids Associated with Sweetpotato Storage and Impacts on Bioactive Properties. Food Chem. 2014;145:717–724. doi: 10.1016/j.foodchem.2013.08.107. [DOI] [PubMed] [Google Scholar]
- 68.Enaru B., Drețcanu G., Pop T.D., Stǎnilǎ A., Diaconeasa Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants. 2021;10:1967. doi: 10.3390/antiox10121967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen C.C., Lin C., Chen M.H., Chiang P.Y. Stability and Quality of Anthocyanin in Purple Sweet Potato Extracts. Foods. 2019;8:393. doi: 10.3390/foods8090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu Y., Deng L., Chen J., Zhou S., Liu S., Fu Y., Yang C., Liao Z., Chen M. An Analytical Pipeline to Compare and Characterise the Anthocyanin Antioxidant Activities of Purple Sweet Potato Cultivars. Food Chem. 2016;194:46–54. doi: 10.1016/j.foodchem.2015.07.133. [DOI] [PubMed] [Google Scholar]
- 71.Luo X., Wang R., Wang J., Li Y., Luo H., Chen S., Zeng X., Han Z. Acylation of Anthocyanins and Their Applications in the Food Industry: Mechanisms and Recent Research Advances. Foods. 2022;11:2166. doi: 10.3390/foods11142166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Putu Eka Widyadharma I., Purwata T.E., Suprapta D.N., Raka Sudewi A.A. Anthocyanin Derived from Purple Sweet Potato Water Extracts Ameliorated Oxidative Stress, Inflammation, Mechanical Allodynia, and Cold Allodynia among Chronic Constriction Injury-Induced Neuropathic Pain in Rats. Open Access Maced. J. Med. Sci. 2020;8:529–536. doi: 10.3889/oamjms.2020.4524. [DOI] [Google Scholar]
- 73.Islam M.S., Yoshimoto M., Terahara N., Yamakawa O. Anthocyanin Compositions in Sweetpotato (Ipomoea batatas L.) Leaves. Biosci. Biotechnol. Biochem. 2002;66:2483–2486. doi: 10.1271/bbb.66.2483. [DOI] [PubMed] [Google Scholar]
- 74.Li G.L., Lin Z., Zhang H., Liu Z., Xu Y., Xu G., Li H., Ji R., Luo W., Qiu Y., et al. Anthocyanin Accumulation in the Leaves of the Purple Sweet Potato (Ipomoea batatas L.) Cultivars. Molecules. 2019;24:3743. doi: 10.3390/molecules24203743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu F., Cai Y.Z., Yang X., Ke J., Corke H. Anthocyanins, Hydroxycinnamic Acid Derivatives, and Antioxidant Activity in Roots of Different Chinese Purple-Fleshed Sweetpotato Genotypes. J. Agric. Food Chem. 2010;58:7588–7596. doi: 10.1021/jf101867t. [DOI] [PubMed] [Google Scholar]
- 76.Su X., Griffin J., Xu J., Ouyang P., Zhao Z., Wang W. Identification and Quantification of Anthocyanins in Purple-Fleshed Sweet Potato Leaves. Heliyon. 2019;5:e01964. doi: 10.1016/j.heliyon.2019.e01964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mano H., Ogasawara F., Sato K., Higo H., Minobe Y. Isolation of a Regulatory Gene of Anthocyanin Biosynthesis in Tuberous Roots of Purple-Fleshed Sweet Potato. Plant Physiol. 2007;143:1252–1268. doi: 10.1104/pp.106.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fenger J.A., Roux H., Robbins R.J., Collins T.M., Dangles O. The Influence of Phenolic Acyl Groups on the Color of Purple Sweet Potato Anthocyanins and Their Metal Complexes. Dye. Pigment. 2021;185:108792. doi: 10.1016/j.dyepig.2020.108792. [DOI] [Google Scholar]
- 79.Jiang T., Mao Y., Sui L., Yang N., Li S., Zhu Z., Wang C., Yin S., He J., He Y. Degradation of Anthocyanins and Polymeric Color Formation during Heat Treatment of Purple Sweet Potato Extract at Different PH. Food Chem. 2019;274:460–470. doi: 10.1016/j.foodchem.2018.07.141. [DOI] [PubMed] [Google Scholar]
- 80.Fan L., Wang Y., Xie P., Zhang L., Li Y., Zhou J. Copigmentation Effects of Phenolics on Color Enhancement and Stability of Blackberry Wine Residue Anthocyanins: Chromaticity, Kinetics and Structural Simulation. Food Chem. 2019;275:299–308. doi: 10.1016/j.foodchem.2018.09.103. [DOI] [PubMed] [Google Scholar]
- 81.Qian B.J., Liu J.H., Zhao S.J., Cai J.X., Jing P. The Effects of Gallic/Ferulic/Caffeic Acids on Colour Intensification and Anthocyanin Stability. Food Chem. 2017;228:526–532. doi: 10.1016/j.foodchem.2017.01.120. [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez L., Muñoz-Bernal Ó.A., Fuentes E., Alvarez-Parrilla E., Palomo I., Wall-Medrano A. Phenolic Profile, Cheminformatics, and Antiplatelet Aggregation Activity of Orange and Purple Sweet Potato (Ipomoea batatas L.) Storage Roots. Food Chem. 2024;454:139794. doi: 10.1016/j.foodchem.2024.139794. [DOI] [PubMed] [Google Scholar]
- 83.Basílio L.S.P., Nunes A., Minatel I.O., Diamante M.S., Di Lázaro C.B., Silva A.C.A.F.E., Vargas P.F., Vianello F., Maraschin M., Lima G.P.P. The Phytochemical Profile and Antioxidant Activity of Thermally Processed Colorful Sweet Potatoes. Horticulturae. 2024;10:18. doi: 10.3390/horticulturae10010018. [DOI] [Google Scholar]
- 84.Jang H.H., Kim H.W., Kim S.Y., Kim S.M., Kim J.B., Lee Y.M. In Vitro and in Vivo Hypoglycemic Effects of Cyanidin 3-Caffeoyl-p-Hydroxybenzoylsophoroside-5-Glucoside, an Anthocyanin Isolated from Purple-Fleshed Sweet Potato. Food Chem. 2019;272:688–693. doi: 10.1016/j.foodchem.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Makori S.I., Mu T.H., Sun H.N. Total Polyphenol Content, Antioxidant Activity, and Individual Phenolic Composition of Different Edible Parts of 4 Sweet Potato Cultivars. Nat. Prod. Commun. 2020;15:1934578X20936931. doi: 10.1177/1934578X20936931. [DOI] [Google Scholar]
- 86.Salawu S.O., Udi E., Akindahunsi A.A., Boligon A.A., Athayde M.L. Antioxidant Potential, Phenolic Profile and Nutrient Composition of Flesh and Peels from Nigerian White and Purple Skinned Sweet Potato (Ipomea batatas L.) Pelagia Res. Libr. Asian J. Plant Sci. Res. 2015;5:14–23. [Google Scholar]
- 87.Shamsudin R., Shaari N., Noor M.Z.M., Azmi N.S., Hashim N. Evaluation of Phytochemical and Mineral Composition of Malaysia’s Purple-Flesh Sweet Potato. Pertanika J. Sci. Technol. 2022;30:2463–2476. doi: 10.47836/pjst.30.4.09. [DOI] [Google Scholar]
- 88.Ranteallo Y., Ahmad M., Syam A., Nilawati A. Identification and Quantification of Minerals and Vitamins of Purple Sweet Potato (Ipomoea batatas) Leave. IOP Conf. Ser. Earth Environ. Sci. 2023;1230:012134. doi: 10.1088/1755-1315/1230/1/012134. [DOI] [Google Scholar]
- 89.Izalin Mohamad Zahari N., Karuppan J., Shah Shaari E., Mohamad K., Othman R., Yaacob Y. Regional Conference on Science, Technology and Social Sciences (RCSTSS 2014) Springer; Singapore: 2016. Quality Attributes of Different Purple Sweet Potato Variety and Sensory Evaluation of Purple Sweet Potato Straight Drink. [Google Scholar]
- 90.Shaari N., Shamsudin R., Nor M.Z.M., Hashim N. Quality Attributes of Malaysia Purple-Fleshed Sweet Potato at Different Peel Condition. Agronomy. 2021;11:872. doi: 10.3390/agronomy11050872. [DOI] [Google Scholar]
- 91.Tshilongo L., Mianda S.M., Seke F., Laurie S.M., Sivakumar D. Influence of Harvesting Stages on Phytonutrients and Antioxidant Properties of Leaves of Five Purple-Fleshed Sweet Potato (Ipomoea batatas) Genotypes. Foods. 2024;13:1640. doi: 10.3390/foods13111640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim H.J., Park W.S., Bae J.Y., Kang S.Y., Yang M.H., Lee S., Lee H.S., Kwak S.S., Ahn M.J. Variations in the Carotenoid and Anthocyanin Contents of Korean Cultural Varieties and Home-Processed Sweet Potatoes. J. Food Compos. Anal. 2015;41:188–193. doi: 10.1016/j.jfca.2015.01.012. [DOI] [Google Scholar]
