Abstract
Collagen peptides and marine collagen are enormous resources currently utilized. This review aims to examine the scientific literature to determine which collagen peptides derived from marine sources and which natural active antioxidants from marine collagen have significant biological effects as health-promoting nutraceuticals. Marine collagen is extracted from both vertebrate and invertebrate marine creatures. For vertebrates, this includes fish skin, bones, scales, fins, and cartilage. For invertebrates, it includes mollusks, echinoderms, crustaceans, and poriferans. The method used involved data analysis to organize information for isolating and identifying marine biocompounds with antioxidant properties. Specifically, amino acids with antioxidant properties were identified, enabling the use of hydrolysates and collagen peptides as natural antioxidant nutraceuticals. The methods of extraction of hydrolyzed collagen and collagen peptides by different treatments are systematized. The structural characteristics of collagen, collagen peptides, and amino acids in fish skin and by-products, as well as in invertebrate organisms (jellyfish, mollusks, and crustaceans), are described. The antioxidant properties of different methods of collagen hydrolysates and collagen peptides are systematized, and the results are comparatively analyzed. Their use as natural antioxidant nutraceuticals expands the range of possibilities for the exploitation of natural resources that have not been widely used until now.
Keywords: marine antioxidant, marine nutraceuticals, marine collagen, marine collagen peptides
1. Introduction
Nutraceuticals have garnered significant attention for their role in alternative treatments for disease prevention and health maintenance. In the European Union (EU), there is specific legislation governing the marketing of functional foods and nutraceuticals, emphasizing their “safety” [1]. The scientific risk assessment is carried out by the European Food Safety Authority [1]. The impact of the COVID-19 pandemic required serious analysis to assess the extent to which dietary supplements and nutraceuticals had potential in the COVID-19 crisis [2,3]. Nutraceuticals are those nutritional products that have additional health benefits [4,5]. Nutraceuticals not only supplement the diet but also contribute to the prophylaxis or treatment of a disorder or disease [6]. Nutraceuticals with antioxidant potential have gained wide interest. In the body, by-products of normal metabolic reactions such as normal cellular respiration and responses to external stimuli on cells generate reactive oxygen species (ROS), which are highly oxidative [7]. Reactive species can be singlet oxygen, hydroxyl radical, superoxide anion, peroxide, and nitrous oxide. Long-term exposure to oxidative stress impairs the biosynthesis of molecules and causes some chondral diseases [8]. Excessive accumulation of ROS damages cell membranes and biological macromolecules, causing damage to tissues and organs, and can generate various pathological conditions such as aging phenomena, arthritis, Alzheimer’s, cancer, and other degenerative diseases [8,9]. To stop such accumulations and maintain the average level of ROS species in the body, antioxidants are needed [10,11]. Synthetic antioxidants including butylated hydroxytoluene (BHT), butylated butylated hydroxyanisole (BHA), and tertiary butylated hydroquinone (TBHQ) are the best known. Although they are compounds with remarkable antioxidant potential, they have shown increased toxicity and their use has begun to be restricted [11]. Under these conditions, natural antioxidants have attracted attention [12]. Compounds with antioxidant capacity from marine resources have gained wide interest, including those from marine fish, seaweed, jellyfish, and mollusks [13,14,15,16]. Among the natural compounds with good antioxidant action and outstanding degradability, the following have stood out: alongside polysaccharides and collagen, gelatin, and collagen peptides [17,18,19]. Native collagen, collagen hydrolysates, and gelatin have gained new potential uses due to their biocompatibility. These include applications as a food source and in various biological and medical domains [20,21,22]. Additionally, they are utilized as biomaterials for medical purposes and in food packaging [23,24]. For a long time, collagen was extracted from terrestrial sources like cattle and pigs. As shown by Lim et al. (2019) due to religious restrictions (Muslims, Hindus, and Jews avoid products from these animals) and the emergence of communicable diseases such as bovine spongiform encephalopathy (BSE), foot-and-mouth disease (FMD), and transmissible spongiform encephalopathy (TSE), which have become prevalent worldwide in recent decades, attempts have been made to search for other sources of collagen [25]. Terrestrial animal products can transmit these diseases (Salvatore et al., 2020) [26]. Thus, collagen from marine resources began to gain great importance. To avoid this risk, Geahchan et al. (2022) and Prelipcean et al. (2022) recommend using marine collagen in wound healing [27,28]. There was an urgent need to identify new alternative sources of collagen. Recent studies on the molecular structure and biochemical properties of fish collagen have shown several similarities to collagen from terrestrial mammals. However, fish collagen has a lower molecular weight and a lower denaturation temperature than mammalian collagen as observed by de Melo Oliveira et al. (2021) and El Blidi et al. (2021) [29,30]. Marine collagen has been studied for applications in different fields: biomaterials, Gallo et al. (2020) and Benayahu et al. (2018); wound healing, Gaspar-Pintilescu et al. (2021) and Cadar et al. (2023); diet use, Paul et al. (2019); and cosmetics, Rodriguez et al. (2018); and antioxidant properties have been reported in several studies by Ballatore et al. (2020), Bashir et al. (2020), Kisling et al. (2019), and Pezeshk et al. (2019) [31,32,33,34,35,36,37,38,39,40]. The marine environment offers a vast resource for isolating collagen and collagen peptides, often wasted as by-products from fish and invertebrate organisms. At present, these resources remain underutilized. In conclusion, the potential of marine-derived collagen antioxidants as valuable nutraceuticals is not fully recognized. This review aims to gather and organize information on techniques for isolating and separating collagen and collagen peptides from marine organisms, both vertebrates and invertebrates, while emphasizing their antioxidant properties. Specifically, it explores the potential of using fish by-products—such as skin, bones, scales, swimming fins, and fish heads—which are rich in collagen and collagen peptides but are currently underutilized globally. The data presented cover the structure and amino acid composition of collagen and their associated antioxidant properties. Results from various analytical methods demonstrate the antioxidant activity of marine collagen products. In addition, the data detail the antioxidant effects of collagen and marine collagen peptides in various medical conditions, supporting their use as natural antioxidant nutraceuticals.
2. Data Collection Method
Literature data covering the period 2015–2024 were collected from databases such as Science Direct, SCOPUS, Google Scholar, and Web of Science, where the keywords “marine collagen”, “marine collagen peptides”, or “marine antioxidant” were used for literature data extraction and analysis.
3. Isolation of Collagen from Marine Resources
3.1. Marine Sources of Collagen
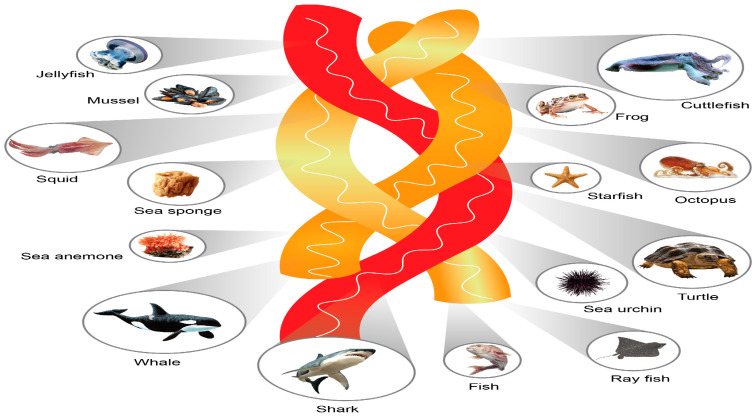
The marine ecosystem encompasses a wide range of habitats, from the surface waters to the deep sea, which host a diverse array of species. These species are a valuable ecological source for obtaining marine collagen with antioxidant properties. The raw materials for marine collagen can be extracted from both vertebrates and invertebrates, including marine fish (such as fish skin and fish waste), poriferans (marine sponges), mollusks (byssus and cephalopods), crustaceans (mantis shrimps), echinoderms (starfish and sea urchins), and coelenterates (jellyfish). Figure 1 shows marine organisms that may be potential sources of marine collagen.
Figure 1.
Marine sources for the preparation of marine collagen.
The diversity and potential were highlighted by de Melo Oliveira et al. (2021) and Rahman (2019) [29,41]. Marine collagen can vary significantly in structure, depending on its source. It is worth noting that marine vertebrates, such as marine fish, possess more intricate skeletal systems with abundant collagen in their bones and skin. This observation is supported by Cherim et al. (2019) and Prajaputra et al. (2024) [42,43]. Currently, a major problem in the fish farming industry is the inadequate management of waste or by-products resulting from improper fish processing, which leads to economic losses and environmental problems.
3.2. Marine Collagen Extraction
3.2.1. Extraction Procedures
Biotechnologies used to extract collagen from marine organisms have been detailed in studies by Prajaputra et al. (2024) and Jafari et al. (2020), who categorized them based on the extracted collagen type. These methods include alkali-soluble collagen (SSC), acid-soluble collagen (ASC), enzymatic methods (PSC), and ultrasonic methods [43,44]. Additionally, Cherim et al. (2017) and Lu et al. (2023) have reported on the isolation and characterization of collagen from marine sources [45,46]. Depending on the chosen extraction method, collagen products vary in different yields and properties. Marine collagen extraction typically involves two primary steps:
-
I.
The pre-treatment stage involves preparing the raw material and eliminating contaminants to ensure the purity of the final product. Marine by-products, including skin, bones, scales, or the head and appendages of marine organisms in the case of invertebrates, are carefully selected. Various compounds, such as pigments, non-collagenous proteins, and unwanted lipids, are removed during this stage, as documented by Ampitiya et al. (2023) [47]. Additionally, other researchers, such as Wang et al. (2018) and Chen et al. (2021) have reported successful removal of adhesive residues using aqueous NaCl solutions of varying concentrations [48,49]. Cumming et al. (2019) reported the removal of inorganic minerals by demineralization with EDTA (ethy-lenediaminetetraacetic acid), as reported [50]. Another option was the use of a 0.5 M HCl solution, which was reported by Xu et al. (2017), Kıyak et al. (2024), and Li C. et al. (2020) [51,52,53]. Sodium chloride, n-butanol, and sodium hypochlorite hexane or hydrogen peroxide solution were used for the removal of dyes and non-collagenous fats, as reported by Wang et al. (2018), and Liu et al. (2019) [48,54]. In 2021, Song et al. reported that fermentation is also an alternative pretreatment that helps to obtain collagen from Nile tilapia skin by the PSC process with very good purity [55].
-
II.
The actual extraction stage can be carried out by specific methods. The most commonly used methods for obtaining collagen are the ASC and PSC methods [43,56].
The ASC procedure is the most widespread. Sirbu et al. in 2019 reported studies on the validation of a quantitative method for the extraction of collagen from the skin of gray mullet fish by the ASC process [57]. For the extraction of collagen from the tissues of marine organisms, acetic acid is the most widely used dilute acid, but other acids can also be used, such as citric acid, lactic acid, or chloroacetic acid. In 2020, Senadheera et al. and in 2021, Shaik et al. showed that organic acids provide higher collagen extraction efficiency than inorganic acids [58,59]. The most widely used ASC extraction method is the one using acetic acid in a 0.5 M concentration, continuously stirred between 24 h and 72 h, for collagen extraction [43,56]. From multiple reported studies, it appears that in order to obtain the best extraction results, the acetic acid concentrations must be adapted to the sample type. Thus, Hadfi et al. (2019) extracted collagen from silver catfish (Pangasius sp.) skin with different concentrations of acetic acid (0.5 M and 0.7 M) and reported yields of 10.9% and 5.47%, respectively [60]. So, there was a higher yield when 0.5 M acetic acid concentrations were used [60]. However, Baderi N.A. et al. (2019) extracted collagen from shortfin scad (Decapterus macrosoma) and reported 1.01% and 1.31% yields when using 0.5 M and 0.7 M acetic acid, respectively, so the yield was higher at 0.7 M acetic acid concentrations [61]. In the following step, the collagen supernatant is obtained by centrifugation, which then has to be precipitated with salt (NaCl). This separates the collagen precipitate. In 2020, Seixas et al. reported these methods along with other procedures for the extraction of collagen from elasmobranch by-products for potential biomaterial use [62]. In 2018, Tanaka et al. isolated collagen from bluefin tuna (Thunnus orientalis) skin, and Tan et al. isolated collagen from channel catfish (Ictalurus punctatus) skin [63,64].
The PSC procedure is also a commonly used process and is based on the reaction of collagen with pepsin. Venkatesan et al. (2017), showed that in this treatment, the enzymes provide increased yields and purity of collagen [65]. Zhao et al. (2018) showed that acid-soluble collagen tends to generate a lower yield, and pepsin extraction increases extraction yield because pepsin cleaves crosslinks in the telopeptide region, thus producing increased collagen solubility in acid [66]. Castaneda-Valbuena et al. (2022) found that treating certain proteins with pepsin reduces their allergenicity, making this treatment suitable for producing collagen hydrolysates or peptides [67]. To obtain collagen hydrolysates, the collagen macromolecules need to be broken down further through processes like basic, acidic, or enzymatic hydrolysis [67]. Asaduzzaman et al. (2020) demonstrated that acidic or basic conditions, along with subcritical water hydrolysis (which avoids toxic solvents and collagen degradation), are preferable for collagen degradation [68]. Pepsin treatments for collagen extraction have been reported by Asaduzzaman et al. (2020) for collagen from mackerel bones (Scomber japonicus) and skin, as well as by Zhang et al. (2017) for frog skin (Rana nigromaculata) using a 0.5 M acetic acid extract containing 0.1% pepsin for 72 h [68,69].
3.2.2. Procedures Applied to the Isolation of Collagen from Invertebrates
In the case of other invertebrate marine organisms, it has been necessary to resort to adapted procedures for collagen extraction. For example, jellyfish collagen is generally precipitated with an aqueous solution of 2.3–2.6 M NaCl.
The collagen precipitate is collected, centrifuged, and solubilized in a 0.5 M acetic acid solution (about three days), followed by salting by dialysis with a NaHPO4 solution. The precipitated collagen is separated by centrifugation, then solubilized in acetic acid and purified by reprecipitation with the addition of solid NaCl to a concentration of 0.9 M. Acid-soluble collagen (ASC) can be digested with pepsin to obtain atelocollagen [19]. In the case of sea urchins, the intact collagen fibrils in the peristomal membranes are different from other types of collagen and cannot be extracted by traditional acid solubilization methods, as this method generally produces it as hydrolyzed gelatin. The shredded native tissue is sequentially treated with a hypotonic solution and a specific decellularization solution to remove both cellular debris and skeletal parts and pigments. After 3–4 days in the β-mercaptoethanol disaggregating solution, collagen fibers are obtained, which are then passed through a filtration step and dialyzed in a 0.5 M EDTA-Na solution [19]. The same protocol is employed for extracting collagen fibers from the aboral arm walls of the starfish. However, an additional step is introduced wherein the samples undergo treatment with 1 mM citric acid between the decellularization and disaggregation solutions. This step is crucial for eliminating calcium carbonate osmosis present in the fresh tissue [19]. In a study conducted by Sun et al. (2021), soluble collagen (ASC), pepsin-soluble collagen (PSC), and water-soluble gelatin (WSG) were extracted from squid (Dosidicus gigas) skin. They found that using the ASC process at 4 °C resulted in the lowest yield of 33.5% [70]. The addition of pepsin (PSC process) increased the collagen yield by approximately 35.0%. The highest yield of 81.9% was achieved through water extraction at 60 °C (WSG). The authors demonstrated that low temperatures can effectively preserve the native helix structures of ASC and PSC. In contrast, heat treatment led to the transformation of collagen into gelatin with uncoordinated and denatured structures [70]. Antioxidant peptides derived from marine fish are obtained by enzymatic hydrolysis methods using different types of enzymes (alkalase, α-chymotrypsin, neutrase, papain, pepsin, and trypsin). Castaneda-Valbuena et al. (2022) showed that the use of optimized buffer systems is required for these enzymes [67]. Separation of peptides is carried out by using chromatographic techniques and ultrafiltration membranes. After collecting the peptide fractions, the lyophilization step follows to obtain purified peptides [67].
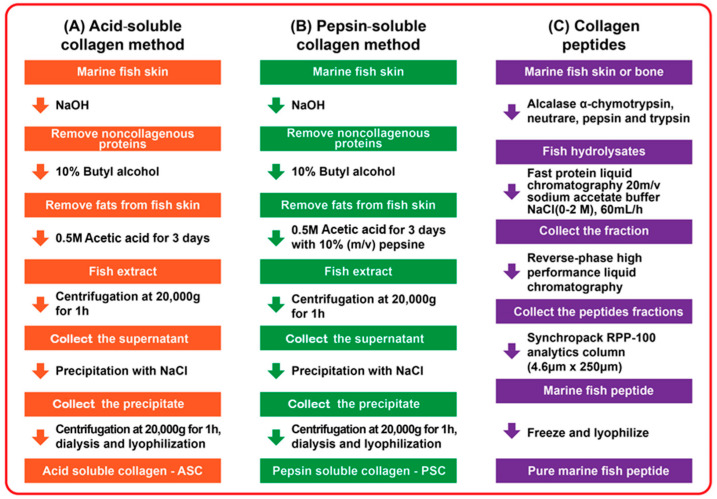
Figure 2 illustrates the commonly employed methods for extracting marine collagen from fish. These include the following: (A) acid treatment, (B) enzymatic treatment, and (C) extraction using pepsins for marine collagen [65]. Additionally, Figure 2 outlines the general procedures for generating collagen peptides from fish skin and bones [65].
Figure 2.
Scheme for obtaining marine collagen through (A) acid-soluble collagen method; (B) pepsin-soluble collagen method and (C) collagen peptides.
3.2.3. Ultrasonic Procedure
The ultrasonic protein extraction process is simple, fast, risk-free, reliable, and financially beneficial. Ultrasonication leads to increased enzyme activity and helps remove temperature-sensitive chemicals. Shaik et al. (2021) studied the effect of ultrasound on collagen extraction in ASC and PSC procedures and showed that the method, being non-invasive, can obtain collagen with an almost intact structure [59]. However, prolonged exposure to ultrasound can lead to a cavitational effect, resulting in elevated temperatures, shear forces, and pressures within the medium. This effect causes the disruption of hydrogen bonds and van der Waals interactions in polypeptide chains, ultimately leading to protein denaturation. Despite these drawbacks, studies such as Shaik et al.’s (2021) have observed the application of ultrasound-assisted ASC and PSC treatments, demonstrating increased yields for collagen extracted from Sharpnose stingray (Dasyatis zugei) using both acid extraction and ultrasound-assisted pepsin extraction while preserving other properties [59]. Zou et al. (2017), Ali et al. (2018), and Petcharat et al. (2021) showed that ultrasound treatment at 20–35 kHz, amplitude 20–100%, pulse 2/2 s–20/20 s, and 200–750 W lasts about 10–30 min or even 0–24 h [71,72,73]. Ali et al. (2018) found that golden carp (Probarbus jullieni) skin extracted with pepsin followed by ultrasonication produced a higher content of amino acids and an increased denaturation temperature, so the combined extraction method maintained the triple helical structure of extracted collagen [72]. Petcharat et al. (2021) performed collagen extraction on clown featherback (Chitala ornata) skin using ultrasonic methods [73]. Pezeshk et al. (2022) confirmed by modern physicochemical methods (X–ray diffraction and FTIR) that collagen from yellowfin tuna skin extracted with ultrasound showed a native undenatured triple catenary helical structure, so ultrasonication did not affect the structural integrity of the collagen [74]. In conclusion, the application of ultrasound in collagen extraction reduces extraction time and can increase both the quality and quantity of extracted collagen at certain extraction amplitudes and times.
3.2.4. Other Methods
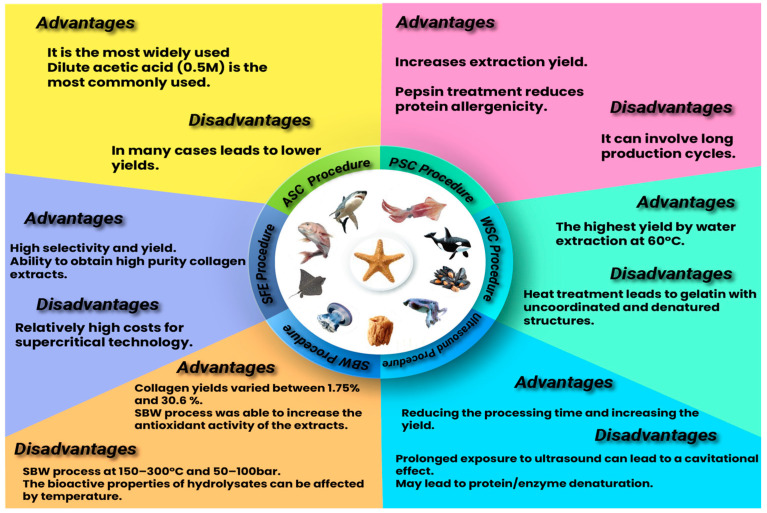
There are alternative methods for extracting collagen from marine resources; however, they are not as popular as ASC, PSC, and ultrasonic treatments [52]. Figure 3 shows the marine collagen extraction procedures with their advantages and disadvantages.
Figure 3.
Advantages and disadvantages of marine collagen extraction procedures.
The WSC procedure has been used to extract collagen from marine invertebrates [70]. This water-soluble collagen (WSC) is produced at 60 °C and is relatively easy to make. However, the process ultimately transforms the collagen into gelatin with uncoordinated and denatured structures, as demonstrated by Sun et al. (2021) [70].
The subcritical water hydrolysis (SBW) procedure represents a green alternative to traditional methods. It involves using water at temperatures between 150 and 300 °C and pressures between 50 and 100 bar. Kıyak et al. (2024) demonstrated that this method has been successfully used for extracting collagen from various fish species and fish by-products [52]. However, a disadvantage of SBW is that the high temperatures may affect the collagen structure [52].
The supercritical fluid extraction (SFE) procedure is an alternative to traditional extraction methods. SFE uses a supercritical fluid, typically CO2, as the extracting solvent to separate components. CO2 is preferred due to its numerous advantages. The primary benefit of SFE is the ability to obtain purified components. Figure 3, as presented by Kıyak et al. (2024), outlines additional advantages and disadvantages of the SFE method [52].
3.3. Data on the Isolation of Marine Collagen
3.3.1. Marine Collagen Isolated from Leather and Marine Fish Waste
Marine fish belong to the vertebrate category, and the raw materials used to isolate collagen from fish are skin, bones, scales, cartilage, and other by-products (such as swimming fins). Fish by-products can vary in composition depending on the size of the fish, the species, and the technology used to process them. Type I collagen obtained from these by-products is preferred. Among the research carried out for the extraction of collagen from skin fish, we list the isolation collagen from Alu—Alu (Sphyraena sp.) by Matarsim et al. (2023) [75]. The extraction of collagen from skins of Asian sea bass and Spanish mackerel (Scomberomorus commerson) was performed by Ampitiya et al. (2023) [47]. Collagen and collagen peptide excision from the skin of round goby fish (Neogobius melanostomus) by Yemisken et al. (2023) and from the skin of silver catfish (Pangasius sp.) by Shaik et al. (2023) have been reported [76,77]. Type I collagen was extracted from other fish by-products, such as unicornfish (Naso reticulatus) bones obtained by Fatiroi et al. (2023) [78]. Research has been reported to isolate collagen from parrotfish (Scarus sordidus) scales by Jaziri et al. (2023) and from Megalonibea (Megalonibea fusca) swim bladders obtained by Mo et al. (2023) [79,80]. Marine collagens were also obtained from the swim bladder of Totoaba (Totoaba macdonaldi) extracted by Cruz-Lopez et al. (2023), from the swim bladder of sea eels (Muraenesox cinereus) extracted by Li H. et al. (2023), and from the cartilage of blue sharks (Prionace glauca) by Pan et al. (2023) [81,82,83]. Research on the extraction of marine collagen from different fish by-products was reported, including from the bones of lizardfish (Saurida tumbil) by Jaziri et al. (2022), and from the tail tendon of skipjack tuna (Katsuwonus pelamis) by Chanmangkang et al. (2022) [84,85]. Marine collagen was isolated from the swim bladder of grass carp (Ctenopharyngodon idella) by Dong et al. (2022), and from the skin of Greenland halibut (Reinhardtius hippoglossoides) by Martins et al. (2022) [86,87]. Other research to obtain marine collagen was done from catfish (Silurus triostegus) skin by Abbas et al. (2022) and from dusky grouper (Epinephelus marginatus) scales by Tziveleka et al. (2022) [88,89]. Collagen was isolated from shark (Prionace glauca) cartilage by Seixas et al. (2020) and from surgeon fish (Huso huso) skin by Atef et al. (2020) [62,90]. Zhang et al. (2022) reported data on gelatin from the cartilage of Siberian sturgeons (Acipenser baerii) [91]. Type I collagen was extracted from the swim bladder of giant croakers (Nibea japonica) by Chen et al. (2019) and from the skin of bigeye tuna (Thunnus obesus) by Ahmed et al. (2019) [92,93]. Kittiphattanabawon et al. (2019) also extracted collagen from Nile tilapia (Oreochromis Niloticus) scales by ASC and PSC procedures [94]. Studies for the extraction of marine collagen from the skin of silver catfish (Chrysichthys nigrodigitatus) were reported by Hukmi et al. (2018) [95]. Iskandar et al. (2018) extracted collagen from the skin of bonylip barb fish (Osteochilus vittatus) [96]. Changfeng C. et al. (2013) characterized collagens from the cartilage of the Scottish hammerhead (Sphyrna lewini), and Zhong-Rui reported data on collagens from the skin and bone of the Spanish mackerel (Scomberomorous niphonius), [97,98]. Hu et al. (2023) reported data on the utilization of peptides from the collagens of monkfish (Lophius litulon) swim bladders [99]. Li et al. (2018) reported studies obtaining collagen from scales of the Miiuy croaker (Miichthys miiuy) [100]. Other studies on the isolation and valorization of collagen from fish and fish derivatives were reported. Nurmila et al. conducted research on the extraction and characterization of antioxidant activities from yellowfin tuna Thunnus albacares skin [101,102]. Studies concerning collagen from skin of grey mullets from the Black Sea were also reported by Cherim et al. in 2019 and in 2017 [103,104]. Collagen extracted from the skin of bluefin tuna (Thunnus orientalis) was reported by Tanaka et al. (2018) [63].
3.3.2. Collagen from Marine Invertebrates
Collagen isolation from invertebrates has been relatively less studied. Sea sponges, sponges or poriferans are part of a category of invertebrates that have been shown to be a potential source of collagen, although they have been little investigated. To date, about 8500 species are known. The class Demospogiae includes Chondrosia reniformis, which has been studied as a potential collagen source by Tassara et al. (2023), Araújo et al. (2021), and Pozzolini et al. (2018) [105,106,107]. Fernandes et al. (2021) reported studies on the biological performance of marine sponge collagen [108]. Parisi et al. (2019) reported on the biological activities of materials derived from spongin, a form of collagen from marine sponges, when incorporated into other materials [109].
Langasco et al. (2017) explored the use and enhancement of the natural collagen-horny skeleton of marine sponges (Porifera, Dictyoceratida) as a biologically based dressing for topical drug delivery [110].
Table 1 shows recent studies with data on the part of the body analyzed, the type of extraction method, the yield obtained for collagen, data on collagen analysis methods for identification, and the type of collagen identified.
Table 1.
Marine collagen isolated from marine vertebrates and invertebrates. Marine species, tissue from marine organism, extraction method, extraction time and yield, physicochemical methods of characterization, and type of isolated collagen.
| Marine Sources Species | Tissue | Type of Extraction Method/Time | Collagen Content % |
Methods for Characterization | Type of Collagen |
References |
|---|---|---|---|---|---|---|
| Vertebrates | ||||||
| Alu alu (Sphyraena sp.) | Skin | ASC 72 h; (4 °C); | 6.77 | SDS-PAGE, SEM, DSC, XRD, ATR-FTIR, | I | [75] |
| Asian sea bass (Lates calcarifer) | Skin | ASC 24 h; (4 °C); | 59.31 | UV spectra, SDS-PAGE, SEM, FTIR, XRD, | I | [47] |
| Seer fish (Scomberomorus commerson) | Skin | ASC 24 h; (4 °C); | 58.21 | FTIR, XRD, SDS-PAGE, UV spectra, SEM | I | [47] |
| Yellowfin tuna (Thunnus albacares) | Skin | ASC 24 h; (4 °C); | 61.26 | UV spectra, FTIR, XRD, SEM | I | [47] |
| Round goby (Neogobius melanostomus) | Skin | ASC 48 h; (4 °C); | 10 | SDS-PAGE, FTIR | I | [76] |
| Silver catfish (Pangasius sp.) | Skin | PSC 48 h; (4 °C); | 26.4 | Solubility, FTIR, SEM, rheology | I | [77] |
| Unicornfish (Naso reticulatus) | Bone | ASC 72 h; (4 °C); | 0.4 | UV spectra, SEM, SDS-PAGE, FTIR, XRD | I | [78] |
| Parrotfish (Scarus sordidus) | Scale | ASC 48 h; (4 °C); PSC 48 h; (4 °C); |
1.17 1 |
ATR-FTIR, SDS-PAGE, UV-Spectra, XRD, DSC, Solubility | I | [79] |
| Megalonibea (Megalonibea fusca) | Swim bladder | ASC 48 h; (4 °C); PSC 48 h; (4 °C); |
33.4 84.8 |
Amino acid analysis, SDS-PAGE, XRD, UV spectra, FTIR, SEM, zeta potential, | I | [80] |
| Totoaba (Totoaba macdonaldi) | Swim bladder | PSC 24 h; (4 °C); | 65 | SDS-PAGE, UV spectra, amino acid analysis, FTIR, XRD, zeta potential | I | [81] |
| Sea eel (Muraenesox cinereus) | Swim bladder | PSC 48 h; (24 °C); | 93.7 | SDS-PAGE, FTIR, SEM, UV spectra | I | [82] |
| Blue shark (Prionace glauca) | Cartilage | PSC 24 h; (4 °C); | 7.69 | SDS-PAGE, SEM, UV spectra, amino acid analysis | II | [83] |
| Lizardfish (Saurida tumbil) | Bone | ASC 72 h; (4 °C); | 1.73 | UV spectra, ATR-FTIR, SDS-PAGE, XRD, DSC, | I | [84] |
| Skipjack tuna (Katsuwonus pelamis) | Tail tendon | ASC 72 h; (4 °C); PSC 27 h; (4 °C); |
8.67 12.04 |
SDS-PAGE, viscosity, SEM, FTIR, DSC | I | [85] |
| Grass carp (Ctenopharyngodon idella) | Swim bladder | PSC 48 h; (4 °C); | 38.9 | FTIR, UV spectra, DSC, SDS-PAGE, | I | [86] |
| Greenland halibut (Reinhardtius hippoglossoides) | Skin | ASC 96 h; (4 °C); | 3.8 | SDS-PAGE, FTIR, amino acid analysis, SEM-EDX, DSC | I | [87] |
| Catfish (Silurus triostegus) | Skin | ASC 72 h; (4 °C); PSC 72 h; (4 °C); |
2.6; 8.24 |
SDS-PAGE, FTIR, HPLC, SEM, solubility, viscosity | I | [88] |
| Dusky grouper (Epinephelus marginatus) | Scale | ASC 72 h; (8 °C); PSC 24 h; (8 °C); |
0.39 1.5 |
SDS-PAGE, SEM, FTIR, XRD, TGA | I | [89] |
| Shark (Prionace glauca) | Cartilage | ASC 48 h; (4 °C); PSC 48 h; (4 °C); |
0.15 3.5 |
SDS-PAGE, UV spectra, DSC, FTIR, amino acid composition, rheology | I | [62] |
| Sturgeon fish (Huso huso) | Skin | ASC 48 h; (4 °C); PSC 48 h; (4 °C); |
9.98 9.08 |
SDS-PAGE, amino acid analysis, FTIR, SEM, DSC | I | [90] |
| Sturgeon (Acipenser baerii) | Gelatin from cartilage | ASC 7 h; pH 9 (45 C); | 28.8 | SDS-PAGE, UV and FTIR, amino acid composition, zeta potential | I | [91] |
| Giant croaker (Nibea japonica) | Swim bladder | ASC 24 h; (4 °C); PSC 8 h; (4 °C); |
11.3 15.35 |
SDS-PAGE, FTIR, SEM, amino acid analysis | I | [92] |
| Bigeye tuna (Thunnus obesus) | Skin | ASC 72 h; (4 °C); PSC 72 h; (4 °C); |
13.5 16.7 |
SDS-PAGE, FTIR, amino acid composition, zeta potential | I | [93] |
| Nile tilapia (Oreochromis niloticus) | Scale | ASC 72 h; (10 °C); PSC 72 h; (10 °C); |
0.77 0.71 |
SDS-PAGE, amino acid composition, FTIR spectra, thermal denaturation temperature, zeta potential | [94] | |
| Silver catfish (Pangasius sp.) | Skin | ASC 24 h; PSC 24 h; |
4.27 2.27 |
FTIR, SEM, solubility | I | [95] |
| Bonylip barb fish (Osteochilus vittatus) | Skin | PSC 8 h; (4 °C); | 6.18 | FTIR, HPLC, viscosity, denaturation values | I | [96] |
| Hammerhead shark (Sphyrna lewini) | Cartilages | ASC 72 h; (4 °C); | 5.64 | SDS-PAGE and peptide mapping, amino acid composition, FTIR, viscosity of collagen, solubility | I | [97] |
| Mackerel (Scomberomorous niphonius) | Skin, bone |
ASC; PSC ASC; PSC |
58.62; 14.43 13.68; 3.48 |
SDS-PAGE, amino acid composition, FTIR, solubility | I | [98] |
| Monkfish (Lophius litulon) | Swim Bladders | ASC 48 h; (4 °C); PSC 48 h; (4 °C); |
4.27 9.54 |
SDS-PAGE. amino acid analysis, mass spectrum UV absorption, solubility | I | [99] |
| Miiuy croaker (Miichthys miiuy) | Scales | ASC 48 h; (4 °C); PSC 48 h; (4 °C); |
0.64 3.87 |
SDS-PAGE, amino acid composition, FTIR, UV, viscosity, solubility, zeta potential, SEM | I | [100] |
| Invertebrate | ||||||
| Sponge (Chondrosia reniformis) (Poriferans) | Sponge tissue | Enzymatic digestion 72 h; (27 °C); |
3.4 | Amino acid analysis, glycosaminoglycan quantification, viscosity, thermal stability | IV | [105] |
| Marine sponges (Chondrilla caribensis) | Sponge tissue | Four protocols ASC, WSC, PSC Lyophilized marine sponge 24 h | P1 (39.2%); P2 (47.2%), P3 (48.2%); P4 (48.3%) |
SEM micrographs; FTIR spectroscopy; circular dichroism | I | [106] |
| Sponge (Chondrosia reniformis) (Poriferans) | Sponge tissue | Enzymatic digestion 24 h (37 °C) |
19 | Glycosaminoglycan quantification, TEM | IV | [107] |
| Common starfish (Asterias rubens) (Echinoderms) | Body wall | ASC + PSC 48 h; (4 °C); | 1.44 | UV spectra, FTIR, SDS-PAGE, amino acid analysis, SEM, solubility | I | [111] |
| Starfish (Asterias pectinifera) (Echinoderms) | Body wall | UAC 1 h; | 3.8 | Amino acid analysis, zeta potential, TEM | I | [112] |
| Sea cucumber (Holothuria cinerascens) (Echinoderms) | Body wall | ASC + PSC 72 h; (4 °C); | 72.2 | SDS-PAGE, FTIR, UV spectra, amino acid composition | I | [113] |
| Sea cucumber (Apostichopus japonicus) | Body wall | PSC 12 h; (4 °C); | 72 h | SDS-PAGE sea cucumber collagen fibrils are heterotypic. Included two clade A fibrillar collagens, one clade B fibrillar collagen, and two FACIT collagens. | Heterotypic | [114] |
| Stomolophus meleagris | Body wall | NaOH for extraction for crude gelatin 24 h and 4 °C | 10.49 | SDS-PAGE, FT-IR and 1H-NMR spectra, amino acid composition | [115] | |
| Jellyfish (Rhopilema esculentum) (Coelenterate) | Umbrella | PSC 72 h; (4 °C); | 4.31 | % I SDS-PAGE, FTIR | I | [116] |
| Jellyfish (Catostylus mosaicus) (Coelenterate) |
Umbrella, oral arm |
ASC 72 h; (4 °C); ASC 72 h; (4 °C); |
1.46 2.24 |
SDS-PAGE, ATR-FTIR, amino acid analysis, raman spectra | I | [117] |
| Jellyfish (Acromitus hardenbergi) | Bell and oral arms |
ASC PSC |
0.09–0.29 0.29–0.39 of lyophilized collagen |
Physicochemical analysis, amino acid composition | I | [118] |
| Jellyfish (Rhopilema esculentum) (Coelenterate) | Tissue | ASC 72 h; (4 °C); PSC 24 h; (4 °C); |
0.12 0.28 |
SDS-PAGE, FTIR, SEM, amino acid analysis | I | [119] |
| Blue mussel (Mytilus edulis) byssus | Body wall | PSC 4 h; (50 °C); | 1.38 | SDS-PAGE, protein determination, RP-HPLC, GP-HPLC | IV | [120] |
| Byssus of Chilean mussels (Mytilus Chilensis) (mollusk) | Mussels | ASC 24 h; (80 °C); PSC 24 h; (80 °C); |
1.8 7.6 |
SDS-PAGE, amino acid analysis | I | [121] |
| Mantis shrimp (Miyakella nepa) (Crustacean) | Muscles | PSC 72 h; (4 °C); | 0.478 | SDS-PAGE, FTIR, solubility | I | [122] |
| Surf clam shell (Coelomactra antiquata) | GSC PSC |
0.59 3.7 |
SDS-PAGE, FT-IR spectra, scanning electron microscopy (SEM), amino acid analysis | I | [123] | |
| Jumbo squid (Dosidicus gigas) | Fins Mantle Arms |
PSC | 17.85 17.65 361.68 |
SDS-PAGE, FT-IR spectra, amino acid composition, OFF GEL electrophoresis | I | [124] |
In addition to marine sponges, echinoderms of the phylum Echinodermata, which includes five distinct classes, were also studied for their collagen. Vate et al. (2023) investigated collagen in the common starfish (Asterias rubens), while Han et al. (2021) studied collagen in the starfish (Asterias pectinifera) [111,112]. Li et al. (2020) extracted a high percentage of collagen, up to 72%, from the sea cucumber Holothuria cinerascens, demonstrating its potential as a marine collagen resource [113]. Tian et al. (2020) also extracted collagen from the sea cucumber Apostichopus japonicus [114]. Another promising source of marine collagen is the Coelenterates. Esparza-Espinoza et al.’s (2019) remarkable research involved extracting collagen from the jellyfish Stomolophus meleagris [115]. Additional studies include those conducted by Felician et al. (2019), who extracted collagen from Rhopilema esculentum, and Rastian et al. (2018), who worked with Catostylus mosaicus jellyfish [116,117]. Khong et al. (2018) isolated collagen from the jellyfish Acromitus hardenbergi, and Cheng (2017) focused on Rhopilema esculentum [118,119]. CunhaNeves et al. (2022) reported studies on blue mussel (Mytilus edulis) byssus collagen hydrolysates, and Rodríguez, F et al. (2017) reported studies on collagen extraction from mussel byssus [120,121].
Hiransuchalert et al. (2021) extracted collagen type I from different mantis shrimp species [122]. Wu et al. (2019) reported studies on collagen isolated from Coelomactra antiquate [123]. Ezquerra-Brauer et al. (2018) reported studies on collagen in jumbo squid (Dosidicus gigas) [124]. The high collagen percentages reported in various studies from Table 1 are as follows: 84.81% (PSC) from the swim bladders of Megalonibea fusca by Mo et al. (2023), 93.7% (PSC) from the sea eel (Muraenesox cinereus) by Li, H. et al. (2023), and 72.2% (PSC) from the sea cucumber (Holothuria cinerascens) by Li, P.H. (2020) [80,82,113].
4. Marine Collagen Structure and Composition
4.1. Structural Characteristics of Collagen and Collagen Peptides
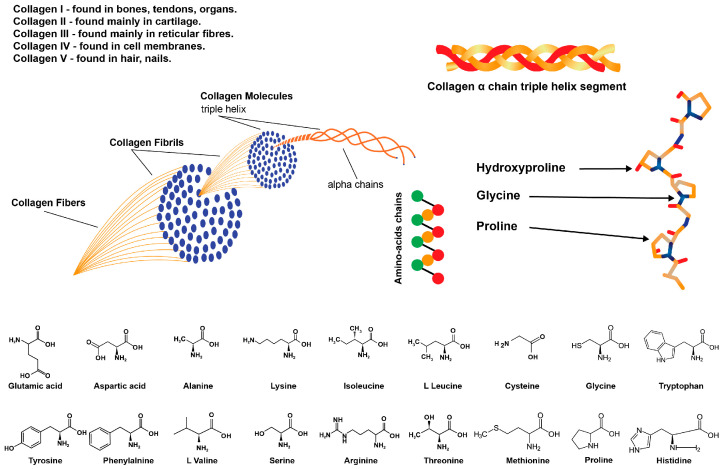
Collagen is a protein found in all living things. This protein has a complex structure consisting of 29 collagen types, as explained by Cherim et al. (2019) and Meyer et al. (2019) [42,125]. In vertebrates, type I collagen is the most abundant type in the body and can be found in bones, skin, tendons, and organs, as explained by Meyer et al. (2019) [125]. Type II collagen is found in cartilage. Type III collagen is present in reticular fibers as well as in blood and skin [125]. In invertebrates, type I and IV collagens are found. By partial denaturation of native collagen, gelatin is obtained, which is a major source of protein biopolymers. Collagen peptides are fragments of collagen with lower molecular masses that are detached from the large triple helix chain. Ryu et al. (2021) showed that proteolytic enzymes can break down proteins into hydrolysates comprising small peptides consisting of 2–20 amino acids [126].
The molecular weight, length, and sequence of peptides, as well as their amino acid composition, influence their bioactive properties; hydrolysates produce amino acid forms that are useful in supporting various human biological functions, as stated by Yathisha et al. (2018) [127]. Zhang et al. (2023) showed the typical collagen structure of fish skin [128]. Al-Shaer et al. (2021) showed that the collagen chain of fish exhibits a Gly-X-Y repeat sequence, where X and Y are generally Pro and Hyp, respectively [129]. Zhu et al. (2020) reported data on type II collagen from the cartilages of skates and sturgeons [130]. Romijn et al. (2019) analyzed the differences between collagen types I and II, and Hu et al. (2022) analyzed the differences generated by the structure of three commercial tuna species with modern methods of analysis [131,132]. Hernández-Ruiz et al. (2023) analyzed the structure of collagen peptide fractions from tilapia (Oreochromis aureus Steindachner, 1864) scales [133]. Figure 4 shows the structure of collagen, collagen peptides, and amino acid chains [34]. Also highlighted are the top five collagen types and the locations where they are most abundant.
Figure 4.
Structure of collagen fibers, collagen fibrils, and amino acid chains. Reprinted with permission from reference [34], 2023, Emin Cadar.
4.2. Amino Acids in Marine Collagen
In vertebrates, different types of collagen show tropocollagen structures. These molecules consist of approximately 35% glycine (Gly), 21% proline (Pro), 11% alanine (Ala), and hydroxyproline (Hyp) [126]. Hydroxyproline at the Y-position is believed to enhance the stability of the helical structure. From a nutritional perspective, amino acids are categorized as essential (EAA), non-essential (NEAA), or conditionally essential (CEAA). The concept of functional amino acids (FAA) has also been introduced; these amino acids are involved in and regulate metabolic pathways that improve health, growth, development, survival, reproduction, neurological metabolic diseases, and infectious diseases [126].
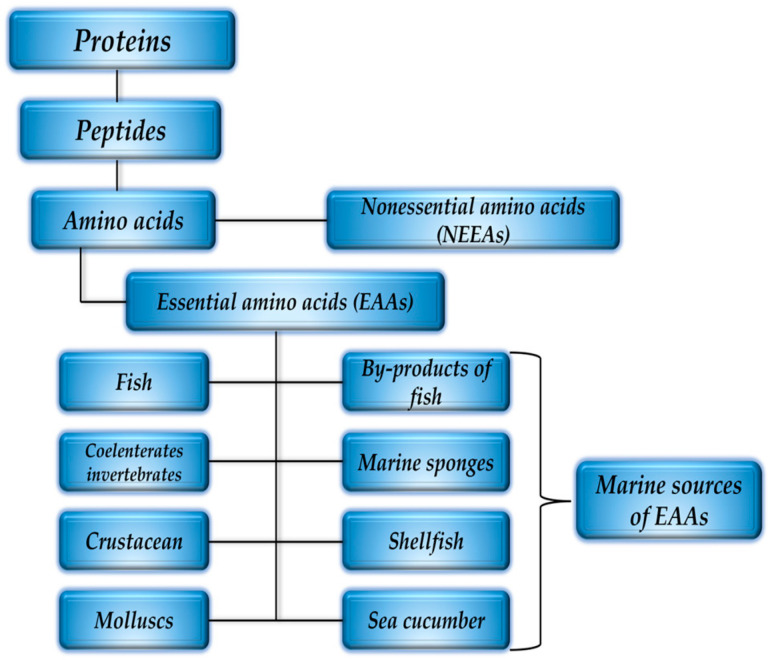
Arg, His, Cys, Lys, Leu, Thr, Met, Trp, Tyr, and Val are EAA; Pro, Glu, Gln, and Gly are CEAA; and Ala, Ser, and Asp are NEAA for human nutrition. In human nutrition, Arg, Cys, Leu, Met, Trp, Tyr, Asp, Glu, Gly, and Pro have been classified as FAA, as shown by Šimat et al. (2020) [7]. Figure 5 shows the potential marine sources of essential amino acids (EAA).
Figure 5.
Amino acids (EAA) in marine-derived collagen and collagen peptides.
4.2.1. Amino Acids from Fish Collagen
The amino acid content of collagen in fish is very different depending on the species of fish, the marine habitat in which it lives, and the pollutants present in marine waters, especially in coastal waters. Research reported on the amino acid content of marine collagen extracted from fish skin and fish by-products shows a different distribution of amino acid types. Blanco et al. (2017) determined the amino acid compositions of collagen from Thunnus albacares fish and found that this skin residue is rich in Gly, Pro, Ala, and Glu [134]. Je et al. (2019) stabilized the amino acid composition of Tilapia fish collagen hydrolysates and found the highest values for Gly, Ala, Pro, and Glu [135]. Garehgheshlagh et al. (2020) studied the Rutilus frisii kutum species and determined that it contained the highest amounts of total amino acids in Gly, Pro, Glu, and Ala [136].
Thuy et al. (2020) reported the highest amounts of the total amino acids found in Gly, Pro, Ala, and Hyp in Pangasianodon hypophthalmu, and for the species Oreochromis niloticus, they reported the order of amino acids in Gly, Pro, and Hyp [137]. Truong et al. (2021) reported the study of amino acids in the species Channa striata and established the following order: Gly, Hyp, Ala, and Glu [138]. Son et al. (2022) reported the amino acid order Gly, Ala, Pro, Arg, and Glu for both the species Pagrus major and Paralichthys olivaceus [139]. Rýglova et al. (2023) provided studies on amino acids from skin collagen of the fish Cyprinus carpio and stabilized the values in the order Gly, Ala, and Pro [140]. Cruz-Lopez et al. (2023) reported the amino acid composition of collagen extracted from the fish Totoaba macdonaldi, with the highest values for Gly, Ala, Pro, and Glu [81]. From the presented analysis, we can see that the main amino acids in most of the collagens in pest skin are Gly, Pro, Ala, Glu, Hyp, and Val. The amino acid Ala, although belonging to the category of non-essential amino acids, is quantitatively found in all collagen extracts from the skin of the marine fish studied. Pro and Ala were the most abundant hydrophobic amino acids in all fish species. It was concluded that hydrophobic amino acids were observed in several peptide sequences with antioxidant properties. Akita et al. (2020) reported studies on the correlation between the content of Pro, Hyp, and Ser and the denaturation temperature of type I collagen with the physiological temperature of marine organisms [141]. The degree of hydroxylation of Pro and Lys is known to influence the thermal stability of collagen [141]. Chinh et al. (2019) reported amino acid sequences of Carp fish scale wastes [142]. From the presented analysis, we can see that the main amino acids in most of the collagens in fish skin are Gly, Pro, Ala, Glu, Hyp, and Val. Pro and Ala were the most abundant hydrophobic amino acids in all fish species, although there were clear differences. Tryptophan (Trp) was not found in all of the species. Table 2 shows the experimental results for the amino acid content of collagen hydrolysates extracted from the skin or swim bladder of the different fish species presented. Regardless of the units of measurement used for reporting these amino acids, Gly consistently appears in the highest amounts across all species analyzed. The values are typically expressed in residues per 1000 residues.
Table 2.