- 93.Xu J., Su X., Lim S., Griffin J., Carey E., Katz B., Tomich J., Smith J.S., Wang W. Characterisation and Stability of Anthocyanins in Purple-Fleshed Sweet Potato P40. Food Chem. 2015;186:90–96. doi: 10.1016/j.foodchem.2014.08.123. [DOI] [PubMed] [Google Scholar]
- 94.Park S.Y., Lee S.Y., Yang J.W., Lee J.S., Oh S.D., Oh S., Lee S.M., Lim M.H., Park S.K., Jang J.S., et al. Comparative Analysis of Phytochemicals and Polar Metabolites from Colored Sweet Potato (Ipomoea batatas L.) Tubers. Food Sci. Biotechnol. 2016;25:283–291. doi: 10.1007/s10068-016-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y., Xu Y., Cao Y., Fang K., Xia W., Jiang Q. Combined Effect of Microwave and Steam Cooking on Phytochemical Compounds and Antioxidant Activity of Purple Sweet Potatoes. Food Sci. Technol. Res. 2017;23:193–201. doi: 10.3136/fstr.23.193. [DOI] [Google Scholar]
- 96.Kim M.Y., Lee B.W., Lee H.U., Lee Y.Y., Kim M.H., Lee J.Y., Lee B.K., Woo K.S., Kim H.J. Phenolic Compounds and Antioxidant Activity in Sweet Potato after Heat Treatment. J. Sci. Food Agric. 2019;99:6833–6840. doi: 10.1002/jsfa.9968. [DOI] [PubMed] [Google Scholar]
- 97.Krochmal-Marczak B., Cebulak T., Kapusta I., Oszmiański J., Kaszuba J., Zurek N. The Content of Phenolic Acids and Flavonols in the Leaves of Nine Varieties of Sweet Potatoes (Ipomoea batatas L.) Depending on Their Development, Grown in Central Europe. Molecules. 2020;25:3473. doi: 10.3390/molecules25153473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jang Y., Koh E. Antioxidant Content and Activity in Leaves and Petioles of Six Sweet Potato (Ipomoea batatas L.) and Antioxidant Properties of Blanched Leaves. Food Sci. Biotechnol. 2019;28:337–345. doi: 10.1007/s10068-018-0481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ooi S.F., Sukri S.A.M., Zakaria N.N.A., Harith Z.T. Carotenoids, Phenolics and Antioxidant Properties of Different Sweet Potatoes (Ipomoea batatas) Varieties. IOP Conf. Ser. Earth Environ. Sci. 2021;756:012077. doi: 10.1088/1755-1315/756/1/012077. [DOI] [Google Scholar]
- 100.Rumbaoa R.G.O., Cornago D.F., Geronimo I.M. Phenolic Content and Antioxidant Capacity of Philippine Sweet Potato (Ipomoea batatas) Varieties. Food Chem. 2009;113:1133–1138. doi: 10.1016/j.foodchem.2008.08.088. [DOI] [Google Scholar]
- 101.Huang Y.C., Chang Y.H., Shao Y.Y. Effects of Genotype and Treatment on the Antioxidant Activity of Sweet Potato in Taiwan. Food Chem. 2006;98:529–538. doi: 10.1016/j.foodchem.2005.05.083. [DOI] [Google Scholar]
- 102.Jia R., Tang C., Chen J., Zhang X., Wang Z. Total Phenolics and Anthocyanins Contents and Antioxidant Activity in Four Different Aerial Parts of Leafy Sweet Potato (Ipomoea batatas L.) Molecules. 2022;27:3117. doi: 10.3390/molecules27103117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Padda M.S., Picha D.H. Antioxidant Activity and Phenolic Composition in “beauregard” Sweetpotato Are Affected by Root Size and Leaf Age. J. Am. Soc. Hortic. Sci. 2007;132:447–451. doi: 10.21273/JASHS.132.4.447. [DOI] [Google Scholar]
- 104.Zeroual A., Sakar E.H., Mahjoubi F., Chaouch M., Chaqroune A., Taleb M. Effects of Extraction Technique and Solvent on Phytochemicals, Antioxidant, and Antimicrobial Activities of Cultivated and Wild Rosemary (Rosmarinus Officinalis L.) from Taounate Region (Northern Morocco) Biointerface Res. Appl. Chem. 2022;12:8441–8452. doi: 10.33263/BRIAC126.84418452. [DOI] [Google Scholar]
- 105.Wang A., Li R., Ren L., Gao X., Zhang Y., Ma Z., Ma D., Luo Y. A Comparative Metabolomics Study of Flavonoids in Sweet Potato with Different Flesh Colors (Ipomoea batatas (L.) Lam) Food Chem. 2018;260:124–134. doi: 10.1016/j.foodchem.2018.03.125. [DOI] [PubMed] [Google Scholar]
- 106.Terahara N. Flavonoids in Foods: A Review. Nat. Prod. Commun. 2015;10:1934578X1501000334. doi: 10.1177/1934578X1501000334. [DOI] [PubMed] [Google Scholar]
- 107.Sun W., Zhang M., Chen H., Zheng D., Fang Z. Effects of Deodorization on the Physicochemical Index and Volatile Compounds of Purple Sweet Potato Anthocyanins (PSPAs) LWT. 2016;68:265–272. doi: 10.1016/j.lwt.2015.12.044. [DOI] [Google Scholar]
- 108.Bechoff A. Ph.D. Thesis. University of Greenwich; London, UK: 2010. Investigating Carotenoid Loss after Drying and Storage of Orange-Fleshed Sweet Potato. [Google Scholar]
- 109.Ayuso P., Quizhpe J., Rosell M.d.l.Á., Peñalver R., Nieto G. Bioactive Compounds, Health Benefits and Food Applications of Artichoke (Cynara Scolymus L.) and Artichoke By-Products: A Review. Appl. Sci. 2024;14:4940. doi: 10.3390/app14114940. [DOI] [Google Scholar]
- 110.Gröber U., Schmidt J., Kisters K. Magnesium in Prevention and Therapy. Nutrients. 2015;7:8199–8226. doi: 10.3390/nu7095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buffington M.A., Abreo K. Hyponatremia: A Review. J. Intensive Care Med. 2016;31:223–236. doi: 10.1177/0885066614566794. [DOI] [PubMed] [Google Scholar]
- 112.Robertson J.I.S. Diuretics, Potassium Depletion and the Risk of Arrhythmias. Eur. Heart J. 1984;5:25–28. doi: 10.1093/eurheartj/5.suppl_A.25. [DOI] [PubMed] [Google Scholar]
- 113.Nieto G., Martínez L., Castillo J., Ros G. Effect of hydroxytyrosol, walnut and olive oil on nutritional profile of Low-Fat Chicken Frankfurters. Eur. J. Lipid Sci. Technol. 2017;119:1600518. doi: 10.1002/ejlt.201600518. [DOI] [Google Scholar]
- 114.Ikanone C.E.O., Oyekan P.O. Effect of Boiling and Frying on the Total Carbohydrate, Vitamin C and Mineral Contents of Irish (Solanun Tuberosum) and Sweet (Ipomea batatas) Potato Tubers. Niger. Food J. 2014;32:33–39. doi: 10.1016/S0189-7241(15)30115-6. [DOI] [Google Scholar]
- 115.Sir Elkhatim K.A., Elagib R.A.A., Hassan A.B. Content of Phenolic Compounds and Vitamin C and Antioxidant Activity in Wasted Parts of Sudanese Citrus Fruits. Food Sci. Nutr. 2018;6:1214–1219. doi: 10.1002/fsn3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valdés F. Vitamina C. Actas Dermosifiliogr. 2006;97:557–568. doi: 10.1016/S0001-7310(06)73466-4. [DOI] [PubMed] [Google Scholar]
- 117.Guclu G., Dagli M.M., Aksay O., Keskin M., Kelebek H., Selli S. Comparative Elucidation on the Phenolic Fingerprint, Sugars and Antioxidant Activity of White, Orange and Purple-Fleshed Sweet Potatoes (Ipomoea batatas L.) as Affected by Different Cooking Methods. Heliyon. 2023;9:e18684. doi: 10.1016/j.heliyon.2023.e18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lv X., Mu J., Wang W., Liu Y., Lu X., Sun J., Wang J., Ma Q. Effects and Mechanism of Natural Phenolic Acids/Fatty Acids on Copigmentation of Purple Sweet Potato Anthocyanins. Curr. Res. Food Sci. 2022;5:1243–1250. doi: 10.1016/j.crfs.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schieber M., Chandel N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014;24:PR453–PR462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 121.Chintha P., Sarkar D., Pecota K., Dogramaci M., Hatterman-Valenti H., Shetty K. Phenolic Bioactive-Linked Antioxidant, Anti-Hyperglycemic, and Antihypertensive Properties of Sweet Potato Cultivars with Different Flesh Color. Hortic. Environ. Biotechnol. 2023;64:877–893. doi: 10.1007/s13580-023-00515-0. [DOI] [Google Scholar]
- 122.Oki T., Sato M., Yoshinaga M., Sakai T., Sugawara T., Terahara N., Suda I. 1, 1-Diphenyl-2-Picrylhydrazyl Radical-Scavenging Capacity and Oxygen Radical Absorbance Capacity of Sweet Potato Cultivars with Various Flesh Colors. J. Jpn. Soc. Food Sci. Technol.—Nippon. Shokuhin Kagaku Kogaku Kaishi. 2009;56:655–659. doi: 10.3136/nskkk.56.655. [DOI] [Google Scholar]