Amino acids from fish collagen from skin and other subproducts.
| Amino Acids | Thunnus albacares | Tilapia Collagen | Rutilus frisii kutum | Pangasianodon hypophthalmus | Oreochromis niloticus |
Channa
striata |
Pagrus
major |
Paralichthys olivaceus |
Cyprinus
carpio |
Totoaba macdonaldi | Totoaba macdonaldi |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | Skin * | Skin ** | Skin * | Skin * | Skin * | Skin * | Skin ** | Skin ** | Skin ** | TSBC ** | Swim bladder *** |
| Essential amino acids (EAAs) | |||||||||||
| Arginine (Arg) | 92.16 ± 2.97 | 7.91 | 70.8 | 53 | 52 | 56 ± 3 | 65.1 ± 1.0 | 60.6 ± 1.9 | 51.7 ± 0.9 | 59 ± 4.47 | 11.58 ± 0.37 |
| Cysteine (Cys) | 0.07 ± 0.00 | - | - | 2 | 2 | 2 ± 1 | 0.9 ± 0.5 | 0.7 ± 0.3 | 1.5 ± 0.1 | - | 0.03 ± 0.01 |
| Glutamic acid (Glu) | 97.89 ± 0.43 | 10.16 | 81.1 | 75 | 71 | 74 ± 4 | 57.9 ± 1.9 | 57.0 ± 0.4 | 74.0 ± 2.1 | 101 ± 1.95 | 9.61 ± 0.14 |
| Glycine (Gly) | 217.22 ± 1.32 | 23.60 | 182.5 | 334 | 332 | 307 ± 7 | 370.7 ± 2.9 | 395.6 ± 1.3 | 318.2 ± 3.6 | 309 ± 3.15 | 29.19 ± 0.31 |
| Histidine (His) | 12.70 ± 0.05 | 1.10 | 8.8 | 6 | 7 | 6 ± 1 | 5.2 ± 0.1 | 6.0 ± 1.0 | 10.7 ± 3.2 | 5 ± 0.34 | 0.47 ± 0.02 |
| Isoleucine (Iso) | 14.26 ± 0.15 | 1.40 | 10.7 | 6 | 7 | 9 ± 2 | 9.3 ± 0.8 | 0.9 ± 0.8 | 13.3 ± 0.8 | 5 ± 0.22 | 0.63 ± 0.01 |
| Leucine (Leu) | 28.28 ± 0.21 | 2.85 | 21.1 | 24 | 22 | 28 ± 3 | 25.1 ± 0.9 | 22.2 ± 0.4 | 26.8 ± 1.1 | 20 ± 0.08 | 1.94 ± 0.05 |
| Lysine (Lys) | 35.37 ± 0.23 | 3.19 | 31.1 | 26 | 28 | 31 ± 2 | 24.3 ± 1.0 | 24.1 ± 0.8 | 29.4 ± 1 | 31 ± 1.63 | 2.52 ± 0.04 |
| Hydroxylysine (Hyl) | - | - | - | 7 | 6 | 6 ± 1 | - | - | - | 5 ± 0.22 | 0.28 ± 0.02 |
| Methionine (Met) | 6.29 ± 0.13 | - | 14.8 | 33 | 34 | 12 ± 1 | 10.7 ± 1.5 | 10.8 ± 0.2 | 12.8 ± 1.1 | 7 ± 0.47 | 1.36 ± 0.03 |
| Phenylalanine (Phe) | 20.75 ± 0.15 | 1.73 | 20.6 | 14 | 16 | 18 ± 2 | 14.4 ± 1.8 | 13.4 ± 1.1 | 14.4 ± 0.5 | 19 ± 0.54 | 1.61 ± 0.03 |
| Proline (Pro) | 114.86 ± 0.45 | 11.01 | 89.6 | 111 | 112 | 126 ± 5 | 89.5 ± 0.7 | 77.85 ± 0.0 | 109.9 ± 2.1 | 122 ± 1.33 | 12.10 ± 0.13 |
| Hydroxiproline (Hyp) | 87.38 ± 0.60 | 8.92 | - | 81 | 83 | 94 ± 5 | 43.3 ± 0.5 | 46.5 ± 0.7 | 70.2 ± 2.1 | 83 ± 1.43 | 5.43 ± 0.05 |
| Threonine (Thr) | 40.00 ± 1.81 | 3.28 | 20.6 | 26 | 24 | 22 ± 2 | 28.0 ± 0.5 | 26.8 ± 0.8 | 26.6 ± 0.7 | 13 ± 2.62 | 1.76 ± 0.06 |
| Tryptophan (Trp) | - | - | - | - | - | - | - | - | - | - | - |
| Tyrosine (Tyr) | 4.42 ± 0.07 | - | 4.4 | 2 | 1 | 5 ± 1 | 5.1 ± 0.6 | 4.0 ± 0.4 | 5.6 ± 2.2 | 2 ± 0.19 | 0.47 ± 0.03 |
| Valine (Val) | 25.64 ± 0.15 | 2.36 | - | 25 | 26 | 26 ± 1 | 17.1 ± 3.4 | 18.0 ± 0.9 | 24.1 ± 0.8 | 16 ± 0.3 | 1.58 ± 0.04 |
| Non-essential amino acids (NEAAs) | |||||||||||
| Alanine (Ala) | 111.78 ± 2.58 | 11.78 | 73.1 | 96 | 98 | 89 ± 5 | 164.5 ± 1.3 | 157.1 ± 1.4 | 119.0 ± 1.2 | 132 ± 1.27 | 12.26 ± 0.13 |
| Aspartic acid (Asp) | 55.40 ± 0.54 | 5.59 | 42.1 | 46 | 47 | 54 ± 3 | 34.0 ± 0.6 | 33.5 ± 0.5 | 53.1 ± 1.1 | 52 ± 1.48 | 5.13 ± 0.05 |
| Serine (Ser) | 35.53 ± 0.25 | 3.54 | 34.8 | 33 | 32 | 35 ± 2 | 34.9 ± 0.7 | 37.2 ± 0.3 | 38.5 ± 0.7 | 23 ± 0.69 | 2.02 ± 0.08 |
| Reference | [134] | [135] | [136] | [137] | [137] | [138] | [139] | [139] | [140] | [81] | [81] |
TSBC (totoaba swim bladder collagen); (Results are expressed in * %; ** Residues/1000 residues, *** g/100 g amino acid).
4.2.2. Amino Acids from Crustacean Collagen
Gly is found to be the amino acid found in all species studied except Rhizostoma pulmo, studied by Cheng et al. (2017), who reported the order Glu, Phe, and Leu [119]. Mequiol et al. (2019) studied Stomalophus meleagris and reported the following order: Gly, Glu, Pro, and Ala [143]. Aziz et al. (2020) reported values for Rhopilema hispidum in the order Gly, Glu, Arg, Pro, Asp, and Ala [144]. Qiu et al. (2020) reported values for amino acids from Nemopilema nomurai in the order Gly, Glu, Ala, Pro, and Asp [145]. Pivnenko et al. (2022) reported amino acids from Rhopilema asamushi in the order Gly, Glu, Pro, Ala, Arg, and Asp [146]. James et al. (2023) reported that amino acids were also found in Rhizostoma pulmo in the order Gly, Glu, Ala, Asp, and Leu [147]. Table 3 shows the results of amino acids found in collagen extracts from marine invertebrates: different species of jellyfish, mollusks, and one species of shrimp. Amino acid values are generally reported in mass percent.
Table 3.
Amino acids from marine crustacean collagen from complete organisms.
| Amino Acids | Rhizostoma pulmo | Stomalophus meleagris |
Rhopilema
hispidum |
Nemopilema nomurai | Rhopilema asamushi | Rhizostoma pulmo |
Rhopilema
esculentum |
Stomolophus
meleagris |
Corbicula japonica (Mollusk) |
Litopenaeus vannamei (Shrimp) |
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | Whole body * | Whole body * | Whole body * | Whole body * | Whole body * | Whole body * | Whole body ** | Whole body * | Whole body * | Whole body *** |
| Essential amino acids (EAAs) | ||||||||||
| Arginine (Arg) | 20 | 52 | 8.84 | 3.87 | 7.2 ± 0.5 | 5.63 | 55.87 | 8.3 ± 0.1 | 4.25 | 11 ± 0.48 |
| Cystine (Cys) | 13 | - | 4.87 | - | 0.6 ± 0.1 | - | 2.4 | 1.1 ± 0.0 | 0.36 | 1.10 ± 0.05 |
| Glutamic acid (Glu) | 152 | 98 | 10.42 | 9.98 | 10.8 ± 1.2 | 13.46 | 103.21 | 13.1 ± 0.1 | 14.48 | 2.78 ± 0.13 |
| Glycine (Gly) | 53 | 309 | 19.21 | 34.82 | 28.8 ± 1.6 | 29.34 | 324.84 | 19.7 ± 0.3 | 6.94 | 15.3 ± 0.66 |
| Histidine (His) | 56 | 2 | 3.28 | 0.29 | 3.0 ± 0.2 | - | - | 1.7 ± 0.1 | 3.66 | 1.14 ± 0.05 |
| Isoleucine (Iso) | 55 | 22 | 2.98 | 1.88 | 2.1 ± 0.4 | - | 11.78 | 2.6 ± 0.0 | 3.95 | 1.55 ± 0.06 |
| Leucine (Leu) | 91 | 34 | 3.79 | 3.09 | 3.2 ± 0.4 | 6.35 | 30.68 | 3.7 ± 0.0 | 8.97 | 2.7 ± 0.11 |
| Lysine (Lys) | 69 | 38 | 3.22 | 2.96 | 4.2 ± 0.6 | 4.62 | 30.22 | 4.3 ± 0.2 | 7.73 | 3.92 ± 0.12 |
| Hydroxylysine (Hyl) | - | 27 | - | - | 2.2 ± 0.5 | - | - | - | - | - |
| Methionine (Met) | 46 | 4 | 2.80 | 0.21 | 0.7 ± 0.1 | - | 8.57 | 1.3 ± 0.0 | 0.16 | 0.9 ± 0.04 |
| Phenylalanine (Phe) | 93 | 10 | 2.16 | 0.9 | 2.2 ± 0.4 | - | 14.86 | 1.5 ± 0.0 | 3.74 | 1.53 ± 0.05 |
| Proline (Pro) | 39 | 82 | 6.93 | 8.16 | 8.4 ± 1.0 | 2.97 | 95.63 | 8.7 ± 0.2 | 6.43 | 6.6 ± 0.31 |
| Hydroxiproline (Hyp) | - | 40 | 5.84 | 6.33 | 3.9 ± 0.7 | 4.82 | 46.86 | 6.9 ± 0.1 | 2 | 0.031 ± 0.001 |
| Threonine (Thr) | 50 | 35 | 4.30 | 3.55 | 3.1 ± 0.3 | 3.18 | 27.33 | 3.9 ± 0.0 | 3.26 | 1.89 ± 0.07 |
| Triptophan (Trp) | - | - | - | - | - | 4.72 | - | 0.3 ± 0.0 | - | 1.12 ± 0.04 |
| Tyrosine (Tyr) | 76 | 6 | 1.71 | 0.11 | 3.9 ± 0.3 | 1.77 | 7.33 | 1.1 ± 0.0 | 3.90 | 2.43 ± 0.08 |
| Valine (Val) | 49 | 35 | 3.23 | 2.74 | 3.1 ± 0.5 | 2.8 | 22.21 | 2.6 ± 0.0 | 7.68 | 2.17 ± 0.09 |
| Non-essential amino acids (NEAAs) | ||||||||||
| Alanine (Ala) | 39 | 82 | 6.11 | 8.36 | 7.8 ± 0.3 | 10.38 | 100.74 | 6.7 ± 0.0 | 5.22 | 5.98 ± 0.26 |
| Aspartic acid (Asp) | 32 | 79 | 6.78 | 7.38 | 7.2 ± 0.8 | 10.91 | 76.86 | 9.0 ± 0.1 | 10.51 | 1.62 ± 0.06 |
| Serine (Ser) | 67 | 45 | 3.43 | 4.89 | 4.7 ± 0.5 | - | 29.85 | 3.3 ± 0.0 | 5.99 | 1.64 ± 0.06 |
| Reference | [119] | [143] | [144] | [145] | [146] | [147] | [148] | [149] | [150] | [151] |
Results are expressed in; * %; ** Residues/1000 residues; *** mg/g dry weight.
Sudirman et al. (2023) studied Rhopilema esculentum and reported for amino acids the order Gly, Glu, Ala, and Pro [148]. Chiarelli et al. (2023) studied Stomolophus meleagris and reported for amino acids the order Gly, Glu, Asp, Pro, and Arg [149]. Tryptophan (Trp) was found to be identified only in Rhizostoma pulmo by James et al. (2023) and in Stomolophus meleagris by Chiarelli et al. (2023) [147,149]. Hydroxylysine (Hyl) was identified only in Stomalophus meleagris by Mequiol et al. (2019) and in Rhopilema asamushi by Pivnenko et al. (2022) [143,146]. Cysteine (Cys) is present in Nemopilema nomurai, reported by Qiu et al. (2020); in Rhizostoma pulmo, reported by James et al. (2023); and in Stomolophus meleagris, reported by Chiarelli et al. (2023) [145,147,149]. It does not show histidine (His) in Rhizostoma pulmo reported by James et al. (2023) nor Rhopilema esculentum, reported by Sudirman et al. (2023) [147,148]. Li N. G. et al. (2018) reported the amino acid content of the mollusk Corbicula japonicasi, with values in the order Glu, Asp, Leu, Lys, and Val [150]. Li X. et al. (2021) reported the amino acid content of the white shrimp Litopenaeus vannamei with higher values for Gly, Arg, Pro, and Ala. It does not show hydroxylysine (Hyl) [151]. The amino acid content of mollusk and shrimp species is much lower than that of jellyfish species. Lima et al. (2019) found that amino acids such as Asp, Gly, and Glu improve wound healing [152]. Hydrophobic amino acids have antioxidant action as they can interact on membrane lipid layers to reach targets and help scavenge radicals [149,152].
5. Antioxidant Activity
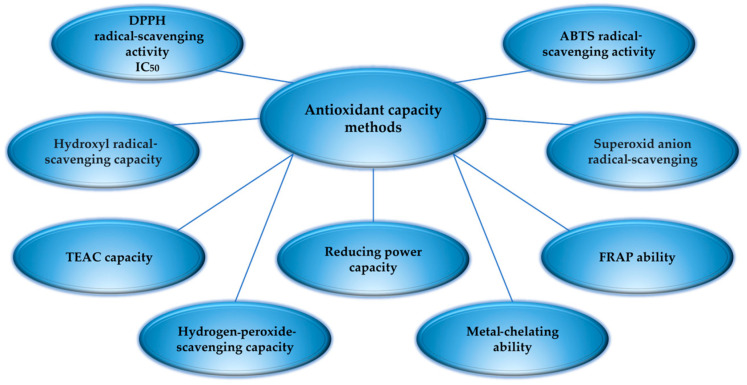
Oxidation is a vital and normal process in vertebrates and humans, whereby free radical species (ROS) are continuously generated in the cellular metabolism. The accumulation of ROS in the body must be kept under control to avoid the diseases they can cause. Oxidative stress is linked to damaging processes such as lipid peroxidation, protein damage, DNA breakdown, or enzyme inactivation. These promote the development of various diseases such as tumor formation or cancer, heart disease, rheumatoid arthritis, or aging. Suo et al. (2022) showed that seventeen ACE inhibitory peptides isolated from the protein hydrolysate of the blue mussel Mytilus eludis could be used as natural ingredients in the development of products with antihypertensive functions [153]. Hydrolysates and collagen peptides from fish by-products have demonstrated antioxidant capacity to reduce oxidative processes and can thus be used to produce functional foods. There were researchers like Nikoo et al. (2021) and Nirmal et al. (2022) who reported that certain hydrophobic amino acid sequences provide antioxidant properties as proton or electron donors or as lipid radical scavengers [154,155]. The antioxidant properties of marine collagen peptides and hydrolysates are influenced by several parameters, such as amino acid composition, chain size and length, or residue/chain sequence [150,154]. Chaoting et al. (2020) emphasized the relationship between peptide structure and the antioxidant activity of peptides isolated from proteins [156]. The relationship between structure and the antioxidant activity of peptides derived from marine by-products was presented by Sila et al. (2016) [157]. Other researchers, such as Phadke et al. (2021) and Nirmal et al. (2023), considered that the molecular weight of peptides influences their antioxidant activity [158,159]. The amino acids Tyr, Met, Hys, Lys, and Trp have strong radical-scavenging activity in oxidative reactions [158]. Nirmal et al. (2023) explained that Hys significantly enhances the antioxidant capacity because protonation of the imidazole ring acts as a hydrogen donor [159]. Azizah et al. (2020) showed that another factor influencing the antioxidant activity of peptides besides amino acid composition is the specificity of the protease used in the hydrolytic process [160]. Nirmal et al. (2023) consider the degree of enzymatic hydrolysis important in assessing the antioxidant activity of proteins and peptide derivatives in fish [159]. The types of enzymatic hydrolysis for several types of enzymes described by Teng et al. (2023) are trypsin, papain, pepsin, alcalase, flavourzyme, protamex, and bromlaine. pH values are 2.0–9.0. Temperatures are 37–55 (°C) and the time is 4 h [161]. The antioxidant capacity can be proven by several methods, as shown in Figure 6.
Figure 6.
Type of methods used to demonstrate antioxidant activity.
By analyzing and summarizing the data presented in Table 4, we can see that the antioxidant activity of collagen and marine collagen were tested by different methods. The DPPH radical-scavenging activity assay method was used to reveal the antioxidant potential in all of the species exemplified in Table 4 [68,147,160,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176]. The antioxidant activity with the highest percentages obtained by DPPH assay were reported by Zamorano-Apodaca et al. (2020), who extracted peptide fractions from mixed by-product: skins, heads, and skeletons from different fish species (different sharks, mullet, guitarfish, ray, weakfish, snapper, squid, seabass, pompano dolphinfish) [167]. The authors showed that the percentages ranged from 67% to 77% at concentrations of 10 mg/m [167]. Antioxidant activity reported by IC50 values that recorded the highest values (IC50 = 8.38 mg/mL) was demonstrated by Asaduzzaman et al. (2020), who performed DPPH assays on amino acids extracted from the bone and skin of the mackerel Scomber japonicas [68]. For the other species of marine organisms reported in Table 4, the antioxidant potential was also reported by various other specific tests. ABTS scavenging activity is a widely used method for demonstrating the antioxidant activity of extracted collagen peptides [68,160,163,165,168,169,170,171,172,175,176]. The highest values by ABTS assay (83.5% at 2.5 mg/mL) were reported on collagenic peptides extracted from Cynoscion guatucupa—stripped weakfish skin—by Lima et al. (2019) [170]. Appreciable values by ABTS assay (81.05% at 500 µg/mL) were also reported by Yang et al. (2020), who analyzed amino acid sequences (Ala-Thr-Val-Tyr) with antioxidant potential from the silky shark Carcharhinus falciformis [168]. Another method for testing antioxidant potential was hydroxyl radical-scavenging activity [161,164,167,169,170,171,173,176]. By the hydroxyl radical-scavenging method, Zamorano-Apodaca et al. (2020) also reported the highest percentages (from 64% to 85% at concentrations of 10 mg/m) attesting to the antioxidant activity of peptide fractions extracted from mixed by-products: skins, heads, and skeletons from different fish species (different sharks, mullet, guitarfish, ray, weakfish, snapper, squid, seabass, pompano dolphinfish) [167]. The superoxide anion radical-scavenging method was also used to reveal antioxidant activity [161,169,171,172,176]. Using superoxide anion radical-scavenging method on invertebrates, the highest values expressed by IC50 (IC50 = 1.55 mg/mL) for collagen from whole tissue in the jellyfish Nemopilema nomurai were reported by Teng et al. (2023), and in vertebrates the highest values (IC50 = 0.91 mg/mL) were reported by Zhang et al. (2019) for the amino acid sequences Pro-Phe-Gly-Pro-Asp from the skin of Japanese Spanish mackerel (Scomberomorus niphonius) [161,169]. FRAP ability is a method successfully used in testing the antioxidant potential for collagen compounds in both vertebrates and invertebrates [162,163,165,166,167,175]. The highest values for the antioxidant activity by the FRAP method (1.4% at 2 mg/mL) were reported by Ahmed et al. (2022) for C- and N-terminal amino acid sequences from Pampus argenteus skins [162]. Table 4 presents the results of antioxidant activity studies conducted by various researchers on different marine species.
Table 4.
Antioxidant activity of various marine species due to the composition of different amino acid sequences tested by different physicochemical methods.
| Fish Species | Source | Amino Acid Sequence/Amino Acid Fraction | Assay Method and Scavenging Rates Results/IC50 Values | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH Radical-Scavenging Activity | ABTS-Scavenging Activity | (OH−) Hydroxyl Radical-Scavenging Activity |
(O2−) Superoxide Anion Radical-Scavenging Activity |
FRAP | Metal-Chelating Activity | ||||
| Pangasius hypopthalmus | skin | Peptide fraction < 3 kDa | - | 45.98% at 50 µmol/g | - | - | - | - | [160] |
| Lutjanus erythropterus | skin | Amino acid sequences with C- and N-terminals | 39.57% at 2 mg/mL | - | - | - | 0.8% at 2 mg/mL | - | [162] |
| Pampus argenteus | skin | Amino acid sequences with C- and N-terminal | 40.89% at 2 mg/mL | - | - | - | 1.4% at 2 mg/mL | - | [162] |
| Thunnus albacares | skin | Peptides (45–245 kDa) | 47.1% at 10 mg/mL | 43.7% at 10 mg/mL | - | - | 0.115% at 10 mg/mL | - | [163] |
| Acipenser baerii | cartilages | Gly-Glu-Tyr-Gly-Phe-Glu | IC50 = 1.27 mg/mL | - | IC50 = 1.16 mg/mL | - | - | - | [164] |
| Pro-Ser-Val-Ser-Leu-Thr | IC50 = 1.05 mg/mL | - | IC5 = 0.97 mg/mL | - | - | - | |||
| Gly-Ile-Glu-Leu-Phe-Pro- | IC50 = 1.38 mg/mL | - | IC50 = 1.63 mg/mL | - | - | - | |||
| Asian sea bass | skin | Amino acid (74 Da–10.175 Da) | 8.97 mmol TE/g | 650.20 mmol TE/g | - | - | 0.36 mmol TE/g | 20.94 mmolTE/g | [165] |
| Rutilus frisii kutum | by-products | Peptide fraction | 67% at 600 g/L | - | - | - | 0.78% at 600 g/L | 61.33% at 600 g/L | [166] |
| Various fish species (different sharks, mullet, guitarfish, ray, weakfish, snapper, squid, seabass, and pompano dolphinfish) | mixed by-products: skins, heads, skeletons |
F1 (≥30 kDa) | 75% at 10 mg/mL | - | 64% at 10 mg/mL | - | 0.127% at 10 mg/mL | - | [167] |
| F2 (10–30 kDa) | 75% at 10 mg/mL; | - | 78% at 10 mg/mL | - | 0.226% at 10 mg/mL | - | |||
| F3 (5–10 kDa) | 68% at 10 mg/mL | - | 84% at 10 mg/mL | - | 0.247% at 10 mg/mL | - | |||
| F4 (1–5 kDa) | 67% at 10 mg/mL | - | 85% at 10 mg/mL | - | 0.309% at 10 mg/mL | - | |||
| F5 (≤1 kDa) | 77% at 10 mg/mL | - | 85% at 10 mg/mL | - | 0.345% at 10 mg/mL | - | |||
| Scomber japonicus | bone | Amino acid (<1650 Da) | IC50 = 8.38 mg/mL | IC50 = 2.61 mg/mL | - | - | - | IC50 = 7.27 mg/mL | [68] |
| skin | Amino acid (<1650 Da) | IC50 = 7.58 mg/mL | IC50 = 2.50 ± 0.05 mg/mL | - | - | - | IC50 = 7.01 mg/mL | ||
| Carcharhinus falciformis | shark skin | Peptide fraction F19 | 45.63% at 1 mg/mL | - | - | - | - | - | [168] |
| shark skin | Ala-Thr-Val-Tyr | - | 81.05% at 500 µg/mL | - | - | - | - | ||
| Scomberomorous niphonius | skin | Pro-Phe-Gly-Pro-Asp | IC50 = 0.80 mg/mL | IC50 = 0.86 mg/mL | IC50 = 0.81 mg/mL | IC50 = 0.91 mg/mL | - | - | [169] |
| Tyr-Gly-Pro-Met | IC50 = 0.72 mg/mL | IC50 = 0.82 mg/mL | IC50 = 0.88 mg/mL | IC50 = 0.73 mg/mL | - | - | |||
| Cynoscion guatucupa—stripped weakfish skin | skin | Peptide sequence with 1263.58 Da |
65.2% at 2.5 mg/mL | 83.5% at 2.5 mg/mL | 69.7% at 2.5 mg/mL | - | - | - | [170] |
| Mustelus griseus | cartilages | Gly-Ala-Glu-Arg-Pro | IC50 = 3.73 mg/mL | IC50 = 0.10 mg/mL | IC50 = 0.25 mg/mL | IC50 = 0.09 mg/mL | - | - | [171] |
| Gly-Glu-Arg-Glu-Ala-Asp | IC50 = 1.87 mg/mL | IC50 = 0.05 mg/mL | IC50 = 0.34 mg/mL | IC50 = 0.33 mg/mL | - | - | |||
| Ala-Glu-Val-Gly | IC50 = 2.30 mg/mL | IC50 = 0.07 mg/mL | IC50 = 0.06 mg/mL | IC50 = 0.18 mg/mL | - | - | |||
| Katsuwonus pelamis | bone | Ser–Ser–Gly–Pro–Pro–Val-Pro–Gly–Pro–Met– | IC50 = 3.149 mM | IC50 = 9.489 mM | - | IC50 = 3.803 mM | - | - | [172] |
| Oreochromis niloticus | scales | S1 (66,430 Da) Peptides | 29.58% at 1 mg/mL; | - | 52.26% at 1 mg/mL | - | - | - | [173] |
| S2 (1335 Da) Peptides | 24.30 %at 1 mg/mL | - | 43.54% at 1 mg/mL | - | - | - | |||
| Rhizostoma pulmo | whole tissue | - | 38.05% at 5 mg/mL | - | - | - | - | - | [147] |
| Nemopilema nomurai | whole tissue | - | IC50 = 1.99 mg/mL | - | IC50 = 0.74 mg/mL | IC50 = 1.55 mg/mL | - | - | [161] |
| Lobonema smithii | whole tissue | - | 8.13% | - | - | - | - | - | [174] |
| oral arms | - | 13.27% | - | - | - | - | - | ||
| Rhopilema hispidum | umbrella | - | 8.40% | - | - | - | - | - | [174] |
| oral arms | - | 10.026% | - | - | - | - | - | ||
| Lobonema smithii | whole tissue | Fraction I (>10 kDa) | IC50 = 3.71 mg/mL | IC50 = 2.91 mg/mL | - | - | 0.65 mmol FeSO4/g | - | [175] |
| Fraction II (10–3 kDa) | IC50 = 0.85 mg/mL | IC50 = 1.15 mg/mL | - | - | 0.27 mmol FeSO4/g | - | |||
| Fraction III (3–1 kDa) | IC50 = 0.95 mg/mL | IC50 = 0.91 mg/mL | - | - | 0.24 mmol FeSO4/g | - | |||
| Fraction IV (<1 kDa) | IC50 = 1.11 mg/mL | IC50 = 0.89 mg/mL | - | - | 0.28 mmol FeSO4/g | - | |||
| Tergillarca granosa | whole tissue | Met-Asp-Leu-Phe-Thr-Glu | IC50 = 0.53 mg/mL | IC50 = 0.96 mg/mL | IC50 = 0.47 mg/mL | IC50 = 0.75 mg/mL | - | - | [176] |
| Trp-Pro-Pro-Asp | IC50 = 0.36 mg/mL | IC50 = 0.54 mg/mL | IC50 = 0.38 mg/mL | IC50 = 0.46 mg/mL | - | - | |||
Antioxidant activity can also be assessed by metal-chelating activity [68,165,166]. The highest values in the metal-chelating method were reported by Khesal et al. (2020) for peptide fractions from by-products from Rutilus frisii kutum [166]. To attest antioxidant activity, some authors have used four different types of methods; for example, Chotphruethipong et al. (2021) tested the antioxidant activity of hydrolyzed collagen from defatted Asian sea bass skin by four methods: DPPH, ABTS, FRAP, and the metal-chelating method [165]. The highest values in the metal-chelating method were reported by Khesal et al. (2020) for peptide fractions from by-products from Rutilus frisii kutum [166]. Using the DPPH, ABTS, and hydroxyl and superoxide anion radical-scavenging methods, Zhang et al. (2019) tested the antioxidant potential of amino acid sequences from mackerel (Scomberomorus niphonius) skin, and Tao et al. (2018) demonstrated the antioxidant activity of amino acid sequences from Mustelus griseus cartilage [169,171]. Also, Yang et al. (2019) reported the antioxidant potential of amino acid sequences from the mollusk Tergillarca granosa by four methods: DPPH, ABTS, and the hydroxyl and superoxide anion radical-scavenging methods [176]. Note from Table 4 that antioxidant activity was only reported by DPPH assay for the collagen extracted from the jellyfish Lobonema smithii and Rhopilema hispidum by Muangrod et al. (2022), and the values for collagen extracted from oral arms are higher than those from whole tissue and respective umbrellas in both jellyfish species [174]. Also, by a single method, ABTS, the antioxidant activity of the peptide fraction < 3 kDa from Pangasius hypopthalmus skin was reported by Azizah et al. [160]. Also, Yang et al. (2019) reported the antioxidant potential of amino acid sequences from the mollusk Tergillarca granosa by four methods: DPPH, ABTS, and the hydroxyl and superoxide anion-scavenging method [176].
The antioxidant activity is attributed to the amino acid sequences in collagen peptides and varies based on the type of enzymatic hydrolysate used for their separation, as shown in Table 5. Zhao et al. (2018) investigated collagen peptides with antioxidant potential by using pepsin for enzymatic hydrolysis [66]. They isolated collagen from the swim bladders of the Miiuy croaker (Miichthys miiuy). Dong et al. (2022) also used pepsin to isolate collagen from the swim bladders of several fish species, including Miichthys miiuy, Labeo rohita, Thunnus albacares, and Silurus triostegus [86]. Zhang et al. (2019) identified amino acid sequences from the skin of Lophius litulon, reporting antioxidant activity tested by various specific methods [169].
Table 5.
Antioxidant activity for different marine species generated by different amino acid sequences evidenced by different specific enzymatic hydrolysis methods and different types of physicochemical analysis methods.
| Fish Species | Source | Amino Acid Sequences/Amino Acid Fraction | Preparation Method | Antioxidant Activity | References |
|---|---|---|---|---|---|
| Miichthys miiuy | Swim bladder | Two chains (α1 and α2) as the major constituents with 115 kDa and 108 kDa | Enzymatic hydrolysis with pepsin | DPPH, ABTS, and hydroxyl radical- and superoxide anion radical-scavenging activity | [66] |
|
Miichthys miiu; Labeo rohita Tunuss albacares; Silurus triostegus |
Swim bladder | Collagen peptides | Enzymatic hydrolysis with pepsin | DPPH, ABTS | [86] |
| Rhizostoma pulmo | Whole body | Peptides with molecular weight < 3 kDa and between 3–10 kDa | Enzymatic hydrolysis with pepsin | DPPH | [147] |
| Lophius litulo | Skin | Amino acid with molecular weight range between 26–130 kDa | Enzymatic hydrolysis with pepsin | DPPH, ABTS, hydroxyl and superoxide anion radical-scavenging | [169] |
| Rhizostoma pulmo | Umbrella and oral arms | Peptide fractions with different ranges: <3 kDa; 3–10 kDa; 10–30 kDa; >30 kDa | Enzymatic hydrolysis with pepsin and collagenase | TEAC, ABTS | [177] |
| Chanos Chanos | Scales | Amino acid peptides with molecular weight < 3 kDa | Enzymatic hydrolysis with pepsin | DPPH, ABTS, lipide peroxidation inhibition, nitric oxide free radical scavenging | [178] |
| Fish (Budu) | Extract fish | Two novel peptides: LDDPVFIH and VAAGRTDAGVH, | Enzymatic hydrolysis pepsin | DPPH, ABTS superoxide anion radical scavenging | [179] |
| Small red scorpinfish Scorpaena notata |
Whole body | Leu-Val-Thr-Gly-Asp-Asp-Lys-Thr-Asn-Leu-Lys Asp-Thr-Gly-Ser-Asp-Lys-Lys-Gln-Leu | Enzymatic hydrolysis with pepsin | DPPH | [180] |
| Hypophthalmichthys molitrix | Skin | Peptides with molecular weight < 1600 Da | Enzymatic hydrolysis with collagenase | DPPH, hydroxyl radical-scavenging activity | [181] |
| Decapterus macarellus | Skin | Collagen peptides | Enzymatic hydrolysis with collagenase | DPPH | [182] |
| Catfish | Skin | Amino acid peptides with 11–135 kDa | Pepsin, collagenase, and trypsin hydrolysis | DPPH, FRAP ability | [183] |
| Caranx ignobilis | Bone | Collagen peptides | Hydrolysis with collagenase enzyme | DPPH, FRAP ability | [184] |
| Thunnus albacares | Skin | Collagen peptides | Enzymatic hydrolysis with alkalase | DPPH, ABTS | [101] |
| Tilapia | Bone | Amino acids: Glu, Lys Gly, and Pro | Enzymatic hydrolysis with alkalase | DPPH, superoxide anion radical scavenging | [185] |
| Cyprinus carpio | Skin | Amino acid fractions: PF1 > 30 kDa; PF2 10–30 kDa; PF3 3–10 kDa and PF4 < 3 kDa | Enzymatic hydrolysis with alkalase | DPPH, hydroxyl radical-scavenging activity, FRAP ability | [186] |
| Theragra chalcogramma | Skin | Amino acid fractions: I < 3 kDa, II 3–10 kDa, III 10–30 kDa, and IV > 30 kDa | Enzymatic hydrolysis with alkalase | TEAC, FRAP ability, nitric oxide free radical scavenging, ORAC | [187] |
| Cynoglossus arel | Skin and scales | Collagen peptides | Enzymatic hydrolysis with alkalase | DPPH, metal-reducing power, metal-chelating activity | [188] |
| Salmon | Scales | Peptide with molecular weight between 219–347 Da | Enzymatic hydrolysis with alkalase | DPPH, ABTS, FRAP ability | [189] |
| Rhopilema hispidum | Whole body | Peptide fractions with molecular weight < 10 kDa, consisting mainly of Gly, Glu, and Arg | Enzymatic hydrolysis with papain | DPPH, metal ion-chelating assays | [174] |
| Sea cucumber Actinopyga lecanora | Stone fish | Ston fish crude protein | Enzymatic hydrolysis with papain | DPPH, ABTS, FRAP ability | [190] |
| Sturgeon fish | Head, skin | Amino acid fractions | Hydrolyzed with papain and bromelain | ABTS, hydroxyl radical-scavenging activity | [191] |
| Lates calcarifer | Skin | Amino acid peptide chain with aromatic and hydrophobic structures |
Enzymatic hydrolysis with papain | Protection against H2O2 damage; nitric oxide free radical scavenging |
[192] |
|
Hypophthalmichthys
molitrix |
Fish waste | Amino acid with 1201.31–1874.01 Da | Enzymatic hydrolysis with papain | ABTS, hydroxyl radical-scavenging activity, lipide peroxidation inhibition |
[193] |
| Actinopyga lecanora | Stone fish | Crude protein: Gly, Glu, Asp, and Ala; papain-digested proteolysate Gly, Glu, Ala, and Asp | Enzymatic hydrolysis with papain in digested proteolysate | DPPH• (IC50 = 0.49 mg/mL), ABTS• (IC50 = 0.36 mg/mL) and FRAP value (0.29 mM FeSO4) |
[194] |
| Nemopilema nomurai | Whole body | Peptides with different molecular weights | Enzymatic hydrolysis with alcalase, protamex, flavourzyme enzymes, papain, pepsin, trypsin, and bromelain | DPPH, FRAP ability, hydroxyl and superoxide anion-scavenging activity | [161] |
| Lobonema smithii | Whole tissue | Amino acids—Gly, Cys, Glx, and Asx; peptide fraction |
Enzymatic hydrolysis with alcalase, flavourzyme, and papain hydrolysis | DPPH, ABTS, FRAP ability | [174,175] |
| Acaudina molpadioides | Whole body | Amino acid from peptides with molecular weight < 1 kDa | Enzymatic hysrolysis with papain, pepsin, trypsin, and neutrase | DPPH, ABTS | [194] |
| Katsuwonus pelamis | Sales | Different peptide fractions: TGP5, TGP7, and TGP9 | Enzymatic hydrolysis with papain, trypsin, and proteases—pepsin, neutrase, and alcalase | DPPH, hydroxyl and superoxide anion radical-scavenging activity | [195] |
| Katsuwonus pelamis | Skin | Amino acids: Gly, Hyp, Pro, and Ala | Enzymatic hydrolysis with trypsin, neutrase, papain, pepsin, and alcalase | DPPH | [196] |
| Pseudosciaena polyactis | Scales | Amino acid peptides with different chains: RCP1, RCP2, RCP3, RCP4, RCP5, and RCP6 | Enzymatic hydrolysis with neutrase, pepsin, papain, trypsin, flavourzyme, and alcalase | DPPH, hydroxyl and superoxide anion radical-scavenging activity | [197] |
| Red lionfish (Pterois volitans L.) |
Fish gutted, skinless fillets | The resulting peptide fractions exhibited high contents of amino acids | Enzymatic hydrolysis with trypsin, pepsin, chymotrypsin, and visceral enzymes | The highest copper-chelating activity, the highest iron-chelating activity, and β-carotene bleaching |
[198] |
| Miichthys miiuy | Swim bladder | Collagen peptides | Enzimatic hydrolysis with pepsin, alcalase, neutrase, papain, and pepsin | DPPH, hydroxyl and superoxide anion radical scavenging | [199] |
| Thunnus obesus | Skin | Amino acids: Arg, Lys, Phe, and Tyr | Enzymatic hydrolysis with bromelain, papain, pepsin, and trypsin | DPPH, reducing power | [200] |
| Mottle skate | Cartilages | Amino acid peptide chains | Enzymatic hydrolysis with trypsin, chymotrypsin, and papain | DPPH, ABTS | [201] |
| Starry triggerfish (Abalistes stellaris) |
Starry triggerfish muscle | Peptides derived from hydrolyzed fish protein | Enzimatic hydrolysis with trypsin | DPPH, ABTS, FRAP ability, and metal-chelating activity | [202] |
| Nibea japonica | Swim bladder | SNNH-1 (collagen peptide)—Gly, Ala, Pro, and Hyp | Enzymatic hydrolysis with neutrase | DPPH, ABTS, hydroxyl radical- and superoxide anion-scavenging activity | [203] |
| Acaudina molpadioides | Whole body | Amino acid from peptides with molecular weight < 1 kDa | Enzymatic hydrolysis with neutrase papain, pepsin, and trypsin | DPPH radical-scavenging activity, ABTS radical-scavenging activity | [194] |
| Channa striata | Scales | Collagen peptides | Enzymatic hydrolysis with protease | DPPH | [204] |
| Chanos Chanos | Skin | Collagen peptides | Enzymatic hydrolysis with protease | DPPH. ABTS | [205] |
| Salmon | Skin | Amino acid fractions: UF1 > 3 kDa and UF2 < 3 kDa | Digestion with protease from Vibrio sp. | DPPH, hydroxyl radical-scavenging activity, protection against H2O2 damage, (ORAC) | [206] |
| Sardine (Sardina pilchardus) |
Head, scales, skin, blood, | SPH amino acid composition | Enzymatic hydrolysis with BSY protease; hydrolysis with 6 M HCl at 110 °C for 24 h. | FRAP ability | [207] |
| Fish Conger myriaster | Skin | Collagen peptides | The diethyl ether extracts of the skin | DPPH | [208] |
| Fish Anguilla japonica | Skin | Collagen peptides | The diethyl ether extracts of the skin | DPPH | [208] |
| Oreochromis niloticus | Skin | Amino acid fractions: I < 1 kDa, II 1–5 kDa, and III > 10 kDa | Extraction with crude enzyme solution from tuna stomach | ABTS, FRAP ability | [209] |
| Malaysian fish sausage (Keropok Lekor) |
By-products | Native collagen and gelatine | Enzymatic by Lactobacillus casei fermentation | DPPH (82.8–88.4%) for fermented FBPs, DPPH (78.9%) for unfermented FBPs |
[210] |
| Johnius dussumieri | Skin | Fractions with different molecular weights: >10 kDa, 5–10 kDa, 3–5 kDa, 1–3 kDa, and <1 kDa |
Hydrolysis with visceral proteases extracted from the gastrointestinal (GI) tract of fish | DPPH, ABTS, FRAP ability, β-carotene bleaching prevention |
[211] |
| Mackerel (Scomber Japonicus) |
Fish | Ten peptides were synthesized | Enzymatic, sub-critical water hydrolysis, gamma irradiation, and chemical hydrolysis | DPPH (36.34%) and the highest SOD-like activity | [38] |
| Bigeye tuna | Skin | Peptides with low molecular weight < 600 Da | Subcritical water hydrolysis | DPPH, ABTS, FRAP ability and metal-chelating activity | [212] |
| Sea bream Sea bass |
By-products, heads, bones | Amino acid profile of residues (gills, heads, and bones) from sea bass and sea bream | Collagen extraction with solvents extraction with pulsed electric fields | DPPH, ABTS, FRAP assay, and ORAC assay | [213] |
| Sander lucioperca and Rutilus rutilus lacustris | Fish lenses | Protein-free extracts from the fish lenses | The presence of ovothiol A (OSH) in the fish lenses of vertebrates | High concentrations of OSH level | [214] |
The antioxidant activity of collagen peptides has also been reported in invertebrates, particularly jellyfish. For instance, James et al. (2023) presented DPPH results for antioxidant activity in Rhizostoma pulmo using pepsin hydrolysis [147]. Similarly, De Domenico et al. (2019) reported antioxidant activity in Rhizostoma pulmo using TEAC and ABTS assays [177]. The pepsin enzymatic hydrolysis process is the most commonly used. By this process, Chen et al. (2018), who extracted collagen from the scales of Chanos chanos, Najafian et al. (2018) from the fish (Budu) and Aissaoui et al. (2017) studied the collagen peptides from the small red scorpionfish Scorpena notate and used different specific methods to highlight the antioxidant potential of marine collagen [178,179,180]. By enzymatic hydrolysis with collagenases, collagen peptides were extracted from the skin of Hypophthalmichthys molitrix by Huang et al. (2023) and from the skin of Decapterus macarellus by Herawati et al. (2022) [181,182]. Antioxidant peptides were extracted by enzymatic hydrolysis with collagenases from catfish skin by Ayat et al. (2021) and from lamuru (Caranx ignobilis) by Nur et al. (2021), and the antioxidant activity of these peptides was revealed [183,184].
By enzymatic hydrolysis with alkalase, hydrolyzed collagen was extracted from tuna (Thunnus albacares) skin by Nurilmala et al. (2020), from Tilapia fish bones by Luo et al. (2022), from Cyprinus carpio skin by Gonzalez et al. (2022), and from Theragra chalcogramma skin by Lee et al. (2022); it showed antioxidant activity tested by specific methods, DPPH, superoxide anion radical scavenging, FRAC ability, TEAC, and ORAC [101,185,186,187]. Table 5 shows the various types of enzymes used in enzymatic hydrolysis and the antioxidant potential of collagen extracts.
Muangrod et al. (2022) and Uptata et al. (2022) studied the antioxidant potential by DPPH, ABTS, and the FRAP ability of peptide fractions extracted from the jellyfish Lobonema smithii by enzymatic hydrolysis with alcalase, flavorzyme, and papain hydrolysis [174,175].
Alkalase hydrolysis has been used to obtain collagen extracts by Viji et al. (2019), who demonstrated the antioxidant activity of collagen peptides from the skin and scales of Cynoglosus arel, and by Sae-leaw et al. (2018), who extracted such collagen with antioxidant properties from salmon scales, with antioxidant activity tested by DPPH, ABTS, and FRAP ability [188,189].
By enzymatic hydrolysis with papain, collagen was extracted and amino acid fractions with antioxidant properties were studied by Muangrod et al. (2022), who studied the jellyfish Rhopilema hispidum; by Jin et al. (2019), who reported data for Sea cucumber Actinopyga lecanora; by Islam et al. (2023), who studied amino acid fractions with antioxidant properties from Sturgeon fish; and by Chotphruethipong et al. (2021), who extracted collagen hydrolysates from the skin of Lates calcarifer [174,190,191,192]. Also, by enzymatic hydrolysis, Iosageanu et al. (2021) extracted collagen peptides from Hypophthalmichthys molitrix; Bordbar et al. (2021) extracted collagens from stonefish (Actinopyga lecanora); and Qiu et al. (2019) extracted collagen peptides from skipjack tuna (Katsuwonus pelamis) scales and conducted studies for the antioxidant potential attested by different methods [193,194,195]. Table 5 presents studies in which the authors present the antioxidant potential of collagen extracts from brown resources attested by different specific methods but emphasize the collagen extraction methods, which, as demonstrated, can influence the extraction yield, the type and purity of extracted components, and the antioxidant properties. We find that most extraction techniques were enzymatic hydrolysis, but also other techniques. Using several types of enzymatic hydrolysis with trypsin, neutrase, protamex, flavorzyme enzymes, trypsin, bromelain, papain, pepsin, and alkalase, different collagen peptides were extracted for which the antioxidant potential was studied. Such were the studies performed by Teng et al. (2023) for collagen from the jellyfish Nemopilema nomurai; by Bordbar et al. (2021), Qiu et al. (2019), and Zhang et al. (2022) for gelatin from the skin of skipjack tuna (Katsuwonus pelamis); and by Wang et al. (2020) for collagen peptides from the scales of red lip Croaker (Pseudosciaena polyactis), who also tested antioxidant activity [161,194,195,196,197]. Other studies for antioxidant activity using enzymatic hydrolysis with multiple enzymes for the extraction of collagen were those reported by Qiu et al. (219) for different collagen peptides extracted from skipjack tuna (Katsuwonus pelamis) scales, and by Chel-Guerrero et al. (2020) for peptide fractions extracted from red lionfish (Pterois volitans L.), who tested the antioxidant potential using different methods [195,198]. Antioxidant activity was also reported by Jin (2019) for collagen from the sea cucumber Acaudina molpadioides and by Zhao et al. (2018) for collagen peptides from the Miichthys miiuy croaker (Miichthys miiuy), both studies folding multiple enzymes to obtain collagen compounds [190,199].
Devita et al. (2021) identified amino acids from Thunnus obessus skin in different enzymatic hydrolyses (with bromelain, papain, pepsin, and trypsin), and Li et al. (2021) reported mottled duck cartilage collagen in several types of hydrolysis (enzymatic hydrolysis with trypsin, chymotrypsin, and papain) and showed the antioxidant activity of the obtained extracts by DPPH, reducing power, and ABTS [200,201]. Sripokara, P. et al., (2019) using enzymatic hydrolysis with trypsin, reported the antioxidant properties of collagen peptides from starry triggerfish (Abalistes stellaris) through several assays: ABTS and DPPH, FRAP ability, and the metal-chelating activity of the hydrolysate sample, which were dose-dependent [202].
Neutrase enzymatic hydrolysis has been used by Bordbar et al. (2021), who extracted collagen from the sea cucumber Acaudina Molpadioides; by Qiu et al. (2019), who extracted gelatine and collagen peptides from skipjack tuna (Katsuwonus pelamis) scales; and by Zheng et al. (2020), who extracted collagen peptides from the swim bladders of the giant croaker (Nibea japonica) and demonstrated their antioxidant activity by different methods, specifically DPPH and ABTS, but also other specific methods [194,195,203]. Using protease enzymatic hydrolysis, Baehaki et al. (2020) extracted collagen peptides from Channa striata skin; Kusumaningtyas et al. (2019) extracted collagen hydrolysates from milkfish (Chanos chanos) skin; Wu et al. (2018) extracted peptides from collagen hydrolysate obtained from Salmon skin; and Vieira et al. (2017) extracted two novel peptides from the head, scales, skin, and blood of sardines (Sardine (Sardina pilchardus), which they tested for their antioxidant activity by specific methods: DPPH, ABTS, and FRAP ability [204,205,206,207].
Using solvents such as diethyl ether extracts to obtain collagens from the skin of the fish Conger myriaster and Anguilla japonica, Santhanam et al. (2022) were able to isolate collagen peptides with antioxidant activity tested by DPPH assay [208]. Jantaratch et al. (2022) reported amino acid fractions from the skin of Oreochromis niloticus in crude enzyme solutions from Tuna stomachs, in which they tested antioxidant activity by ABTS and FRAP [209]. Rashid et al. (2023) obtained fish protein hydrolysates from Malaysian fish salami (Keropok Lekor) using enzymatic methods by Lactobacillus casei fermentation and evaluated their antioxidant and antibacterial activity [210]. Dara et al. (2020) utilized hydrolysis with visceral proteases extracted from the gastrointestinal tracts of fish and demonstrated the antioxidant activity of peptide fractions obtained from Johnius dusumieris skin using DPPH, ABTS, and FRAP assays [211].
One other extraction method, hydrolysis of subcritical water for the production of bioactive peptides with antioxidant properties, has been used by Bashir et al. (2020), who identified antioxidant peptides from the mackerel (Scomber Japonicus), and by Ahmed et al. (2018), who identified bioactive peptides from tuna skin collagen [38,212]. Franco et al. (2020) explored the antioxidant properties of collagen with a specific method: DPPH, ABTS, and FRAP assays for collagen extracted from sea bream and sea bass by-products utilized solvents in pulsed electric fields [213]. Yanshole et al. (2019) reported interesting studies on the presence of ovothiol A (OSH) in the lenses of Sander lucioperca and Rutilus rutilus lacustris fish [214]. Their study shows that high concentrations of OSH levels in fish are seasonally variable [214].
6. Antioxidant Applications of Nutraceuticals Based on Collagen, Gelatin, and Collagen Peptides
Nutraceuticals are specialized products consumed with food to provide health benefits beyond basic nutrition. These products come in various forms, like tablets, capsules, powders, and beverages. Functional proteins, a subset of nutraceuticals, are complex mixtures of biologically active proteins that support normal immune function. With a global shift towards healthier lifestyles, there has been a significant investment in nutritional products [215]. According to global reports, the functional protein market is expected to reach USD 7.98 billion by 2026, growing at a CAGR of 6.93% from 2019 [216].
Collagen hydrolysates and peptides derived from marine sources are notable nutraceuticals due to their biological activities.
6.1. Anti-Cancer Activity
Antitumor and antioxidant activity were reported by Mizarpour et al. (2020) on studies done with hydrolysates from Barred mackerel skin, which were screened for cytotoxic activity against human MCF-7 cell line cells [217]. Nine fractions obtained by hydrolysis of fish gelatin were tested, of which the F1 fraction was found to have very good antioxidant and anti-carcinogenic activities [217]. Yaghoubzadeh et al. (2019) reported research on hydrolyzed proteins and collagen peptide fractions with molecular masses less than 3 kDa obtained from Rainbow trout fish, in which they evidenced antioxidant and anticancer activities in human colorectal carcinoma HCT-16 [218]. Lu et al. (2017) reported the activity of two peptides extracted from cod fish skin that had essential actions in various invasive processes, inhibiting MMP-1, p-ERK, and p-p38 [219]. Ramesh et al. (2021) identified the antitumor cytotoxic activity of Leji-malides (A-D), which are unique 24-membered polyene macrolides found in the species Eudistoma cf. rigida [220]. Meanwhile, Ganesan et al. (2020) and Hu et al. (2012) reported both in vitro and in vivo antitumor activity on HELA and HT-29 cell lines, along with the antioxidant activity of polypeptides with a molecular weight of 20,419 Da extracted from the bivalve mollusca Archa subcrenata [215,221]. Their findings showed that the tumor growth inhibition rates of P2 were 26.4%, 41.4%, and 46.4% for hepatoma cells H-22 and 34.0%, 45.8%, and 60.1% for sarcoma cells in S-180 tumor-bearing mice [215,221]. Figure 7 illustrates the common conditions for which nutraceutical antioxidants containing collagen hydrolysates, gelatins, or collagen peptides from marine sources are recommended.
Figure 7.
Diseases treated with antioxidant nutraceuticals that have in their compositions peptides and collagen hydrolysates.