- 123.Esatbeyoglu T., Rodríguez-Werner M., Schlösser A., Winterhalter P., Rimbach G. Fractionation, Enzyme Inhibitory and Cellular Antioxidant Activity of Bioactives from Purple Sweet Potato (Ipomoea batatas) Food Chem. 2017;221:447–456. doi: 10.1016/j.foodchem.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 124.Ryan M.J., Jackson J.R., Hao Y., Leonard S.S., Alway S.E. Inhibition of Xanthine Oxidase Reduces Oxidative Stress and Improves Skeletal Muscle Function in Response to Electrically Stimulated Isometric Contractions in Aged Mice. Free Radic. Biol. Med. 2011;51:38–52. doi: 10.1016/j.freeradbiomed.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He F., Ru X., Wen T. Molecular Sciences NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020;21:4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ye J., Xiangjun A.E., Ae M., Ae C.Y., Wang C. Effect of Purple Sweet Potato Anthocyanins on B-Amyloid-Mediated PC-12 Cells Death by Inhibition of Oxidative Stress. Neurochem. Res. 2010;35:357–365. doi: 10.1007/s11064-009-0063-0. [DOI] [PubMed] [Google Scholar]
- 127.Insanu M., Amalia R., Fidrianny I. Potential Antioxidative Activity of Waste Product of Purple Sweet Potato (Ipomoea batatas Lam) Pak. J. Biol. Sci. 2022;25:681–687. doi: 10.3923/pjbs.2022.681.687. [DOI] [PubMed] [Google Scholar]
- 128.Chen C.-M., Lin Y.-L., Oliver C.-Y., Phd C., Hsu C.-Y., Shieh M.-J., Liu J.-F., Phd R.D. Consumption of Purple Sweet Potato Leaves Decreases Lipid Peroxidation and DNA Damage in Humans. Asia Pac. J. Clin. Nutr. 2008;17:408–414. [PubMed] [Google Scholar]
- 129.Chang W.-H., Chen Msc C.-M., Hu S.-P., Kan N.-W., Chiu C.-C., Liu J.-F. Effect of Purple Sweet Potato Leaf Consumption on the Modulation of the Antioxidative Status in Basketball Players during Training. Asia Pac. J. Clin. Nutr. 2007;16:455–461. [PubMed] [Google Scholar]
- 130.Zhang Y., Niu F., Sun J., Xu F., Yue R. Purple Sweet Potato (Ipomoea batatas L.) Color Alleviates High-Fat-Diet-Induced Obesity in SD Rat by Mediating Leptin’s Effect and Attenuating Oxidative Stress. Food Sci. Biotechnol. 2015;24:1523–1532. doi: 10.1007/s10068-015-0196-7. [DOI] [Google Scholar]
- 131.Chang W.-H., Hu S.-P., Huang Y.-F., Yeh T.-S., Liu J.-F. Effect of Purple Sweet Potato Leaves Consumption on Exercise-Induced Oxidative Stress and IL-6 and HSP72 Levels. J. Appl. Physiol. 2010;109:1710–1715. doi: 10.1152/japplphysiol.00205.2010. [DOI] [PubMed] [Google Scholar]
- 132.Kano M., Takayanagi T., Harada K., Makino K., Ishikawa F. Antioxidative Activity of Anthocyanins from Purple Sweet Potato, Ipomoera batatas Cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005;69:979–988. doi: 10.1271/bbb.69.979. [DOI] [PubMed] [Google Scholar]
- 133.Yang Z.-W., Tang C.-E., Zhang J.-L., Zhou Q., Zhang Z.-C. Stability and Antioxidant Activity of Anthocyanins from Purple Sweet Potato (Ipomoea batatas L. Cultivar Eshu No. 8) Subjected to Simulated in Vitro Gastrointestinal Digestion. Int. J. Food Sci. Technol. 2019;54:2604–2614. doi: 10.1111/ijfs.14172. [DOI] [Google Scholar]
- 134.Tu W., Wang H., Li S., Liu Q., Sha H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019;10:637–651. doi: 10.14336/AD.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hwang Y.P., Choi J.H., Yun H.J., Han E.H., Kim H.G., Kim J.Y., Park B.H., Khanal T., Choi J.M., Chung Y.C., et al. Anthocyanins from Purple Sweet Potato Attenuate Dimethylnitrosamine-Induced Liver Injury in Rats by Inducing Nrf2-Mediated Antioxidant Enzymes and Reducing COX-2 and INOS Expression. Food Chem. Toxicol. 2011;49:93–99. doi: 10.1016/j.fct.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 136.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C.B., Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jin Q., Liu T., Qiao Y., Liu D., Yang L., Mao H., Ma F., Wang Y., Peng L., Zhan Y. Oxidative Stress and Inflammation in Diabetic Nephropathy: Role of Polyphenols. Front. Immunol. 2023;14:1185317. doi: 10.3389/fimmu.2023.1185317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kumar S., Pandey A.K., Lu K.P., Sastre J. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013;2013:16. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shibata H., Sakamoto Y., Oka M., Kono Y. Natural Antioxidant, Chlorogenic Acid, Protects against DNA Breakage Caused by Monochloramine. Biosci. Biotechnol. Biochem. 1999;63:1295–1297. doi: 10.1271/bbb.63.1295. [DOI] [PubMed] [Google Scholar]
- 140.Xu W., Liu L., Hu B., Sun Y., Ye H., Ma D., Zeng X. TPC in the Leaves of 116 Sweet Potato (Ipomoea batatas L.) Varieties and Pushu 53 Leaf Extracts. J. Food Compos. Anal. 2010;23:599–604. doi: 10.1016/j.jfca.2009.12.008. [DOI] [Google Scholar]
- 141.Gan L.J., Yang D., Shin J.A., Kim S.J., Hong S.T., Lee J.H., Sung C.K., Lee K.T. Oxidative Comparison of Emulsion Systems from Fish Oil-Based Structured Lipid versus Physically Blended Lipid with Purple-Fleshed Sweet Potato (Ipomoea batatas L.) Extracts. J. Agric. Food Chem. 2012;60:467–475. doi: 10.1021/jf203708y. [DOI] [PubMed] [Google Scholar]
- 142.Trefts E., Gannon M., Wasserman D.H. The liver. Curr. Biol. Mag. 2017;27:R1147–R1151. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Devarbhavi H., Asrani S.K., Arab J.P., Nartey Y.A., Pose E., Kamath P.S. Global Burden of Liver Disease: 2023 Update. J. Hepatol. 2023;79:516–537. doi: 10.1016/j.jhep.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 144.Qian H., Chao X., Williams J., Fulte S., Li T., Yang L., Ding W.X. Autophagy in Liver Diseases: A Review. Mol. Aspects Med. 2021;82:100973. doi: 10.1016/j.mam.2021.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li C., Feng Y., Li J., Lian R., Qin L., Wang C. Extraction, Purification, Structural Characterization, and Hepatoprotective Effect of the Polysaccharide from Purple Sweet Potato. J. Sci. Food Agric. 2023;103:2196–2206. doi: 10.1002/jsfa.12239. [DOI] [PubMed] [Google Scholar]
- 146.Wang W., Li J., Wang Z., Gao H., Su L., Xie J., Chen X., Liang H., Wang C., Han Y. Oral Hepatoprotective Ability Evaluation of Purple Sweet Potato Anthocyanins on Acute and Chronic Chemical Liver Injuries. Cell Biochem. Biophys. 2014;69:539–548. doi: 10.1007/s12013-014-9829-3. [DOI] [PubMed] [Google Scholar]
- 147.Ding X., Fan S. Purple Sweet Potato Polysaccharide Ameliorates Concanavalin A-Induced Hepatic Injury by Inhibiting Inflammation and Oxidative Stress. Phytomedicine. 2024;129:155652. doi: 10.1016/j.phymed.2024.155652. [DOI] [PubMed] [Google Scholar]
- 148.Sun J., Zhou B., Tang C., Gou Y., Chen H., Wang Y., Jin C., Liu J., Niu F., Kan J., et al. Characterization, Antioxidant Activity and Hepatoprotective Effect of Purple Sweetpotato Polysaccharides. Int. J. Biol. Macromol. 2018;115:69–76. doi: 10.1016/j.ijbiomac.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 149.Zhang M., Pan L.J., Jiang S.T., Mo Y.W. Protective Effects of Anthocyanins from Purple Sweet Potato on Acute Carbon Tetrachloride-Induced Oxidative Hepatotoxicity Fibrosis in Mice. Food Agric. Immunol. 2016;27:157–170. doi: 10.1080/09540105.2015.1079589. [DOI] [Google Scholar]
- 150.Hwang Y.P., Choi J.H., Han E.H., Kim H.G., Wee J.H., Jung K.O., Jung K.H., Kwon K.I., Jeong T.C., Chung Y.C., et al. Purple Sweet Potato Anthocyanins Attenuate Hepatic Lipid Accumulation through Activating Adenosine Monophosphate-Activated Protein Kinase in Human HepG2 Cells and Obese Mice. Nutr. Res. 2011;31:896–906. doi: 10.1016/j.nutres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 151.Kang H., Lee S.G. Protective Effect of Purple Sweet Potato Leaf (Ipomoea batatas Linn Convolvulaceae) against Alcohol-Induced Liver Damage in Mice. Trop. J. Pharm. Res. 2021;20:301–308. doi: 10.4314/tjpr.v20i2.12. [DOI] [Google Scholar]