Ganesan et al. (2020) and Beaulieu et al. (2013) reported antitumor activity against various cancer cell lines. They observed mortality rates of 81%, 85%, 89%, and 90% in cell lines BT549 (breast carcinoma), HCT15 (colon carcinoma), A549 (type II lung epithelial), and PC3 (prostate cancer), respectively, at a concentration of 44 mg/mL [215,222]. This activity was attributed to 50 kDa fractions containing 56% of the proteins rich in the amino acids Thr, Pro, and Gly, sourced from the mussel Mitylus edulis [215,222]. Additionally, Wali et al. (2019) and Ruiz-Torres et al. (2017) highlighted the specific anticancer properties of coral derivatives. These compounds exhibit anti-inflammatory, anticancer, and antioxidant activities, suggesting potential for cancer treatment [223,224].
6.2. Antidiabetic Activity
Xu et al. (2022) reported studies on Gly-Pro-type peptides, containing 4–9 amino acid residues, obtained by enzymatic hydrolysis of tilapia Oreocchromis niloticus skin gelatin using seven proteases: papain, bromelain, neutrase, alkalase, protamex, flavorzyme, and trypsin [225]. Some proteases showed differences in peptide release, with the authors concluding that papain released strong dipeptidyl peptidase IV (DPP-IV)-inhibitory peptides to the greatest extent from Tilapia fish skin [225]. Wang et al. (2015) reported studies performed on gelatin hydrolysates on different fish from both cold and warm water [226]. They demonstrated that peptide fractions with MW < 1.5 kDa obtained from Halibut and Tilapia fish presented remarkable DPP-IV inhibitory activity of 38.2% and 51.9%, respectively, at a sample concentration of 1 mg solid/mL and re-performed in vivo antihyperglycemic experiments on streptozotocin-induced diabetic rats, demonstrating improved glucose tolerance. Better results were reported for the amino-acid-rich warm-water fish gelatin from Tilapia fish as a more potent antihyperglycemic agent compared to the gelatin hydrolysate from Halibut, due to its superior amino acid content [226].
6.3. Antiobesity Activity
Wang et al. (2020) reported studies on collagen peptides with molecular weights ranging from 500 to 5000 Da, extracted from an enzymatic hydrolysate of Walleye Pollock skin, that had efficient effects against obesity in mice fed a high-fat diet [227]. The results show that collagen peptide extracts from Walleye pollock are a potential agent in the development of an adjuvant for the treatment of obesity and associated metabolic diseases [227].
Raksha et al. (2023) reported studies of collagen peptides extracted from the jellyfish Diplulmaris antarctica that have action in preventing and treating obesity caused by a high-calorie diet and in curing other pathologies associated with increased oxidative stress [228].
6.4. Osteoarthritis and Bone Diseases
Luo et al. (2022) characterized low-molecular-weight collagen peptides, primarily composed of Gly, Ala, and Pro, extracted from Atlantic salmon bone. They evaluated these peptides’ effects on chondrocytes induced by interleukin 1β (IL-1β) and assessed their efficacy and safety as anti-osteoarthritis agents through biomarker testing. The goal was to develop a dietary supplement that could delay arthritis development and support anti-inflammatory cartilage regeneration [229].
6.5. Cardiovascular Diseases
Hypertension has recently become a major global problem. In recent decades, there has been increasing interest in natural ACE-inhibitory peptides from food. These include by-products from fish skin: collagen, collagen hydrolysates, and collagen peptides, which are an important source of ACE-inhibitory peptides. Cui L. et al. (2023) studied antiplatelet peptides in collagen hydrolysates from silver carp skin that were enriched using macroporous resins. The results showed the yield and antiplatelet activity of the 20% ethanol fraction with an IC50 of 2.03 mg/mL, which recommended the use of fish antiplatelet peptides as functional foods [230].
In most cardiovascular diseases, atherosclerosis occurs, which is inflammation of the blood vessels. Liu H. et al. (2022) demonstrated through research on collagen hydrolysates from Atlantic salmon fish skin (Salmo salar) that they possess potent anti-inflammatory activity, protective activity against endothelial cell injury, antioxidant activity, and anti-platelet aggregation activity in vitro [231]. Also, collagen hydrolysates from Salmon fish showed combined effects on the regulation of serum biomarkers of inflammation (IL-6 and TNF-α), on endothelial injury (MCP-1), activating platelets (TXB2 and PF4), and regulating oxidative stress. It can be a dietary supplement for the prevention of atherosclerosis [231]. Abdelhedi et al. (2017) conducted comparative studies on gelatin hydrolysates extracted from black-barred halfbeak (Hemiramphus far) hides using different acidic, alkaline, and enzymatic hydrolysis treatments [232]. Their research demonstrated the high antioxidant potential of fish collagen hydrolysates and highlighted the ACE-inhibitory activity of peptides as a promising nutraceutical product for various cardiovascular diseases [232].
Similarly, Aissaoui et al. (2017) studied collagenous hydrolysates from scorpion fish (Scorpaena notata) red fish heads obtained through enzymatic treatments. The authors showed that these peptides exhibit high inhibitory activity against the angiotensin-I-converting enzyme, with IC50 values of 0.98, 1.69, and 1.44 µm. They also concluded that fish by-products can be exploited as nutraceuticals against oxidative stress and hypertension [233]. Thuanthong et al. (2017) and Liu et al. (2019) have highlighted the importance of Oreochromis niloticus and Pinctada fucata martensii in cardiovascular treatments [234,235].
Zhong et al. (2018) optimized the enzymatic hydrolysis process to separate bioactive peptides with ACE-inhibitory activity from sea cucumber (Stichopus japonicus) gonads. The peptides were identified showing the highest ACE-inhibitory activity (IC50 of 260.22 µM) and cytotoxicity to Caco-2 cells [236]. Zhang et al. (2018) studied peptides extracted from the hydrolysates of jellyfish gonads (Rhopilema esculentum Kishi-nouye) using neutral proteases. These peptides with the SY amino acid sequence demonstrated both good ACE-inhibitory and antioxidant activities [237]. This purified dipeptide is recommended as a functional food material for its antioxidant properties and ACE-inhibitory activity [237].
6.6. Anti-Alzheimer’s Activity and Neurodegenerative Diseases
Alzheimer’s disease is a neurodegenerative disease that occurs due to the progressive loss of neurons. Abuine et al. (2019) and Choi et al. (2015) showed that the prevalence of neurodegenerative diseases increased with increasing life expectancy [238,239]. Abuine et al. (2019) and Lee et al. (2015) reported as mechanisms of action the inhibitory effect of β-secretase attributed to the peptide sequence QGYRPLRGPEFL [238,240].
The neuroprotective effect and antioxidant activity of protein extracts from the skin of grass carp (Ctenopharyngodon idella) has also been shown by Abuine et al. (2019) and Cai et al. (2015), who showed that PYSFK-, GFGPZL-, and VGGRPP-type peptides showed important neuroprotective activity [238,241]. Abuine et al. (2019) and Xu et al. (2015) showed neuroprotective effects in Alzheimer’s disease with the presentation of collagen peptides from catfish (Oncorhynchus keta) [238,242]. Ganesan et al. (2020) and Pangestuti et al. (2013) reported neuroprotective effects against Ab42-induced neuronal death in PC12 cells by collagen peptides extracted from the sea horse (Hippocampus trimaculatus): GTZDZLDK [215,243].
6.7. Oral Diseases
One of the most prevalent diseases of the oral cavity is oral mucosal ulcers, which manifest as severe burning pain and difficulty chewing, drinking, and even speaking. Gao et al. (2022) evidenced the role of collagens from marine resources in the healing of oral cavity wounds [244]. They demonstrated that low-molecular-weight collagen peptides from Tilapia fish skin play a role in the healing of traumatic oral ulcers in rats [244]. Xu et al. (2021) proved through research on periodontal membrane cell culture experiments of hydrolyzed Tilapia fish collagen that it has the function of regenerating periodontal tissue in vitro [245]. Tilapia fish collagen has been used in the production of composite membranes as nanofibers, together with bioactive glass and chitosan. Zhou, T et al. (2017) made a biomimetic fish collagen/bioactive glass/chitosan (Col/BG/CS) nanofiber composite membrane to study the biological effects on human periodontal ligament cells (HPDLCs) [246]. The results of Tang et al. (2015) suggested that tilapia scale collagen might be a potential alternative to type I collagen for use in oral diseases [247]. Liu C et al. (2015) suggested for the first time that hydrolyzed tilapia fish collagen (HFC) can be used for periodontal tissue regeneration and is a promising bioactive ingredient for biomaterials used in alveolar bone regeneration [248].
6.8. Wound Healing Activity
Chotphruethipong et al. (2021) proved antioxidant and anti-inflammatory activities in wound healing of sea bass (Lates calcarifer) collagen hydrolysates conjugated with epigallocatechin gallate through the inhibition of nitric oxide production and tumor necrosis factor-α in RAW264.7 cells [192]. Also, Chotphruethipong et al. in 2021 reported studies on two collagenous peptides (PO and POG) isolated from the skin of Asian sea bass (Lates calcarifer), which showed antioxidant effects [249]. Sivarman et al. (2021) showed that the collagen peptide induces cell growth and migration of fibroblast cells and facilitates the wound healing process. They recommended the use of these peptides as a functional ingredient for nutraceuticals used in wound healing [250]. Chen et al. (2019) demonstrated the existence of collagen peptides from a collagen sponge extracted from the bladder of Nibea japonica with the GAPO sequence, which produced accelerated wound healing [92]. Mice treated with sponge collagen had significantly reduced interleukins. These have potential applications for wound healing.
6.9. Anti-Inflammatory Activity
Sivaraman et al. (2021) reported obvious anti-inflammatory effects generated by peptide fractions with molecular weights of 1–3 kDa extracted from the skin of the fish Clarias batrachus and Pangasius pangasius. Peptide fractions from these two fish species showed a suppression of inflammatory proteins (TNF-α, IL-6, NF-κB, and p-IκB). Due to these properties, the collagen hydrolysates of these fish species can be functional foods, and purified fractions can be used as nutraceuticals with anti-inflammatory properties [250].
6.10. Anti-Aging and Skin Protection Activity
Skin aging occurs under the action of intrinsic (e.g., aging) and extrinsic (e.g., smoking and UV) factors. UV irradiation consists of UV-A, UV-B, and UV-C. Fu et al. (2022) showed that UV-B radiation is responsible for the largest proportion of photoaging, mainly by inducing epidermal and superficial dermal damage [251]. They demonstrated that UV-B irradiation can cause excessive production of reactive oxygen species (ROS) and a range of skin damage through several signaling pathways, such as the stimulation of mitogen-activated protein kinase (MAPK) activity [251]. Xia et al. (2021) reported on natural bioactive peptides with anti-aging effects. They detailed the molecular mechanisms involved [252]. Maia Campos et al. (2021) evaluated the clinical efficacy of low-dose oral supplements of fish cartilage hydrolysate [253]. After a 90-day treatment period, there was a significant reduction in wrinkles and an increase in dermis echogenicity compared to the placebo and baseline values [253].
Table 6 systematizes the biological activities of collagen hydrolysates, collagen peptides, and amino acid sequences from various marine organisms with results in the treatment of various diseases.
Table 6.
The biological activities of collagen hydrolysates, collagen peptides, and amino acid sequences from different marine organisms with results in the treatment of different diseases are systematized.
| Marin Organism Type | Species | Organ/Protein/Peptide Fraction/Amino Acid Sequence | Biological Activity | Results in Treating Diseases | References |
|---|---|---|---|---|---|
| Fish | Barred mackerel | Skin fish gelatin/highest percentages of amino acid GPAQRID | Anticancer MCF-7 line cells Antioxidant (DPPH) | Nine fractions were obtained from the hydrolysis of fish skin gelatin. Fraction F1 was the most active fraction for antioxidant and cytotoxic activity against MCF-7-line cells | [217] |
| Fish (cold-water) |
Rainbow trout | Skin—the collagen peptide fractions < 3 KDa | Anticancer HCT—116 cell line Antioxidant | The isolated peptide fractions from skin on HCT-116 cancer cells have cytotoxic properties. Skin hydrolysate showed antioxidant properties | [218] |
| Fish | Cod | Skin/two peptides: GEIGPSGGRGKP GKDGDAGPK and GFSGLDGAKGD |
MMP are used for processes of angiogenesis and tumor metastasis | MMP inhibitory activity two peptides were found to exhibit a significant inhibition of MMP-1, p-ERK, and p-p38 | [219] |
| Tunicate | Eudistoma cf. rigida | Lejimalides (A-D) unique 24-membered polyene macrolides | Antitumor | In vitro cytotoxic activity | [220] |
| Mollusca bivalva | Arca subcrenata | A polypeptide fraction with a MW of 20,491.0 Da | Antitumor activity in vitro and in vivo; antioxidant action | Purified polypeptides were treated for HeLa and for HT-29 | [215] [221] |
| Mussel | Mytilus edulis | The 50 kDa fraction contains 56% of the proteins with important amino acids | Anti-proliferative agent | The 50 kDa fraction showed immortalized cancerous cell lines and mortality rate against BT549 cell lines, HCT15, A549, and PC3 | [215] [219] |
| Fish | Tilapia fish Oreochromis niloticus | Skin/Gly-Pro-type peptides | Diabetes and DPP-IV-inhibitory activities | The DPP-IV-inhibitory activity of synthetic Gly-Pro was studied. It is used to treat people with type 2 diabetes. | [225] |
| Fish | Hippoglossus stenolepis | Skin/SPGSSGPQGFTG, GPVGPAGNPGANGLN, and PPGPTGPRGQPGNIGF |
Diabetes | Displayed in vitro DPP-IV-inhibitory activity and were used for an in vivo antihyperglycemic experiment | [226] |
| Fish | Oreochromis niloticus | Skin/IPGDPGPPGPPGP, LPGERGRPGAPGP, and GPKDRGLPGPPGRDGM |
Diabetes | Antihyperglycemic activity | [226] |
| Fish | Walleye pollock | Skin/collagen peptides | Anti-obesity activity | Attenuated obesity and modulated gut microbiota; | [227] |
| Jellyfish | Diplulmaris antarctica | Hydrolyzed collagen peptides | Anti-obesity activity | decrease the body weight by decreasing the level of fasting blood glucose | [228] |
| Fish—Atlantic Salmon |
Salmo salar | Bone—small-molecular-weight peptide rich in Gly-X-Y structure | Osteoarthritis | Showed potential to be a novel and safe dietary supplement for helping anti-inflammatory activities, ultimately hindering osteoarthritis development | [229] |
| Fish | Silver carp | Skin—collagen hydrolysates OG or PG | Cardiovascular diseases antiplatelet peptides | The results showed that the antiplatelet peptides have increased antioxidant capacity beneficial in cardiovascular disease | [230] |
| Fish | Salmo Salar | Skin—multifunctional peptides FAGPPGGDGQPGAK and IAGPAGPRGPSGPA. |
Atherosclerosis, antiplatelet aggregation and antioxidation activity | Atherosclerosis treatment protects against endothelial injury, platelet aggregation, and thrombosis. Aids anti-inflammation and endothelial damaging protection | [231] |
| Fish | Saurida elongata | Skin—RYRP | Hypertension | ACE-inhibitory activity with an IC50 value of 52 µM. | [185] |
| Fish | Hemiramphus far | Skin—collagen peptides | Hypertension | ACE-inhibitory activity 80.76% at 1 mg/mL | [232] |
| Fish | Scorpaena notata | By-product—PHSRSKGFPGP, GZKSVPQVR, and VQGKSPBV |
Hypertension | ACE-inhibitory and antioxidant activity; IC50 values of 0.98, 1.69, and 1.44 μM | [233] |
| Fish Nile Talpia |
Oreochromis niloticus | Skin—GIV, GAPGF, GFAGPA, SGDIGFPGPK, and GIPGPIGPPGRP MW less than 1.2 kDa |
Moderate hypertension | The resulting hydrolysate had an ACE-inhibitory activity (IC50) of 1.2 mg/mL, which was slightly reduced by simulated gastrointestinal digestion. | [234] |
| Pearl oyster |
Pinctada fucata
martensii |
HLHT and GWA | Hypertension | ACE inhibitory | [235] |
| Sea cucumber | Stichopus japonicus | Peptide fractions: EIYR, LF, and NAPHMR | Hypertension | ACE-inhibitory activity IC50 of 260.22 μM | [236] |
| Jellyfish |
Rhopilema esculentum
kishinouye |
Peptide fraction: SY | Hypertension | ACE-inhibitory activity IC50 of 1164.179 μM | [237] |
| Fish | Skate Raja kenojei | Skin—ZGYRPLRGPQFL | Anti-Alzheimer’s, neuroprotective | Inhibitory peptides for β-secretase have been identified such as Pro-Glu-Phe-Leu. | [238] [240] |
| Cyprinidae Fish |
Ctenopharyngodon idella | Skin—PYSFK—MW: 640.74 Da, GFGPZL—MW: 618.89 Da, VGGRPP—MW: 484.56 Da |
Anti-Alzheimer’s, neuroprotective, antioxidant activity |
Neuroprotection and antioxidant activity of these three peptide sequences | [238] [241] |
| Fish | Oncorhynchus keta | Skin—polypeptides | Anti-Alzheimer’s, neuroprotective |
Anti-acetylcholinesterase, increasing hippocampus phosphorylation | [238] [242] |
| Seahorse | Hippocampus trimaculatus | Amino acids GTZDZLDK MW 906.4 Da |
Neuroprotective | Neuroprotective effects against Ab42-induced neuronal death in PC12 cells | [215] [243] |
| Fish | Tilapia | Skin—collagen peptides | Oral mucosal ulcers | The effects of collagen peptides on the healing of oral mucosal ulcers in a rat model were macroscopically and microscopically analyzed in vivo | [244] |
| Fish | Tilapia scales | Collagen peptides | Oral and maxillofacial tissue regeneration | In vitro periodontal tissue regeneration | [245] |
| Fish | Sea bass Lates calcarifer | Hydrolyzed collagen from sea bass | Wound healing, antioxidant, and anti-obesity activities | HC and the HC-EGCGG conjugate showed anti-inflammatory activity by inhibiting the production of nitric oxide and tumor necrosis factor-α in lipopolysaccharide-induced RAW264.7 cells | [192] |
| Fish | Sea bass Lates calcarifer | Skin—antioxidant peptides (PO, POG) | Wound healing | Antioxidant peptides from Asian sea bass skin containing Pro-Hyp and Pro-Hyp-Gly increase wound healing by accelerating fibroblast mobility to injured tissue | [249] |
| Fish | Nibea japonica | Swim bladders—collagen peptides with GAPO | Wound-healing activity and antioxidant activity | Collagen peptides from collagen sponge produced accelerated wound healing. Mice treated with the collagen sponge had significantly reduced levels of interleukins | [92] |
| Fish | Clarias batrachus, Pangasius pangasius | Skin—peptide fractions in the range of 1–3 kDa molecular weight | Anti-inflammatory activity | The suppression of inflammatory proteins (TNF-α, IL6, NFκB, and p-IκB) by the peptide fractions confirmed the anti-inflammatory activity | [250] |
| Fish | Thunnus obesus | Collagen peptides from skin (TSCP) and from bones (TBCP) with <1 kDa | UV protector of skin, photoaging | Excellent anti-photoaging activity, improving cell viability and inhibiting skin water loss. Attenuated skin photoaging | [251] |
| Fish | Black pomfret (Parastromateus niger) |
Peptides | Anti-aging effects | Natural bioactive peptides have been shown to improve the effects of skin aging due to free-radical-scavenging activity and anti-aging peptides | [252] |
| Fish | Hydrolyzed fish cartilage | Collagen peptides | Anti-aging activity | Measurements of skin wrinkles, dermis echogenicity and thickness, and morphological and structural characteristics of the skin were performed | [253] |
| Fish | Salmo salar | By-products—TPQVHIAVDKF | Anti-allergic activity | Subsequently, the novel eleven-amino-acid peptide, identified as TPEVHIAVDKF, was found to exert anti-allergic activity | [254] |
| Fish | By-product from fish scales | Fish scale peptides and other compounds like hydroxyapatite | Treating malnutrition | Each component has multiple beneficial properties for the human body as an antioxidant in the treatment of malnutrition | [255] |
| Fish | Pacific cod | Skin gelatin; GPAGPHGPPGKDGR, AGPHGPPGKDGR, and AGPAGPAGAR |
Iron deficiency treatment | GPAGPHGPPGKDGR and AGPHGPPGKDGR supplied additional iron binding sites. This study suggests a potential application of gelatin-derived peptides as novel carriers to combat iron deficiency | [256] |
6.11. Other Diseases
6.11.1. Anti-Allergic Activity
Wang et al. (2020) studied the by-products of Atlantic salmon (Salmo salar) and extracted enzymatic hydrolysate collagenic peptides from them [254]. Their research identified six fractions, with fraction C6 demonstrating the strongest antiallergic activity [254]. Additionally, they isolated a novel eleven-amino-acid peptide, TPEVHIAVDKF, which showed antiallergic properties. This study suggests that Atlantic salmon by-products could be a valuable source of new ingredients for food and pharmaceutical products aimed at managing food allergies [254].
6.11.2. Treating Malnutrition
Salindeho et al. (2022) reported studies on fish scale peptides mixed with hydroxyapatite and chitin and showed that each component has multiple beneficial properties for the human body as an antioxidant, in the treatment of malnutrition, as a hypocholesterol-lowering agent, and in bone metabolism [255].
6.11.3. Iron Deficiency Treatment
Wu et al. (2015) reported studies on Pacific cod gelatin and showed that several amino acids can be bound by iron ions. This study suggests a potential application of gelatin-derived peptides as novel carriers to combat iron deficiency [256].
7. Conclusions
The present study highlights the significance of marine-derived collagen compounds and marine resources for obtaining collagen and collagen peptides from both invertebrates and vertebrates. Based on the literature, enzymatic hydrolysis of collagen, which releases peptides and peptide moieties, is an efficient method for obtaining natural antioxidant compounds from marine sources. Various tests, such as DPPH and ABTS scavenging activity, hydroxyl and superoxide anion radical-scavenging activity, FRAP capacity, and metal-chelating activity, have demonstrated antioxidant activity. However, there are limited data on the beneficial effects of these isolated collagen peptide fractions on human health in in vivo studies for alternative treatments. This review has shown that marine collagen antioxidants from different vertebrate and invertebrate species can be involved in treatments for cancer, diabetes, obesity, osteoarthritis, cardiological conditions, and Alzheimer’s disease. Additionally, collagen antioxidants are used in bone tissue regeneration and osteoarthritis, antihypertensive and neurodegenerative diseases, oral and dental diseases, cell regeneration against oxidative stress, skin lesion healing and protection, anti-inflammatory and anti-allergic responses, and iron deficiency treatments.
The global consumption of marine products has increased due to the use of marine by-products, which are rich in bioactive components that enhance human health by creating novel nutraceutical compounds with antioxidant properties. The objective of fully exploiting marine resources can also be achieved through the efficient and effective use of fish by-products (skin, bones, scales, fins, and fish heads) which contain significant amounts of collagen and collagen peptides. This paper supports the growing utilization of marine antioxidant biocompounds. However, it is often unclear what kind of water or cultural environment certain by-products originate from, raising concerns about the efficacy and safety of these nutraceuticals for human health. Therefore, further research is needed to identify barriers and ensure successful production of antioxidant nutraceuticals from marine resources in the food, pharmaceutical, and biomedical industries.