- 152.Cho A.S., Jeon S.M., Kim M.J., Yeo J., Seo K.I., Choi M.S., Lee M.K. Chlorogenic Acid Exhibits Anti-Obesity Property and Improves Lipid Metabolism in High-Fat Diet-Induced-Obese Mice. Food Chem. Toxicol. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 153.Shi H., Dong L., Jiang J., Zhao J., Zhao G., Dang X., Lu X., Jia M. Chlorogenic Acid Reduces Liver Inflammation and Fibrosis through Inhibition of Toll-like Receptor 4 Signaling Pathway. Toxicology. 2013;303:107–114. doi: 10.1016/j.tox.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 154.Liu L., Zhang C., Zhai M., Yu T., Pei M., Du P., Li A., Yan J., Li C., Zhang G. Effect of Chlorogenic Acid on Lipid Metabolism in 3T3-L1 Cells Induced by Oxidative Stress. Food Biosci. 2023;51:102330. doi: 10.1016/j.fbio.2022.102330. [DOI] [Google Scholar]
- 155.Ngai M., Mariko T., Kishimoto Y., Iizuka M., Saita E., Toyozaki M., Kamiya T., Ikeguchi M., Kondo K. Sweet Potato (Ipomoea batatas L.) Leaves Suppressed Oxidation of Low Density Lipoprotein (LDL) in Vitro and in Human Subjects. J. Clin. Biochem. Nutr. 2011;48:203–208. doi: 10.3164/jcbn.10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao J.G., Yan Q.Q., Xue R.Y., Zhang J., Zhang Y.Q. Isolation and Identification of Colourless Caffeoyl Compounds in Purple Sweet Potato by HPLC-DAD-ESI/MS and Their Antioxidant Activities. Food Chem. 2014;161:22–26. doi: 10.1016/j.foodchem.2014.03.079. [DOI] [PubMed] [Google Scholar]
- 157.Tang Y., Cai W., Xu B. Profiles of Phenolics, Carotenoids and Antioxidative Capacities of Thermal Processed White, Yellow, Orange and Purple Sweet Potatoes Grown in Guilin, China. Food Sci. Hum. Wellness. 2015;4:123–132. doi: 10.1016/j.fshw.2015.07.003. [DOI] [Google Scholar]
- 158.Lekmine S., Boussekine S., Akkal S., Martín-García A.I., Boumegoura A., Kadi K., Djeghim H., Mekersi N., Bendjedid S., Bensouici C., et al. Investigation of Photoprotective, Anti-Inflammatory, Antioxidant Capacities and LC–ESI–MS Phenolic Profile of Astragalus gombiformis Pomel. Foods. 2021;10:1937. doi: 10.3390/foods10081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim D., Kim Y., Kim Y. Effect of Purple Sweet Potato Using Different Cooking Methods on Cytoprotection against Ethanol-Induced Oxidative Damage through Nrf2 Activation in HepG2 Cells. Antioxidants. 2023;12:1650. doi: 10.3390/antiox12081650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chao P.Y., Huang Y.P., Hsieh W. Bin Inhibitive Effect of Purple Sweet Potato Leaf Extract and Its Components on Cell Adhesion and Inflammatory Response in Human Aortic Endothelial Cells. Cell Adhes. Migr. 2013;7:237–245. doi: 10.4161/cam.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sugata M., Lin C.Y., Shih Y.C. Anti-Inflammatory and Anticancer Activities of Taiwanese Purple-Fleshed Sweet Potatoes (Ipomoea batatas L. Lam) Extracts. Biomed Res. Int. 2015;2015:768093. doi: 10.1155/2015/768093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang T., Zhou J., Liu W., Tao W., He J., Jin W., Guo H., Yang N., Li Y. The Anti-Inflammatory Potential of Protein-Bound Anthocyanin Compounds from Purple Sweet Potato in LPS-Induced RAW264.7 Macrophages. Food Res. Int. 2020;137:109647. doi: 10.1016/j.foodres.2020.109647. [DOI] [PubMed] [Google Scholar]
- 163.Sun J., Chen H., Kan J., Gou Y., Liu J., Zhang X., Wu X., Tang S., Sun R., Qian C., et al. Anti-Inflammatory Properties and Gut Microbiota Modulation of an Alkali-Soluble Polysaccharide from Purple Sweet Potato in DSS-Induced Colitis Mice. Int. J. Biol. Macromol. 2020;153:708–722. doi: 10.1016/j.ijbiomac.2020.03.053. [DOI] [PubMed] [Google Scholar]
- 164.Sun R., Kan J., Cai H., Hong J., Jin C., Zhang M. In Vitro and in Vivo Ameliorative Effects of Polyphenols from Purple Potato Leaves on Renal Injury and Associated Inflammation Induced by Hyperuricemia. J. Food Biochem. 2022;46:e14049. doi: 10.1111/jfbc.14049. [DOI] [PubMed] [Google Scholar]
- 165.Subawa I.W., Astawa P., Bakta I.M., Astawa I.N.M., Krisna G.A. Purple Sweet Potato (Ipomoea batatas L.) Extract Effects on Levels of Inflammatory Markers and Chondrocyte Count in Gout Arthritis Wistar Rat Model. Foot Ankle Surg. 2023;29:611–615. doi: 10.1016/j.fas.2023.07.007. [DOI] [PubMed] [Google Scholar]
- 166.Lee S.L., Chin T.Y., Tu S.C., Wang Y.J., Hsu Y.T., Kao M.C., Wu Y.C. Purple Sweet Potato Leaf Extract Induces Apoptosis and Reduces Inflammatory Adipokine Expression in 3T3-L1 Differentiated Adipocytes. Evid.-Based Complement. Altern. Med. 2015;2015:126302. doi: 10.1155/2015/126302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Solihah I., Herlina H., Munirah E., Haryanti H., Amalia M., Rasyid R.S.P., Suciati T., Fatma F. The Hypoglycemic Effect of Purple Sweet Potato Leaf Fractions in Diabetic Rats. J. Adv. Pharm. Educ. Res. 2023;13:64–72. doi: 10.51847/rQSvc5gZwg. [DOI] [Google Scholar]
- 168.Martínez-Zamora L., Penalver R., Ros G., Nieto G. Olive tree derivatives and Hydroxytyrosol: Their potential effects on human health and its use as functional ingredient in meat. Foods. 2021;10:2611. doi: 10.3390/foods10112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen L., Yang Y., Zhang J., Feng L., Zhou Q. Diacylated Anthocyanins from Purple Sweet Potato (Ipomoea batatas L.) Attenuate Hyperglycemia and Hyperuricemia in Mice Induced by a High-Fructose/High-Fat Diet. J. Zhejiang Univ.-Sci. B. 2005;24:587–601. doi: 10.1631/jzus.B2200587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mi W., Hu Z., Zhao S., Wang W., Lian W., Lu P., Shi T. Purple Sweet Potato Anthocyanins Normalize the Blood Glucose Concentration and Restore the Gut Microbiota in Mice with Type 2 Diabetes Mellitus. Heliyon. 2024;10:e31784. doi: 10.1016/j.heliyon.2024.e31784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee C.-L., Lee S.-L., Chen C.-J., Chen H.-C., Kao M.-C., Liu C.-H., Chen J.-Y., Lai Y.-T., Wu Y.-C. Characterization of Secondary Metabolites from Purple Ipomoea batatas Leaves and Their Effects on Glucose Uptake. Molecules. 2016;21:745. doi: 10.3390/molecules21060745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wira Mahadita G., Suastika K. Purple Sweet Potato Tuber Extract Lowers Mallondialdehyde and Improves Glycemic Control in Subjects with Type 2 Diabetes Mellitus. Glob. Adv. Res. J. Med. Med. Sci. 2016;5:208–213. [Google Scholar]
- 173.Li J., Shi Z., Mi Y. Purple Sweet Potato Color Attenuates High Fat-Induced Neuroinflammation in Mouse Brain by Inhibiting Mapk and NF-ΚB Activation. Mol. Med. Rep. 2018;17:4823–4831. doi: 10.3892/mmr.2018.8440. [DOI] [PubMed] [Google Scholar]
- 174.Widyastuti K., Mahadewa T.G.B., Suprapta D.N., Sudewi A.A.R. Effect of Providing Purple Sweet Potato Water Extract on Tumor Necrosis Factor-α Levels, Protein 53 Expression, Glial Fibrillary Acidic Protein Expression, Brain-Derived Neurotrophic Factor Levels, and Spatial Working Memory in Rats with d-Galactose Induction. Dement. Neuropsychol. 2022;16:228–236. doi: 10.1590/1980-5764-DN-2021-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kang H., Kwak Y.G., Koppula S. Protective Effect of Purple Sweet Potato (Ipomoea batatas Linn, Convolvulaceae) on Neuroinflammatory Responses in Lipopolysaccharide-Stimulated Microglial Cells. Trop. J. Pharm. Res. 2014;13:1257–1263. doi: 10.4314/tjpr.v13i8.9. [DOI] [Google Scholar]
- 176.Lu J., Wu D.M., Zheng Y.L., Hu B., Zhang Z.F. Purple Sweet Potato Color Alleviates D-Galactose-Induced Brain Aging in Old Mice by Promoting Survival of Neurons via PI3K Pathway and Inhibiting Cytochrome C-Mediated Apoptosis. Brain Pathol. 2010;20:598–612. doi: 10.1111/j.1750-3639.2009.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Made Oka Adnyana I., Raka Sudewi A., Purwa Samatra D., Suprapta D. Neuroprotective Effects of Purple Sweet Potato Balinese Cultivar in Wistar Rats With Ischemic Stroke. Open Access Maced. J. Med. Sci. 2018;6:1959–1964. doi: 10.3889/oamjms.2018.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shan Q., Lu J., Zheng Y., Li J., Zhou Z., Hu B., Zhang Z., Fan S., Mao Z., Wang Y.-J., et al. Purple Sweet Potato Color Ameliorates Cognition Deficits and Attenuates Oxidative Damage and Inflammation in Aging Mouse Brain Induced by D-Galactose. J. Biomed Biotechnol. 2009;2009:564737. doi: 10.1155/2009/564737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun H., Zhang P., Zhu Y., Lou Q., He S. Antioxidant and Prebiotic Activity of Five Peonidin-Based Anthocyanins Extracted from Purple Sweet Potato (Ipomoea batatas (L.) Lam.) Sci. Rep. 2018;8:5018. doi: 10.1038/s41598-018-23397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tang C., Sun J., Zhou B., Jin C., Liu J., Kan J., Qian C., Zhang N. Effects of Polysaccharides from Purple Sweet Potatoes on Immune Response and Gut Microbiota Composition in Normal and Cyclophosphamide Treated Mice. Food Funct. 2018;9:937–950. doi: 10.1039/C7FO01302G. [DOI] [PubMed] [Google Scholar]
- 181.Zhang X., Yang Y., Wu Z., Weng P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota in Vitro. J. Agric. Food Chem. 2016;64:2582–2590. doi: 10.1021/acs.jafc.6b00586. [DOI] [PubMed] [Google Scholar]
- 182.Kilua A., Han K.H., Fukushima M. Effect of Polyphenols Isolated from Purple Sweet Potato (Ipomoea batatas Cv. Ayamurasaki) on the Microbiota and the Biomarker of Colonic Fermentation in Rats Fed with Cellulose or Inulin. Food Funct. 2020;11:10182–10192. doi: 10.1039/D0FO02111C. [DOI] [PubMed] [Google Scholar]
- 183.Kilua A., Nomata R., Nagata R., Fukuma N., Shimada K., Han K.-H., Fukushima M. Purple Sweet Potato Polyphenols Differentially Influence the Microbial Composition Depending on the Fermentability of Dietary Fiber in a Mixed Culture of Swine Fecal Bacteria. Nutrients. 2019;11:1495. doi: 10.3390/nu11071495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang Z.C., Zhou Q., Yang Y., Wang Y., Zhang J.L. Highly Acylated Anthocyanins from Purple Sweet Potato (Ipomoea batatas L.) Alleviate Hyperuricemia and Kidney Inflammation in Hyperuricemic Mice: Possible Attenuation Effects on Allopurinol. J. Agric. Food Chem. 2019;67:6202–6211. doi: 10.1021/acs.jafc.9b01810. [DOI] [PubMed] [Google Scholar]
- 185.Hwa K.S., Chung D.-M., Chung Y.C., Chun H.K. Hypouricemic Effects of Anthocyanin Extracts of Purple Sweet Potato on Potassium Oxonate-Induced Hyperuricemia in Mice. Phytother. Res. 2011;25:1415–1417. doi: 10.1002/ptr.3421. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Z.C., Wang H.B., Zhou Q., Hu B., Wen J.H., Zhang J.L. Screening of Effective Xanthine Oxidase Inhibitors in Dietary Anthocyanins from Purple Sweet Potato (Ipomoea batatas L. Cultivar Eshu No.8) and Deciphering of the Underlying Mechanisms in Vitro. J. Funct. Foods. 2017;36:102–111. doi: 10.1016/j.jff.2017.06.048. [DOI] [Google Scholar]
- 187.Zhang Z.C., Su G.H., Luo C.L., Pang Y.L., Wang L., Li X., Wen J.H., Zhang J.L. Effects of Anthocyanins from Purple Sweet Potato (Ipomoea batatas L. Cultivar Eshu No. 8) on the Serum Uric Acid Level and Xanthine Oxidase Activity in Hyperuricemic Mice. Food Funct. 2015;6:3045–3055. doi: 10.1039/C5FO00499C. [DOI] [PubMed] [Google Scholar]
- 188.Guo L., Liu J., Yang Y., Zeng Y., Yuan F., Zhong F., Jin Y., Wan R., Liu W. Purple Sweet Potato Anthocyanins Elicit Calcium Overload-Induced Cell Death by Inhibiting the Calcium-Binding Protein S100A4 in Acute Lymphoblastic Leukemia. Food Biosci. 2021;42:101214. doi: 10.1016/j.fbio.2021.101214. [DOI] [Google Scholar]
- 189.Vishnu V.R., Renjith R.S., Mukherjee A., Anil S.R., Sreekumar J., Jyothi A.N. Comparative Study on the Chemical Structure and in Vitro Antiproliferative Activity of Anthocyanins in Purple Root Tubers and Leaves of Sweet Potato (Ipomoea batatas) J. Agric. Food Chem. 2019;67:2467–2475. doi: 10.1021/acs.jafc.8b05473. [DOI] [PubMed] [Google Scholar]
- 190.Ji C., Zhang Z., Zhang B., Chen J., Liu R., Song D., Li W., Lin N., Zou X., Wang J., et al. Purification, Characterization, and in Vitro Antitumor Activity of a Novel Glucan from the Purple Sweet Potato Ipomoea batatas (L.) Lam. Carbohydr. Polym. 2021;257:117605. doi: 10.1016/j.carbpol.2020.117605. [DOI] [PubMed] [Google Scholar]
- 191.Meng M., Sun Y., Qi Y., Xu J., Sun J., Bai Y., Han L., Han R., Hou L., Sun H. Structural Characterization and Induction of Tumor Cell Apoptosis of Polysaccharide from Purple Sweet Potato (Ipomoea batatas (L.) Lam) Int. J. Biol. Macromol. 2023;235:123799. doi: 10.1016/j.ijbiomac.2023.123799. [DOI] [PubMed] [Google Scholar]
- 192.Martínez L., Ros G., Nieto G. Effect of natural extracts obtained from food industry by-products on nutritional quality and shelf life of chicken nuggets enriched with organic Zn and Se pro-vided in broiler diet. Poult. Sci. 2020;99:1491–1501. doi: 10.1016/j.psj.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Asadi K., Ferguson L.R., Philpott M., Karunasinghe N. Cancer-Preventive Properties of an Anthocyanin-Enriched Sweet Potato in the APC MIN Mouse Model. J. Cancer Prev. 2017;22:135–146. doi: 10.15430/JCP.2017.22.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ryu H.W., Lee S.U., Lee S., Song H.H., Son T.H., Kim Y.U., Yuk H.J., Ro H., Lee C.K., Hong S.T., et al. 3-Methoxy-Catalposide Inhibits Inflammatory Effects in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Cytokine. 2017;91:57–64. doi: 10.1016/j.cyto.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 195.Kaser A., Zeissig S., Blumberg R.S. Genes and Environment: How Will Our Concepts on the Pathophysiology of IBD Develop in the Future? Dig. Dis. 2010;28:395–405. doi: 10.1159/000320393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Martínez J., Nieto G., Castillo J., Ros G. Influence of in vitro gastrointestinal digestion and/or grape seed extract addition on antioxidant capacity of meat emulsions. LWT Food Sci. Technol. 2014;59:834–840. doi: 10.1016/j.lwt.2014.07.048. [DOI] [Google Scholar]
- 197.Díaz P., Nieto G., Bañón S., Garrido M.D. Determination of shelf life of sousvide salmon (Salmo salar) based on sensory attributes. J. Food Sci. 2009;74:371–376. doi: 10.1111/j.1750-3841.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- 198.De Aguiar Cipriano P., Kim H., Fang C., Paula Venancio V., Mertens-Talcott S.U., Talcott S.T. In Vitro Digestion, Absorption and Biological Activities of Acylated Anthocyanins from Purple Sweet Potatoes (Ipomoea batatas) Food Chem. 2022;374:131076. doi: 10.1016/j.foodchem.2021.131076. [DOI] [PubMed] [Google Scholar]