Abbreviations
| ASC | Acid-soluble collagen |
| PSC | Pepsin-soluble collagen |
| WSG | Water-soluble gelatin |
| ROS | Reactive oxygen species |
| FTIR | Fourier-transform infrared spectroscopy |
| HPLC | High-performance liquid chromatography |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid |
| FRAP | Ferric ion reducing antioxidant power |
| ACE | Angiotensin-converting enzyme |
| IL | Interleukin |
| MMP | Matrix metalloproteinase |
Author Contributions
Conceptualization: E.C., I.P. and R.S.; methodology: E.C., A.-M.I., C.L.T., A.-M.P., A.-M.L.D. and C.P.; writing—original draft preparation: E.C., A.-M.P., A.-M.L.D., C.P., A.-M.I. and C.L.T.; writing—review and editing: R.S., A.-M.P., A.-M.L.D., C.P. and E.C.; supervision: E.C. and R.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vettorazzi A., López de Cerain A., Sanz-Serrano J., Gil A.G., Azqueta A. European Regulatory Framework and Safety Assessment of Food-Related Bioactive Compounds. Nutrients. 2020;12:613–629. doi: 10.3390/nu12030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djaoudene O., Romano A., Bradai Y.D., Zebiri F., Ouchene A., Yousfi Y., Amrane-Abider M., Sahraoui-Remini Y., Madani K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic and Health Effects. Nutrients. 2023;15:3320–3344. doi: 10.3390/nu15153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lordan R., Rando H.M., COVID-19 Review Consortium. Greene C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. mSystems. 2021;6:1–22. doi: 10.1128/mSystems.00122-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garza-Juárez A., Pérez-Carrillo E., Arredondo-Espinoza E.U., Islas J.F., Benítez-Chao D.F., Escamilla-García E. Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases. Foods. 2023;12:3262–3280. doi: 10.3390/foods12173262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AlAli M., Alqubaisy M., Aljaafari M.N., AlAli A.O., Baqais L., Molouki A., Abushelaibi A., Lai K.S., Lim S.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules. 2021;26:2540–2568. doi: 10.3390/molecules26092540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z., Ren Z., Zhang J., Chuang C.C., Kandaswamy E., Zhou T., Zuo L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018;9:477–491. doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simat V., Elabed N., Kulawik P., Ceylan Z., Jamroz E., Yazgan H., Čagalj M., Regenstein J.M., Özogul F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs. 2020;18:627–667. doi: 10.3390/md18120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Bonaduce D. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y.Z., Wang Y.M., Pan X., Chi C.F., Wang B. Antioxidant Mechanisms of the Oligopeptides (FWKVV and FMPLH) from Muscle Hydrolysate of Miiuy Croaker against Oxidative Damage of HUVECs. Oxid. Med. Cell Longev. 2021;2021:9987844. doi: 10.1155/2021/9987844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirończuk-Chodakowska I., Witkowska A.M., Zujko M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018;63:68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo J.M., Munekata P.E.S., Gomez B., Barba F.J., Mora L., Perez-Santaescolastica C., Toldra F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018;79:136–147. doi: 10.1016/j.tifs.2018.07.003. [DOI] [Google Scholar]
- 12.Ye H., Xin T., Weidong Z., Yi C., Qiang Y., Jianhua X. Food-derived bioactive peptides: Production, biological activities, opportunities and challenges. J. Future Foods. 2022;2:294–306. doi: 10.1016/j.jfutfo.2022.08.002. [DOI] [Google Scholar]
- 13.Vladkova T., Georgieva N., Staneva A., Gospodinova D. Recent Progress in Antioxidant Active Substances from Marine Biota. Antioxidants. 2022;11:439–566. doi: 10.3390/antiox11030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadar E., Negreanu-Pirjol T., Sirbu R., Dragan A.M.L., Negreanu-Pirjol B.S., Axente E.R., Ionescu A.M. Biocompounds from Green Algae of Romanian Black Sea Coast as Potential Nutraceuticals. Processes. 2023;11:1750. doi: 10.3390/pr11061750. [DOI] [Google Scholar]
- 15.Rahman A., Silva T.H. Collagens from Marine Organisms towards Biomedical Applications. Mar. Drugs. 2022;20:170–174. doi: 10.3390/md20030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macedo M.W.F.S., Cunha N.B., Carneiro J.A., Costa R.A., Alencar S.A., Cardoso M.H., Franco O.L., Dias S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021;8:667764. doi: 10.3389/fmars.2021.667764. [DOI] [Google Scholar]
- 17.Felician F.F., Xia C., Qi W., Xu H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018;15:e1700557. doi: 10.1002/cbdv.201700557. [DOI] [PubMed] [Google Scholar]
- 18.Sable R., Parajuli P., Jois S. Peptides, peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Mar. Drugs. 2017;15:124–161. doi: 10.3390/md15040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppola D., Oliviero M., Vitale G.V., Lauritano C., D’Ambra I., Iannace S., De Pascale D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs. 2020;18:214–237. doi: 10.3390/md18040214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlicevic M., Maestri E., Marmiroli M. Marine bioactive peptides—An overview of generation, structure and application with a focus on food sources. Mar. Drugs. 2020;18:424–445. doi: 10.3390/md18080424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaspar-Pintiliescu A., Stefan L.M., Anton E.D., Berger D., Matei C., Negreanu-Pirjol T., Moldovan L. Physicochemical and Biological Properties of Gelatin Extracted from Marine Snail Rapana venosa. Mar. Drugs. 2019;17:589–603. doi: 10.3390/md17100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benayahu D., Pomeraniec L., Shemesh S., Heller S., Rosenthal Y., Rath-Wolfson L., Benayahu Y. Biocompatibility of a Marine Collagen-Based Scaffold In Vitro and In Vivo. Mar. Drugs. 2020;18:420–431. doi: 10.3390/md18080420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv L.C., Huang Q.Y., Ding W., Xiao X.H., Zhang H.Y., Xiong L.X. Fish Gelatin: The Novel Potential Applications. J. Funct. Foods. 2019;63:103581. doi: 10.1016/j.jff.2019.103581. [DOI] [Google Scholar]
- 24.Bhuimbar M.V., Bhagwat P.K., Dandge P.B. Extraction and characterization of acid soluble collagen from fish waste: Development of collagen-chitosan blend as food packaging film. J. Environ. Chem. Eng. 2019;7:102983. doi: 10.1016/j.jece.2019.102983. [DOI] [Google Scholar]
- 25.Lim Y.S., Ok Y.J., Hwang S.Y., Kwak J.Y., Yoon S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs. 2019;17:467–499. doi: 10.3390/md17080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvatore L., Gallo N., Natali M.L., Campa L., Lunetti P., Madaghiele M., Blasi F.S., Corallo A., Capobianco L., Sannino A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;113:110963. doi: 10.1016/j.msec.2020.110963. [DOI] [PubMed] [Google Scholar]
- 27.Geahchan S., Baharlouei P., Rahman A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs. 2022;20:61–77. doi: 10.3390/md20010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prelipcean A.-M., Iosageanu A., Gaspar-Pintiliescu A., Moldovan L., Craciunescu O., Negreanu-Pirjol T., Negreanu-Pirjol B., Mitran R.A., Marin M., D’Amora U. Marine and Agro-Industrial By-Products Valorization Intended for Topical Formulations in Wound Healing Applications. Materials. 2022;15:3507–3523. doi: 10.3390/ma15103507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Melo Oliveira V., Assis C.R.D., Costa B.D.A.M., de Araújo Neri R.C., Monte F.T.D., da Costa Vasconcelos H.M.S., França R.C.P., Santos J.F., de Souza Bezerra R., Porto A.L.F. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021;1224:19023. doi: 10.1016/j.molstruc.2020.129023. [DOI] [Google Scholar]
- 30.El Blidi O., El Omari N., Balahbib A., Ghchime R., Ibrahimi A., Bouyahya A., Chokairi O., Barkiyou M. Extraction Methods, Characterization and Biomedical Applications of Collagen: A Review. Biointerface Res. Appl. Chem. 2021;11:13587–13613. doi: 10.33263/BRIAC115.1358713613. [DOI] [Google Scholar]
- 31.Gallo N., Natali M.L., Sannino A., Salvatore L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020;11:79–106. doi: 10.3390/jfb11040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benayahu D., Sharabi M., Pomeraniec L., Awad L., Haj-Ali R., Benayahu Y. Unique collagen fibers for biomedical applications. Mar. Drugs. 2018;16:102–113. doi: 10.3390/md16040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaspar-Pintiliescu A., Anton E.D., Iosageanu A., Berger D., Matei C., Mitran R.A., Negreanu-Pirjol T., Craciunescu O., Moldovan L. Enhanced Wound Healing Activity of Undenatured Type I Collagen Isolated from Discarded Skin of Black Sea Gilthead Bream (Sparus aurata) Conditioned as 3D Porous Dressing. Chem. Biodivers. 2021;18:e2100293. doi: 10.1002/cbdv.202100293. [DOI] [PubMed] [Google Scholar]
- 34.Cadar E., Pesterau A.M., Sirbu R., Negreanu-Pirjol B.S., Tomescu C.L. Jellyfishs—Significant marine resources with potential in wound healing process. A Review. Mar. Drugs. 2023;21:201–228. doi: 10.3390/md21040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul C., Leser S., Oesser S. Significant Amounts of Functional Collagen Peptides Can Be Incorporated in the Diet While Maintaining Indispensable Amino Acid Balance. Nutrients. 2019;11:1079. doi: 10.3390/nu11051079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez M.I.A., Rodriguez L.G.B., Sánchez M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018;17:20–26. doi: 10.1111/jocd.12450. [DOI] [PubMed] [Google Scholar]
- 37.Ballatore M.B., Bettiol M.D.R., Vanden Braber N.L., Aminahuel C.A., Rossi Y.E., Petroselli G., Erra-Balsells R., Cavaglieri L.R., Montenegro M.A. Antioxidant and cytoprotective effect of peptides produced by hydrolysis of whey protein concentrate with trypsin. Food Chem. 2020;319:126472. doi: 10.1016/j.foodchem.2020.126472. [DOI] [PubMed] [Google Scholar]
- 38.Bashir K.M.I., Sohn J.H., Kim J.S., Choi J.S. Identification and Characterization of Novel Antioxidant Peptides from Mackerel (Scomber japonicus) Muscle Protein Hydrolysates. Food Chem. 2020;323:126809. doi: 10.1016/j.foodchem.2020.126809. [DOI] [PubMed] [Google Scholar]
- 39.Kisling A., Lust R.M., Katwa L.C. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019;228:30–34. doi: 10.1016/j.lfs.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Pezeshk S., Ojagh S.M., Rezaei M., Shabanpour B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicrob. Proteins. 2019;11:1015–1022. doi: 10.1007/s12602-018-9483-y. [DOI] [PubMed] [Google Scholar]
- 41.Rahman M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs. 2019;17:118–130. doi: 10.3390/md17020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherim M., Mustafa A., Cadar E., Lupașcu N., Paris S., Sirbu R. Collagen Sources and Areas of Use. Eur. J. Med. Nat. Sci. 2019;2:8–13. doi: 10.26417/ejis.v4i1.p122-128. [DOI] [Google Scholar]
- 43.Prajaputra V., Isnaini N., Maryam S., Ernawati E., Deliana F., Haridhi H.A., Fadli N., Sofyatuddin K., Sri A., Nurfadillah N., et al. Exploring marine collagen: Sustainable sourcing, extraction methods, and cosmetic applications. S. Afr. J. Chem. Eng. 2024;47:197–211. doi: 10.1016/j.sajce.2023.11.006. [DOI] [Google Scholar]
- 44.Jafari H., Lista A., Siekapen M.M., Ghaffari-Bohlouli P., Nie L., Alimoradi H., Shavandi A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers. 2020;12:2230–2266. doi: 10.3390/polym12102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cherim M., Sirbu R., Belu I. Isolation of Collagen from Marine Resources from the Black Sea. Curr. Health Sci. J. 2017;43:301–305. doi: 10.12865/CHSJ.43.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu W.C., Chiu C.S., Chan Y.J., Mulio A.T., Li P.H. Characterization and biological properties of marine by-product collagen through ultrasound-assisted extraction. Aquac. Rep. 2023;29:101514. doi: 10.1016/j.aqrep.2023.101514. [DOI] [Google Scholar]
- 47.Ampitiya A.G.D.M., Gonapinuwala S.T., Fernando C.A.N., De Croos M.D.S.T. Extraction and characterisation of type I collagen from the skin offcuts generated at the commercial fish processing centres. J. Food Sci. Technol. 2023;60:484–493. doi: 10.1007/s13197-022-05630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J., Pei X., Liu H., Zhou D. Extraction and characterization of acid-soluble and pepsin-soluble collagen from skin of loach (Misgurnus anguillicaudatus) Int. J. Biol. Macromol. 2018;106:544–550. doi: 10.1016/j.ijbiomac.2017.08.046. [DOI] [PubMed] [Google Scholar]
- 49.Chen X., Jin W., Chen D., Dong M., Xin X., Li C., Xu Z. Collagens made from giant salamander (Andrias davidianus) skin and their odorants. Food Chem. 2021;361:130061. doi: 10.1016/j.foodchem.2021.130061. [DOI] [PubMed] [Google Scholar]
- 50.Cumming M.H., Hall B., Hofman K. Isolation and Characterisation of Major and Minor Collagens from Hyaline Cartilage of Hoki (Macruronus novaezelandiae) Mar. Drugs. 2019;17:223–239. doi: 10.3390/md17040223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu S., Yang H., Shen L., Li G. Purity and yield of collagen extracted from southern catfish (Silurus meridionalis Chen) skin through improved pretreatment methods. Int. J. Food Prop. 2017;20:S141–S153. doi: 10.1080/10942912.2017.1291677. [DOI] [Google Scholar]
- 52.Kıyak B.D., Çınkır N.I., Çelebi Y., Malçok S.D., Koç G.C., Adal S., Yüksel A.N., Süfer Ö., Karabacak A.Ö., Ramniwas S., et al. Advanced technologies for the collagen extraction from food waste—A review on recent progress. Microchem. J. 2024;201:110404. doi: 10.1016/j.microc.2024.110404. [DOI] [Google Scholar]
- 53.Li C., Song W., Wu J., Lu M., Zhao Q., Fang C., Wang W., Park Y.D., Qian G.Y. Thermal stable characteristics of acid- and pepsin-soluble collagens from the carapace tissue of Chinese soft-shelled turtle (Pelodiscus sinensis) Tissue Cell. 2020;67:101424. doi: 10.1016/j.tice.2020.101424. [DOI] [PubMed] [Google Scholar]
- 54.Liu W., Zhang Y., Cui N., Wang T. Extraction and characterization of pepsin- solubilized collagen from snakehead (Channa argus) skin: Effects of hydrogen peroxide pretreatments and pepsin hydrolysis strategies. Process. Biochem. 2019;76:194–202. doi: 10.1016/j.procbio.2018.10.017. [DOI] [Google Scholar]
- 55.Song Z., Liu H., Chen L., Chen L., Zhou C., Hong P., Deng C. Characterization and comparison of collagen extracted from the skin of the Nile tilapia by fermentation and chemical pretreatment. Food Chem. 2021;340:128139. doi: 10.1016/j.foodchem.2020.128139. [DOI] [PubMed] [Google Scholar]
- 56.Cherim M., Sirbu R., Erimia C.L., Mustafa A., Tomescu A. Obtaining of Collagen Biomaterials and Their Use in the Medical Field. Eur. J. Med. Nat. Sci. 2020;3:31–39. doi: 10.26417/ejis.v4i2.p31-39. [DOI] [Google Scholar]
- 57.Sirbu R., Stanciu G., Cadar E., Tomescu A., Cherim M. Validation of a Quantitative Analysis Method for Collagen Extracted from Grey Mullet Marine Fish. Rev. Chim. 2019;70:835–842. doi: 10.37358/RC.19.3.7016. [DOI] [Google Scholar]
- 58.Senadheera T.R.L., Dave D., Shahidi F. Sea cucumber derived type I collagen: A comprehensive review. Mar. Drugs. 2020;18:471–515. doi: 10.3390/md18090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaik M.I., Asrul E.N.F., Sarbon N.M. Functional properties of sharpnose stingray (Dasyatis zugei) skin collagen by ultrasonication extraction as influenced by organic and inorganic acids. Biocatal. Agric. Biotechnol. 2021;35:102103. doi: 10.1016/j.bcab.2021.102103. [DOI] [Google Scholar]
- 60.Hadfi N., Sarbon N. Physicochemical properties of silver catfish (Pangasius sp.) skin collagen as influenced by acetic acid concentration. Food Res. 2019;3:783–790. doi: 10.26656/fr.2017.3(6).130. [DOI] [Google Scholar]
- 61.Baderi N.A., Sarbon N. Microstructure, extractability and physicochemical properties of shortfin scad (Decapterus macrosoma) bone collagen as influenced by acetic acid concentration. Int. Food Res. J. 2019;26:451–458. [Google Scholar]
- 62.Seixas M.J., Martins E., Reis R.L., Silva T.H. Extraction and characterization of collagen from elasmobranch byproducts for potential biomaterial use. Mar. Drugs. 2020;18:617–635. doi: 10.3390/md18120617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka T., Takahashi K., Tsubaki K., Hirata M., Yamamoto K., Biswas A., Moriyama T., Kawamura Y. Isolation and characterization of acid-soluble bluefin tuna (Thunnus orientalis) skin collagen. Fish. Aquat. Sci. 2018;21:7–15. doi: 10.1186/s41240-018-0084-1. [DOI] [Google Scholar]
- 64.Tan Y., Chang S.K.C. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018;242:147–155. doi: 10.1016/j.foodchem.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 65.Venkatesan J., Anil S., Kim S.K., Shim M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs. 2017;15:143–154. doi: 10.3390/md15050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao W.H., Chi C.F., Zhao Y.Q., Wang B. Preparation, Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Swim Bladders of Miiuy Croaker (Miichthys miiuy) Mar. Drugs. 2018;16:161–180. doi: 10.3390/md16050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castaneda-Valbuena D., Berenguer-Murcia A., Fernandez-Lafuente R., Morellon-Sterling R., Tacias-Pascacio V.G. Biological activities of peptides obtained by pepsin hydrolysis of fishery products. Process. Biochem. 2022;120:53–63. doi: 10.1016/j.procbio.2022.05.029. [DOI] [Google Scholar]
- 68.Asaduzzaman A.K.M., Getachew A.T., Cho Y.J., Park J.S., Haq M., Chun B.S. Characterization of pepsin-solubilised collagen recovered from mackerel (Scomber japonicus) bone and skin using subcritical water hydrolysis. Int. J. Biol. Macromol. 2020;148:1290–1297. doi: 10.1016/j.ijbiomac.2019.10.104. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J., Duan R. Characterisation of acid-soluble and pepsin-solubilised collagen from frog (Rana nigromaculata) skin. Int. J. Biol. Macromol. 2017;101:638–642. doi: 10.1016/j.ijbiomac.2017.03.143. [DOI] [PubMed] [Google Scholar]
- 70.Sun B., Li C., Mao Y., Qiao Z., Jia R., Huang T., Yang W. Distinctive characteristics of collagen and gelatin extracted from Dosidicus gigas skin. Int. J. Food Sci. Technol. 2021;56:3443–3454. doi: 10.1111/ijfs.14968. [DOI] [Google Scholar]
- 71.Zou Y., Wang L., Cai P., Li P., Zhang M., Sun Z., Sun C., Xu W., Wang D. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. Int. J. Biol. Macromol. 2017;105:1602–1610. doi: 10.1016/j.ijbiomac.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Ali A.M.M., Kishimura H., Benjakul S. Extraction efficiency and characteristics of acid and pepsin soluble collagens from the skin of golden carp (Probarbus jullieni) as affected by ultrasonication. Process. Biochem. 2018;66:237–244. doi: 10.1016/j.procbio.2018.01.003. [DOI] [Google Scholar]
- 73.Petcharat T., Benjakul S., Karnjanapratum S., Nalinanon S. Ultrasound-assisted extraction of collagen from clown featherback (Chitala ornata) skin: Yield and molecular characteristics. J. Sci. Food Agric. 2021;101:648–658. doi: 10.1002/jsfa.10677. [DOI] [PubMed] [Google Scholar]
- 74.Pezeshk S., Rezaei M., Abdollahi M. Impact of ultrasound on extractability of native collagen from tuna by-product and its ultrastructure and physicochemical attributes. Ultrason. Sonochem. 2022;89:106129. doi: 10.1016/j.ultsonch.2022.106129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matarsim N.N., Jaziri A.A., Shapawi R., Mokhtar R.A.M., Noordin W.N.M., Huda N. Type I collagen from the skin of Barracuda (Sphyraena sp.) prepared with different organic acids: Biochemical, microstructural and functional properties. J. Funct. Biomater. 2023;14:87. doi: 10.3390/jfb14020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yemisken E., Jim’enez-Rosado M., Perez-Puyana V., Sancar S., Bektas S., Yildiz T., Eryilmaz L., Romero A. Alternative sources of marine bioactive compounds from the Black Sea: Isolation and characterization of fish skin collagen from Neogobius melanostomus (Pallas 1814) (Perciformes: Gobiidae) Reg. Stud. Mar. Sci. 2023;60:102887. doi: 10.1016/j.rsma.2023.102887. [DOI] [Google Scholar]
- 77.Shaik M.I., Md Nor I.N., Sarbon N.M. Effect of extraction time on the extractability and physicochemical properties of pepsin—Soluble collagen (PCS) from the skin of silver catfish (Pangasius sp.) Gels. 2023;9:300–314. doi: 10.3390/gels9040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fatiroi N.S., Jaziri A.A., Shapawi R., Mokhtar R.A.M., Noordin W.N.M., Huda N. Biochemical and microstructural characteristics of collagen biopolymer from unicornfish (Naso reticulatus Randall, 2001) bone prepared with various acid types. Polymers. 2023;15:1054–1070. doi: 10.3390/polym15041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaziri A.A., Shapawi R., Mokhtar R.A.M., Noordin W.N.M., Huda N. Extraction and characterization of type I collagen from parrotfish (Scarus sordidus Forsskål, 1775) scale solubilized with the aid of acetic acid and pepsin. Int. J. Biomater. 2023;2023:7312447. doi: 10.1155/2023/7312447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mo C., Wang Q., Li G., Dong W., Liang F., Wu C., Wang Z., Wang Y. Extraction and characterization of pepsin-and acid-soluble collagen from the swim bladders of Megalonibea fusca. Mar. Drugs. 2023;21:159–174. doi: 10.3390/md21030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cruz-Lopez H., Rodríguez-Morales S., Enríquez-Paredes L.M., Villarreal-Gomez L.J., True C., Olivera-Castillo L., Fernandez-Velasco D.A., Lopez L.M. Swim bladder of farmed Totoaba macdonaldi: A source of value-added collagen. Mar. Drugs. 2023;21:173–187. doi: 10.3390/md21030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H., Tian J., Cao H., Tang Y., Huang F., Yang Z. Preparation of enzymesoluble swim bladder collagen from Sea Eel (Muraenesox cinereus) and evaluation its wound healing capacity. Mar. Drugs. 2023;21:525–543. doi: 10.3390/md21100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan Z., Ge B., Wei M., Elango J., Wu W. Isolation and biochemical properties of type II collagen from blue shark (Prionace glauca) cartilage. Mar. Drugs. 2023;21:260–276. doi: 10.3390/md21050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaziri A.A., Shapawi R., Mohd-Mokhtar R.A., Noordin W.N.M., Huda N. Biochemical analysis of collagens from the bone of lizardfish (Saurida tumbil Bloch, 1795) extracted with different acids. PeerJ. 2022;10:e13103. doi: 10.7717/peerj.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chanmangkang S., Wangtueai S., Pansawat N., Tepwong P., Panya A., Maneerote J. Characteristics and properties of acid-and pepsin-solubilized collagens from the tail tendon of Skipjack Tuna (Katsuwonus pelamis) Polymers. 2022;14:5329–5348. doi: 10.3390/polym14235329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong Y., Dai Z. Physicochemical, structural and antioxidant properties of collagens from the swim bladder of four fish species. Mar. Drugs. 2022;20:550–564. doi: 10.3390/md20090550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martins E., Fernandes R., Alves A.L., Sousa R.O., Reis R.L., Silva T.H. Skin byproducts of Reinhardtius hippoglossoides (Greenland Halibut) as ecosustainable source of marine collagen. Appl. Sci. 2022;12:11282. doi: 10.3390/app122111282. [DOI] [Google Scholar]
- 88.Abbas A.A., Shakir K.A., Walsh M.K. Functional properties of collagen extracted from catfish (Silurus triostegus) waste. Foods. 2022;11:633–647. doi: 10.3390/foods11050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tziveleka L.A., Kikionis S., Karkatzoulis L., Bethanis K., Roussis V., Ioannou E. Valorization of fish waste: Isolation and characterization of acid-and pepsin-soluble collagen from the scales of mediterranean fish and fabrication of collagen-based nanofibrous scaffolds. Mar. Drugs. 2022;20:664–680. doi: 10.3390/md20110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atef M., Ojagh S.M., Latifi A.M., Esmaeili M., Udenigwe C.C. Biochemical and structural characterization of sturgeon fish skin collagen (Huso huso) J. Food Biochem. 2020;44:e13256. doi: 10.1111/jfbc.13256. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Z., Wang Y.-M., Qiu Y.-T., Chi C.-F., Luo H.-Y., Wang B. Gelatin From Cartilage of Siberian Sturgeon (Acipenser baerii) on Ultraviolet-A Injured Human Skin Fibroblasts. Front. Mar. Sci. 2022;9:925407. doi: 10.3389/fmars.2022.925407. [DOI] [Google Scholar]
- 92.Chen Y., Jin H., Yang F., Jin S., Liu C., Zhang L., Huang J., Wang S., Yan Z., Cai X., et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol. 2019;138:483–491. doi: 10.1016/j.ijbiomac.2019.07.111. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed R., Haq M., Chun B.S. Characterization of marine derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus) Int. J. Biol. Macromol. 2019;135:668–676. doi: 10.1016/j.ijbiomac.2019.05.213. [DOI] [PubMed] [Google Scholar]
- 94.Kittiphattanabawon P., Sriket C., Kishimura H., Benjakul S. Characteristics of Acid and Pepsin Solubilized Collagens from Nile Tilapia (Oreochromis niloticus) Scale. Emir. J. Food and Agric. 2019;31:95–101. doi: 10.9755/ejfa.2019.v31.i2.1911. [DOI] [Google Scholar]
- 95.Hukmi N.M.M., Sarbon N.M. Isolation and characterization of acid soluble collagen (ASC) and pepsin soluble collagen (PSC) extracted from silver catfish (Pangasius sp.) skin. Int. Food Res. J. 2018;25:1785–1791. [Google Scholar]
- 96.Iskandar J., Rizal A. Characteristics of Physical-Chemical Properties of Collagen Extracted from the Skin of Bonylip Barb Fish (Osteochilus vittatus) World Appl. Sci. J. 2018;36:78–84. [Google Scholar]
- 97.Changfeng C., Wang B., Zhong-rui L., Hong-Yu L., Guo-Fang D. Characterization of acid-soluble collagens from the cartilages of scalloped hammerhead (Sphyrna lewini), red stingray (Dasyatis akajei), and skate (Raja porosa) Food Sci. Biotechnol. 2013;22:909–916. doi: 10.1007/s10068-013-0163-0. [DOI] [Google Scholar]
- 98.Li Z.-R., Wang B., Chi C.-F., Zhang Q.-H., Gong Y.-D., Tang J.-J., Luo H.-Y., Ding G.-F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius) Food Hydrocoll. 2013;31:103–113. doi: 10.1016/j.foodhyd.2012.10.001. [DOI] [Google Scholar]
- 99.Hu Y.-D., Xi Q.-H., Kong J., Zhao Y.-Q., Chi C.-F., Wang B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from the Collagens of Monkfish (Lophius litulon) Swim Bladders: Isolation, Characterization, Molecular Docking Analysis and Activity Evaluation. Mar. Drugs. 2023;21:516–534. doi: 10.3390/md21100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li L.-Y., Zhao Y.-Q., He Y., Chi C.-F., Wang B. Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Scales of Miiuy Croaker (Miichthys miiuy) Mar. Drugs. 2018;16:394–413. doi: 10.3390/md16100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nurilmala M., Hizbullah H.H., Karnia E., Kusumaningtyas E., Ochiai Y. Characterization and Antioxidant Activity of Collagen, Gelatin, and the Derived Peptides from Yellowfin Tuna (Thunnus albacares) Skin. Mar. Drugs. 2020;18:98–110. doi: 10.3390/md18020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nurilmala M., Pertiwi R.M., Nurhayati T., Fauzi S., Batubara I., Ochiai Y. Characterization of collagen and its hydrolysate from yellowfin tuna Thunnus albacares skin and their potencies as antioxidant and antiglycation agents. Fish. Sci. 2019;85:591–599. doi: 10.1007/s12562-019-01303-5. [DOI] [Google Scholar]
- 103.Cherim M., Sirbu R., Tomescu A., Popa M.F., Cadar E. Comparative Studies on the Physico-chemical Characteristics of Bio-materials with Collagen from Calf and Fish Skins from Black Sea. Mater. Plast. 2019;56:179–185. doi: 10.37358/MP.19.1.5147. [DOI] [Google Scholar]
- 104.Cherim M., Stanciu G., Rasit E.Y., Cadar E. Capitalization of marine resources from black sea by obtaining and characterizing collagen from Grey Mullet. J. Sci. Arts. 2017;4:795–802. [Google Scholar]
- 105.Tassara E., Orel B., Ilan M., Cavallo D., Dodero A., Castellano M., Vicini S., Giovine M., Pozzolini M. Seasonal molecular difference in fibrillar collagen extracts derived from the marine sponge Chondrosia reniformis (Nardo, 1847) and their impact on its derived biomaterials. Mar. Drugs. 2023;21:210–229. doi: 10.3390/md21040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Araújo T.A.T., de Souza A., Santana A.F., Braga A.R.C., Custódio M.R., Simões F.R., Araújo G.M., Miranda A., Alves F., Granito R.N. Comparison of Different Methods for Spongin-like Collagen Extraction from Marine Sponges (Chondrilla caribensis and Aplysina fulva): Physicochemical Properties and In Vitro Biological Analysis. Membranes. 2021;11:522–538. doi: 10.3390/membranes11070522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pozzolini M., Scarfì S., Gallus L., Castellano M., Vicini S., Cortese K., Gagliani M.C., Bertolino M., Costa G., Giovine M. Production, characterization and biocompatibility evaluation of collagen membranes derived from marine sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs. 2018;16:111–141. doi: 10.3390/md16040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fernandes K.R., Parisi J.R., Cruz M.A., Gabbai-Armelin P.R., Tashiro de Araújo T.A. Characterization and Biological Performance of Marine Sponge Collagen. Braz. Arch. Biol. Technol. 2021;64:e21200592. doi: 10.1590/1678-4324-2021200592. [DOI] [Google Scholar]
- 109.Parisi J., Fernandes K., Avanzi I., Dorileo B., Santana A., Andrade A., Gabbai-Armelin P., Fortulan C., Trichês E., Granito R. Incorporation of collagen from marine sponges (spongin) into hydroxyapatite samples: Characterization and in vitro biological evaluation. Mar. Biotechnol. 2019;21:30–37. doi: 10.1007/s10126-018-9855-z. [DOI] [PubMed] [Google Scholar]
- 110.Langasco R., Cadeddu B., Formato M., Lepedda A.J., Cossu M., Giunchedi P., Pronzato R., Rassu G., Manconi R., Gavini E. Natural collagenic skeleton of marine sponges in pharmaceutics: Innovative biomaterial for topical drug delivery. Mater. Sci. Eng. C. 2017;70:710–720. doi: 10.1016/j.msec.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 111.Vate N.K., Strachowski P., Undeland I., Abdollahi M. Structural and functional properties of collagen isolated from lumpfish and starfish using isoelectric precipitation vs. salting out. Food Chem. X. 2023;18:100646. doi: 10.1016/j.fochx.2023.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han S.B., Won B., Yang S.C., Kim D.H. Asterias pectinifera derived collagen peptide-encapsulating elastic nanoliposomes for the cosmetic application. J. Ind. Eng. Chem. 2021;98:289–297. doi: 10.1016/j.jiec.2021.03.039. [DOI] [Google Scholar]
- 113.Li P.H., Lu W.C., Chan Y.J., Ko W.C., Jung C.C., Le Huynh D.T., Ji Y.X. Extraction and characterization of collagen from sea cucumber (Holothuria cinerascens) and its potential application in moisturizing cosmetics. Aquaculture. 2020;515:734590. doi: 10.1016/j.aquaculture.2019.734590. [DOI] [Google Scholar]
- 114.Tian M., Xue C., Chang Y., Shen J., Zhang Y., Li Z., Wang Y. Collagen fibrils of sea cucumber (Apostichopus Japonicus) are heterotypic. Food Chem. 2020;316:126272. doi: 10.1016/j.foodchem.2020.126272. [DOI] [PubMed] [Google Scholar]
- 115.Esparza-Espinoza D.M., del Carmen Santacruz-Ortega H., Plascencia-Jatomea M., Aubourg S.P., Salazar-Leyva J.A., Rodríguez-Felix F., Ezquerra-Brauer J.M. Chemical-Structural Identification of Crude Gelatin from Jellyfish (Stomolophus meleagris) and Evaluation of Its Potential Biological Activity. Fishes. 2023;8:246–261. doi: 10.3390/fishes8050246. [DOI] [Google Scholar]
- 116.Felician F.F., Yu R.H., Li M.Z., Li C.J., Chen H.Q., Jiang Y., Tang T., Qi W.Y., Xu H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019;22:12–20. doi: 10.1016/j.cjtee.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rastian Z., Pütz S., Wang Y., Kumar S., Fleissner F., Weidner T., Parekh S. Type I Collagen from Jellyfish Catostylus mosaicus for Biomaterial Applications. ACS Biomater. Sci. Eng. 2018;4:2115–2125. doi: 10.1021/acsbiomaterials.7b00979. [DOI] [PubMed] [Google Scholar]
- 118.Khong N.M., Yusoff F.M., Jamilah B., Basri M., Maznah I., Chan K.W. Improved collagen extraction from jellyfish (Acromitus hardenbergi) with increased physical-induced solubilization processes. Food Chem. 2018;251:41–50. doi: 10.1016/j.foodchem.2017.12.083. [DOI] [PubMed] [Google Scholar]
- 119.Cheng X., Shao Z., Li C., Yu L., Raja M.A., Liu C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum Kishinouye for use in hemostatic applications. PLoS ONE. 2017;12:e0169731. doi: 10.1371/journal.pone.0169731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.CunhaNeves A., Harnedy-Rothwell P.A., FitzGerald R.J. In vitro angiotensin-converting enzyme and dipeptidyl peptidase-IV inhibitory, and antioxidant activity of blue mussel (Mytilus edulis) byssus collagen hydrolysates. Eur. Food Res. Technol. 2022;248:1721–1732. doi: 10.1007/s00217-022-04000-3. [DOI] [Google Scholar]
- 121.Rodríguez F., Moran L., Gonzalez G., Troncoso E., Zúniga R.N. Collagen extraction from mussel byssus: A new marine collagen source with physicochemical properties of industrial interest. J. Food Sci. Technol. 2017;54:1228–1238. doi: 10.1007/s13197-017-2566-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hiransuchalert R., Oonwiset N., Imarom Y., Chindudsadeegul P., Laongmanee P., Arnupapboon S. Extraction and characterization of pepsin-soluble collagen from different mantis shrimp species. Fish. Aquat. Sci. 2021;24:406–414. doi: 10.47853/FAS.2021.e42. [DOI] [Google Scholar]
- 123.Wu J., Guo X., Liu H., Chen L. Isolation and comparative study on the characterization of guanidine hydrochloride soluble collagen and pepsin soluble collagen from the body of surf clam shell (Coelomactra antiquata) Foods. 2019;8:11. doi: 10.3390/foods8010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ezquerra-Brauer J.M., Márquez-Ríos E., López-Corona B.E., Ocaño-Higuera V.M., Ramírez-Guerra H.E., Cota-Arriola O. Physicochemical changes of pepsin-solubilized and insoluble collagen in jumbo squid (Dosidicus gigas) muscle after cooking process. Int. J. Food Prop. 2018;21:821–834. doi: 10.1080/10942912.2018.1477159. [DOI] [Google Scholar]
- 125.Meyer M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online. 2019;18:24–98. doi: 10.1186/s12938-019-0647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ryu B., Shin K.H., Kim S.K. Muscle Protein Hydrolysates and Amino Acid Composition in Fish. Mar. Drugs. 2021;19:377–389. doi: 10.3390/md19070377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yathisha U.G., Bhat I., Karunasagar I., Mamatha B.S. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2018;59:2363–2374. doi: 10.1080/10408398.2018.1452182. [DOI] [PubMed] [Google Scholar]
- 128.Zhang X., Wang J., Zhang Q., Fan Y., Zhang H., Ahmad K., Hou H. Distribution, Typical Structure and Self-Assembly Properties of Collagen from Fish Skin and Bone. Molecules. 2023;28:6529–6543. doi: 10.3390/molecules28186529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al-Shaer A., Lyons A., Ishikawa Y., Hudson B.G., Boudko S.P., Forde N.R. Sequence-dependent Mechanics of Collagen Reflect its Structural and Functional Organization. Biophys. J. 2021;120:4013–4028. doi: 10.1016/j.bpj.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu L., Li J., Wang Y., Sun X., Li B., Poungchawanwong S., Hou H. Structural Feature and Self-assembly Properties of Type II Collagens from the Cartilages of Skate and Sturgeon. Food Chem. 2020;331:127340. doi: 10.1016/j.foodchem.2020.127340. [DOI] [PubMed] [Google Scholar]
- 131.Romijn E.I., Finnøy A., Lilledahl M.B. Analyzing the Feasibility of Discriminating between Collagen Types I and II using Polarization-resolved Second Harmonic Generation. J. Biophotonics. 2019;12:e201800090. doi: 10.1002/jbio.201800090. [DOI] [PubMed] [Google Scholar]
- 132.Hu L., Zhang H., Hu Z., Chin Y., Zhang X., Chen J., Liu D., Hu Y. Comparative Proteomics Analysis of Three Commercial Tuna Species through SWATH-MS based Mass Spectrometry and Chemometrics. Food Control. 2022;141:109162. doi: 10.1016/j.foodcont.2022.109162. [DOI] [Google Scholar]
- 133.Hernández-Ruiz K.L., López-Cervantes J., Sánchez-Machado D.I., Campas-Baypoli O.N., Quintero-Guerrero A.A., de Lourdes Grijalva-Delgado M., Chávez-Almanza A.F. Collagen Peptide Fractions from Tilapia (Oreochromis aureus Steindachner, 1864) Scales: Chemical Characterization and Biological Activity. Food Biosci. 2023;53:102658. doi: 10.1016/j.fbio.2023.102658. [DOI] [Google Scholar]
- 134.Blanco M., Vasquez J.A., Perez-Martin R.I., Sotelo C.G. Hydrolysates of Fish Skin Collagen: An Opportunity from Valorizing Fish Industry Byproducts. Mar. Drugs. 2017;15:131–146. doi: 10.3390/md15050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Je H.J., Han Y.K., Lee H.G., Bae I.Y. Anti-aging potential of fish collagen hydrolysates subjected to simulated gastrointestinal digestion and Caco-2 cell permeation. J. Appl. Biol. Chem. 2019;62:101–107. doi: 10.3839/jabc.2019.015. [DOI] [Google Scholar]
- 136.Garehgheshlagh N.S., Fatemi M.J., Jamili S., Sharifi A.M., Nourani M.R. Isolation and characterization of acid-soluble collagen from the skin of Rutilus Frisii Kutum (Kamensky) of the Caspian Sea. Iranian J. Fish. Sci. 2020;19:768–779. doi: 10.22092/ijfs.2019.118957. [DOI] [Google Scholar]
- 137.Thuy T.M.L., Nguyen V.M., Tran T.T., Takahashi K., Osako K. Comparison of acid-soluble collagen characteristic from three important freshwater fish skins in Mekong Delta Region, Vietnam. J. Food Biochem. 2020;44:e13397. doi: 10.1111/jfbc.13397. [DOI] [PubMed] [Google Scholar]
- 138.Truong T.M.T., Nguyen V.M., Tran T.T., Le T.M.T. Characterization of Acid-Soluble Collagen from Food Processing By-Products of Snakehead Fish (Channa striata) Processes. 2021;9:1188–1198. doi: 10.3390/pr9071188. [DOI] [Google Scholar]
- 139.Son S.A., Shin E.S., Park Y.M., Ma A., Yang H., Kim S.H., Shin T.S. Composition of Collagen Extracted from the Skin of Three Different Varieties of Fish. J. Korean Soc. Food Sci. Nutr. 2022;51:71–81. doi: 10.3746/jkfn.2022.51.1.71. [DOI] [Google Scholar]
- 140.Rýglova S., Braun M., Suchy T., Hribal M., Zaloudkov M., Vistejnova L. The investigation of batch-to-batch variabilities in the composition of isolates from fish and mammalian species using different protocols. Food Res. Int. 2023;169:112798. doi: 10.1016/j.foodres.2023.112798. [DOI] [PubMed] [Google Scholar]
- 141.Akita M., Nishikawa Y., Shigenobu Y., Ambe D., Morita T., Morioka K., Adachi K. Correlation of proline, hydroxyproline and serine content, denaturation temperature and circular dichroism analysis of type I collagen with the physiological temperature of marine teleosts. Food Chem. 2020;329:126775. doi: 10.1016/j.foodchem.2020.126775. [DOI] [PubMed] [Google Scholar]
- 142.Chinh N.T., Manh V.Q., Trung V.Q., Lam T.D., Huynh M.D., Tung N.Q., Trinh N.D., Hoang T. Characterization of Collagen Derived From Tropical Freshwater Carp Fish Scale Wastes and Its Amino Acid Sequence. Nat. Prod. Commun. 2019;14:1–12. doi: 10.1177/1934578X19866288. [DOI] [Google Scholar]
- 143.Merquiol L., Romano G., Ianora A., D’Ambra I. Biotechnological applications of scyphomedusae. Mar. Drugs. 2019;17:604–630. doi: 10.3390/md17110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aziz N.A.A., Salim N., Zarei M., Saari N., Yusoff F.M. Extraction, anti-tyrosinase, and antioxidant activities of the collagen hydrolysate derived from Rhopilema hispidum. Prep. Biochem. Biotechnol. 2020;51:44–53. doi: 10.1080/10826068.2020.1789991. [DOI] [PubMed] [Google Scholar]
- 145.Qiu L., Wang B., Zou S., Wang Q., Zhang L. Isolation and characterization of collagen from the jellyfish Nemopilema nomurai. J. Pharm. Pract. 2020;38:509–515. doi: 10.12206/j.issn.1006-0111.202008078. [DOI] [Google Scholar]
- 146.Pivnenko T.N., Kovalev A.N., Pozdnyakova Y.M., Esipenkoa R.V. The Composition of Collagen-Containing Preparations from Rhopilema asamushi Uchida Jellyfish and Assessment of the Safety of their External Use. Appl. Biochem. Microbiol. 2022;58:864–872. doi: 10.1134/S0003683822070043. [DOI] [Google Scholar]
- 147.James S., Tilvi S., Khandeparker R., Sreepada R.A., Thakur N., Gauthankar M. Jellyfish Rhizostoma pulmo collected off Goa Coast (India) as a rich source of tryptophan containing collagen and its enhanced antioxidant potential. J. Food Sci. Technol. 2023;60:2825–2834. doi: 10.1007/s13197-023-05800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sudirman S., Chen C.Y., Chen C.K., Felim J., Kuo H.P., Kong Z.L. Fermented jellyfish (Rhopilema esculentum) collagen enhances antioxidant activity and cartilage protection on surgically induced osteoarthritis in obese rats. Front. Pharmacol. 2023;14:1117893. doi: 10.3389/fphar.2023.1117893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chiarelli P.G., Chen J., Pegg R.B., Solval K.M. Demineralization enhances the physicochemical properties of hydrolyzed collagen powders derived from cannonball jellyfish (Stomolophus meleagris) Food Biosci. 2023;56:1103183. doi: 10.1016/j.fbio.2023.103183. [DOI] [Google Scholar]
- 150.Li N.G., Kalenik K. Amino-acid composition of soft tissues of bivalve mollusk Corbicula japonica. Chem. Nat. Compd. 2018;54:1031–1032. doi: 10.1007/s10600-018-2546-1. [DOI] [Google Scholar]
- 151.Li X., Han T., Zheng S., Wu G. Nutrition and Function of Amino Acids in Aquatic Crustaceans. In: Wu G., editor. Amino Acids in Nutrition and Health, Advances in Experimental Medicine and Biology. Volume 1285. Springer; Cham, Switzerland: 2021. pp. 169–189. [DOI] [PubMed] [Google Scholar]
- 152.Lima M.M., Vanier N.L., Dias A.R.G., Zavareze E., Prentice C., Moreira A.D. Whitemouth croaker (Micropogonias furnieri) protein hydrolysates: Chemical composition, molecular mass distribution, antioxidant activity and amino acid profile. Int. Food Res. J. 2019;26:247–254. [Google Scholar]
- 153.Suo S.K., Zhao Y.Q., Wang Y.M., Pan X.Y., Chi C.F., Wang B. Seventeen novel angiotensin converting enzyme (ACE) inhibitory peptides from the protein hydrolysate of Mytilus edulis: Isolation, identification, molecular docking study, and protective function on HUVECs. Food Funct. 2022;13:7831–7846. doi: 10.1039/D2FO00275B. [DOI] [PubMed] [Google Scholar]
- 154.Nikoo M., Regenstein J.M., Noori F., Piri G.S. Autolysis of rainbow trout (Oncorhynchus mykiss) by-products: Enzymatic activities, lipid and protein oxidation, and antioxidant activity of protein hydrolysates. LWT-Food Sci. Technol. 2021;140:110702. doi: 10.1016/j.lwt.2020.110702. [DOI] [Google Scholar]
- 155.Nirmal N.P., Santivarangkna C., Rajput M.S., Benjakul S., Maqsood S. Valorization of fish byproducts: Sources to end-product applications of bioactive protein hydrolysate. Compr. Rev. Food Sci. Food Saf. 2022;21:1803–1842. doi: 10.1111/1541-4337.12917. [DOI] [PubMed] [Google Scholar]
- 156.Chaoting W., Jixian Z., Haihui Z., Yuqing D., Haile M. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020;105:308–322. doi: 10.1016/j.tifs.2020.09.019. [DOI] [Google Scholar]
- 157.Sila A., Bougatef A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods. 2016;21:10–26. doi: 10.1016/j.jff.2015.11.007. [DOI] [Google Scholar]
- 158.Phadke G.G., Rathod N.B., Ozogul F., Elavarasan K., Karthikeyan M., Shin K.H., Kim S.K. Exploiting of secondary raw materials from fish processing industry as a source of bioactive peptide-rich protein hydrolysates. Mar. Drugs. 2021;19:480–502. doi: 10.3390/md19090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nirmal N.P., Rajput M.S., Rathod N.B., Mudgil P., Pati S., Bono G., Nalinanon S., Li L., Maqsood S. Structural characteristic and molecular docking simulation of fish protein-derived peptides: Recent updates on antioxidant, anti-hypertensive and anti-diabetic peptides. Food Chem. 2023;405:134737. doi: 10.1016/j.foodchem.2022.134737. [DOI] [PubMed] [Google Scholar]
- 160.Azizah N., Ochiai Y., Nurilmala M. Collagen peptides from Pangasius fish skin as antioxidants. Earth Environ. Sci. 2020;404:12055. doi: 10.1088/1755-1315/404/1/012055. [DOI] [Google Scholar]
- 161.Teng L., Wang X., Yu H., Li R., Geng H., Xing R., Liu S., Li P. Jellyfish Peptide as an Alternative Source of Antioxidant. Antioxidants. 2023;12:742–759. doi: 10.3390/antiox12030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ahmed M., Anand A., Verma A.K., Patel R. In-vitro self-assembly and antioxidant properties of collagen type I from Lutjanus erythropterus, and Pampus argenteus skin. Biocatal. Agric. Biotechnol. 2022;43:102412. doi: 10.1016/j.bcab.2022.102412. [DOI] [Google Scholar]
- 163.Heidari M.G., Rezaei M. Extracted pepsin of trout waste and ultrasound-promoted method for green recovery of fish collagen. Sustain. Chem. Pharm. 2022;30:100854. doi: 10.1016/j.scp.2022.100854. [DOI] [Google Scholar]
- 164.Sheng Y., Qiu Y.T., Wang Y.M., Chi C.F., Wang B. Novel Antioxidant Collagen Peptides of Siberian Sturgeon (Acipenser baerii) Cartilages: The Preparation, Characterization, and Cytoprotection of H2O2 Damaged Human Umbilical Vein Endothelial Cells (HUVECs) Mar. Drugs. 2022;20:325–345. doi: 10.3390/md20050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chotphruethipong L., Binlateh T., Hutamekalin P., Sukketsiri W., Aluko R.E., Benjakul S. In vitro antioxidant and wound-healing activities of hydrolyzed collagen from defatted Asian sea bass skin as influenced by different enzyme types and hydrolysis processes. RSC Adv. 2021;11:18144–18151. doi: 10.1039/D1RA03131G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Khesal M.A., Sharifan A., Hoseini E., Ghavami A. Optimization of Enzymatic Hydrolysis Conditions of Caspian kutum (Rutilus frisii kutum) By-product for Production of Bioactive Peptides with Antioxidative Properties. Int. J. Pept. Res. Therap. 2020;26:1829–1838. doi: 10.1007/s10989-019-09981-6. [DOI] [Google Scholar]
- 167.Zamorano-Apodaca J.C., García-Sifuentes C.O., Carvajal-Millán E., Vallejo-Galland B., Scheuren-Acevedo S.M., Lugo-Sánchez M.E. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chem. 2020;331:127350. doi: 10.1016/j.foodchem.2020.127350. [DOI] [PubMed] [Google Scholar]
- 168.Yang Q., Cai X., Yan A., Tian Y., Du M., Wang S. A specific antioxidant peptide: Its properties in controlling oxidation and possible action mechanism. Food Chem. 2020;327:126984. doi: 10.1016/j.foodchem.2020.126984. [DOI] [PubMed] [Google Scholar]
- 169.Zhang J.B., Zhao Y.Q., Wang Y.M., Chi C.F., Wang B. Eight Collagen Peptides from Hydrolysates Fraction of Spanish Mackerel Skins: Isolation, Identification, and In Vitro Antioxidant Activity Evaluation. Mar. Drugs. 2019;17:224–237. doi: 10.3390/md17040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lima K.O., Costa de Quadros C., Rocha M., Gomes de Lacerda J.T.J., Juliano M.A., Dias M., Mendes M.A., Prentice C. Bioactivity and bioaccesibility of protein hydrolyzates from industrial byproducts of Stripped weakfish (Cynoscion guatucupa) Food Sci. Technol. 2019;111:408–413. doi: 10.1016/j.lwt.2019.05.043. [DOI] [Google Scholar]
- 171.Tao J., Zhao Y.Q., Chi C.F., Wang B. Bioactive Peptides from Cartilage Protein Hydrolysate of Spotless Smoothhound and Their Antioxidant Activity In Vitro. Mar. Drugs. 2018;16:100. doi: 10.3390/md16040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ding D., Bowei D., Chao Z., Fakhar Z., Yaqin H. Isolation and identification of an antioxidant collagen peptide from skipjack tuna (Katsuwonus pelamis) bone. RSC Adv. 2019;9:27032–27041. doi: 10.1039/C9RA04665H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xia G., Zhang X., Dong Z., Shen X. Comparative Study on the Antioxidant Activity of Peptides from Pearl Oyster (Pinctada martensii) Mantle Type V Collagen and Tilapia (Oreochromis niloticus) Scale Type I Collagen. J. Ocean Univ. China. 2017;16:1175–1182. doi: 10.1007/s11802-017-3323-7. [DOI] [Google Scholar]