- 199.Wang Y.J., Zheng Y.L., Lu J., Chen G.Q., Wang X.H., Feng J., Ruan J., Sun X., Li C.X., Sun Q.J. Purple Sweet Potato Color Suppresses Lipopolysaccharide-Induced Acute Inflammatory Response in Mouse Brain. Neurochem. Int. 2010;56:424–430. doi: 10.1016/j.neuint.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 200.Phull A.R., Kim S.J. Fucoidan as Bio-Functional Molecule: Insights into the Anti-Inflammatory Potential and Associated Molecular Mechanisms. J. Funct. Foods. 2017;38:415–426. doi: 10.1016/j.jff.2017.09.051. [DOI] [Google Scholar]
- 201.Al Bander Z., Nitert M.D., Mousa A., Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health. 2020;17:7618. doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nie Y., Lin Q., Luo F. Effects of Non-Starch Polysaccharides on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017;18:1372. doi: 10.3390/ijms18071372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hwang S.J., Kim Y.-W., Park Y., Lee H.-J., Kim K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014;63:81–90. doi: 10.1007/s00011-013-0674-4. [DOI] [PubMed] [Google Scholar]
- 204.Astrid Petersmann A., Müller-Wieland D., Müller U.A., Landgraf R., Nauck M., Freckmann G., Heinemann L., Schleicher E. Definition, Classification and Diagnosis. Exp. Clin. Endocrinol. Diabetes. 2019;127:S1–S7. doi: 10.1055/a-1018-9078. [DOI] [PubMed] [Google Scholar]
- 205.Harreiter J., Roden M. Diabetes Mellitus: Definition, Classification, Diagnosis, Screening and Prevention (Update 2023) Wien. Klin. Wochenschr. 2023;135:6–15. doi: 10.1007/s00508-022-02122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Banday M.Z., Sameer A.S., Nissar S. Pathophysiology of Diabetes: An Overview. Avicenna J. Med. 2020;10:174–188. doi: 10.4103/ajm.ajm_53_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.European Medicines Agency . Guideline on Clinical Investigation of Medicinal Products in the Treatment or Prevention of Diabetes Mellitus. European Medicines Agency; London, UK: 2012. [Google Scholar]
- 208.Luiz de Brito Alves J., Istri Sri Arisanti C., Made Agus Gelgel Wirasuta I., Musfiroh I., Hainida Khairul Ikram E., Muchtaridi M. Mechanism of Anti-Diabetic Activity from Sweet Potato (Ipomoea batatas): A Systematic Review. Foods. 2023;12:2810. doi: 10.3390/foods12142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nizamutdinova I.T., Jin Y.C., Chung J., Shin S.C., Lee S.J., Seo H.G., Lee J.H., Chang K.C., Kim H.J. The Anti-Diabetic Effect of Anthocyanins in Streptozotocin-Induced Diabetic Rats through Glucose Transporter 4 Regulation and Prevention of Insulin Resistance and Pancreatic Apoptosis. Mol. Nutr. Food Res. 2009;53:1419–1429. doi: 10.1002/mnfr.200800526. [DOI] [PubMed] [Google Scholar]
- 210.Kritsilis M., Rizou S.V., Koutsoudaki P.N., Evangelou K., Gorgoulis V.G., Papadopoulos D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018;19:2937. doi: 10.3390/ijms19102937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Agnello L., Ciaccio M. Neurodegenerative Diseases: From Molecular Basis to Therapy. Int. J. Mol. Sci. 2022;23:12854. doi: 10.3390/ijms232112854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cheslow L., Snook A.E., Waldman S.A. Biomarkers for Managing Neurodegenerative Diseases. Biomolecules. 2024;14:398. doi: 10.3390/biom14040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Van Schependom J., D’haeseleer M. Advances in Neurodegenerative Diseases. J. Clin. Med. 2023;12:1709. doi: 10.3390/jcm12051709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dugger B.N., Dickson D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ruz C., Alcantud J.L., Montero F.V., Duran R., Bandres-Ciga S. Proteotoxicity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020;21:5646. doi: 10.3390/ijms21165646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kwon H.S., Koh S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kowalczyk T., Sitarek P., Zaa C.A., Marcelo Á.J., An Z., Medina-Franco J.L., Velasco-Velázquez M.A. Anthocyanins: Molecular Aspects on Their Neuroprotective Activity. Biomolecules. 2023;13:1598. doi: 10.3390/biom13111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mattioli R., Francioso A., Mosca L., Silva P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules. 2020;25:3809. doi: 10.3390/molecules25173809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ullah R., Khan M., Shah S.A., Saeed K., Kim M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients. 2019;11:1195. doi: 10.3390/nu11061195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gibson G.R., Hutkins R., Ellen Sanders M., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 221.Tang C., Han J., Chen D., Zong S., Liu J., Kan J., Qian C., Jin C. Recent Advances on the Biological Activities of Purple Sweet Potato Anthocyanins. Food Biosci. 2023;53:102670. doi: 10.1016/j.fbio.2023.102670. [DOI] [Google Scholar]
- 222.Cheng H., Zhang D., Wu J., Liu J., Zhou Y., Tan Y., Feng W., Peng C. Interactions between Gut Microbiota and Polyphenols: A Mechanistic and Metabolomic Review. Phytomedicine. 2023;119:154979. doi: 10.1016/j.phymed.2023.154979. [DOI] [PubMed] [Google Scholar]
- 223.Mu J., Xu J., Wang L., Chen C., Chen P. Anti-Inflammatory Effects of Purple Sweet Potato Anthocyanin Extract in DSS-Induced Colitis: Modulation of Commensal Bacteria and Attenuated Bacterial Intestinal Infection. Food Funct. 2021;12:11503–11514. doi: 10.1039/D1FO02454J. [DOI] [PubMed] [Google Scholar]
- 224.Zhang D., Liu J., Cheng H., Wang H., Tan Y., Feng W., Peng C. Interactions between Polysaccharides and Gut Microbiota: A Metabolomic and Microbial Review. Food Res. Int. 2022;160:111653. doi: 10.1016/j.foodres.2022.111653. [DOI] [PubMed] [Google Scholar]
- 225.Kwon C., Ediriweera M.K., Kim Cho S. Interplay between Phytochemicals and the Colonic Microbiota. Nutrients. 2023;15:1989. doi: 10.3390/nu15081989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yamauchi T., Ueda T. Primary Hyperuricemia Due to Decreased Renal Uric Acid Excretion. Nippon. Rinsho. Jpn. J. Clin. Med. 2008;66:679–681. [PubMed] [Google Scholar]
- 227.Fathallah-Shaykh S.A., Cramer M.T. Uric Acid and the Kidney. Pediatr. Nephrol. 2014;29:999–1008. doi: 10.1007/s00467-013-2549-x. [DOI] [PubMed] [Google Scholar]
- 228.Maiuolo J., Oppedisano F., Gratteri S., Muscoli C., Mollace V. Regulation of Uric Acid Metabolism and Excretion. Int. J. Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 229.Yan J., Zhang G., Hu Y., Ma Y. Effect of Luteolin on Xanthine Oxidase: Inhibition Kinetics and Interaction Mechanism Merging with Docking Simulation. Food Chem. 2013;141:3766–3773. doi: 10.1016/j.foodchem.2013.06.092. [DOI] [PubMed] [Google Scholar]
- 230.Lin S., Zhang G., Liao Y., Pan J. Inhibition of Chrysin on Xanthine Oxidase Activity and Its Inhibition Mechanism. Int. J. Biol. Macromol. 2015;81:274–282. doi: 10.1016/j.ijbiomac.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 231.Qian X., Wang X., Luo J., Liu Y., Pang J., Zhang H., Xu Z., Xie J., Jiang X., Ling W. Hypouricemic and Nephroprotective Roles of Anthocyanins in Hyperuricemic Mice. Food Funct. 2019;10:867–878. doi: 10.1039/C8FO02124D. [DOI] [PubMed] [Google Scholar]
- 232.Brown J.S., Amend S.R., Austin R.H., Gatenby R.A., Hammarlund E.U., Pienta K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023;21:1142–1147. doi: 10.1158/1541-7786.MCR-23-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.National Cancer Institute Cancer Statistics. [(accessed on 19 June 2024)]; Available online: https://www.cancer.gov/about-cancer/understanding/statistics.