- 174.Muangrod P., Charoenchokpanich W., Roytrakul S., Rungsardthong V., Vatanyoopaisarn S., Charoenlappanit S., Wonganu B., Thumthanaruk B. Effect of pepsin on antioxidant and antibacterial activity of protein hydrolysate from salted jellyfish (Lobonema smithii and Rhopilema hispidum) by-products. E3S Web Conf. 2022;355:02013. doi: 10.1051/e3sconf/202235502013. [DOI] [Google Scholar]
- 175.Upata M., Siriwoharn T., Makkhun S., Yarnpakdee S., Regenstein J.M., Wangtueai S. Tyrosinase Inhibitory and Antioxidant Activity of Enzymatic Protein Hydrolysate from Jellyfish (Lobonema smithii) Foods. 2022;11:615–633. doi: 10.3390/foods11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang X.R., Qiu Y.T., Zhao Y.Q., Chi C.F., Wang B. Purification and Characterization of Antioxidant Peptides Derived from Protein Hydrolysate of the Marine Bivalve Mollusk Tergillarca granosa. Mar. Drugs. 2019;17:251–267. doi: 10.3390/md17050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Domenico S., De Rinaldis G., Paulmery M., Piraino S., Leone A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs. 2019;17:134–155. doi: 10.3390/md17020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen Y.P., Liang C.H., Wu H.T., Pang H.Y., Chen C., Wang G.H., Chan L.P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. 2018;55:2310–2317. doi: 10.1007/s13197-018-3148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Najafian L., Babji A.S. Purification and Identification of Antioxidant Peptides from Fermented Fish Sauce (Budu) Purification and Identification of Antioxidant Peptides From. J. Aquat. Food Prod. Technol. 2018;28:14–24. doi: 10.1080/10498850.2018.1559903. [DOI] [Google Scholar]
- 180.Aissaoui N., Abidi F., Hardouin J., Abdelkafi Z., Marrakchi N., Jouenne T., Marzouki M.N. Two novel peptides with angiotensin I converting enzyme inhibitory and antioxidative activities from Scorpaena notata muscle protein hydrolysate. Biotechnol. Appl. Biochem. 2017;64:201–210. doi: 10.1002/bab.1478. [DOI] [PubMed] [Google Scholar]
- 181.Huang J.J., Li H.I., Xiong G.G., Cai J., Liao T., Zu X.Y. Extraction, identification and anti-photoaging activity evaluation of collagen peptides from silver carp (Hypophthalmichthys molitrix) skin. Food. Sci. Technol. 2023;173:114384. doi: 10.1016/j.lwt.2022.114384. [DOI] [Google Scholar]
- 182.Herawati E., Akhsanitaqwim Y., Agnesia P., Listyawati S., Pangastuti A., Ratriyanto A. In Vitro Antioxidant and Antiaging Activities of Collagen and Its Hydrolysate from Mackerel Scad Skin (Decapterus macarellus) Mar. Drugs. 2022;20:516–529. doi: 10.3390/md20080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ayat A.A., Shakir K.A. Functional Properties of Catfish Skin Collagen Hydrolysates. Iraqi J. Agric. Sci. 2021;52:1528–1540. doi: 10.36103/ijas.v52i6.1494. [DOI] [Google Scholar]
- 184.Nur S., Wierson Y., Yulia Y., Sami J.F., Megawati M., Andi N.A., Marwati M., Gani S.A. Characterization, antioxidant and α-Glucosidase inhibitory activity of Collagen Hydrolysate from Lamuru (Caranx ignobilis) Fishbone. Sains Malays. 2021;50:2329–2341. doi: 10.17576/jsm-2021-5008-16. [DOI] [Google Scholar]
- 185.Luo J., Yao X., Soladoye O.P., Zhang Y., Fu Y. Phosphorylation modification of collagen peptides from fish bone enhances their calcium-chelating and antioxidant activity. Food Sci. Technol. 2022;155:112978. doi: 10.1016/j.lwt.2021.112978. [DOI] [Google Scholar]
- 186.González-Serrano D.J., Hadidi M., Varcheh M., Jelyani A.Z., Moreno A., Lorenzo J.M. Bioactive Peptide Fractions from Collagen Hydrolysate of Common Carp Fish Byproduct: Antioxidant and Functional Properties. Antioxidants. 2022;11:509–520. doi: 10.3390/antiox11030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee J.E., Noh S.-K., Kim M.J. Effects of Enzymatic- and Ultrasound-Assisted Extraction on Physicochemical and Antioxidant Properties of Collagen Hydrolysate Fractions from Alaska Pollack (Theragra chalcogramma) Skin. Antioxidants. 2022;11:2112–2124. doi: 10.3390/antiox11112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Viji P., Phannendra T.S., Jesmi D., Rao B.M., Das P.H.D., George N. Functional and Antioxidant Properties of Gelatin Hydrolysates Prepared from Skin and Scale of Sole Fish. J. Aquat. Food Prod. Technol. 2019;28:976–986. doi: 10.1080/10498850.2019.1672845. [DOI] [Google Scholar]
- 189.Sae-leaw T., Benjakul S. Antioxidant activities of hydrolysed collagen from salmon scale ossein prepared with the aid of ultrasound. Int. J. Food Sci. Technol. 2018;53:2786–2795. doi: 10.1111/ijfs.13891. [DOI] [Google Scholar]
- 190.Jin H.X., Xu H.P., Li Y., Zhang Q.W., Xie H. Preparation and Evaluation of Peptides with Potential Antioxidant Activity by Microwave Assisted Enzymatic Hydrolysis of Collagen from Sea Cucumber Acaudina Molpadioides Obtained from Zhejiang Province in China. Mar. Drugs. 2019;17:169–183. doi: 10.3390/md17030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Islam M.R., Li W., Ogata Y., Yoshioka T., Ura K., Yasuaki T. Production and antioxidant activity of peptides from sturgeon head. Sustain. Chem. Pharm. 2023;31:100944. doi: 10.1016/j.scp.2022.100944. [DOI] [Google Scholar]
- 192.Chotphruethipong L., Binlateh T., Hutamekalin P., Sukketsiri W., Aluko R.E., Benjakul S. Hydrolyzed collagen from defatted sea bass skin and its conjugate with epigallocatechin gallate: In vitro antioxidant, anti-inflammatory, wound-healing and anti-obesity activities. Food Biosci. 2021;43:101303. doi: 10.1016/j.fbio.2021.101303. [DOI] [Google Scholar]
- 193.Iosageanu A., Ilie D., Craciunescu O., Seciu-Grama A.-M., Oancea A., Zarnescu O., Moraru I., Oancea F. Effect of Fish Bone Bioactive Peptides on Oxidative, Inflammatory and Pigmentation Processes Triggered by UVB Irradiation in Skin Cells. Molecules. 2021;26:2691–2714. doi: 10.3390/molecules26092691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bordbar S., Chay S.Y., Ebrahimpour A., Zarei M., Saari N. Profiling of antioxidative proteolysate enzymatically hydrolysed from stone fish (Actinopyga lecanora) Int. Food Res. J. 2021;28:848–859. doi: 10.47836/ifrj.28.4.21. [DOI] [Google Scholar]
- 195.Qiu Y.T., Wang Y.M., Yang X.R., Zhao Y.Q., Chi C.F., Wang B. Gelatin and antioxidant peptides from gelatin hydrolysate of skipjack tuna (Katsuwonus pelamis) scales: Preparation, Identification and activity evaluation. Mar. Drugs. 2019;17:565–572. doi: 10.3390/md17100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang S.Y., Zhao Y.Q., Wang Y.M., Yang X.R., Chi C.F., Wang B. Gelatins and antioxidant peptides from Skipjack tuna (Katsuwonus pelamis) skins: Purification, characterization, and cytoprotection on ultraviolet-A injured human skin fibroblasts. Food Biosci. 2022;50:102138. doi: 10.1016/j.fbio.2022.102138. [DOI] [Google Scholar]
- 197.Wang W.Y., Zhao Y.Q., Zhao G.X., Chi C.F., Wang B. Antioxidant Peptides from Collagen Hydrolysate of Redlip Croaker (Pseudosciaena polyactis) Scales: Preparation, Characterization, and Cytoprotective Effects on H2O2-Damaged HepG2 Cells. Mar. Drugs. 2020;18:156–175. doi: 10.3390/md18030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chel-Guerrero L., Estrella-Millán Y., Betancur-Ancona D., Aranda-González I., Castellanos-Ruelas A., Gallegos-Tintoré S. Antioxidant, chelating, and angiotensin-converting enzyme inhibitory activities of peptide fractions from red lionfish (Pterois volitans L.) muscle protein hydrolysates. Int. Food Res. J. 2020;27:224–233. [Google Scholar]
- 199.Zhao W.H., Luo Q.B., Pan X., Chi C.F., Sun K.L., Wang B. Preparation, identification, and activity evaluation of ten antioxidant peptides from protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy) J. Funct. Foods. 2018;47:503–511. doi: 10.1016/j.jff.2018.06.014. [DOI] [Google Scholar]
- 200.Devita L., Nurilmala M., Lioe H.N., Suhartono M.T. Chemical and Antioxidant Characteristics of Skin-Derived Collagen Obtained by Acid-Enzymatic Hydrolysis of Bigeye Tuna (Thunnus obesus) Mar. Drugs. 2021;19:222–241. doi: 10.3390/md19040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li W., Kobayashi T., Meng D., Miyamoto N., Tsutsumi N., Ura K., Takagi Y. Free radical scavenging activity of type II collagen peptides and chondroitin sulfate oligosaccharides from by-products of mottled skate processing. Food Biosci. 2021;41:100991. doi: 10.1016/j.fbio.2021.100991. [DOI] [Google Scholar]
- 202.Sripokar P., Benjakulb S., Klomklaoc S. Antioxidant and functional properties of protein hydrolysates obtained from starry triggerfish muscle using trypsin from albacore tuna liver. Biocatal. Agric. Biotechnol. 2019;17:447–454. doi: 10.1016/j.bcab.2018.12.013. [DOI] [Google Scholar]
- 203.Zheng J., Tian X., Xu B., Yuan F., Gong J., Yang Z. Collagen peptides from swim bladders of giant croaker (Nibea japonica) and their protective effects against H2O2 -induced oxidative damage toward human umbilical vein endothelial cells. Mar. Drugs. 2020;18:430. doi: 10.3390/md18080430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Baehaki A., Widiastuti I., Nainggolan C., Gofar N. Antioxidant activities of snakehead (Channa striata) fish skin: Peptides hydrolysis using protease TP2 isolate from swamp plant silage. Potravinarstvo Slovak J. Food Sci. 2020;14:379–384. doi: 10.5219/1264. [DOI] [Google Scholar]
- 205.Kusumaningtyas E., Nurilmala M., Sibarani D. Antioxidant and antifungal activities of collagen hydrolysates from skin of milkfish (Chanos chanos) hydrolyzed using various bacillus proteases. Earth Environ. Sci. 2019;278:012040. doi: 10.1088/1755-1315/278/1/012040. [DOI] [Google Scholar]
- 206.Wu R.B., Wu C.L., Liu D., Yang X.H., Huang J.F., Zhang J., Liao B., He H.L. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018;248:346–352. doi: 10.1016/j.foodchem.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 207.Vieira E., Ferreira I.M.P.L.V.O. Antioxidant and antihypertensive hydrolysates obtained from by-products of cannery sardine and brewing industries. Int. J. Food Prop. 2017;20:662–673. doi: 10.1080/10942912.2016.1176036. [DOI] [Google Scholar]
- 208.Santhanam R., Ramesh S., Nivedhitha S., Balasundari S. Pharmaceuticals and Nutraceuticals from fish and Their Activities. In: Santhanam R., Ramesh S., Nivedhitha S., Balasundari S., editors. Pharmaceuticals and Nutraceuticals from Fish and Fish Wastes. 1st ed. Taylor & Francis Group; London, UK: 2022. pp. 7–14. [DOI] [Google Scholar]
- 209.Jantaratch N., Worawattanamateekul W., Hinsui J. Antioxidant Properties of Tilapia Skin Collagen Peptides. Jantaratch Worawattanamateekul Hinsui. 2022;8:102–111. [Google Scholar]
- 210.Rashid N.Y.A., Musaalbakri A.M., Khairul F.P., Nazamid S., Fadzlie W.F.W. Evaluation of antioxidant and antibacterial activities of fish protein hydrolysate produced from Malaysian fish sausage (Keropok Lekor) by-products by indigenous Lactobacillus casei fermentation. J. Clean. Prod. 2022;347 doi: 10.1016/j.jclepro.2022.131303. [DOI] [Google Scholar]
- 211.Dara P.K., Elavarasan K., Shamasundar B.A. Characterization of antioxidant and surface-active properties of gelatin hydrolysates obtained from croaker fish skin. Int. Aqua. Res. 2020;12:116–126. doi: 10.22034/IAR(20).2020.1892203.1006. [DOI] [Google Scholar]
- 212.Ahmed R., Chun B.S. Subcritical water hydrolysis for the production of bioactive peptides from tuna skin collagen. J. Supercrit. Fluids. 2018;141:88–96. doi: 10.1016/j.supflu.2018.03.006. [DOI] [Google Scholar]
- 213.Franco D., Munekata P.E.S., Agregan R., Bermidez R., Lopez-Pedrouso M., Pateiro M., Lorenzo J.M. Application of Pulsed Electric Fields for Obtaining Antioxidant Extracts from Fish Residues. Antioxidants. 2020;9:90. doi: 10.3390/antiox9020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yanshole V.Y., Yanshole L.V., Zelentsova E.A., Tsentalovich Y.P. Ovothiol A is the Main Antioxidant in Fish Lens. Metabolites. 2019;9:95. doi: 10.3390/metabo9050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ganesan A.R., Mohanram M.S.G., Balasubramanian B., Kim I.H., Seedevi P., Mohan K., Kanagasabai S., Arasu M.V.A., Al-Dhabi N.A., Ignacimuthu S. Marine invertebrates’ proteins: A recent update on functional property. J. King Saud Univ. Sci. 2020;32:1496–1502. doi: 10.1016/j.jksus.2019.12.003. [DOI] [Google Scholar]
- 216.Newswire G. Global Functional Proteins Market Is Expected to Reach USD 7.98 Billion by 2026: Fior Markets. 2019. [(accessed on 23 July 2024)]. Available online: https://www.globenewswire.com/en/news-release/2019/07/09/1880105/0/en/Global-Functional-Proteins-Market-is-Expected-to-Reach-USD-7-98-Billion-by-2026-Fior-Markets.html.
- 217.Mirzapour-Kouhdasht A., Moosavi-Nasab M., Krishnaswamy K., Khalesi M. Optimization of gelatin production from Barred mackerel by-products: Characterization and hydrolysis using native and commercial proteases. Food Hydrocoll. 2020;108:105970–105984. doi: 10.1016/j.foodhyd.2020.105970. [DOI] [Google Scholar]
- 218.Yaghoubzadeh Z., Peyravii G.F., Kaboosi H., Safari R., Fattahi E. Antioxidant activity and anticancer effect of bioactive peptides from rainbow trout (Oncorhynchus mykiss) skin hydrolysate. Int. J. Pept. Res. Ther. 2019;26:625–632. doi: 10.1007/s10989-019-09869-5. [DOI] [Google Scholar]
- 219.Lu J., Hou H., Fan Y., Yang T., Li B. Identification of MMP-1 inhibitory peptides from cod skin gelatin hydrolysates and the inhibition mechanism by MAPK signaling pathway. J. Funct. Foods. 2017;33:251–260. doi: 10.1016/j.jff.2017.03.049. [DOI] [Google Scholar]
- 220.Ramesh C., Tulasi B.R., Raju M., Thakur N., Dufossé L. Marine natural products from tunicates and their associated microbes. Mar. Drugs. 2021;19:308. doi: 10.3390/md19060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hu X., Song L., Huang L., Zheng Q., Yu R. Antitumor Effect of a Polypeptide Fraction from Arca subcrenata in Vitro and in Vivo. Mar. Drugs. 2012;10:2782–2794. doi: 10.3390/md10122782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Beaulieu L., Thibodeau J., Bonnet C., Bryl P., Carbonneau M.-E. Evidence of Anti-Proliferative Activities in Blue Mussel (Mytilus edulis) By-Products. Mar. Drugs. 2013;11:975–990. doi: 10.3390/md11040975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wali F., Majid S., Rasool S., Shehada S.B., Abdulkareem S.K., Firdous A., Beigh S., Shakeel S., Mushtaq S., Akbar I., et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019;27:767–777. doi: 10.1016/j.jsps.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ruiz-Torres V., Encinar JAWali A., Herranz-Lopez M., Perez-Sanchez A., Galiano V., Barrajon-Catalan E., Micol V. An updated review on marine anticancer compounds: The use of virtual screening for the discovery of small-molecule cancer drugs. Molecules. 2017;22:1037–1074. doi: 10.3390/molecules22071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xu Q., Zheng L., Huang M., Zhao M. Exploring structural features of potent dipeptidyl peptidase IV (DPP-IV) inhibitory peptides derived from tilapia (Oreochromis niloticus) skin gelatin by an integrated approach of multivariate analysis and Gly-Probased peptide library. Food Chem. 2022;397:13382. doi: 10.1016/j.foodchem.2022.133821. [DOI] [PubMed] [Google Scholar]
- 226.Wang T.Y., Hsieh C.H., Hung C.C., Jao C.L., Chen M.C., Hsu K.C. Fish skin gelatin hydrolysates as dipeptidyl peptidase IV inhibitors and glucagon-like peptide1 stimulators improve glycaemic control in diabetic rats: A comparison between warm- and cold-water fish. J. Funct. 2015;19:330–340. doi: 10.1016/j.jff.2015.09.037. [DOI] [Google Scholar]
- 227.Wang S., Lv Z., Zhao W., Wang L., He N. Collagen peptide from Walleye pollock skin attenuated obesity and modulated gut microbiota in high-fat diet-fed mice. J. Funct. Foods. 2020;74:104194. doi: 10.1016/j.jff.2020.104194. [DOI] [Google Scholar]
- 228.Raksha N., Halenova T., Vovk T., Kostyuk O., Synelnyk T., Andriichuk T., Maievska T., Savchuk O., Ostapchenko L. Anti-obesity effect of collagen peptides obtained from Diplulmaris antarctica, a jellyfish of the Antarctic region. Croat. Med. J. 2023;64:21–28. doi: 10.3325/cmj.2023.64.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Luo X., Liu W., Zhao M., Liu T., Xiong E., Lei L., Jia F., Feng F. A novel Atlantic salmon (Salmo salar) bone collagen peptide delays osteoarthritis development by inhibiting cartilage matrix degradation and anti-inflammatory. Food Res. Int. 2022;162:112148. doi: 10.1016/j.foodres.2022.112148. [DOI] [PubMed] [Google Scholar]
- 230.Cui L., Li B. Enrichment of antiplatelet peptides and removal of fishy odor from silver carp skin collagen hydrolysates by macroporous resins: pH value of loading sample affects the peptides separation. Food Chem. 2023;411:135481. doi: 10.1016/j.foodchem.2023.135481. [DOI] [PubMed] [Google Scholar]
- 231.Liu H., Yang Y., Liu Y., Cui L., Fu L., Li B. Various bioactive peptides in collagen hydrolysate from Salmo salar skin and the combined inhibitory effects on atherosclerosis in vitro and in vivo. Food Res. Int. 2022;157:111281. doi: 10.1016/j.foodres.2022.111281. [DOI] [PubMed] [Google Scholar]
- 232.Abdelhedi O., Nasri R., Mora L., Toldra F., Nasri M., Jridi M. Collagenous proteins from black-barred halfbeak skin as a source of gelatin and bioactive peptides. Food Hydrocoll. 2017;70:123–133. doi: 10.1016/j.foodhyd.2017.03.030. [DOI] [Google Scholar]
- 233.Aissaoui N., Abidi F., Hardouin J., Abdelkafi Z., Marrakchi N., Jouenne T., Marzouki M.N. ACE Inhibitory and Antioxidant Activities of Novel Peptides from Scorpaena Notata By-Product Protein Hydrolysate. Int. J. Pept. Res. Ther. 2017;23:13–23. doi: 10.1007/s10989-016-9536-6. [DOI] [PubMed] [Google Scholar]
- 234.Thuanthong M., De Gobba C., Sirinupong N., Youravong W., Otte J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from Nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods. 2017;36:243–254. doi: 10.1016/j.jff.2017.07.011. [DOI] [Google Scholar]
- 235.Liu P., Lan X., Yaseen M., Wu S., Feng X., Zhou L., Sun J., Liao A., Liao D., Sun L. Purification, Characterization and Evaluation of Inhibitory Mechanism of ACE Inhibitory Peptides from Pearl Oyster (Pinctada fucata martensii) Meat Protein Hydrolysate. Mar. Drugs. 2019;17:463. doi: 10.3390/md17080463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhong C., Sun L.C., Yan L.J., Lin Y.C., Liu G.M., Cao M.J. Production, Optimisation and Characterisation of Angiotensin Converting Enzyme Inhibitory Peptides from Sea Cucumber (Stichopus japonicus) Gonad. Food Funct. 2018;9:594–603. doi: 10.1039/C7FO01388D. [DOI] [PubMed] [Google Scholar]
- 237.Zhang Q., Song C., Zhao J., Shi X., Sun M., Liu J., Zhu B. Separation and characterization of antioxidative and angiotensin converting enzyme inhibitory peptide from jellyfish gonad hydrolysate. Molecules. 2018;23:94. doi: 10.3390/molecules23010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Abuine R., Rathnayake A.U., Byun H.G. Biological activity of peptides purified from fish skin hydrolysates. Fish Aquatic Sci. 2019;22:1–14. doi: 10.1186/s41240-019-0125-4. [DOI] [Google Scholar]
- 239.Choi D.Y., Choi H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharmacal Res. 2015;38:139–170. doi: 10.1007/s12272-014-0503-5. [DOI] [PubMed] [Google Scholar]
- 240.Lee J.K., Li-Chan E.C.Y., Byun H.G. Characterization of β-secretase inhibitory peptide purified from skate skin protein hydrolysate. Eur. Food Res. Technol. 2015;240:129–136. doi: 10.1007/s00217-014-2314-9. [DOI] [Google Scholar]
- 241.Cai L., Wu X., Zhang Y., Li X., Ma S., Li J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods. 2015;16:234–242. doi: 10.1016/j.jff.2015.04.042. [DOI] [Google Scholar]
- 242.Xu L., Dong W., Zhao J., Xu Y. Effect of marine collagen peptides on physiological and neurobehavioral development of male rats with perinatal asphyxia. Mar. Drugs. 2015;13:3653–3671. doi: 10.3390/md13063653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pangestuti R., Ryu B., Himaya S. Optimization of hydrolysis conditions, isolation, and identification of neuroprotective peptides derived from seahorse Hippocampus trimaculatus. Amino Acids. 2013;45:369–381. doi: 10.1007/s00726-013-1510-4. [DOI] [PubMed] [Google Scholar]
- 244.Gao Q., Shang Y., Zhoui W., Deng S., Peng C. Marine collagen peptides: A novel biomaterial for the healing of oral mucosal ulcers. Dent. Mater. J. 2022;41:850–859. doi: 10.4012/dmj.2021-323. [DOI] [PubMed] [Google Scholar]
- 245.Xu N., Peng X.L., Li H.R., Liu J.X., Cheng J.S.Y., Qi X.Y., Ye S.J., Gong H.L., Zhao X.H., Yu J., et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021;8:702108. doi: 10.3389/fnut.2021.702108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhou T., Liu X., Sui B., Liu C., Mo X., Sun J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017;12:055004. doi: 10.1088/1748-605X/aa7b55. [DOI] [PubMed] [Google Scholar]
- 247.Tang J., Saito T. Biocompatibility of novel type I collagen purified from tilapia fish scale: An in vitro comparative study. BioMed Res. Int. 2015;2015:139476. doi: 10.1155/2015/139476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Liu C., Sun J. Hydrolyzed tilapia fish collagen induces osteogenic differentiation of human periodontal ligament cells. Biomed. Mater. 2015;10:065020. doi: 10.1088/1748-6041/10/6/065020. [DOI] [PubMed] [Google Scholar]
- 249.Chotphruethipong L., Sukketsiri W., Aluko R.E., Sae-Leaw T., Benjakul S. Effect of hydrolyzed collagen from defatted Asian sea bass (Lates calcarifer) skin on fibroblast proliferation, migration and antioxidant activities. J. Food Sci. Technol. 2021;58:541–551. doi: 10.1007/s13197-020-04566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sivarman K., Shanthi C. Role of fish collagen hydrolysate in attenuating inflammation—An in vitro study. J. Food Biochem. 2021;45:e13876. doi: 10.1111/jfbc.13876. [DOI] [PubMed] [Google Scholar]
- 251.Fu Y., Li C., Wang Q., Gao R., Cai X., Wang S., Zhang Y. The protective effect of collagen peptides from bigeye tuna (Thunnus obesus) skin and bone to attenuate UVB-induced photoaging via MAPK and TGF-β signaling pathways. J. Funct. Foods. 2022;93:105101. doi: 10.1016/j.jff.2022.105101. [DOI] [Google Scholar]
- 252.Xia E., Zhu X., Gao X., Ni J., Guo H. Antiaging Potential of Peptides from Underused Marine Bioresources. Mar. Drugs. 2021;19:513. doi: 10.3390/md19090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Maia Campos P.M.B.G., Franco R.S.B., Kakuda L., Cadioli G.F., Costa G.M.D., Bouvret E. Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Morphological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study. Molecules. 2021;26:4880. doi: 10.3390/molecules26164880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wang K., Siddanakoppalu P.N., Ahmed I., Pavase T.R., Lin H., Li Z. Purification and Identification of Anti-Allergic Peptide from Atlantic Salmon (Salmo salar) Byproduct Enzymatic Hydrolysates. J. Funct. Foods. 2020;72:104084. doi: 10.1016/j.jff.2020.104084. [DOI] [Google Scholar]
- 255.Salindeho N., Mokolensang J.F., Manu L., Taslim N.A., Nurkolis F., Gunawan W.B., Yusuf M., Mayulu N., Tsopmo A. Fish scale rich in functional compounds and peptides: A potential nutraceutical to overcome undernutrition. Front. Nutr. 2022;9:1072370. doi: 10.3389/fnut.2022.1072370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wu W., Li B., Hou H., Zhang H., Zhao X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J. Funct. Foods. 2017;35:418–427. doi: 10.1016/j.jff.2017.06.013. [DOI] [PubMed] [Google Scholar]