- 234.Chen P.N., Chu S.C., Chiou H.L., Chiang C.L., Yang S.F., Hsieh Y.S. Cyanidin 3-Glucoside and Peonidin 3-Glucoside Inhibit Tumor Cell Growth and Induce Apoptosis in Vitro and Suppress Tumor Growth in Vivo. Nutr. Cancer. 2009;53:232–243. doi: 10.1207/s15327914nc5302_12. [DOI] [PubMed] [Google Scholar]
- 235.Yu X., Zhou C., Yang H., Huang X., Ma H., Qin X., Hu J. Effect of Ultrasonic Treatment on the Degradation and Inhibition Cancer Cell Lines of Polysaccharides from Porphyra Yezoensis. Carbohydr. Polym. 2015;117:650–656. doi: 10.1016/j.carbpol.2014.09.086. [DOI] [PubMed] [Google Scholar]
- 236.Jiang C., Xiong Q., Li S., Zhao X., Zeng X. Structural Characterization, Sulfation and Antitumor Activity of a Polysaccharide Fraction from Cyclina Sinensis. Carbohydr. Polym. 2015;115:200–206. doi: 10.1016/j.carbpol.2014.08.095. [DOI] [PubMed] [Google Scholar]
- 237.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tsai T.C., Huang H.P., Chang K.T., Wang C.J., Chang Y.C. Anthocyanins from Roselle Extract Arrest Cell Cycle G2/M Phase Transition via ATM/Chk Pathway in P53-Deficient Leukemia HL-60 Cells. Environ. Toxicol. 2017;32:1290–1304. doi: 10.1002/tox.22324. [DOI] [PubMed] [Google Scholar]
- 239.Li W.L., Yu H.Y., Zhang X.J., Ke M., Hong T. Purple Sweet Potato Anthocyanin Exerts Antitumor Effect in Bladder Cancer. Oncol. Rep. 2018;40:73–82. doi: 10.3892/or.2018.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tang S., Kan J., Sun R., Cai H., Hong J., Jin C., Zong S. Anthocyanins from Purple Sweet Potato Alleviate Doxorubicin-Induced Cardiotoxicity in Vitro and in Vivo. J. Food Biochem. 2021;45:e13869. doi: 10.1111/jfbc.13869. [DOI] [PubMed] [Google Scholar]
- 241.Hanieh H., Gerile C., Narabara K., Gu Z., Abe A., Kondo Y. In Vivo Immunomodulatory Effects of Dietary Purple Sweet Potato after Immunization in Chicken. Anim. Sci. J. 2010;81:116–121. doi: 10.1111/j.1740-0929.2009.00715.x. [DOI] [PubMed] [Google Scholar]
- 242.Miyazaki K., Makino K., Iwadate E., Deguchi Y., Ishikawa F. Anthocyanins from Purple Sweet Potato Ipomoea batatas Cultivar Ayamurasaki Suppress the Development of Atherosclerotic Lesions and Both Enhancements of Oxidative Stress and Soluble Vascular Cell Adhesion Molecule-1 in Apolipoprotein E-Deficient Mice. J. Agric. Food Chem. 2008;56:11485–11492. doi: 10.1021/jf801876n. [DOI] [PubMed] [Google Scholar]
- 243.Ju R., Zheng S., Luo H., Wang C., Duan L., Sheng Y., Zhao C., Xu W., Huang K. Purple Sweet Potato Attenuate Weight Gain in High Fat Diet Induced Obese Mice. J. Food Sci. 2017;82:787–793. doi: 10.1111/1750-3841.13617. [DOI] [PubMed] [Google Scholar]
- 244.Kim O.K., Nam D.E., Yoon H.G., Baek S.J., Jun W., Lee J. Immunomodulatory and Antioxidant Effects of Purple Sweet Potato Extract in LP-BM5 Murine Leukemia Virus-Induced Murine Acquired Immune Deficiency Syndrome. J. Med. Food. 2015;18:882–889. doi: 10.1089/jmf.2014.3274. [DOI] [PubMed] [Google Scholar]
- 245.Dong G., Xu N., Wang M., Zhao Y., Jiang F., Bu H., Liu J., Yuan B., Li R. Anthocyanin Extract from Purple Sweet Potato Exacerbate Mitophagy to Ameliorate Pyroptosis in Klebsiella Pneumoniae Infection. Int. J. Mol. Sci. 2021;22:11422. doi: 10.3390/ijms222111422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Baek S.J., Hammock B.D., Hwang I.K., Li Q., Moustaid-Moussa N., Park Y., Safe S., Suh N., Yi S.S., Zeldin D.C., et al. Natural Products in the Prevention of Metabolic Diseases: Lessons Learned from the 20th Kast Frontier Scientists Workshop. Nutrients. 2021;13:1881. doi: 10.3390/nu13061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ngcobo A., Mianda S.M., Seke F., Sunette L.M., Sivakumar D. Phytonutritional Composition and Antioxidant Properties of Southern African, Purple-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam.) Storage Roots. Antioxidants. 2024;13:338. doi: 10.3390/antiox13030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xie Y., Liu Q., Mao C., Pang H., Ye P., Cui B., Chen X., Fu H., Wang Y., Wang Y. Radio Frequency Drying and Puffing of Composite Purple Sweet Potato Chips. J. Food Compos. Anal. 2024;125:105736. doi: 10.1016/j.jfca.2023.105736. [DOI] [Google Scholar]
- 249.Nurdjanah S., Nurdin S.U., Astuti S., Manik V.E. Chemical Components, Antioxidant Activity, and Glycemic Response Values of Purple Sweet Potato Products. Int. J. Food Sci. 2022;2022:7708172. doi: 10.1155/2022/7708172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rahmi Y., Kurniawati A.D., Widyanto R.M., Ariestiningsih A.D., Aisyi A.Z.A.F., Ruchaina A.N., Sihombing E.V., Istira F.B., Nafsiyah I., Permatasari K.D., et al. The Sensory, Physical and Nutritional Quality Profiles of Purple Sweet Potato and Soy-Based Snack Bars for Pregnant Women. J. Public Health Res. 2021;10:jphr-2021. doi: 10.4081/jphr.2021.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wijaya H., Slay A., Abdullah N. Ice Cream Products Made from Processed Purple Sweet Potatoes: A Product Organoleptic Study. IOP Conf. Ser. Earth Environ. Sci. 2021;807:042074. doi: 10.1088/1755-1315/807/4/042074. [DOI] [Google Scholar]
- 252.Basílio L.S.P., Vanz Borges C., Minatel I.O., Vargas P.F., Tecchio M.A., Vianello F., Lima G.P.P. New Beverage Based on Grapes and Purple-Fleshed Sweet Potatoes: Use of Non-Standard Tubers. Food Biosci. 2022;47:101626. doi: 10.1016/j.fbio.2022.101626. [DOI] [Google Scholar]
- 253.Im Y.R., Kim I., Lee J. Phenolic Composition and Antioxidant Activity of Purple Sweet Potato (Ipomoea batatas (l.) Lam.): Varietal Comparisons and Physical Distribution. Antioxidants. 2021;10:462. doi: 10.3390/antiox10030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Amoah I., Cobbinah J.C., Yeboah J.A., Essiam F.A., Lim J.J., Tandoh M.A., Rush E. Edible Insect Powder for Enrichment of Bakery Products—A Review of Nutritional, Physical Characteristics and Acceptability of Bakery Products to Consumers. Future Foods. 2023;8:100251. doi: 10.1016/j.fufo.2023.100251. [DOI] [Google Scholar]
- 255.Olson R., Gavin-Smith B., Ferraboschi C., Kraemer K. Food Fortification: The Advantages, Disadvantages and Lessons from Sight and Life Programs. Nutrients. 2021;13:1118. doi: 10.3390/nu13041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lin S. Dietary Fiber in Bakery Products: Source, Processing, and Function. Adv. Food Nutr. Res. 2022;99:37–100. doi: 10.1016/BS.AFNR.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 257.Bhatnagar P., Gururani P., Parveen A., Gautam P., Chandra Joshi N., Tomar M.S., Nanda M., Vlaskin M.S., Kumar V. Algae: A Promising and Sustainable Protein-Rich Food Ingredient for Bakery and Dairy Products. Food Chem. 2024;441:138322. doi: 10.1016/j.foodchem.2023.138322. [DOI] [PubMed] [Google Scholar]
- 258.Kourkouta L., Koukourikos K., Iliadis C., Ouzounakis P., Monios A., Tsaloglidou A. Bread and Health. J. Pharm. Pharmacol. 2017;5:821–826. doi: 10.17265/2328-2150/2017.11.005. [DOI] [Google Scholar]
- 259.Rezende L.V., Paraizo W.B., de Santos J.D.A., Amorim K.A., Goulart G.A.S., Becker F.S., Damiani C. Study of the Drying Process of the Purple-Fleshed Sweet Potato (Ipomoea batatas (L.) Lam) in Spouted Bed. Food Sci. Technol. 2024;44 doi: 10.5327/fst.00180. [DOI] [Google Scholar]
- 260.Cui R., Zhu F. Changes in Structure and Phenolic Profiles during Processing of Steamed Bread Enriched with Purple Sweetpotato Flour. Food Chem. 2022;369:130578. doi: 10.1016/j.foodchem.2021.130578. [DOI] [PubMed] [Google Scholar]
- 261.Santiago D.M., Matsushita K., Tsuboi K., Yamada D., Murayama D., Kawakami S., Shimada K., Koaze H., Yamauchi H. Texture and Structure of Bread Supplemented with Purple Sweet Potato Powder and Treated with Enzymes. Food Sci. Technol. Res. 2015;21:537–548. doi: 10.3136/fstr.21.537. [DOI] [Google Scholar]
- 262.Kweman N.T., Julianti E., Romauli N.D.M. Physicochemical Characteristics and Glycemic Index of Bread Made from Purple Sweet Potato Flour, Starch, Fiber from Solid Waste of Starch Processing. IOP Conf. Ser. Earth Environ. Sci. 2021;924:012040. doi: 10.1088/1755-1315/924/1/012040. [DOI] [Google Scholar]
- 263.Zhu F., Sun J. Physicochemical and Sensory Properties of Steamed Bread Fortified with Purple Sweet Potato Flour. Food Biosci. 2019;30:100411. doi: 10.1016/j.fbio.2019.04.012. [DOI] [Google Scholar]
- 264.Laila U., Rochmadi, Pudjiraharti S. Microencapsulation of Purple-Fleshed Sweet Potato Anthocyanins with Chitosan-Sodium Tripolyphosphate by Using Emulsification-Crosslinking Technique. J. Math. Fundam. Sci. 2019;51:29–46. doi: 10.5614/j.math.fund.sci.2019.51.1.3. [DOI] [Google Scholar]
- 265.Herminiati A., Kurniasari R.A., Cahyadi W. Optimization of Formula Cookies Based on Purple Sweet Potato Flour (Ipomoea batatas L.) Using I-Optimal Mixture Design Method. BIO Web Conf. 2023;69:03002. doi: 10.1051/bioconf/20236903002. [DOI] [Google Scholar]
- 266.Liu Y.-N., Jeong D.-H., Jung J.-H., Kim H.-S. Quality Characteristics and Antioxidant Activities of Cookies Added with Purple Sweet Potato Powder. Korean J. Food Cook. Sci. 2013;29:275–281. doi: 10.9724/kfcs.2013.29.3.275. [DOI] [Google Scholar]
- 267.Chung H.J. Influence of Purple Sweet Potato Powder Addition on the Quality Characteristics and Oxidative Stability of Cookies. J. Food Sci. Nutr. 2009;14:60–65. doi: 10.3746/jfn.2009.14.1.060. [DOI] [Google Scholar]
- 268.Muhammad R., Ikram E.H.K., Sharif M.S.M., Nor N.M. The Physicochemical Analysis and Anthocyanin Level of Malaysian Purple Sweet Potato Cracker. Curr. Res. Nutr. Food Sci. 2022;10:1030–1045. doi: 10.12944/CRNFSJ.10.3.19. [DOI] [Google Scholar]
- 269.Julianti E., Lubis Z., Limanto S. Utilization of Purple Sweet Potato Flour, Starch, and Fibre in biscuits Making. Int. Conf. Food Bio-Ind. 2019;443:012047. doi: 10.1088/1755-1315/443/1/012047. [DOI] [Google Scholar]
- 270.Manalu M., Rumida, Julianti E., Romauli N.D.M. Composites Flour Formulation Made from Yellow Pumpkin, Purple Sweet Potato, Corn, and Wolf-Herring Flour for Replacement of Wheat Flour on Low- and High- Moisture Foods Part I: Cookies and Muffin. Food Humanit. 2024;2:100261. doi: 10.1016/j.foohum.2024.100261. [DOI] [Google Scholar]
- 271.Bakar M.F.A., Ranneh Y., Kamil N.F.M. Development of High Fiber Rich Antioxidant Biscuits from Purple and Orange Sweet Potato Peels. Food Res. 2022;6:12–19. doi: 10.26656/fr.2017.6(1).036. [DOI] [Google Scholar]
- 272.Shewry P.R., Halford N.G., Belton P.S., Tatham A.S. The Structure and Properties of Gluten: An Elastic Protein from Wheat Grain. Philos. Trans. R. Soc. B Biol. Sci. 2002;357:133–142. doi: 10.1098/rstb.2001.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Huang Y., Zhou S., Zhao G., Ye F. Destabilisation and Stabilisation of Anthocyanins in Purple-Fleshed Sweet Potatoes: A Review. Trends Food Sci. Technol. 2021;116:1141–1154. doi: 10.1016/j.tifs.2021.09.013. [DOI] [Google Scholar]
- 274.Nieto G. Incorporation of by-products of rosemary and thyme in the diet of ewes: Effect on the fatty acid profile of lamb. Eur. Food Res. Technol. 2013;236:379–389. doi: 10.1007/s00217-012-1892-7. [DOI] [Google Scholar]
- 275.Nieto G., Bañón S., Garrido M.D. Incorporation of thyme leaves in the diet of pregnant and lactating ewes: Effect on the fatty acid profile of lamb. Small Rumin. Res. 2012;105:140–147. doi: 10.1016/j.smallrumres.2011.11.016. [DOI] [Google Scholar]
- 276.Aziz A.A., Padzil A.M., Muhamad I.I. Effect of Incorporating Purple-Fleshed Sweet Potato in Biscuit on Antioxidant Content, Antioxidant Capacity and Colour Characteristics. Malays. J. Anal. Sci. 2018;22:667–675. doi: 10.17576/mjas-2018-2204-13. [DOI] [Google Scholar]